Abstract
Migration to lymph nodes and secretion of cytokines are critical functions of mature dendritic cells (DCs); however, these 2 functions are not necessarily linked. This is the first report showing that quantitative differences in identical signaling pathways determine DC migration and cytokine secretion. Using different polymerized forms of CD40 ligand, we demonstrate that the strength and persistence of CD40 signaling can induce either function. Induction of monocyte-derived DC (MoDC) migration required a weak and transient CD40 signal, whereas strong and persistent CD40 signaling blocked migration and biased toward cytokine secretion. In contrast to MoDCs, CD40 activation of CD1c+ peripheral blood DCs (PBDCs) induced a nonpersistent, intracellular signaling profile resulting in migratory-type DCs unable to secrete interleukin-12p70 (IL-12p70). Extracellular signal-regulated kinase 1/2 (ERK1/2) and p38K activation synergistically mediated cytokine secretion, whereas migration was enhanced by p38K activation but reduced by persistent ERK1/2 activity. This model of signal strength and persistence also applied when stimulating DCs with intact microbes. Thus, a novel concept emerges in which the type of immune response induced by DCs is tuned by the strength and persistence of DC activating signals.
Introduction
Dendritic cells (DCs) respond to specific environmental signals in distinct and coordinated ways that reveal a remarkable capacity to process and transduce information. A wide variety of distinct classes of activation stimuli induce the maturation of immature monocyte-derived DCs (MoDCs). In this regard, different classes of activation stimuli have been shown to dramatically alter the genetic profile of MoDCs,1 resulting in complex changes in the expression profiles of a variety of proteins and the functional behavior of the DCs. However, within this apparent functional plasticity, critical DC functions such as uptake and transport of antigen into lymph nodes, interaction with T cells, and secretion of cytokines must somehow be maintained to ensure effective immune responses are induced.
We and others recently reported that critical DC functions, such as migration of MoDCs toward CCR7 ligands, which guide DCs into lymph nodes,2-4 and secretion of interleukin-12p70 (IL-12p70), are not necessarily linked.5 Rather, migration was induced when MoDCs were activated in the presence of prostaglandin E2 (PGE2), an effect mediated by adenosine 3′,5′ cyclic monophosphate (cAMP) and protein kinase A (PKA) pathways.5,6 These migratory-type MoDCs secreted little or no IL-12p70. In contrast, CD40 ligand trimers (CD40L3) (which cross-link multiple CD40 molecules on the DC surface) induced MoDCs to secrete high levels of IL-12p70, particularly when combined with specific soluble cofactors, such as interferon-γ (IFN-γ),7,8 IL-4,9 or IL-1β.7,10 Interestingly, although expressing similar levels of CCR7 as migratory-type DCs, CD40L3-stimulated MoDCs were poor migratory cells. Thus, it appeared that the capacity for migration and cytokine secretion characterized 2 distinct functional DC phenotypes: migratory-type and proinflammatory-type DCs.5 Complementing our in vitro data, Kabashima et al recently provided in vivo evidence for a regulatory function of PGE2 on the development of proinflammatory and migratory DCs.11,12 Mice deficient in the PGE2 receptor EP4 developed more severe colitis upon challenge with dextran sodium sulfate and showed reduced migration of Langerhans cells into the draining lymph nodes.
However, blood circulating CD1c+ peripheral blood DCs (PBDCs) differed from MoDCs in this respect. CD1c+ PBDCs spontaneously acquired a migratory phenotype in culture and secreted negligible levels of IL-12p70 following activation with various classes of stimuli.13 This led us to speculate that the in vivo stimulation history of CD1c+ PBDCs may restrict them to express a migratory-type functional profile upon activation.
The decision as to whether maturing DCs in tissues migrate to draining lymph nodes to induce T-cell responses or remain at the site of inflammation to condition the microenvironment via secretion of cytokines may represent a critical crossroad for the induction of effective immune reactions. Because DCs respond to a variety of stimuli, common pathways may exist, which are utilized by different surface receptors and which ultimately result in similar functional outcomes. However, these functional pathways have yet to be defined.
The present study analyzed signal transduction pathways and their functional consequences in MoDCs and CD1c+ PBDCs in response to activation with either CD40L3 or the weaker signaling CD40L monomer (CD40L1). This was also compared with DC stimulation with a completely different class of stimulus, such as microbial contact. We found that the strength and persistence of signaling induced by these differing types of activation stimuli, as measured by the level of extracellular signal-regulated kinase 1/2 (ERK1/2) and p38K phosphorylation and nuclear factor–κB (NF-κB) binding activity, were more predictive determinants of the functional profile that MoDCs expressed than the type of stimulus itself. Weak and transient signaling induced a migratory-type functional profile, whereas strong and sustained signaling was required for IL-12p70 production. Given that these principles applied to unrelated classes of stimuli such as CD40L or contact with intact Escherichia coli, they may therefore reflect a more general rule for the regulation of DC migration and cytokine secretion. In addition, our model provides an explanation for the predominant expression of a migratory-type functional profile by CD1c+ PBDCs,13 given that for this DC population CD40 ligation only induced a transient signaling profile. This is the first report showing that quantitative differences in identical signaling pathways are determinants of DC function and might account for functional differences between different DC types.
Materials and methods
Media
DCs were cultured in RPMI 1640 (Sigma, Taufkirchen, Germany) supplemented with 60 mg/L penicillin G, 12.6 mg/L streptomycin, 2 mM l-glutamine, 1% nonessential amino acids, and 10% heat-inactivated fetal calf serum (FCS) (Sigma) in a 5% CO2 incubator.
Monoclonal antibodies, enzyme-linked immunosorbent assay (ELISA) kits, cytokines, and chemokines
Flow cytometric analysis of DCs was performed using the following monoclonal antibodies (mAbs): fluorescein isothiocyanate (FITC)–conjugated immunoglobulin G1 (IgG1) isotype control, phycoerythrin (PE)–conjugated IgG1 isotype control, anti-CD86/B70/B7-2–FITC (PharMingen/Becton Dickinson, San Diego, CA), and anti-CD83–PE (PharMingen/Becton Dickinson). Cytokine enzyme-linked immunosorbent assay (ELISA) kits (Opteia) for IL-6 and IL-12p70 were purchased from PharMingen/Becton Dickinson. The following cytokines were added to DC cultures: recombinant human granulocyte-macrophage colony-stimulating factor (rhGM-CSF) (40 ng/mL; Immunex, Seattle, WA), recombinant human tumor necrosis factor-α (rhTNF-α) (10 ng/mL), rhIL-4 (500 U/mL), interferon-α2a (IFN-α2a) (2000 IU/mL), and IFN-γ (1000 IU/mL) (all from PromoCell, Heidelberg, Germany). CD40L trimer (1 μg/mL final concentration) was a gift from Immunex, an Amgen company. CD40L monomers and the enhancing anti-FLAG M1 mAb were purchased from Alexis (Grünstadt, Germany). PGE2 (1 μM final concentration) was purchased from ICN Biomedicals (Aurora, OH). CCL21 (6Ckine) was purchased from PromoCell and used as the chemokine in migration assays (40 ng/mL). The mitogen-activated protein kinase kinase (MEK) inhibitor PD98059 and the p38K inhibitor as well as the NF-κB inhibitors MG-132 and Bay11-7985 were purchased from Calbiochem (Merck Biosciences, Darmstadt, Germany).
MoDCs
Peripheral blood mononuclear cells (PBMCs) were obtained from buffy coat preparations from healthy donors from the Red Cross Blood Bank (Heidelberg, Germany) and used to produce MoDCs. CD14+ monocytes (5 × 105) were affinity purified using the magnetic-activated cell separation (MACS) CD14 isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany) and cultured in 1 mL RPMI, 10% FCS, GM-CSF (40 ng/mL), and IL-4 (500 U/mL) in 24-well plates. By day 7, MoDCs represented more than 90% of cultured cells. On day 7, all wells were washed and readjusted to a concentration of 1 × 105 DCs per milliliter. Maturation-inducing factors were added on day 7, and cells and supernatants were harvested on day 9 for functional assessment. All cytokines and stimuli examined for their ability to stimulate DC functional maturation in the present study have previously been tested in dose titration analyses, and the concentrations used in the figures represent those found to be optimal.5
Isolation of CD1c+ PBDCs
Peripheral blood mononuclear cells were isolated from buffy coats of healthy volunteers provided by the Red Cross Blood Bank (Melbourne, Australia) by Ficoll-Paque density gradient centrifugation (Amersham Biosciences, Uppsala, Sweden). CD1c+ PBDCs were sorted by positive selection using the BDCA-1 (CD1c) isolation kit following the manufacturer's instructions (Miltenyi Biotec, Auburn, CA). Purity of CD1c+ CD11c+ HLA-DR–positive cells after 2 rounds of positive selection was more than 97%. CD1c+ PBDCs (5 × 105 per protocol) were stimulated with CD40L trimers (1 μg/mL) and IFN-γ for 30 minutes or 24 hours following a 24-hour culture period in the presence of GM-CSF/IL-4. Subsequently, PBDCs were harvested, washed, resuspended at concentrations of 5 × 106/mL in Western sample buffer, and snap frozen as described above.
Cytokine ELISAs
Cytokine secretion by stimulated MoDCs was measured by cytokine ELISAs. IL-6 and IL-12p70 ELISAs were performed on supernatants of MoDC cultures according to the manufacturer's instructions using Maxisorp plates (Nunc, Wiesbaden, Germany). The horseradish peroxidase (HRP) substrate was tetramethylbenzidine (TMB) peroxidase (BD PharMingen, Heidelberg, Germany); the color reaction was terminated by adding 100 μL orthophosphoric acid (1 M). Plates were read in a Sunrise microplate reader (Tecan, Salzburg, Austria).
Migration assays
MoDCs matured with the indicated stimuli for 36 to 48 hours were harvested from their wells, washed, and tested for migration toward CCL21 chemokine using the transwell assay. Briefly, lower chambers of transwell plates (3.0 μm pore size; Costar, Corning, NY) were filled with 350 μL RPMI/10% FCS with or without CCL21 (6Ckine; 40 ng/mL). A total of 1 × 104 to 2 × 104 DCs were added in 50 μL RPMI/10%FCS into the upper chamber, and cells were incubated at 37° C for 3 to 4 hours. Cells in the lower chambers were harvested, concentrated to 50 μL volumes in Eppendorf tubes, and counted with a hemocytometer. Migration for all stimulation conditions was performed in duplicate wells.
Western blot analysis
MoDCs activated for 30 minutes to 48 hours with the indicated protocols were harvested, washed, resuspended at concentrations of 5 × 106/mL in Western sample buffer (100 mM Tris [tris(hydroxymethyl)aminomethane]–HCl [pH 6.8], 4% sodium dodecyl sulfate [SDS], 0.2% bromophenol blue, 20% glycerol, 200 mM dithiothreitol [DTT]), and snap frozen in –80° C. Prior to use, lysates were thawed, heated for 3 minutes to 96° C, homogenized with a sonicator, and 10 mL extract per lane separated onto 10% SDS–polyacrylamide gel electrophoresis (SDS-PAGE) followed by electroblotting. Blocking was performed in phosphate-buffered saline (PBS) plus 5% nonfat milk powder for 2 hours. Membranes were incubated with the following primary antibodies in blocking buffer plus 0.1% Tween 20 overnight at 4° C: antiphospho-ERK1/2 (Thr202/Tyr204, 1:2000; Cell Signaling Technology, New England Biolabs, Frankfurt am Main, Germany), antiphospho-p38K (1:2000; Cell Signaling Technology), anti-ERK1/2 (1:2000; Santa Cruz Biotechnology, Heidelberg, Germany), and anti-p38K (1:1000; Santa Cruz Biotechnology). After washing, secondary antibodies were applied in blocking buffer for 2 hours at room temperature: antirabbit HRP for antiphospho-mAb (1:3000; Cell Signaling Technology) and goat-antirabbit HRP for nonphosphorylated mAb (1:2000; Santa Cruz Biotechnology). Membranes were washed followed by detection of immunoreactive proteins using the enhanced chemiluminescence (ECL) Western blot system (Santa Cruz Biotechnology); exposure times ranged from 30 seconds to 15 minutes.
NF-κB EMSA and NF-κB supershifts
Electrophoretic mobility shift assay (EMSA) was performed as previously described14 using 10 μg cellular extract. Binding of NF-κB to 1 ng radiolabeled NF-κB consensus oligonucleotides (5′-AGT TGA GGG GAC TTT CCC AGG C-3′; 50 000 cpm) was induced for 20 minutes at room temperature in 10 mmol/L HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) (pH 7.5), 0.5 mmol/L EDTA (ethylenediaminetetraacetic acid), 100 mmol/L KCl, 2 mmol/L DTT, 2% glycerol, 4% Ficoll, 0.25% Nonidet P-40 (NP-40), 1 mg/mL bovine serum albumin (BSA), and 0.1 μg/μL poly(dIdC). Protein DNA complexes were separated from the unbound DNA probe by electrophoresis through 5% native polyacrylamide gels containing 3.25% glycerol and 0.5 × TBE (Tris-borate-EDTA). Specificity of binding was ascertained by competition with a 160-fold molar excess of unlabeled NF-κB consensus oligonucleotides. Characterization of the NF-κB subunits contributing to the observed shift was achieved by applying 2.5 μg anti-p65, anti-p50, anti-cRel, and anti-RelB antibodies (Santa Cruz Biotechnology) into the binding reactions with nuclear extracts of isolated mononuclear cells.
Results
Different polymerized CD40L preparations differentially induce MoDC migration and cytokine secretion
Maturation signals determine the expression of distinct MoDC functions, such as migration to lymph node–directing chemokines or secretion of cytokines (eg, IL-12p70 and IL-6). These 2 distinct functions may be controlled either by the coordinated action of several surface receptors or by specific differences in the signal transduction pathway employed by the same surface receptor.
CD40L is a membrane-expressed TNF family member that optimally functions by cross-linking and signaling through multiple CD40 molecules on the recipient cell.15 Signaling through CD40 can be induced by polymerized forms of soluble, recombinant CD40L. Recombinant CD40L is generated as a soluble trimer (with a c-terminal leucine zipper, CD40L3) or as a soluble monomer that is subsequently polymerized with an anti-FLAG mAb (enhancer) against a c-terminal FLAG domain (CD40L1). Both CD40L preparations are equivalent at inducing the phenotypic maturation of MoDCs as assessed by the up-regulation of surface CD83 and CD86 expression (n = 6, data not shown).
CD40L3 is also a potent inducer of cytokine production (particularly in the presence of cofactors such as IFN-γ, IL-4, or IL-1β) but does not induce migration toward CCL21, despite the up-regulation of the corresponding chemokine receptor CCR7.5 In contrast, CD40L1 stimulation induced migration of MoDCs to CCL21 (Figure 1A). Interestingly, MoDCs stimulated with CD40L3 expressed equivalent CCR7 levels (as assessed by fluorescence-activated cell sorting [FACS]) to those of the more migratory-type MoDCs simulated with CD40L1 (n = 3, data not shown), arguing against the possibility that the differences in migratory function related to differing levels of CCR7 receptor expression. These surprisingly differing functional effects of CD40L3 and CD40L1 were seen irrespective of the presence or absence of IFN-γ (n = 5, data not shown). Maximal migration was induced in response to PGE2-containing stimuli,5,16,17 which induced very little cytokine production (Figure 1A).
Migration and cytokine secretion by mature MoDCs differ in response to differentially polymerized CD40L preparations. MoDCs were generated from CD14+ monocytes within 5 to 7 days of culture in the presence of GM-CSF and IL-4. Immature MoDCs (1 × 105 to 3 × 105/mL) were washed and resuspended in culture medium. The following cytokines were added as indicated for an additional 36 to 48 hours: GM-CSF (20 ng/mL), TNF-α (10 ng/mL), IFN-α (2000 IU/mL), PGE2 (1 μM), CD40L1 ([CD40L monomers] 1 μg/mL; enhancer, 1 μg/mL), CD40L3 ([CD40L trimers] 1 μg/mL), and IFN-γ (1000 U/mL). (A) Migration assays were performed in duplicate using 3 μm pore size transwell plates. Migration to medium in the absence or presence of CCL21 (6Ckine, 40 ng/mL) in the lower well is shown as mean values ± SEM of 14 experiments. Supernatants were harvested and (B) IL-12p70 and (C) IL-6 production measured by ELISA (mean values ± SEM, n = 11). *P < .05; **P < .01 as compared with activation with CD40L3 plus IFN-γ.
Migration and cytokine secretion by mature MoDCs differ in response to differentially polymerized CD40L preparations. MoDCs were generated from CD14+ monocytes within 5 to 7 days of culture in the presence of GM-CSF and IL-4. Immature MoDCs (1 × 105 to 3 × 105/mL) were washed and resuspended in culture medium. The following cytokines were added as indicated for an additional 36 to 48 hours: GM-CSF (20 ng/mL), TNF-α (10 ng/mL), IFN-α (2000 IU/mL), PGE2 (1 μM), CD40L1 ([CD40L monomers] 1 μg/mL; enhancer, 1 μg/mL), CD40L3 ([CD40L trimers] 1 μg/mL), and IFN-γ (1000 U/mL). (A) Migration assays were performed in duplicate using 3 μm pore size transwell plates. Migration to medium in the absence or presence of CCL21 (6Ckine, 40 ng/mL) in the lower well is shown as mean values ± SEM of 14 experiments. Supernatants were harvested and (B) IL-12p70 and (C) IL-6 production measured by ELISA (mean values ± SEM, n = 11). *P < .05; **P < .01 as compared with activation with CD40L3 plus IFN-γ.
Cytokine secretion also differed in response to the 2 CD40L preparations. Significantly higher IL-12p70 levels were induced in MoDCs stimulated with CD40L3 plus IFN-γ (Figure 1B), while CD40L1 plus IFN-γ induced larger amounts of IL-6 (Figure 1C). Thus, the 2 differentially polymerized forms of CD40L, which target the same surface receptor, induced different functional profiles in MoDCs.
CD40L3 and CD40L1 induce distinct patterns of ERK1/2, p38K, and NF-κB activation in MoDCs
We next examined whether the functional differences induced by CD40L3 and CD40L1 were due to targeting of different signaling pathways or whether they reflected differences in the signal strength or kinetics of the same pathways. Three independent signaling pathways activated during cell stress or immune response induction were examined: ERK1/2, p38K, and NF-κB.18-20
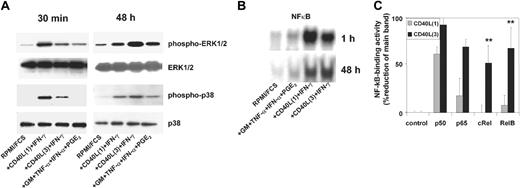
Interestingly, the activity and kinetics of all 3 signaling pathways differed substantially between CD40L3 and CD40L1 (Figure 2). CD40L1 induced the rapid phosphorylation of ERK1/2 and p38K as early as 30 to 60 minutes after stimulation. In contrast, CD40L3 induced slower kinetics of mitogen-activated protein kinase (MAPK) phosphorylation (ERK1/2 and p38K) but reached higher levels by 48 hours than those achieved by CD40L1 (Figure 2A). The NF-κB binding activity following stimulation with CD40L3 or CD40L1 paralleled that of the MAPK pathways (Figure 2B). Interestingly, CD40L3 stimulated all 4 NF-κB family members (p50, p65, RelB, and cRel) in MoDCs by 48 hours. In contrast, CD40L1 only stimulated p50 and p65 activity in MoDCs with little or no activation of RelB or cRel at this time point. These results suggest that CD40L1 induces an early and strong but transient activation signal, whereas CD40L3 induces an initially weaker but persistent activation signal.
Kinetics and strength of activation of ERK1/2, p38 MAPK, and NF-κB in MoDCs in response to different CD40L preparations. MoDCs were stimulated as described in the legend to Figure 1. Cells were harvested at the indicated time points following stimulation, gently washed, and resuspended in lysis buffer. (A) Phosphorylation of ERK1/2 and p38 MAPK 30 minutes and 48 hours after activation (representative of at least 4 experiments). (B) NF-κB binding activity in cellular protein extracts of migratory-type and proinflammatory-type MoDCs. Electrophoretic mobility shift assay (EMSA) was performed 1 hour (n = 1) and 48 hours (n = 3) after stimulation. Supershift: comparison of binding activity of NF-κB family members in CD40L1- and CD40L3-activated MoDCs after 48 hours. (C) NF-κB binding activity as percentage reduction of optical density in the main band in the presence of the specified antibody relative to the control band without antibody. Results are shown as mean values ± SEM of 3 experiments; *P < .05 comparing CD40L1 and CD40L3.
Kinetics and strength of activation of ERK1/2, p38 MAPK, and NF-κB in MoDCs in response to different CD40L preparations. MoDCs were stimulated as described in the legend to Figure 1. Cells were harvested at the indicated time points following stimulation, gently washed, and resuspended in lysis buffer. (A) Phosphorylation of ERK1/2 and p38 MAPK 30 minutes and 48 hours after activation (representative of at least 4 experiments). (B) NF-κB binding activity in cellular protein extracts of migratory-type and proinflammatory-type MoDCs. Electrophoretic mobility shift assay (EMSA) was performed 1 hour (n = 1) and 48 hours (n = 3) after stimulation. Supershift: comparison of binding activity of NF-κB family members in CD40L1- and CD40L3-activated MoDCs after 48 hours. (C) NF-κB binding activity as percentage reduction of optical density in the main band in the presence of the specified antibody relative to the control band without antibody. Results are shown as mean values ± SEM of 3 experiments; *P < .05 comparing CD40L1 and CD40L3.
DC migration is inhibited by the persistence of activation stimuli
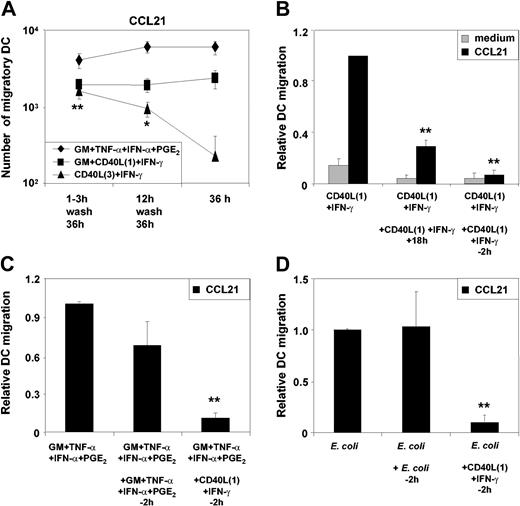
Because our results revealed distinct differences in the signaling profile for MAPK and NF-κB in MoDCs in response to the 2 CD40L preparations, we investigated whether the strength and persistence of the CD40L stimulus was responsible for regulating the functional profile of MoDCs. To assess the influence of signal persistence, all stimuli were washed out of DC cultures after 3 hours or 12 hours of stimulation. MoDCs were then cultured in medium alone for a total of 36 to 48 hours. As shown in Figure 3A, transient stimulation of MoDCs with CD40L3 induced efficient migration to CCL21, whereas prolonged stimulation reduced migratory function over time. In contrast, migration of MoDCs was induced by CD40L1 with either transient or prolonged exposure. Similarly, migration induced by the cytokine cocktail consisting of GM-CSF, TNF-α, IFN-α, and PGE2 also required only a transient exposure to the activation factors. These findings suggest that migration is induced in response to weak and transient stimuli and is blocked if CD40 signaling persists. This is further supported by our observation that repeated addition of CD40L1 to MoDCs cultures to prolong CD40 signaling reduced the number of migratory DCs. In particular, addition of CD40L1 only 2 hours prior to the migration assay completely blocked MoDC migration (Figure 3B). The inhibition of migration by CD40 signaling was also observed for MoDCs stimulated with either GM-CSF plus TNF-α plus IFN-α plus PGE2 (Figure 3C) or E coli (Figure 3D). However, restimulating MoDCs with the same initiating stimulus (ie, GM-CSF plus TNF-α plus IFN-α plus PGE2 or E coli) 2 hours prior to the migration assay had no influence on the capacity of MoDCs to migrate to CCL21 (Figure 3C-D).
Persistence of different classes of stimuli modulates MoDCmigration. MoDCs were activated with the indicated stimuli. Cells were washed twice 3 hours or 12 hours after stimulation, and cell cultures were continued in RPMI plus 10% FCS without cytokines for a further 36 to 48 hours. MoDCs containing the various stimuli throughout the 36-hour culture period were cultured in parallel as controls. (A) Migration toward CCL21 of transiently or continuously stimulated MoDCs (mean ± SEM of 9 experiments, *P < .05; **P < .01 comparing CD40L3 plus IFN-γ stimulation over 3 hours and 12 hours with 36-hour stimulation). (B) CD40L1 plus IFN-γ was added either once or repeatedly at 0, 6, and 18 hours (+18h) or at 0 hours and for a second time 2 hours prior to the migration assay (–2h) (mean ± SEM of 4 experiments, **P < .05 as compared with single stimulation). (C) GM-CSF plus TNF-α plus IFN-α plus PGE2 was added either once or for a second time 2 hours prior to the migration assay (–2h). Alternatively, CD40L1 plus IFN-γ was added 2 hours prior to the migration assay into cultures initially stimulated with GM plus TNF-α plus IFN-α plus PGE2 (mean ± SEM of 4 experiments, **P < .01 as compared with single stimulation). (D) Intact E coli was prepared as indicated in “Materials and methods,” and 20 μL of the E coli suspension was added to 1 mL of culture. Intact E coli was added either once or for a second time 2 hours prior to the migration assay (–2h). Alternatively, CD40L1 plus IFN-γ was added 2 hours prior to the migration assay into cultures initially stimulated with intact E coli (mean ± SEM of 4 experiments, **P < .01 as compared with single stimulation).
Persistence of different classes of stimuli modulates MoDCmigration. MoDCs were activated with the indicated stimuli. Cells were washed twice 3 hours or 12 hours after stimulation, and cell cultures were continued in RPMI plus 10% FCS without cytokines for a further 36 to 48 hours. MoDCs containing the various stimuli throughout the 36-hour culture period were cultured in parallel as controls. (A) Migration toward CCL21 of transiently or continuously stimulated MoDCs (mean ± SEM of 9 experiments, *P < .05; **P < .01 comparing CD40L3 plus IFN-γ stimulation over 3 hours and 12 hours with 36-hour stimulation). (B) CD40L1 plus IFN-γ was added either once or repeatedly at 0, 6, and 18 hours (+18h) or at 0 hours and for a second time 2 hours prior to the migration assay (–2h) (mean ± SEM of 4 experiments, **P < .05 as compared with single stimulation). (C) GM-CSF plus TNF-α plus IFN-α plus PGE2 was added either once or for a second time 2 hours prior to the migration assay (–2h). Alternatively, CD40L1 plus IFN-γ was added 2 hours prior to the migration assay into cultures initially stimulated with GM plus TNF-α plus IFN-α plus PGE2 (mean ± SEM of 4 experiments, **P < .01 as compared with single stimulation). (D) Intact E coli was prepared as indicated in “Materials and methods,” and 20 μL of the E coli suspension was added to 1 mL of culture. Intact E coli was added either once or for a second time 2 hours prior to the migration assay (–2h). Alternatively, CD40L1 plus IFN-γ was added 2 hours prior to the migration assay into cultures initially stimulated with intact E coli (mean ± SEM of 4 experiments, **P < .01 as compared with single stimulation).
DC cytokine secretion is modulated by the persistence and strength of activation factors
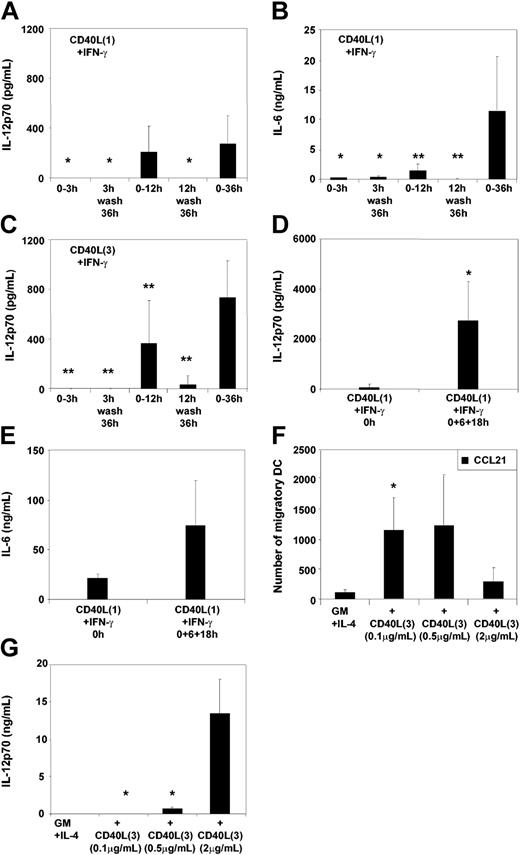
The impact of prolonged stimulation upon the induction of cytokine secretion was next assessed. IL-12p70 and IL-6 production by MoDCs was measured following 3 hours, 12 hours, or 36 to 48 hours of continuous stimulation. Furthermore, MoDCs were washed after 3 and 12 hours of stimulation and then cultured in fresh medium for a total of 36 to 48 hours (Figure 4A-C). Interestingly, withdrawal of CD40L3 or CD40L1 after 12 hours of stimulation resulted in cessation of either IL-12p70 or IL-6 secretion by MoDCs (Figure 4A-C). On the other hand, repeated addition of CD40L1 resulted in increased secretion of IL-12p70 and IL-6 by MoDCs (Figure 4D-E). These findings suggest that secretion of cytokines requires persistent CD40 signaling and can rapidly be shut down in the absence of stimulation. To further test the hypothesis that signal strength determines the functional profile of MoDCs, cytokine production and migratory capacity of MoDCs in response to graded doses of CD40L were analyzed. As expected from the previous findings, reduction of the CD40L3 concentration resulted in an increased migratory capacity and reduced secretion of IL-12p70 (Figure 4F-G). To mimic persistent expression of CD40L on cellular surfaces (such as T cells and other cell types), we examined the effect of a CD40L trimer–transfected baby hamster kidney (BHK) cell line upon stimulation of immature MoDCs. In contrast to the nontransfected wild-type cell line, which did not activate MoDCs, the CD40L-transfected cell line induced high levels of IL-12p70 and IL-6 secretion but did not induce migration, analogous to the effects seen with CD40L3 but not with CD40L1 (n = 3, data not shown).
Persistence and strength of stimuli modulate MoDC cytokine secretion. MoDCs were stimulated as in the legend to Figure 3. DC cultures were washed twice 3 hours and 12 hours after addition of the indicated stimuli, supernatants were analyzed for IL-12p70 and IL-6, and cultures continued in RPMI plus 10% FCS without cytokines for a further 36 to 48 hours. Cultures containing the various stimuli throughout the 36-hour culture period were run in parallel as controls. Secretion of (A) IL-12p70 and (B) IL-6 by MoDCs stimulated either transiently or continuously with CD40L1 plus IFN-γ or (C) IL-12p70 secretion following transient or continuous stimulation with CD40L3 plus IFN-γ (mean ± SEM of 6 to 9 experiments, *P < .05; **P < .01 as compared with 36-hour to 48-hour stimulation). (D) IL-12p70 or (E) IL-6 secretion of MoDCs stimulated once or repeatedly (0, 6, 18 hours) with CD40L1 plus IFN-γ (mean ± SD of 4 experiments, *P < .05 as compared with single stimulation). Effect of titrating CD40L3 in the presence of 1000 U/mL IFN-γ on (F) migration toward CCL21 and (G) IL-12p70 secretion (mean ± SEM of 9 experiments, *P < .05 as compared with 2 μg/mL CD40L3).
Persistence and strength of stimuli modulate MoDC cytokine secretion. MoDCs were stimulated as in the legend to Figure 3. DC cultures were washed twice 3 hours and 12 hours after addition of the indicated stimuli, supernatants were analyzed for IL-12p70 and IL-6, and cultures continued in RPMI plus 10% FCS without cytokines for a further 36 to 48 hours. Cultures containing the various stimuli throughout the 36-hour culture period were run in parallel as controls. Secretion of (A) IL-12p70 and (B) IL-6 by MoDCs stimulated either transiently or continuously with CD40L1 plus IFN-γ or (C) IL-12p70 secretion following transient or continuous stimulation with CD40L3 plus IFN-γ (mean ± SEM of 6 to 9 experiments, *P < .05; **P < .01 as compared with 36-hour to 48-hour stimulation). (D) IL-12p70 or (E) IL-6 secretion of MoDCs stimulated once or repeatedly (0, 6, 18 hours) with CD40L1 plus IFN-γ (mean ± SD of 4 experiments, *P < .05 as compared with single stimulation). Effect of titrating CD40L3 in the presence of 1000 U/mL IFN-γ on (F) migration toward CCL21 and (G) IL-12p70 secretion (mean ± SEM of 9 experiments, *P < .05 as compared with 2 μg/mL CD40L3).
Signal strength and persistence induced by intact bacteria determine MoDC function
To assess if the same rules of signal strength and persistence apply for other classes of activation stimuli, we investigated the effect of graded doses of E coli on the functional properties of MoDCs. Adding E coli over a broad concentration range demonstrated that with low concentrations MoDCs firstly down-regulated their capacity to secrete IL-12p70, which was followed by a decrease of IL-6 production. In contrast, the migratory capacity was not negatively affected (Figure 5A,C,E). Similarly, removing the stimulus by washing MoDCs 3 hours after exposure to E coli also significantly reduced cytokine production but not migration (Figure 5B,D,F). Indeed, migratory-type MoDCs (ie, low producers of IL-12p70 and IL-6) similar to those generated with PGE2-containing cytokine combinations were generated when MoDCs were transiently stimulated with low concentrations of E coli.
Strength and persistence of stimulation with intactE colimodulateMoDC migration and cytokine secretion. MoDCs were stimulated with intact E.coli as indicated. One half of the cultures were washed twice 3 hours after addition of Ecoli and then cultured in RPMI/FCS until 48 hours (mean ± SEM of 8 experiments are shown [n = 4 for E coli 1:1000]). (A) Migration toward CCL21 and (C) IL-12p70 secretion or (E) IL-6 secretion of MoDCs activated with varying concentrations of intact E coli. (*P < .05; **P < .01 as compared with E coli 1:1). Effect of washing MoDCs following 3 hours of activation with varying concentrations of live E coli on (B) migration toward CCL21 and (D) IL-12p70 secretion or (F) IL-6 secretion. Cultures were continued in RPMI/FCS for a total of 48 hours. Mean ± SEM of 8 experiments are shown (n = 4 for E coli 1:1000), *P < .05; **P < .01 compared with unwashed MoDCs (normalized to 1).
Strength and persistence of stimulation with intactE colimodulateMoDC migration and cytokine secretion. MoDCs were stimulated with intact E.coli as indicated. One half of the cultures were washed twice 3 hours after addition of Ecoli and then cultured in RPMI/FCS until 48 hours (mean ± SEM of 8 experiments are shown [n = 4 for E coli 1:1000]). (A) Migration toward CCL21 and (C) IL-12p70 secretion or (E) IL-6 secretion of MoDCs activated with varying concentrations of intact E coli. (*P < .05; **P < .01 as compared with E coli 1:1). Effect of washing MoDCs following 3 hours of activation with varying concentrations of live E coli on (B) migration toward CCL21 and (D) IL-12p70 secretion or (F) IL-6 secretion. Cultures were continued in RPMI/FCS for a total of 48 hours. Mean ± SEM of 8 experiments are shown (n = 4 for E coli 1:1000), *P < .05; **P < .01 compared with unwashed MoDCs (normalized to 1).
Migratory and proinflammatory MoDCs represent irreversibly committed mature DC populations
To assess if the commitment of MoDCs to a migratory or proinflammatory phenotype is reversible or reflects a terminal differentiation step, MoDCs were washed 24 hours after stimulation. MoDCs received a second activation stimulus either immediately afterward or following a 24-hour rest period. One of the 2 restimulation strategies was applied: Either the cytokines GM-CSF, TNF-α, IFN-α, and PGE2 were added and the migratory capacity assessed after 48 hours, or MoDCs were stimulated with E coli, and IL-6 and IL-12p70 secretion was measured (Table 1).
Previously activated MoDCs represent irreversibly committed mature DC populations
Initial activation protocol . | IL-6, ng/mL . | IL-12p70, pg/mL . |
|---|---|---|
| Plus E coli 24 h after activation* | ||
| No cytokines | 104 ± 32 | 3046 ± 289 |
| GM+T+N+P | 0 | 34 ± 34 |
| CD40L3+IFN-γ | 14 ± 10 | 901 ± 150 |
| CD40L1+IFN-γ | 3.8 ± 2.5 | 453 ± 155 |
| Plus E coli following 24 h of activation plus 24 h of rest† | ||
| No cytokines | 200 ± 0 | 497 ± 138 |
| GM+T+N+P | 4.4 ± 3 | 0 |
| CD40L3+IFN-γ | 73 ± 42 | 0 |
| CD40L1+IFN-γ | 63 ± 46 | 0 |
Initial activation protocol . | IL-6, ng/mL . | IL-12p70, pg/mL . |
|---|---|---|
| Plus E coli 24 h after activation* | ||
| No cytokines | 104 ± 32 | 3046 ± 289 |
| GM+T+N+P | 0 | 34 ± 34 |
| CD40L3+IFN-γ | 14 ± 10 | 901 ± 150 |
| CD40L1+IFN-γ | 3.8 ± 2.5 | 453 ± 155 |
| Plus E coli following 24 h of activation plus 24 h of rest† | ||
| No cytokines | 200 ± 0 | 497 ± 138 |
| GM+T+N+P | 4.4 ± 3 | 0 |
| CD40L3+IFN-γ | 73 ± 42 | 0 |
| CD40L1+IFN-γ | 63 ± 46 | 0 |
Cytokine secretion by MoDCs initially activated with GM-CSF plus TNF-α plus IFN-α plus PGE2 (GM+T+N+P), CD40L1 plus IFN-γ, or CD40L3 plus IFN-γ (protocols as in the legend to Figure 1). Twenty-four hours after activation, DCs were washed twice and resuspended in RPMI/FCS. The indicated activation stimuli (no cytokines or live E coli [20 μL/mL]) were added either immediately (*) or following a 24-hour rest period in medium without cytokines (†). Mean values ± SEM of 4 experiments are shown.
After the initial stimulation, MoDCs expressed an irreversible functional profile. Thus, migration remained the characteristic of initially migratory-type MoDCs, even if the cells were allowed to recover from the initial stimulus for 24 hours (Figure 6). In addition, migratory-type MoDCs were incapable of secreting cytokines in response to E coli (Table 1), as reported previously.5 Not only was IL-6 and IL-12p70 secretion induced by CD40L abrogated after stimulus removal (Figure 4), but IL-12p70 secretion could no longer be induced by subsequent restimulation with intact E coli (Table 1). These results demonstrate that migratory-type or inflammatory-type MoDCs are terminally differentiated DC populations and that these functions are mutually exclusive.
The initial activation stimulus commits MoDCs irreversibly to expressa specific functional phenotype. Migration of MoDCs initially stimulated with either GM-CSF plus TNF-α plus IFN-α plus PGE2 or with CD40L1 plus IFN-γ or with CD40L3 plus IFN-γ. MoDCs were washed twice 24 hours after stimulation. The indicated activation stimuli (no cytokines or GM-CSF plus TNF-α plus IFN-α plus PGE2) were added either (A) immediately or (B) following a 24-hour rest period in medium without cytokines (mean ± SEM of 4 experiments are shown, *P < .05 as compared with no cytokine condition).
The initial activation stimulus commits MoDCs irreversibly to expressa specific functional phenotype. Migration of MoDCs initially stimulated with either GM-CSF plus TNF-α plus IFN-α plus PGE2 or with CD40L1 plus IFN-γ or with CD40L3 plus IFN-γ. MoDCs were washed twice 24 hours after stimulation. The indicated activation stimuli (no cytokines or GM-CSF plus TNF-α plus IFN-α plus PGE2) were added either (A) immediately or (B) following a 24-hour rest period in medium without cytokines (mean ± SEM of 4 experiments are shown, *P < .05 as compared with no cytokine condition).
CD40L3 stimulation induces transient ERK1/2 phosphorylation in CD1c+ PBDCs and migratory MoDCs, correlating with their migratory functional profile
In humans, CD1c+ DC precursors can be isolated from peripheral blood. In contrast to MoDCs, CD1c+ PBDCs mature spontaneously during in vitro culture, rapidly acquire migratory function in the absence of PGE2-containing stimuli, and are low cytokine producers.13,21 Because cytokine production requires sustained activation signals, we investigated whether differences in the persistence of signaling may explain their predominantly migratory functional profile.
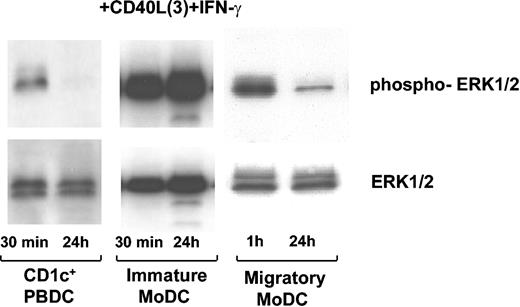
MoDCs activated with CD40L3 plus IFN-γ exhibited strong and persistent ERK1/2 phosphorylation after 24 hours. In contrast, migratory CD1c+ PBDCs displayed intracellular phosphorylation of ERK1/2 after 30 minutes of stimulation with CD40L3 plus IFN-γ, but this returned to background levels by 24 hours (n = 3) (Figure 7). Furthermore, repeated stimulation could not prolong the activity of ERK1/2 in CD1c+ PBDCs (n = 1, not shown). As with migratory CD1c+ PBDCs, MoDCs committed to a migratory functional profile following stimulation with TNF-α, IFN-α, and PGE2 (Figure 7 and Table 1) displayed a transient ERK phosphorylation profile. Therefore, cell type–specific differences in CD40 signaling might account for functional differences observed in different DC subsets.
Nonpersistent ERK1/2 phosphorylation in CD1c+PBDCs and migratory MoDCs activated with CD40L3plus IFN-γ. CD1c+ PBDCs were isolated from PBMCs. Following a 24-hour culture in the presence of GM-CSF plus IL-4, CD1c+ PBDCs (5 × 105 per condition) were stimulated with CD40L3 plus IFN-γ for 30 minutes or 24 hours. Subsequently, CD1c+ PBDCs were harvested, washed, resuspended at concentrations of 5 × 106/mL in Western sample buffer, and snap frozen. ERK1/2 phosphorylation of 1 representative experiment is shown (n = 3 for CD1c+ PBDCs). Immature MoDCs were stimulated with CD40L3 plus IFN-γ directly following the 7-day culture period (n = 5). Migratory MoDCs were initially stimulated with GM-CSF plus TNF-α plus IFN-α plus PGE2 for 24 hours and then washed, and CD40L3 plus IFN-γ was added as indicated (n = 4).
Nonpersistent ERK1/2 phosphorylation in CD1c+PBDCs and migratory MoDCs activated with CD40L3plus IFN-γ. CD1c+ PBDCs were isolated from PBMCs. Following a 24-hour culture in the presence of GM-CSF plus IL-4, CD1c+ PBDCs (5 × 105 per condition) were stimulated with CD40L3 plus IFN-γ for 30 minutes or 24 hours. Subsequently, CD1c+ PBDCs were harvested, washed, resuspended at concentrations of 5 × 106/mL in Western sample buffer, and snap frozen. ERK1/2 phosphorylation of 1 representative experiment is shown (n = 3 for CD1c+ PBDCs). Immature MoDCs were stimulated with CD40L3 plus IFN-γ directly following the 7-day culture period (n = 5). Migratory MoDCs were initially stimulated with GM-CSF plus TNF-α plus IFN-α plus PGE2 for 24 hours and then washed, and CD40L3 plus IFN-γ was added as indicated (n = 4).
Inhibition of ERK1/2 in response to CD40 ligation induces a migratory phenotype in MoDCs
To examine the impact of the signaling pathways examined in Figure 2 on the functional development of MoDCs, we used specific inhibitors for ERK1/2 (PD98059), p38K (p38K inhibitor), and NF-κB (MG-132 22-24 and Bay11-7085 25,26 ). Specificity and efficiency of the MAPK inhibitors is shown in Figure 8A. Our results show that p38K mediates migration (Figure 8C), whereas inhibition of ERK1/2 significantly enhanced the migratory capacity of MoDCs toward CCL21 (Figure 8D). In contrast with their antagonistic regulation of migratory function, both p38K and ERK1/2 synergistically enhanced secretion of IL-6 (Figure 8E) and IL-12p70 (Figure 8F). Blocking NF-κB with MG-132 or Bay11 induced apoptosis (data not shown). Although it is possible that members of this family of transcription factors also influence cytokine secretion and migration, we were not able to establish this due to the predominant apoptotic effects of these 2 pan–NF-κB inhibitors. Thus, ERK1/2 and p38K synergized on cytokine secretion but had antogonistic effects on the induction of migration of MoDCs.
Synergistic and antagonistic effects of ERK1/2 and p38 MAPKmodulate MoDC migration and cytokine secretion. MoDCs were stimulated as described in the legend to Figure 1. Additionally, PD98059 (inhibitor of ERK1/2) or p38 MAPK inhibitor (p38i) was added as indicated. Cellular lysates were prepared after 2 and 24 hours. (A) Inhibition of phosphorylation of the p38K substrates MAPKAPK-2 and hsp-27 but not of p38K by 10 μM p38K inhibitor (p38Ki). One representative of at least 3 experiments is shown. (B) Inhibition of ERK1/2 phosphorylation but not of p38K phosphorylation by the MEK inhibitor PD98059. One representative of at least 5 experiments is shown. (C) Inhibition of migration of MoDCs activated for 48 hours with the indicated protocols by p38Ki (10 μM) (mean ± SEM of 4 to 8 experiments, **P < .01). (D) Enhanced migration of MoDCs activated for 48 hours in the presence of PD98059 (30 μM) (mean ± SEM of 4 to 8 experiments, *P < .05). (E) Reduction of IL-6 secretion and (F) reduction of IL-12p70 secretion by MoDCs activated with intact E coli for 48 hours in the absence or presence of p38Ki (10 μM), PD98059 (30 μM), or both (mean ± SEM of 7 experiments, *P < .05, **P < .01).
Synergistic and antagonistic effects of ERK1/2 and p38 MAPKmodulate MoDC migration and cytokine secretion. MoDCs were stimulated as described in the legend to Figure 1. Additionally, PD98059 (inhibitor of ERK1/2) or p38 MAPK inhibitor (p38i) was added as indicated. Cellular lysates were prepared after 2 and 24 hours. (A) Inhibition of phosphorylation of the p38K substrates MAPKAPK-2 and hsp-27 but not of p38K by 10 μM p38K inhibitor (p38Ki). One representative of at least 3 experiments is shown. (B) Inhibition of ERK1/2 phosphorylation but not of p38K phosphorylation by the MEK inhibitor PD98059. One representative of at least 5 experiments is shown. (C) Inhibition of migration of MoDCs activated for 48 hours with the indicated protocols by p38Ki (10 μM) (mean ± SEM of 4 to 8 experiments, **P < .01). (D) Enhanced migration of MoDCs activated for 48 hours in the presence of PD98059 (30 μM) (mean ± SEM of 4 to 8 experiments, *P < .05). (E) Reduction of IL-6 secretion and (F) reduction of IL-12p70 secretion by MoDCs activated with intact E coli for 48 hours in the absence or presence of p38Ki (10 μM), PD98059 (30 μM), or both (mean ± SEM of 7 experiments, *P < .05, **P < .01).
Discussion
Migration of DCs from peripheral sites to lymph nodes and the production of cytokines, such as IL-12, are critical DC functions for the induction and regulation of immune responses. Previously, we and others reported that these 2 functions are differentially regulated and mutually exclusive in MoDCs.5,6 In the present study we demonstrate that migration and cytokine secretion can be regulated through identical receptor/ligand systems, such as CD40/CD40L, and depend on the strength and persistence of the ligand-induced signal. Interestingly, this concept also applied to other receptor systems, such as those employed by DCs during pathogen recognition (eg, Toll-like receptors). Thus, a novel concept emerges in which signal strength and persistence are 2 critical determinants of specific DC functions.
The kinetics of acquisition of migratory function and cytokine secretion by MoDCs were found to be substantially different. Migratory function was acquired and maintained over a long period following weak or transient signaling through CD40 ligation (such as provided by CD40L1) or pathogen encounter. In contrast, secretion of IL-12p70 required persistent signaling over at least 12 hours (such as provided by CD40L3) and was rapidly and irreversibly abrogated if the stimulus was cleared. Although IL-6 production was induced early after activation, prolonged secretion of this cytokine had a similar requirement for persistent stimulation as observed for IL-12p70. Differences in MoDC migratory capacity induced by the differing forms of CD40L were not due to induction of differing levels of CCR7 expression because the MoDCs matured by either form of CD40L expressed equivalent levels of CCR7. This suggests that the different migratory profiles more likely reflect differences at the level of signal transduction pathways. It is also possible that the blunted migratory function of CD40L3-matured MoDCs could be due to (a) desensitization of CCR7 or (b) abrogation of the CCL21 gradient by CCL21 production by the MoDCs. Regarding the first possibility, CD40L trimer–activated DCs washed and rested in fresh medium so that they no longer secreted cytokines (unless restimulated) remained nonmigratory cells when subsequently exposed to GM plus TNF-α plus IFN-α plus PGE2 (Figure 6B). Assuming that desensitization of CCR7 is not permanent but reversible after removal of the CCR7 ligand (as observed for other receptor classes), these data would indicate that desensitization is not the major mechanism responsible for lack of migratory function. Regarding abrogation of the CCL21 gradient by autocrine CCL21 production by the MoDCs, although we have been able to detect CCL21 in the supernatants of CD40L3-matured MoDCs (by ELISA), these levels were substantially lower (less than 1 ng/mL range) than the levels of chemokine used in the assay (40 ng/mL) and thus were unlikely sufficient to abrogate the CCL21 gradients arising from the lower chamber (data not shown).
Interestingly, for CD1c+ PBDCs, CD40 ligation induced only transient ERK1/2 signaling, which correlated with CD1c+ PBDCs predominantly migrating to lymph node–directing chemokines and a poor ability to secrete IL-12p70.5,11 Because migratory-type MoDCs displayed a similar functional profile to CD1c+ PBDCs (ie, low producers of cytokines upon subsequent stimulation and only transiently phosphorylated ERK), we hypothesize that both CD1c+ PBDCs and migratory MoDCs have been instructed to express an intracellular signaling program that is refractory to persistent signaling. Therefore, cell type–specific and maturational stage–specific differences in signaling through identical pathways may predispose DCs to express specific functions.
Given that CD40L is not only expressed by activated CD4+ T helper cells but also by inflamed smooth muscle cells, vascular endothelial cells, macrophages, eosinophils, activated platelets, as well as DCs themselves (reviewed by Luft et al7 ), the findings of the current paper may more closely reflect interactions between immature, sentinel DCs with neighboring stromal and endothelial cells or innate effectors during inflammation in the periphery than with naive T cells in lymph nodes. In this regard, naive T cells only express CD40L after interaction with mature DCs that have migrated into draining lymph nodes following maturation in the periphery. However, in patients with autoimmune diseases such as Sjögren syndrome,27 rheumatoid arthritis,28 inflammatory bowel disease,29 or systemic lupus erythematosus,30 aberrant CD40L expression by T cells as well as by non–T-cell populations has been reported. Perhaps under specific pathologic conditions, a primary T-cell role is physiologically relevant, but this is less likely under steady state conditions or during the initial moments following pathogen detection.
Our hypothesis for the modulation of DC function by the strength and persistence of stimulation finds parallels in T-cell biology. Here, T-helper cell differentiation toward either Th-1– or Th-2–type cells has also been proposed to be regulated by the strength (and persistence) of T-cell receptor signaling.31 Furthermore, it has recently been shown that Th-2 differentiation requires transient ERK activation, whereas sustained ERK activation induces Th-1 differentiation in naive T cells.32 The present study demonstrates that MoDC migration and cytokine secretion diverged at the level of ERK1/2 activation because inhibition of ERK1/2 during DC activation resulted in reduced cytokine levels while simultaneously increasing migratory capacity. This suggests that ERK signaling above or below a certain threshold may regulate the expression of specific cell functions in a variety of cell types.33 Thus, the concept of strength and persistence of receptor stimulation seems to represent a more generic principle of operation in the immune system.
Persistent signaling may serve the purpose of conditioning the local microenvironment during inflammation or pathogen invasion, where large amounts of IL-12p70 activate innate effector cells, such as natural killer (NK) cells. In contrast, DCs migrating out of epicenters of infection to draining lymph nodes are likely to enter an environment devoid of the initial activating stimulus. This loss of signal persistency will attenuate the cytokine-secreting capacity, probably to levels more appropriate for the priming and stimulation of antigen-specific T cells and thus avoiding the activation of irrelevant bystander T cells. This hypothesis is supported by our observation that migratory-type DCs are poor producers of IL-12p70 even when subsequently stimulated with potent inducers of IL-12p70 (ie, intact E coli). Similarly, MoDCs producing high levels of IL-12 were unable to acquire migratory capacity and, therefore, lymph node–homing potential when subsequently stimulated with potent inducers of migration (ie, PGE2-containing stimuli). These findings suggest that DCs are terminally instructed after activation at peripheral sites to fulfill 1 of the 2 mutually exclusive functions—that is, to migrate to lymph nodes for efficient T-cell interaction or to condition the microenvironment by producing large quantities of cytokines. Accordingly, mice deficient in the PGE2 receptor EP4 respond to inflammatory stimuli with excessive inflammation and show reduced migration of Langerhans cells.11,12
These findings have important implications for the clinical use of MoDCs as vaccine adjuvants. The functional profile of the DCs is likely to influence the outcome of the vaccination. Migratory-type DCs are more efficient in transporting antigen to lymph nodes for efficient presentation to T cells. In contrast, proinflammatory-type DCs could be powerful tools for conditioning the microenvironment (eg, in the vicinity of tumors) and thereby enhance innate and antigen-specific effector cell function at these sites.
The observation that cytokine secretion is to a large degree dependent on the persistence of the activating stimulus may provide a new perspective on the pathophysiology of chronic inflammation and autoimmune disease. For instance, the persistence of bacterial infections, such as Borrelia burgdorferi34 and chlamydia,14,35 as well as CD40/CD40L overexpression in tissues36-39 have been associated with chronic disease. Such persisting stimuli are likely to promote persistent cytokine secretion, which eventually leads to tissue damage. Interrupting persistent signaling with specific inhibitors of signal transduction pathways may provide new therapeutic tools for the management of chronic inflammation and autoimmune disease.
Prepublished online as Blood First Edition Paper, April 27, 2004; DOI 10.1182/blood-2003-12-4146.
Supported in part by grants from the Deutsche Forschungsgemeinschaft (PPN [SFB 405; Na 138/5-3]), a program grant from the Australian National Health and Medical Research Council (NH&MRC), and the Ludwig Institute for Cancer Research. M.S. is supported by a grant of the Dr Mildred Scheel Stiftung.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Immunex, an Amgen company, Seattle, WA, for provision of CD40L trimer.

![Figure 1. Migration and cytokine secretion by mature MoDCs differ in response to differentially polymerized CD40L preparations. MoDCs were generated from CD14+ monocytes within 5 to 7 days of culture in the presence of GM-CSF and IL-4. Immature MoDCs (1 × 105 to 3 × 105/mL) were washed and resuspended in culture medium. The following cytokines were added as indicated for an additional 36 to 48 hours: GM-CSF (20 ng/mL), TNF-α (10 ng/mL), IFN-α (2000 IU/mL), PGE2 (1 μM), CD40L1 ([CD40L monomers] 1 μg/mL; enhancer, 1 μg/mL), CD40L3 ([CD40L trimers] 1 μg/mL), and IFN-γ (1000 U/mL). (A) Migration assays were performed in duplicate using 3 μm pore size transwell plates. Migration to medium in the absence or presence of CCL21 (6Ckine, 40 ng/mL) in the lower well is shown as mean values ± SEM of 14 experiments. Supernatants were harvested and (B) IL-12p70 and (C) IL-6 production measured by ELISA (mean values ± SEM, n = 11). *P < .05; **P < .01 as compared with activation with CD40L3 plus IFN-γ.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/4/10.1182_blood-2003-12-4146/6/m_zh80160465140001.jpeg?Expires=1769346986&Signature=pPeueab1TAb5R4fswaZ3WSGalJIeWHjY7tef1YdbV7apPoFG2XOsEgQ-jICuZpP-NB~7g45gIni0XXRL5qSIxjYB6vZCf7EJbuMJOEW~m-~HwHBpfaZYoYTlqCEtNUg~mbxbYD7d6QxpFHzy4moAQUNOAn6RZlfgK2VjwyKQjCwe5vWm0h~-ggzqWzbzgouOlJbnobgTdnFgw9B6~cdFqfufc3UWNzKxuNkQKQHiOIS4SkERPB6ArDIDCPKo~1tvlN2GRwpda12NtuBsUqteT8hgjI7O50oF2qMAU0VzR1fzLmfiZdJo10JmdEqyouuj5O8DHkgENYT0LAPeQE84vA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Strength and persistence of stimulation with intact E coli modulate MoDC migration and cytokine secretion. MoDCs were stimulated with intact E.coli as indicated. One half of the cultures were washed twice 3 hours after addition of E coli and then cultured in RPMI/FCS until 48 hours (mean ± SEM of 8 experiments are shown [n = 4 for E coli 1:1000]). (A) Migration toward CCL21 and (C) IL-12p70 secretion or (E) IL-6 secretion of MoDCs activated with varying concentrations of intact E coli. (*P < .05; **P < .01 as compared with E coli 1:1). Effect of washing MoDCs following 3 hours of activation with varying concentrations of live E coli on (B) migration toward CCL21 and (D) IL-12p70 secretion or (F) IL-6 secretion. Cultures were continued in RPMI/FCS for a total of 48 hours. Mean ± SEM of 8 experiments are shown (n = 4 for E coli 1:1000), *P < .05; **P < .01 compared with unwashed MoDCs (normalized to 1).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/4/10.1182_blood-2003-12-4146/6/m_zh80160465140005.jpeg?Expires=1769346986&Signature=A~aUWdM8t30L5lrEkvFTshx8swmE9smFUpnYaFeJJ2r2aI7otzvx76HqhNlxmMyR9xfgihTn4y~hhCTXHH0VKsWN32AuaS7r9nqMj4YIkoIwBSb3AfFkN03T6Pqw6IJVjvuX1XwicsIwg32Kv~JE~YVlsyYj-sEngPMkWhRLgxNo09ZNVooVwoZIvKyDTF4P0fDBswO8bDN8yQQK~sD-ZyHowHCgxiO3zZQxSuIGgVeMv-NezJFDu-Yl6UbYjce6r4eV~jO2dT4MTRvQeASm9TL0387acKD7u6Hrm9i3L67CJT7oLac4g7wm0~m8oNG16yROWNMQrt-EMPDYKsYqXg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal