Abstract
Hematopoietic stem cells (HSCs) are a subset of bone marrow cells that are capable of self-renewal and of giving rise to all types of blood cells. However, the mechanisms involved in controlling the number and abilities of HSCs remain largely unknown. The Indian hedgehog (Ihh) signal has an essential role in inducing hematopoietic tissue during embryogenesis. We investigated the roles of the Ihh in coculture with CD34+ cells and human stromal cells. Ihh mRNA was expressed in primary and telomerized human (hTERT) stromal cells, and its receptor molecules were detected in CD34+ cells. Ihh gene transfer into hTERT stromal cells enhanced their hematopoietic supporting potential, which was elevated compared with control stromal cells, as indicated by the colony-forming units in culture (CFU-Cs) (26-fold ± 2-fold versus 59-fold ± 3-fold of the initial cell number; mixed colony-forming units [CFU-Mix's], 63-fold ± 37-fold versus 349-fold ± 116-fold). Engraftments of nonobese diabetic/severe combined immunodeficiency–ß2m–/– (NOD/SCID–ß2m–/–) repopulating cells (RCs) expanded on Ihh stromal cells were significantly higher compared with control coculture results, and engraftment was neutralized by addition of an antihedgehog antibody. Limiting dilution analysis indicated that NOD/SCID–ß2m–/– RCs proliferated efficiently on Ihh stromal cells, compared with control stromal cells. These results indicate that Ihh gene transfer could enhance the primitive hematopoietic support ability of human stromal cells.
Introduction
Ex vivo expansion of hematopoietic cells is used not only for tumor purging1 and gene transfer,2 but also to amplify the number of hematopoietic stem cells (HSCs)/hematopoietic progenitor cells, especially in umbilical cord blood (CB) grafts. The use of CB as a source of HSCs is increasing because there is less incidence of graft-versus-host disease after CB transplantation.3 This allows use of grafts with greater human leukocyte antigen disparity and therefore increases the number of grafts available for transplantation. However, absolute HSC dose is a limiting factor in the use of CB as a graft for adult transplant recipients.4 For this reason, many approaches to ex vivo expansion of HSCs have been attempted, and valuable information has been gathered regarding methods to retain stem cell functions during ex vivo culture.5-7
Until recently, interactions between stromal and hematopoietic cells were viewed as essential to the maintenance of stem cell properties, such as the ability to reconstitute the hematopoietic system in vivo after transplantation or in vitro in long-term cultures.8,9 Several studies have shed light on the molecular mechanisms involved in stromal HSC support ability. Some processes are mediated by growth factors including interleukin-3 (IL-3), IL-6, thrombopoietin (TPO), Flk-2/Flt-3 ligand (FL), and stem cell factor (SCF),10 and other processes involve adhesive molecules including Notch, mKirre,11 and connexin 43.12 It has also been demonstrated that some soluble factors such as bone morphogenetic protein and the wingless- and int-related protein (Wnts) family, which play important roles in the generation of cell polarity and the specification of cell fate during embryogenesis, are involved in postnatal hematopoiesis.13 Accordingly, increasing attention has been focused on the roles of some embryonic factors in the bone marrow (BM) microenvironment.14
Recently, it has been revealed that primitive hematopoiesis and angiogenesis in the developing yolk sac are induced by a family of soluble proteins, Indian hedgehog (Ihh), which are released from a layer of visceral endoderm.15-17 Subsequently, it was shown that endochondral bone development is modulated by Ihh released from chondrocytes, which are progeny of bone marrow stromal stem cells.18 These results raised the possibility that Ihh proteins not only are involved in bone formation, but are also in the bone marrow microenvironment. More recently, it was reported that a member of the hedgehog family, sonic hedgehog (Shh), which was known to be a key signaling component in patterning of neural cord,19 stimulates proliferation of CB CD34+ hematopoietic cells.20 However, it was demonstrated that Shh and Ihh work on different but partially overlapping aspects of embryogenesis. In addition, although both share the receptor patched (Ptc) and use smoothened (Smo) as a signal-transduction molecule, hedgehog family members do not exhibit identical bioactivities.21,22 Thus, it has been uncertain whether Ihh could be involved in regulation of HSCs.
In the present study, we screened bone marrow stromal cells for the expression of Ihh and detected it in human stromal cells and CD34+ cells. Moreover, Smo was expressed in human stromal cells and in the CD34+ fraction, but not in the CD34– fraction. On the basis of these findings, we analyzed the role of Ihh in hematopoietic support using a coculture system with human stromal cells and CD34+ hematopoietic cells.
Materials and methods
Human bone marrow stromal cells
Human BM was obtained by aspiration from the posterior iliac crest of healthy adult volunteers after informed consent. The study was approved by the Institutional Review Board at our university. Preparation of primary stromal cells was described previously.23 Telomerized human stromal cells (hTERT stromal cells) that exhibited a prolonged life span with the same hematopoietic progenitor support as primary stromal cells were employed to establish gene-modified stromal cells.23 Human stromal cells were cultured in long-term culture (LTC) medium containing minimum essential medium–α (Sigma Chemical, St Louis, MO), 12.5% horse serum (Gibco BRL, Rockville, MD), 12.5% fetal calf serum (Gibco BRL), 1 × 10–6 M hydrocortisone (Sigma Chemical), and 1 × 10–4 M β-mercaptoethanol (Sigma Chemical) at 37° C under 5% CO2 in a humidified atmosphere.
Purification of human CB CD34+ cells
CB was obtained following normal full-term deliveries after informed consent was obtained. After sedimentation of the red blood cells in the CB with the same volume of 6% (wt/vol) hydroxyethyl starch at room temperature for 30 minutes, low-density (less than 1.077 g/mL) mononuclear cells (MNCs) were separated by Ficoll-Paque Plus (Pharmacia, Uppsala, Sweden). CD34+ cell purification was conducted by positive selection with a MACS Direct CD34 Progenitor Cell Isolation Kit (Miltenyi Biotech, Bergish-Gladbach, Germany) according to the manufacturer's instructions.
Retroviral vectors and gene transduction
The retroviral vector pRx-IRES-hrGFP was constructed by replacing the neomycin-resistant gene of pRx-IRES-neo24 with humanized Renillareniformis green fluorescent protein (hrGFP) fragment (approximately 717 base pair [bp]) from the phrGFP-C plasmid (Stratagene, La Jolla, CA). After the ligation mixture containing pRX-IRES-hvGFP was transformed into DH5α-competent cells (Life Technologies, Grand Island, NY), the colonies containing pRx-IRES-hrGFP were confirmed by minipreparation (Bio-Rad, Hercules, CA) and restriction endonuclease digestion.
A cDNA corresponding to a biologically active N-terminal polypeptide fragment of the Ihh protein, containing a secretion-signal peptide (Ihh-N), was used as described previously.25 We attempted to construct a retroviral expression vector containing Ihh-N cDNA and transduce it into hTERT stromal cells to overexpress the Ihh gene (Figure 1A). Ihh-N cDNA was cloned by reverse transcriptase–polymerase chain reaction (RT-PCR) from CB-derived RNA. Cloning primers (5′-AGGATCCACCATGTCTCCCGCCCGGCTCCGGCCCCGACTGCACTTC-3′,5′-GAGCGGCCGCTTAGCCGCCCGTCTTGGCTGCGGCCGAGTG-3′, containing added BamH1 and NotI sites) were used to perform PCR. The PCR product was ligated into the pGEM-T easy vector (Life Technologies) to generate pGEM-T easy-Ihh-N.
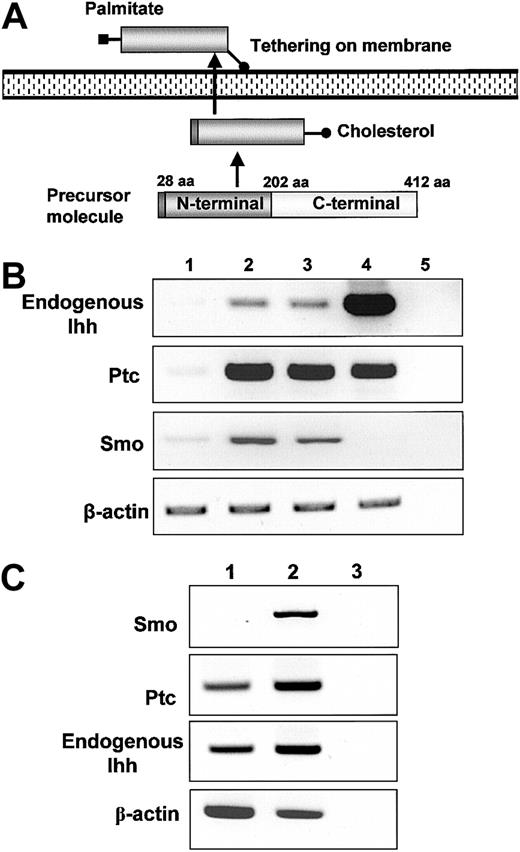
Screening for mRNA expression of mesodermal growth factors and their receptor. (A) Processing of endogenous Ihh in mammalian cells. Palmitoyl modification of the N-terminal fragment was essential for potentiating hedgehog activity. This occurs after autocatalytic processing of the signal peptide in cells. The C-terminal fragment of the Indian hedgehog protein displays an autoproteolytic activity and a cholesterol transferase activity, whereas the C-terminal product has no signaling activity. The cholesterol moiety of Ihh contributes to restriction of the spatial distribution of Ihh protein to the cell surface. (B) The mRNA expression of Ihh and receptor molecules. For detection of Ihh, a primer pair specific for endogenous Ihh was used. Lane 1, human umbilical vein endothelial cells (HUVECs); lane 2, primary stromal cells; lane 3, hTERT stromal cells; lane 4, cord blood; lane 5, H2O. (C) The mRNA expression of hedgehog receptor molecules in CB: lane 1, total cord blood RT+; lane 2 CD34+RT+, lane 3 CD34+RT–.
Screening for mRNA expression of mesodermal growth factors and their receptor. (A) Processing of endogenous Ihh in mammalian cells. Palmitoyl modification of the N-terminal fragment was essential for potentiating hedgehog activity. This occurs after autocatalytic processing of the signal peptide in cells. The C-terminal fragment of the Indian hedgehog protein displays an autoproteolytic activity and a cholesterol transferase activity, whereas the C-terminal product has no signaling activity. The cholesterol moiety of Ihh contributes to restriction of the spatial distribution of Ihh protein to the cell surface. (B) The mRNA expression of Ihh and receptor molecules. For detection of Ihh, a primer pair specific for endogenous Ihh was used. Lane 1, human umbilical vein endothelial cells (HUVECs); lane 2, primary stromal cells; lane 3, hTERT stromal cells; lane 4, cord blood; lane 5, H2O. (C) The mRNA expression of hedgehog receptor molecules in CB: lane 1, total cord blood RT+; lane 2 CD34+RT+, lane 3 CD34+RT–.
To construct pRx-Ihh-N-IRES-hrGFP, pGEM-T easy-Ihh-N was double-digested with BamHI and NotI (New England Biolabs, Beverly, MA) and ligated into the BamHI/NotI site in pRx-IRES-hrGFP. Viral supernatant was produced from phoenix-AMPHO cells (American Type Culture Collection, Manassa, VA) after transfection of purified plasmid DNA (Qiagen, Tokyo, Japan) in Lipofectamine transfection reagent (Life Technologies). The viral supernatant containing Rx-IRES-hrGFP or Rx-Ihh-N-IRES-hrGFP was used to infect hTERT stromal cells. The hTERT stromal cells receiving an empty vector, Rx-IRES-hrGFP, were employed as controls. After gene transduction of Rx-IRES-hrGFP or Rx-Ihh-IRES-hrGFP, control or Ihh-transduced stromal cells (Ihh stromal cells) were sorted by detection of GFP expression, with the use of FACSCalibur (Becton Dickinson, Mountain View, CA).
Analysis of expression of Ihh and related genes by RT-PCR and immunoblotting
For RT-PCR analysis, total RNA was prepared from cells by means of the RNeasy kit (Qiagen). Then, 1 μg total RNA was reverse transcribed by SuperScriptII (Invitrogen, Tokyo, Japan) and amplified by means of the AccuTaq LA Polymerase Mix (Sigma) with primers specific for the vector-derived Ihh (5′-TGCGGGCCGGGTCGGGTGGTG-3′ and 5′-GCCGCCCGTCTTGGCTGC-3′) or endogenous Ihh (5′-CTTCCGGGCCACATTTGCCAGCCA-3′ and 5′-GAGACGCCCCAGGCGGTAGAGCA-3′); Ptc (5′-CTGTTGGCATAGGAGTGGAGTTCACC-3′ and 5′-CTGCTGGGCCTCGTAGTGCCGAAGC-3′); Smo (5′-CAGAACATCAAGTTCAACAGTTCAGGC-3′ and 5′-ATAGGTGAGGACCACAAACCAAACCACACC-3′); or, as a control, human β-actin (5′-GCTCGTCGTCGACAACGGCTC-3′ and 5′-CAAACATGATCTGGGTCATCTTCTC-3′). The PCR amplification was carried out with the use of 35 cycles of 94° C for 30 seconds, 58° C for 30 seconds, and 72° C for 60 seconds. The PCR products were separated on a 2% agarose gel.
For immunoblot analysis, 20 μL supernatant or 50 μg lysate from control and Ihh stromal cells was subjected to electrophoresis on a 4%/20% sodium dodecyl sulfate (SDS)–polyacrylamide gradient gel and transferred to a polyvinylidene difluoride (PVDF) membrane by means of a semidry transfer apparatus (Bio-Rad Laboratories, Tokyo, Japan). Cell lysate was prepared in a buffer containing 50 mM Tris (tris(hydroxymethyl)aminomethane)–HCl (pH 7.4), 1% Nonidet P40 (NP40), 150 mM NaCl, and a protease inhibitor mixture (Roche Diagnostics, Tokyo, Japan). Anti-Ihh antibody (Ab) was prepared from hybridoma 5E1, purchased from the University of Iowa hybridoma bank (Iowa City, IA). Proteins were visualized with the enhanced chemiluminescence (ECL) method (Amersham Pharmacia Biotech).
Coculture of CB CD34+ cells with control or Ihh stromal cells
First, 40 000 control or Ihh stromal cells were plated in 25 cm2 flasks in LTC medium. When the stromal cells reached greater than 90% confluence, the cells were washed 5 times with phosphate-buffered saline (PBS) before the addition of CB CD34+ cells. Then, 5 000 CB CD34+ cells were seeded on a monolayer of either control stromal or Ihh stromal cells that had been pre-established in 5 mL serum-free medium (X-VIVO 10; BioWhittaker, Walkersville, MD), supplemented with 50 ng/mL human TPO, 10 ng/mL human SCF, and 50 ng/mL human FL (all R&D Systems, Minneapolis, MN) at 37° C under 5% CO2. After 1 week of coculture, 5 mL fresh complete medium containing the same concentrations of cytokines was added, and the coculture was continued for 1 week. At the end of the second week of coculture, hematopoietic cells that had expanded on stromal cells were collected by pipetting. Adherent hematopoietic cells on the stromal layer were removed with the use of PBS, and dissociated hematopoietic cells were mixed with nonadherent cells. Stromal layers were treated further with 2 cycles of pipetting. To avoid the presence of stromal cells in the resulting cell suspensions, a 30-minute adhesion procedure was performed at 37° C as described previously.23,26 The coculture was maintained by adding 5 mL fresh medium containing cytokines on day 28. This allowed us to observe the formation of cobblestone areas (CAs) from hematopoietic cells that transmigrated beneath the layer of stromal cells. The number of CAs that included more than 5 cells was counted on day 28 of coculture. For a neutralization study, 0, 3, 10, 30, and 100 μg/mL antihedgehog blocking Ab 5E1 or vehicle was added to the coculture systems every 3 days.17,27
Analysis of clonogenic cells
The total numbers of colony-forming unit in culture (CFU-C) and mixed colony-forming unit (CFU-Mix) cells in uncultured CD34+ cells or in cocultured preparations were evaluated as described previously.23,28 Aliquots of cells were cultured in quadruplicate with the use of 35-mm tissue culture dishes in 1 mL MethoCult GF H4434V (Stem Cell Technologies, Vancouver, Canada). After 14 days of culture in a humidified environment at 37° C under 5% CO2, colonies consisting of 50 or more cells were scored under a microscope.
Transplantation into NOD/SCID–ß2m–/– mice
Six- to 10-week-old nonobese diabetic/severe combined immunodeficiency–ß2m–/– (NOD/SCID–ß2m–/–) mice were bred from breeding pairs originally obtained from Jackson Laboratory (Bar Harbor, ME). All animals were handled under sterile conditions and maintained in microisolators. All animal experiments were performed in accordance with institutional guidelines approved by the Animal Care Committee of Sapporo Medical University (Sapporo, Japan). First, 5000 CB CD34+ cells were cocultured for 2 weeks on a layer of either control stromal or Ihh stromal cells in the presence of TPO, SCF, and FL, with or without 100 μg/mL antihedgehog blocking Ab. After 2 weeks of coculture, nonadherent hematopoietic cells were removed, followed by trypsinization of adherent cell layers. To avoid the presence of allogeneic stromal cells in the resulting cell suspensions, a 30-minute adhesion procedure was performed at 37° C. Hematopoietic cells from this suspension were mixed with the nonadherent cells. The percentage of contamination of gene-modified GFP-expressing stromal cells in the hematopoietic cells was judged to be less than 0.01% by microscopy and flow cytometry. The hematopoietic cells thus obtained were injected through the lateral tail vein into mice that had been irradiated with 300 cGy (Softex, Tokyo, Japan). Cells were cotransplanted with 5 × 106 irradiated (1500 cGy) peripheral blood (PB) MNCs from healthy volunteers as carrier cells. The mice were killed 8 or 13 weeks after transplantation by cervical dislocation, and BM MNCs were harvested from NOD/SCID–ß2m–/– mice. The presence of human hematopoietic cells was confirmed by flow cytometric analysis with the use of antihuman-specific CD45 as described below, and also by detection of human genomic DNA with the use of genomic PCR specific for human ALU repetitive sequences.23
Immunophenotyping of expanded hematopoietic cells and the progeny of NOD/SCID–ß2m–/– repopulating cells
Aliquots of cells were stained with fluorescein isothiocyanate (FITC)– and/or phycoerythrin (PE)–conjugated monoclonal Abs, including the isotype control Abs (Immunotech, Westbrook, MI). The cells obtained from mice were blocked in PBS with 10% normal mouse serum (Pharmingen, San Diego, CA) and 1 μL Fc block reagent (Pharmingen) at 4° C for 10 minutes prior to staining. 10– We incubated 106 cells with FITC-conjugated CD34 or CD45, along with PE-conjugated CD34, CD38, CD41, CD90, Glycophorin A (Immunotech), CD19, CD11b, or CD3 (Dako Japan, Kyoto, Japan) at 4° C for 30 minutes; cells were then washed twice with PBS containing 0.1% bovine serum albumin (BSA). The cells were analyzed by flow cytometric analysis by means of an EPICS XL flow cytometer with EXPO32 analysis software (Coulter, Tokyo, Japan).
Statistical analysis
Results are expressed as the mean ± standard deviation (SD). Significance was assessed by either the Student t test or the Mann-Whitney U test.
Results
Expression of hedgehog-related genes in CB, primary, and hTERT stromal cells
It has been revealed that Ihh is involved not only in primitive hematopoiesis,29 but also in endochondral bone development. Ihh is expressed in prehypertrophic and hypertrophic chondrocytes of the mouse embryo, and these cells are progeny of stromal stem cells.18 On the basis of these reports, we assumed there was a possibility that Ihh was expressed in human stromal cells and that it contributes to hematopoiesis. As expected, the Ihh mRNA was expressed in both primary and hTERT stromal cells, and higher expression levels were detected in CB cells (Figure 1B). However, mRNA levels were lower when HUVECs were used as endothelial cells. Furthermore, the receptor-complex proteins Ptc and Smo were expressed in the human primary and hTERT stromal cells (Figure 1C). We also examined the expression of Shh, which is involved in the development of neural and thymic tissues during embryogenesis19,30 and has been reported to enhance ex vivo expansion of HSCs.20 However, the expression of Shh was not detectable in either primary or hTERT stromal cells, although its expression was easily detected in thymus and HUVECs (data not shown). These results suggested that the expression of Ihh in human CD34+ cells and stromal cells is more significant than the expression of Shh.
We proceeded to analyze the expression of Ptc and Smo in hematopoietic cells. Interestingly, although expression of the Ptc mRNA was detectable in both whole CB and in the CB CD34+ fraction (95% pure), the expression of Smo mRNA was detected only in CD34+ cells (Figure 1C), suggesting that Ihh affects CD34+ cells via its receptor complex. On the basis of these findings, we hypothesized that hematopoietic cells and stromal cells could interact via Ihh proteins. To obtain insight into the roles of Ihh, we initially conducted retrovirus-mediated gene transfer of Ihh gene into hTERT stromal cells and examined the effect of Ihh on hematopoietic support of hTERT stromal cells.
Establishment of a human stromal cell line expressing high levels of Ihh
We examined whether Ihh stromal cells and control stromal cells receiving an empty vector could maintain their character as stromal cells. Control and Ihh stromal cells exhibited morphologic characteristics similar to those of the primary stromal cells and expressed stromal surface antigens such as CD105 (SH2) and CD73 (SH3) (Figure 2A). In addition, the doubling time (DT) of the Ihh stromal cells was somewhat slower (approximately 7 days) compared with control stromal cells (approximately 6 days).
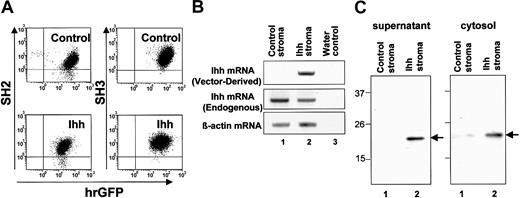
Schematic representation of processing of endogenous Ihh and expression of Ihh in gene-modified human stromal cells. (A) Expression of SH2, SH3, and GFP in control and Ihh stromal cells. Control or Ihh-transduced stromal cells (Ihh stromal cells) were sorted after gene transduction. Postsorting purities of the GFP+ control and Ihh stromal cells were 96.7% and 93.3%, respectively. Cells were gated on the basis of forward and side light scatter to exclude debris. (B) Expression of Ihh mRNA analyzed by RT-PCR specific for vector-derived Ihh and endogenous Ihh: lane 1, control stromal cells; lane 2, Ihh stromal cells; lane 3, H2O. (C) Immunoblot analysis of Ihh in supernatant (1) and cytosol (2) of human stromal cells. The LTC medium of the stromal cells was replaced in the serum-free X-VIVO10 medium after washing with PBS, and supernatants were collected for 72 hours after the culture. Ihh was immunolabeled with the 5E1 antihedgehog antibody. Lane 1, control stromal cells; lane 2, Ihh stromal cells.
Schematic representation of processing of endogenous Ihh and expression of Ihh in gene-modified human stromal cells. (A) Expression of SH2, SH3, and GFP in control and Ihh stromal cells. Control or Ihh-transduced stromal cells (Ihh stromal cells) were sorted after gene transduction. Postsorting purities of the GFP+ control and Ihh stromal cells were 96.7% and 93.3%, respectively. Cells were gated on the basis of forward and side light scatter to exclude debris. (B) Expression of Ihh mRNA analyzed by RT-PCR specific for vector-derived Ihh and endogenous Ihh: lane 1, control stromal cells; lane 2, Ihh stromal cells; lane 3, H2O. (C) Immunoblot analysis of Ihh in supernatant (1) and cytosol (2) of human stromal cells. The LTC medium of the stromal cells was replaced in the serum-free X-VIVO10 medium after washing with PBS, and supernatants were collected for 72 hours after the culture. Ihh was immunolabeled with the 5E1 antihedgehog antibody. Lane 1, control stromal cells; lane 2, Ihh stromal cells.
Next, expression of Ihh proteins in the stromal cells was assessed by RT-PCR and immunoblot analysis. The expression of vector-derived Ihh mRNA could be easily detected in Ihh stromal cells, but not in control stromal cells, although expression of endogenous Ihh mRNA was detected in both control and Ihh stromal cells (Figure 2B). Furthermore, although the expression of cytosolic Ihh protein was higher in Ihh stromal cells than in control stromal cells, the secretion of Ihh protein into the supernatant was detectable in Ihh stromal cells, but not in control stromal cells. This result may be due to the lack of a cholesterol moiety in the transgenic Ihh protein. The cholesterol contributes to a tether on the stromal membrane (Figures 1A and 2C). These results indicated that the transgene of Ihh-N was highly expressed in Ihh stromal cells. Thus, using these gene-modified stromal cells, we conducted comparative experiments as described in the following section.
Hematopoietic progenitor support by control and Ihh stromal cells in vitro
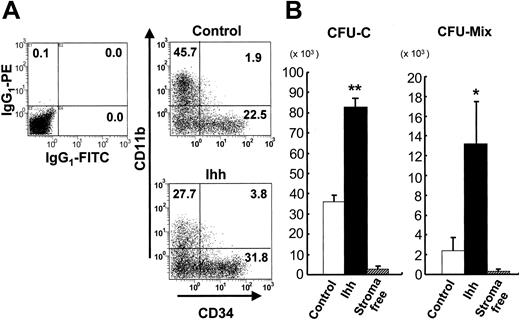
First, we analyzed the expression of surface markers on expanded hematopoietic cells that had been cocultured with either control or Ihh stromal cells. No GFP+ stromal cells could be detected by isotope control staining, suggesting that GFP+ stromal cells could be removed by an adhesion procedure, described in “Materials and methods.” The percentage of CD34+ cells in cocultures with Ihh stromal cells was significantly higher than the percentage in cocultures with control stromal cells (32.2 ± 2.9 versus 23.1 ± 3.1; P < .05), as shown in Figure 3A, and the percentage of CD11b+ cells that were expanded on Ihh stromal cells was lower than that of similar cells that were expanded on control stromal cells (28.0 ± 1.7 versus 46.1 ± 2.3; P < .05). However, there was no difference in surface expression of CD3, CD19, GPA, CD38, or CD41 (data not shown). These results suggested that Ihh stromal cells preferentially supported CD34+ cells with reduced differentiation of the granulocyte-macrophage lineage.
Analysis of surface antigens and the number of clonogenic cells after 2 weeks' expansion of CB-derived CD34+ cells on control or Ihh stromal cells. (A) Expression of surface antigens on ex vivo–expanded hematopoietic cells. The x-axis indicates CD34 labeled with a FITC-conjugated monoclonal Ab. The y-axis indicates CD11b labeled with a PE-conjugated monoclonal Ab. Positivity for a surface antigen was defined with the use of the isotype control monoclonal Ab. Data shown are from 1 representative experiment of 4 showing similar results. IgG1 indicates immunoglobulin G1. (B) Ex vivo expansion of clonogenic cells in CB CD34+ cells for 2 weeks. Values indicate the number of clonogenic cells for Ihh stroma versus control stroma. *P < .05 versus control. **P < .01 versus control. The number of CFU-Cs and CFU-Mix's before the culture were 1412 ± 369 and 37.5 ± 7.8, respectively, out of the 5000 CB CD34+ cells. The results are expressed as mean ± standard deviation. Data represent 8 independent experiments, each done in quadruplicate.
Analysis of surface antigens and the number of clonogenic cells after 2 weeks' expansion of CB-derived CD34+ cells on control or Ihh stromal cells. (A) Expression of surface antigens on ex vivo–expanded hematopoietic cells. The x-axis indicates CD34 labeled with a FITC-conjugated monoclonal Ab. The y-axis indicates CD11b labeled with a PE-conjugated monoclonal Ab. Positivity for a surface antigen was defined with the use of the isotype control monoclonal Ab. Data shown are from 1 representative experiment of 4 showing similar results. IgG1 indicates immunoglobulin G1. (B) Ex vivo expansion of clonogenic cells in CB CD34+ cells for 2 weeks. Values indicate the number of clonogenic cells for Ihh stroma versus control stroma. *P < .05 versus control. **P < .01 versus control. The number of CFU-Cs and CFU-Mix's before the culture were 1412 ± 369 and 37.5 ± 7.8, respectively, out of the 5000 CB CD34+ cells. The results are expressed as mean ± standard deviation. Data represent 8 independent experiments, each done in quadruplicate.
Next, we assessed the expansion of clonogenic cells after ex vivo expansion of CB CD34+ cells with or without control or Ihh stromal cells. The numbers of CFU-Cs and CFU-Mix's detected 2 weeks after the start of coculture with either control or Ihh stromal cells were significantly higher than those found in cultures grown under stromal-free conditions (Figure 3B). Notably, the number of CFU-Cs and CFU-Mix's in cocultures with Ihh stromal cells was higher than that found in cocultures with control stromal cells. Because hematopoietic cells were sporadically observed beneath the Ihh stromal cell layer as well as beneath the control stromal cells, the cocultures were continued by adding cytokines. At 28 days, some of the hematopoietic cells beneath the stromal cells had divided and exhibited a cobblestone appearance. The number of CAs below Ihh stromal cells was similar to the number found below control stromal cells (68.8 ± 16.5 versus 77.1 ± 14.4 per 5000 of CD34+ cells). This suggests that although CA formation below stromal cells was not affected by expression of Ihh, the ability of hematopoietic progenitor cell support above the Ihh stromal cells was remarkably enhanced.
Blocking of Ihh by the monoclonal Ab 5E1
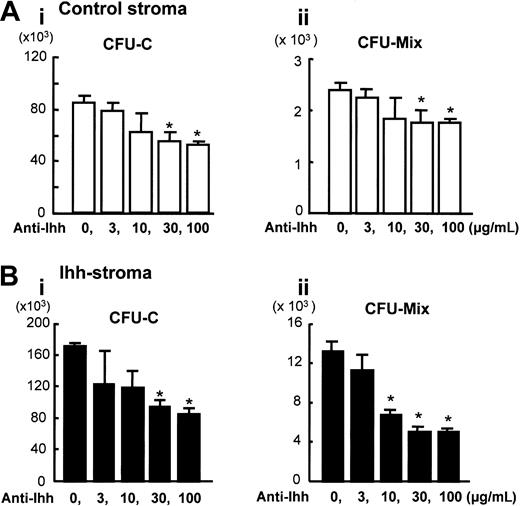
We conducted a blocking study using the neutralizing antihedgehog Ab 5E1, to clarify whether the increased hematopoietic support ability is a direct effect of Ihh. Previous studies using this same antibody have shown its specificity and neutralizing capacity to hedgehog signaling in Ihh proteins.27 We investigated whether the number of hematopoietic cells that expanded on either control or Ihh stromal cells was reduced by the addition of 0 to 100 μg/mL antihedgehog Ab. The number of CFU-Cs and CFU-Mix's expanded on the control stromal layer was significantly reduced by the antihedgehog Ab treatment in a dose-dependent manner, and reduction of the number of colonies leveled out (Figure 4A). These results suggest that the expression level of endogenous Ihh in a control coculture system plays a significant role in inducing expansion of hematopoietic clonogenic cells. In cocultures with Ihh stromal cells, a significant reduction in the number of hematopoietic cells expanded on Ihh stromal cells was observed with respect to the CFU-Cs and CFU-Mix's in the presence of an antihedgehog blocking antibody (Figure 4B). However, the number of hematopoietic progenitor cells did not reach the number of hematopoietic progenitor cells that had expanded on control stromal cells at the same concentration of Ab. One possible explanation of these results is that the dose of antihedgehog blocking antibody is not sufficient to inhibit the activity of Ihh proteins completely. Another possible explanation is that Ihh may affect human stromal cells via a hedgehog receptor complex in a paracrine and autocrine fashion, and an indirect effect elicited by hedgehog expression may be prolonged even in the presence of antihedgehog Ab.
Blocking of Ihh by 5E1 monoclonal Ab in coculture with control or Ihh stromal cells. Expanded hematopoietic cells were harvested at 2 weeks and analyzed. The x-axis indicates the concentration of blocking Ab 5E1, and the y-axis indicates the number of cells. Ai and Bi indicate the total number of CFU-Cs; Aii and Bii, the total number of CFU-Mix's. (A) Ex vivo expansion on control stromal cells. (B) Ex vivo expansion on Ihh stromal cells. *P < .05 versus vehicle (0 μg/mL Ab 5E1) (Student t test). Data represent 4 independent experiments, each done in quadruplicate. Results are expressed as means ± SD.
Blocking of Ihh by 5E1 monoclonal Ab in coculture with control or Ihh stromal cells. Expanded hematopoietic cells were harvested at 2 weeks and analyzed. The x-axis indicates the concentration of blocking Ab 5E1, and the y-axis indicates the number of cells. Ai and Bi indicate the total number of CFU-Cs; Aii and Bii, the total number of CFU-Mix's. (A) Ex vivo expansion on control stromal cells. (B) Ex vivo expansion on Ihh stromal cells. *P < .05 versus vehicle (0 μg/mL Ab 5E1) (Student t test). Data represent 4 independent experiments, each done in quadruplicate. Results are expressed as means ± SD.
It has been reported that expansion of hematopoietic progenitor cells does not always correlate with high engraftment rates of repopulating cells.2 Hence, we further examined the engraftment of repopulating cells (RCs) in NOD/SCID–ß2m–/– mice as a substitute for the in vivo human repopulating cell assay to evaluate the expansion of HSCs.
Engraftment of NOD/SCID–ß2m–/– RCs cocultured with stromal cells
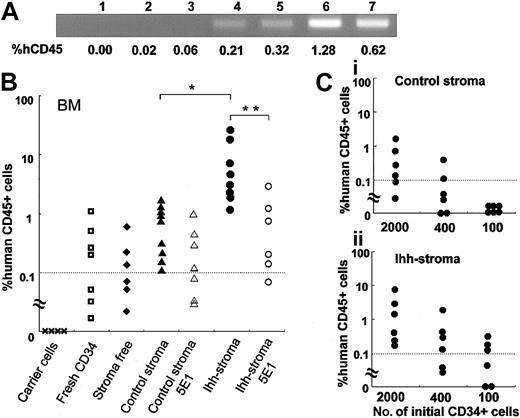
First, 5000 fresh CB CD34+ cells, or all of the expanded hematopoietic cells generated after 2 weeks of coculture with stroma-free, control, or Ihh stromal cells, were transplanted into irradiated NOD/SCID–ß2m–/– mice (the average number of expanded hematopoietic cells injected per mouse was 3.8 × 105, 2.5 × 106, and 3.2 × 106, respectively). Simultaneously, 5 × 106 irradiated PB MNCs were cotransplanted, to approximately equalize the total number of transplanted cells.31 The presence of human cells in the BM of the NOD/SCID–ß2m–/– mice was evaluated by flow cytometry and by PCR analysis to detect ALU sequences. We have previously reported that the cut-off level for detecting the presence of CD45+ human cells in NOD/SCID–ß2m–/– mice was around 0.1%.23 Consistent with our previous report, human ALU gene sequences could be detected by PCR amplification when the proportion of hCD45 was more than 0.21% (Figure 5A, lanes 4-7), while they were not detectable at an hCD45 percentage of 0.06% (Figure 5A, lane 3). Thus, we set the cut-off level at 0.1%. When fresh CB CD34+ cells or hematopoietic cells that had been expanded under stromal-free conditions were transplanted into NOD/SCID–ß2m–/– mice, the reconstitution of human cells was not always observed, but was detected in only 43% (3 of 7) or 50% (3 of 6) of the NOD/SCID–ß2m–/– mice (Figure 5B). These results were consistent with our previous reports showing that the reconstitution frequency of human cells in BM of NOD/SCID mice was not elevated after 2 weeks of expansion in the presence of SCF, TPO, and FL without human stromal cells.23 In clear contrast, when hematopoietic cells that had been expanded on control stromal cells were transplanted into NOD/SCID–ß2m–/– mice, the reconstitution of human hematopoietic cells was detected in all of the NOD/SCID–ß2m–/– mice (9 of 9), although the percentage of hCD45+ cells in the BM of mice receiving transplants of hematopoietic cells that had been cocultured with control stromal cells was similar to that of mice receiving transplants of fresh CB CD34+ cells (Figure 5B). In addition, gene-modified GFP-expressing stromal cells were not detected in BM and spleen of NOD/SCID–ß2m–/– mice, as confirmed by isotype control staining (data not shown). Interestingly, reconstitution of human hematopoietic cells that had been expanded on Ihh stromal cells was observed in all NOD/SCID–ß2m–/– mice (9 of 9) 8 weeks after transplantation, and the percentage of hCD45+ cells in the BM of mice with transplants of hematopoietic cells that had been cocultured with Ihh stromal cells was higher than that with control stromal cells (Figure 5B). Furthermore, the increase in the percentage of hCD45+ cells detected in NOD/SCID–ß2m–/– mice receiving transplants of hematopoietic cells after ex vivo culture with Ihh stromal cells could be reduced if cultures were completed by adding a blocking Ab specific for the Ihh protein (Figure 5B). In addition, although the percentage of hCD45+ cells detected in NOD/SCID–ß2m–/– mice receiving transplants of hematopoietic cells after ex vivo culture with control stromal cells was not reduced in the presence of antihedgehog blocking Ab, the frequency of reconstitution of human cells in BM of NOD/SCID–ß2m–/– mice receiving transplants of hematopoietic cells expanded on Ihh stromal cells was reduced. These results suggest that Ihh protein directly contributes to the higher engraftment in NOD/SCID–ß2m–/– mice observed for hCD45+ cells that had been cocultured with human stromal cells.
Analysis of human genome and the percentage of hCD45 in NOD/SCID–ß2m–/– mice. Analysis of human genome and the percentage of hCD45 in NOD/SCID–ß2m–/– mice. (A) PCR amplification of human ALU sequences was performed to confirm the sequence found in the human genome. Mice (n = 3) with transplants of accessory cells only (lanes 1-3); mice (n = 5) with transplants of fresh CD34+ cells (lanes 4-7). The percentage of hCD45 indicates the percentage of the human CD45+ hematopoietic cells in the BM of mice with transplants. (B) Analyses of hCD45+ cells in the BM of NOD/SCID–ß2m–/– mice with transplants. Irradiated carrier cells, fresh CD34+ cells, or CD34+ cells that had been expanded on control stromal cells or Ihh stromal cells, were transplanted into NOD/SCID–ß2m–/– mice. Mice were killed 8 weeks after transplantation, and the BM cells were analyzed by flow cytometry. Data are shown for carrier cells; fresh CD34+ cells; CD34+ cells that had been expanded ex vivo in the absence of stromal cells for 2 weeks; CD34+ cells that had been expanded on control stromal cells for 2 weeks; CD34+ cells that had been expanded on control stromal cells for 2 weeks in the presence of anti-Ihh blocking Ab; CD34+ cells that had been expanded on Ihh stromal cells for 2 weeks; and CD34+ cells that had been expanded on Ihh stromal cells for 2 weeks in the presence of anti-Ihh blocking Ab. A dotted line indicates the cut-off level (0.1%) for successful engraftment of human hematopoietic cells. *P < .05 versus control stromal cells. **P < .05 versus Ihh stromal cells (Mann-Whitney U test). Data represent a summary of 2 separate experiments. (C) Engraftment of a graded dose of CD34+ cells at the start of culture and their progeny after 2 weeks. Percentage of engraftment of human CD45+ cells that had been expanded with control stromal cells (i), or Ihh stromal cells (ii) is shown. Dotted lines indicate cutoff level (0.1%).
Analysis of human genome and the percentage of hCD45 in NOD/SCID–ß2m–/– mice. Analysis of human genome and the percentage of hCD45 in NOD/SCID–ß2m–/– mice. (A) PCR amplification of human ALU sequences was performed to confirm the sequence found in the human genome. Mice (n = 3) with transplants of accessory cells only (lanes 1-3); mice (n = 5) with transplants of fresh CD34+ cells (lanes 4-7). The percentage of hCD45 indicates the percentage of the human CD45+ hematopoietic cells in the BM of mice with transplants. (B) Analyses of hCD45+ cells in the BM of NOD/SCID–ß2m–/– mice with transplants. Irradiated carrier cells, fresh CD34+ cells, or CD34+ cells that had been expanded on control stromal cells or Ihh stromal cells, were transplanted into NOD/SCID–ß2m–/– mice. Mice were killed 8 weeks after transplantation, and the BM cells were analyzed by flow cytometry. Data are shown for carrier cells; fresh CD34+ cells; CD34+ cells that had been expanded ex vivo in the absence of stromal cells for 2 weeks; CD34+ cells that had been expanded on control stromal cells for 2 weeks; CD34+ cells that had been expanded on control stromal cells for 2 weeks in the presence of anti-Ihh blocking Ab; CD34+ cells that had been expanded on Ihh stromal cells for 2 weeks; and CD34+ cells that had been expanded on Ihh stromal cells for 2 weeks in the presence of anti-Ihh blocking Ab. A dotted line indicates the cut-off level (0.1%) for successful engraftment of human hematopoietic cells. *P < .05 versus control stromal cells. **P < .05 versus Ihh stromal cells (Mann-Whitney U test). Data represent a summary of 2 separate experiments. (C) Engraftment of a graded dose of CD34+ cells at the start of culture and their progeny after 2 weeks. Percentage of engraftment of human CD45+ cells that had been expanded with control stromal cells (i), or Ihh stromal cells (ii) is shown. Dotted lines indicate cutoff level (0.1%).
We next conducted limiting dilution analysis to more carefully investigate whether increased engraftment of hCD45+ cells detected in NOD/SCID–ß2m–/– mice resulted from the expansion of NOD/SCID–ß2m–/– RCs. The cultured hematopoietic cells that had been derived from 2000, 400, or 100 CD34+ cells expanded on control stromal cells were transplanted (the average number of expanded hematopoietic cells injected per mouse was 1.0 × 106, 2.1 × 105, or 5.2 × 104, respectively), and at limiting dilution, engraftment was observed in 4 of 6 mice, 2 of 6 mice, and 0 of 6 mice, respectively. On the other hand, when the cultured hematopoietic cells that had been derived from 2000, 400, or 100 CD34+ cells expanded on Ihh stromal cells were transplanted (the average number of cells injected per mouse was 1.3 × 10,6 2.6 × 10,5 and 6.5 × 10,4 respectively), engraftment was observed in 6 of 6 mice, 4 of 6 mice, and 3 of 6 mice, respectively. A higher frequency of reconstitution of human cells in BM of NOD/SCID–ß2m–/– mice receiving transplants of hematopoietic cells expanded on Ihh stromal cells was observed, as compared with reconstitution rates of cells expanded on control stromal cells, when a lower cell dose was transplanted. The degree of expansion on Ihh stromal cells was estimated to be 6.6-fold greater than that of control stromal cells, according to the Poisson predicted frequency. These results suggest that Ihh stromal cells significantly amplified the NOD/SCID–ß2m–/– RCs in CB CD34+ cells compared with control stromal cells.
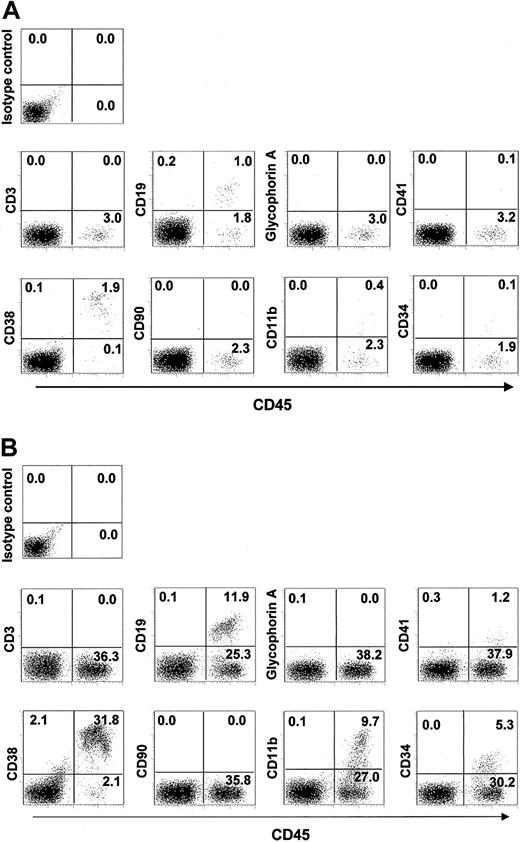
We further examined the surface markers on hematopoietic cells that had differentiated from NOD/SCID–ß2m–/– RCs expanded on Ihh stromal cells, and compared them with markers on cells that were expanded on control stromal cells. A representative flow cytometric analysis of highly reconstituted mice is shown in Figure 6A (control stroma) and 6B (Ihh stroma). No GFP+ stromal cells in BM (Figure 6) were detected by isotope control staining, indicating that no GFP+ stromal cells were engrafted in BM of NOD/SCID–ß2m–/– mice. Most cells were CD19+ B-lymphoid or CD11b myeloid-lineage cells, but CD3+ cells were not detected. The expression pattern of surface antigens on hematopoietic cells that had been cocultured with Ihh stromal cells (Figure 6B) was substantially similar to that of hematopoietic cells that had been cocultured with control stromal cells (Figure 6A). This suggested that Ihh stromal cells, as well as control stromal cells, support NOD/SCID–ß2m–/– lymphomyeloid RCs 8 weeks after tranplantation. However, engraftment of NOD/SCID–ß2m–/– RCs was drastically diminished by 13 weeks after transplantation of hematopoietic cells that had been expanded on either Ihh stromal cells or control stromal cells, although engraftment of human hematopoietic cells that were expanded on Ihh stromal cells is higher than that for cells expanded on control stromal cells (percentage of CD45+ cells, 3.28 ± 0.60 versus not detected). These results suggest that Ihh stromal cells dominantly support human repopulating RCs that were seen in NOD/SCID–ß2m–/– mice for up to 8 weeks following transplantation.
Flow cytometric analysis of lineage markers on human hematopoietic cells engrafted in NOD/SCID– ß2m–/– mice. CD34+ cells were expanded for 2 weeks on control stromal cells (panel A), or on Ihh stromal cells (panel B), and then transplanted into NOD/SCID–ß2m–/– mice. Hematopoietic cells were immunolabeled with a FITC-conjugated hCD45 antibody to ensure human origin, and with a PE-conjugated antibody specific for the indicated lineage marker. Data shown are from 1 representative experiment of 4 showing similar results. Numbers in each quadrant indicate percentages of hematopoietic cells.
Flow cytometric analysis of lineage markers on human hematopoietic cells engrafted in NOD/SCID– ß2m–/– mice. CD34+ cells were expanded for 2 weeks on control stromal cells (panel A), or on Ihh stromal cells (panel B), and then transplanted into NOD/SCID–ß2m–/– mice. Hematopoietic cells were immunolabeled with a FITC-conjugated hCD45 antibody to ensure human origin, and with a PE-conjugated antibody specific for the indicated lineage marker. Data shown are from 1 representative experiment of 4 showing similar results. Numbers in each quadrant indicate percentages of hematopoietic cells.
Discussion
In the present study, Ihh and its receptor molecules were expressed in stromal cells and/or in CB CD34+ cells. Gene transfer of Ihh augments the support ability of human stromal cells for progenitor cells, as well as for NOD/SCID–ß2m–/– RCs, via direct effects elicited by the Ihh proteins.
Recently, evidence for the proliferative effect of hedgehog proteins, which have been examined primarily in the case of the sonic hedgehog protein, has accumulated for a variety of cell types, including T lymphocytes,32 HSCs, and ectoderm-derived cells such as neural progenitors and keratinocytes.32-34 Hedgehog family proteins have also been shown to function in distinct aspects of embryogenesis, and the tissue distribution of individual members is largely separated or else only partially overlapping.21,22 Thus, it has been unclear which specific hedgehogs function in the BM microenvironment. In the present study, the expression of Ihh, but not Shh, was detected in bone marrow stromal cells and in stromal cells that express high levels of Ihh protein efficiently and that expand primitive hematopoietic cells. Furthermore, these proliferative effects were neutralized by an antihedgehog blocking Ab. These results indicate that Ihh contributes to the proliferation of primitive hematopoietic cells. Moreover, the proliferative effect of hematopoietic cells, even in coculture with control stromal cells, was neutralized with the same blocking Ab, suggesting that expression of Ihh in either CD34+ cells or human stromal cells contributes to the proliferation of HSCs in general coculture conditions. Because the expression level of Ihh in CD34+ cells is higher than that of control stromal cells, Ihh expressed in CD34+ cells may be more significant than that released from human stromal cells.
It was demonstrated by Glimm et al35 that NOD/SCID–ß2m–/– mice are sequentially engrafted by 2 distinct populations of transplantable human short-term repopulating hematopoietic cells (STRCs) in addition to long-term repopulating hematopoietic cells (LTRCs), which equivalently engraft either NOD/SCID mice or NOD/SCID–ß2m–/– mice. One of these populations is myeloid-restricted STRC (STRC-M), which produces the large and rapid but transient burst of erythroid dominant cells seen in the first 3 weeks after transplantation. The other population is dual myeloid- and lymphoid-repopulating STRC (STRC-ML), which engrafts 6 to 8 weeks after transplantation but declines rapidly by 13 weeks. In the present study, we observed a higher frequency of reconstitution of human cells in the BM of NOD/SCID–ß2m–/– mice that had received transplants of hematopoietic cells expanded on Ihh stromal cells 8 weeks after transplantation, but this declined drastically by 13 weeks. It was therefore reasonable to assume that Ihh stromal cells dominantly expand STRC-MLs. It was shown that STRCs are markedly higher in mobilized peripheral blood as compared with BM and CB, and this elevated STRC content of mobilized peripheral blood could explain the apparently faster rates of hematologic recovery that they typically achieve clinically.35,36 Therefore, coculture with Ihh stromal cells and CB leads to expansion of STRCs, and this could compensate for delayed recovery of myelo-suppression after CB transplantation.3 Further study using larger animal models will be required to elucidate whether the expanded STRCs actually contribute to early reconstitution of the BM.
Finally, aberrant hedgehog-pathway activation, which can result from mutations in the hedgehog receptor complex, has been associated with proliferative diseases, including basal cell carcinoma,37 medulloblastoma,38 and small-cell lung cancer.39 Furthermore, the molecular basis by which hedgehog accelerates the cell cycle, via the induction of cyclin D and cyclin E, has been reported recently.40 Nevertheless, Ihh stromal cells, in which Ihh should work in a paracrine and/or autocrine fashion, did not exhibit growth acceleration. Moreover, they did not lose contact inhibition either in serum-free conditions or in LTC medium. The morphologic characteristics and surface antigens, including SH2 and SH3, of Ihh stromal cells did not change compared with the control stromal cells (Figure 2A). There were no indications that Ihh stromal cells undergo transformation. Furthermore, tumor formation of Ihh stromal cells, as well as control stromal cells, was not observed in any tissue after transplantation through intravenous and intraperitoneal injection (data not shown). Transformation and tumorigenesis of cells may be induced by continuous hedgehog signaling, and this may in turn be a consequence of point mutations in signaling components.41 In addition, the outcome of hedgehog signaling on cell growth may be different for various cell types.
In conclusion, transfer of the Ihh gene can confer enhanced hematopoietic support ability to human stromal cells. These finding may be useful for understanding the role of hedgehog signaling in the hematopoietic microenvironment, and there are potential medical applications of Ihh in the regulation of hematopoiesis.
Prepublished online as Blood First Edition Paper, April 22, 2004; DOI 10.1182/blood-2003-09-3347.
Supported in part by a grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan (H.H.), and a grant from the Ministry of Health, Labor and Welfare of Japan (M.K.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Hiroshi Isogai, DVM, PhD; Ms Noriko Kawano; Mr Mitsuhiro Sasaki; and the staff of the animal facility at Sapporo Medical University for care of the NOD/SCID–ß2m–/– mice colony. We also thank Ms Sumiyo Asakura and Ms Hanae Inoue for cloning and preparation of the Ihh expression vectors.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal