Abstract
Intensity of the preparative regimen is an important component of allogeneic transplantations for myelodysplasia (MDS) or acute myelogenous leukemia (AML). We compared outcomes after a truly nonablative regimen (120 mg/m2 fludarabine, 4 g/m2 cytarabine, and 36 mg/m2 idarubicin [FAI]) and a more myelosuppressive, reduced-intensity regimen (100 to 150 mg/m2 fludarabine and 140 or 180 mg/m2 melphalan [FM]). We performed a retrospective analysis of 94 patients with MDS (n = 26) and AML (n = 68) treated with FM (n = 62) and FAI (n = 32). The FAI group had a higher proportion of patients in complete remission (CR) at transplantation (44% versus 16%, P = .006), patients in first CR (28% versus 3%, P = .008), and HLA-matched sibling donors (81% versus 40%, P = .001). Median follow-up is 40 months. FM was significantly associated with a higher degree of donor cell engraftment, higher cumulative incidence of treatment-related mortality (TRM; P = .036), and lower cumulative incidence of relapse-related mortality (P = .029). Relapse rate after FAI and FM was 61% and 30%, respectively. Actuarial 3-year survival rate was 30% after FAI and 35% following FM. In a multivariate analysis of patient- and treatment-related prognostic factors, progression-free survival was improved after FM, for patients in CR at transplantation, and for those with intermediate-risk cytogenetics. Survival was improved for patients in CR at transplantation. In conclusion, FM provided better disease control though at a cost of increased TRM and morbidity.
Introduction
High-dose chemoradiotherapy with allogeneic bone marrow transplantation has been extensively used in young patients with acute myeloid leukemia (AML) and high-risk myelodysplastic syndromes (MDS). Approximately 50% to 70% of patients can achieve long-term disease control when undergoing transplantation in early phases of the disease, with transplantation-related deaths accounting for 20% to 30% of treatment failures.1 Allogeneic progenitor cell transplantation (APCT) has been limited to young patients due to the increased risk of regimen-related toxicities and graft versus host disease (GVHD) that occurs with increasing age. Improvements in supportive care have now enabled many centers to increase the age limit forAPCT, but few centers are performing transplantations in patients older than 55 to 60 years of age.2 These patients, however, represent a significant fraction of subjects with these diseases, with low response and cure rates with conventional chemotherapy3 and poor tolerance to myeloablative conditioning regimens. Thus, novel transplantation therapies need to be explored to increase the applicability of this procedure for most patients with myeloid leukemias.
The definition of a truly nonablative regimen is one that can be given routinely without stem cell support, with neutrophil recovery within 28 days, and one in which mixed chimerism can be routinely detected early after transplantation.4,5 A variety of nonablative transplantation regimens have been proposed. Some of these regimens are minimally myelosuppressive and therefore are truly nonablative.6-8 Other dose-reduced regimens cannot be safely administered without stem cell support; these latter regimens have been termed “reduced-intensity” conditioning regimens and usually involve a combination of a purine analog (primarily fludarabine) with an alkylating agent (usually melphalan or busulfan).9,10 These reduced-intensity regimens are generally considered to include less than 16 mg/kg busulfan or less than 10 Gy total body irradiation (TBI). They have been usually associated with prompt engraftment of donor cells procured from both HLA-matched related and unrelated donors, while the truly nonablative regimens have been associated with a varying degree of mixed chimerism and a higher risk of primary and secondary graft failures.5,10
At MD Anderson Cancer Center, both reduced-intensity and truly nonablative regimens for the treatment of myeloid leukemias have been explored. To determine whether in AML and MDS a reduced-intensity conditioning regimen would result in lower relapse rates than a truly nonablative regimen, a retrospective analysis of transplantation outcomes was performed to address this question. Herein are the results of that analysis.
Patients and methods
Eligibility
Patients were included in this study if they had either AML or high-risk MDS and had undergone an allogeneic progenitor cell transplantation from an HLA-compatible donor with either a truly nonablative regimen of fludarabine, cytarabine (araC), and idarubicin (FAI) or with a reducedintensity conditioning regimen with fludarabine in combination with melphalan 140 or 180 mg/m2 (FM140 or FM180).
Although FAI and FM protocols coexisted in our institution for several years with overlapping eligibility criteria, FAI was intended to be the treatment of choice for older patients with sibling donors and who were in remission, with a high risk of relapse. This was due to the low intensity of the regimen, perceived as adequate for patients with early disease. The FAI protocol started accruing patients in June 1995. Eligibility criteria included availability of a sibling donor (HLA-A, -B, and -DRB1 matched or with 1 mismatch), age older than 55 years, or presence of organ dysfunction precluding treatment with high-dose radiation or chemotherapy. No unrelated donors were allowed.
The first FM study started accrual in February 1996 and the second study, comparing FM140 and FM180, in November 1997. The 2 doses of melphalan were investigated in an attempt to minimize toxicities observed with FM180. The lower age limit for participation in the FM studies was 55 years, but younger patients with organ dysfunction that made them ineligible for high-dose treatment protocols were also eligible. Both related and unrelated donors were allowed. Patients with more advanced disease were preferably treated with FM in order to provide higher dose intensity in the preparative regimen.
Patient and treatment characteristics
From June 1995 until December 2000, a total of 94 patients with AML or high-risk MDS underwent APCT with FAI or with FM. Their characteristics are summarized in Table 1.
Characteristics of patients treated with FAI or FM
Characteristics . | FAI . | FM . | P . |
|---|---|---|---|
| No. patients | 32 | 62 | |
| Average age, y (range) | 61 (27-74) | 54 (22-75) | .001 |
| No. female (%)/no. male (%) | 18 (56)/14 (44) | 29 (47)/33 (53) | .514 |
| Average mos. from diagnosis to treatment (range) | 10 (1.1-48.6) | 12 (0.9-173.6) | .662 |
| No. prior therapies (range) | 2 (0-4) | 2 (0-8) | .399 |
| Diagnosis, no. (%) | |||
| AML | 26 (81) | 42 (68) | |
| MDS | 6 (19) | 20 (32) | .253 |
| Stage at transplantation, no. (%) | |||
| Remission | 14 (44) | 10 (16) | .006 |
| Not in remission | 18 (56) | 52 (84) | |
| First remission at transplantation, no. (%) | 9 (28) | 2 (3) | .0008 |
| Donor type, no. (%) | |||
| Matched sibling | 26 (81) | 25 (40) | |
| Mismatched related | 6 (19) | 8 (13) | <.001 |
| Matched unrelated | 0 (0) | 29 (47) | |
| Stem cell source, no. (%) | |||
| Bone marrow | 27 (84) | 26 (42) | <.001 |
| Peripheral blood | 5 (16) | 36 (58) |
Characteristics . | FAI . | FM . | P . |
|---|---|---|---|
| No. patients | 32 | 62 | |
| Average age, y (range) | 61 (27-74) | 54 (22-75) | .001 |
| No. female (%)/no. male (%) | 18 (56)/14 (44) | 29 (47)/33 (53) | .514 |
| Average mos. from diagnosis to treatment (range) | 10 (1.1-48.6) | 12 (0.9-173.6) | .662 |
| No. prior therapies (range) | 2 (0-4) | 2 (0-8) | .399 |
| Diagnosis, no. (%) | |||
| AML | 26 (81) | 42 (68) | |
| MDS | 6 (19) | 20 (32) | .253 |
| Stage at transplantation, no. (%) | |||
| Remission | 14 (44) | 10 (16) | .006 |
| Not in remission | 18 (56) | 52 (84) | |
| First remission at transplantation, no. (%) | 9 (28) | 2 (3) | .0008 |
| Donor type, no. (%) | |||
| Matched sibling | 26 (81) | 25 (40) | |
| Mismatched related | 6 (19) | 8 (13) | <.001 |
| Matched unrelated | 0 (0) | 29 (47) | |
| Stem cell source, no. (%) | |||
| Bone marrow | 27 (84) | 26 (42) | <.001 |
| Peripheral blood | 5 (16) | 36 (58) |
FAI indicates fludarabine, araC, and idarubicin; FM, fludarabine and melphalan.
All subjects signed written informed consents and were treated under MD Anderson Cancer Center protocols approved by the institutional review board. Patients received 1 of 3 preparative regimens. The truly nonablative regimen consisted of a combination of fludarabine 30 mg/m2 given intravenously daily for 4 days followed 4 hours later by an infusion of cytarabine 1 g/m2. In addition to these 2 agents, patients also received idarubicin 12 mg/m2 intravenously daily for 3 days. Patients receiving the reduced-intensity conditioning regimen received either fludarabine 25 to 30 mg/m2 for 4 to 5 days in combination with melphalan 140 or 180 mg/m2 total dose
GVHD prophylaxis consisted of a combination of tacrolimus (FK506, Prograf, Fujisawa, Deerfield, IL) or cyclosporine and methotrexate 5 mg/m2 intravenously on days 1, 3, 6, and 11 after transplantation. Tacrolimus or cyclosporine levels were monitored 3 times a week and kept at therapeutic ranges of 5 to 15 ng/dL for tacrolimus and 150 to 300 ng/dL for cyclosporine during the first 50 days and then tapered at the discretion of the primary physician depending on donor type, disease status at time of transplantation, presence or absence of GVHD, and presence of residual donor cells as documented by chimerism or cytogenetic analysis.
Antibacterial, antifungal, and antiviral prophylaxis consisted of trimethoprim-sulfamethoxazole for Pneumocystis carinii prophylaxis, acyclovir or valacyclovir for herpes simplex prophylaxis, and surveillance cytomegalorivus (CMV) antigenemia testing for all patients with preemptive use of ganciclovir in the event of a positive antigenemia test. All patients received filgrastim (granulocyte colony-stimulating factor [G-CSF], Neupogen, Amgen, Thousand Oaks, CA) 5 mg/kg subcutaneously daily from day +7 until achievement of an absolute neutrophil count above 1.5 × 109/L for 3 days. Patients who developed grade 2 or greater acute GVHD received methylprednisolone at least 0.5 mg/kg every 6 hours and, if possible, were enrolled on treatment protocols for GVHD. Packed red blood cells were administered to maintain hemoglobin levels greater or equal to 80 g/L (8 g/dL). Platelet transfusions were administered to keep the platelet count at a level of greater than or equal to 10 × 109/L. All blood products were filtered and irradiated.
Donor stem cells or bone marrow were procured using standard mobilization protocols and pheresis techniques. The stem cells or bone marrow from all related donors were collected at the University of Texas MD Anderson Cancer Center, and they were processed according to current institutional guidelines and protocols. All healthy donors signed written informed consent for the procedure. Bone marrow procured from unrelated donors was obtained through the National Marrow Donor Program (NMDP) according to applicable guidelines at the time of procurement. As required by the NMDP, donors gave consent at the donor center after an extensive screening and information process.
Statistical considerations
The major end points of this retrospective analysis were to evaluate the 2 regimens in terms of progression-free survival (PFS) after allogeneic transplantation, nonrelapse mortality, and relapse mortality. Relapse was defined by either cytogenetic or morphologic criteria. Secondary end points included engraftment, chimerism, and overall survival (OS).
We used the methods of Kaplan and Meier11 to estimate the median OS and the median PFS. The Cox12 proportional hazards regression model was then used to test the statistical significance of several potential prognostic factors for OS and for PFS. This modeling was done in a univariate fashion. From this model we estimated the hazard ratio for each potential prognostic factor with a 95% confidence interval. All potential prognostic factors, excluding those measured after treatment, were then included in a saturated model, and backward elimination was used to remove factors from the model based on the likelihood ratio test in the multiple regression analysis. We kept the variable to indicate the treatment regimen in the model, even though it may not have been statistically significant, because it was our aim to estimate the effect of the treatment regimen while adjusting for the other potential prognostic factors. We also included in the multivariate model other well-known patient- and treatment-related prognostic factors such as cytogenetics, disease status at transplantation, use of a matched sibling donor, and recipient age, although some of these are not statistically significant in our model. The median age was selected as the cut point for age in the model.
For disease response (complete remission [CR] or continuous CR [CCR] versus no CR or CCR) and chimerism (100% donor versus less than 100%) we performed a landmark analysis. All patients who died before study day 30 were removed from the analysis of overall survival, and all patients who died or had progressive disease before study day 30 were excluded from the analysis of PFS. OS and PFS were then measured from study day 30.
Non–relapse-related mortality and relapse-related mortality were considered competing risks for mortality. The cumulative incidence of non–relapserelated mortality and relapse-related mortality was calculated using the methods of Gooley et al.13
Patient characteristics were compared among treatment groups by the χ2 test or Fisher exact test for categorical variables and by the t test or Wilcoxon test for variables measured on a continuous scale. PFS and overall survival were defined as previously described.11,14 The 2-sided log-rank test was used to test the univariate association between variables and PFS, overall survival, and nonrelapse mortality. Acute and chronic GVHD were evaluated as time-dependent covariates. Engrafted patients surviving more than 100 days were considered to be at risk for chronic GVHD, while all engrafted patients were considered at risk for acute GVHD. All P values presented are 2-sided. Statistical analyses were carried out using SAS 1999-2001 (SAS Institute, Cary, NC) and S-PLUS 2000 (MathSoft, Seattle, WA) software.
Human leukocyte antigen (HLA) typing
HLA typing for class I antigens was performed using standard serologic techniques. Low-resolution molecular typing using hybridization techniques of amplified sampled DNA with sequence-specific oligonucleotide probes, followed by high-resolution molecular typing using polymerase chain reaction in the sampled DNA with sequence-specific primers, was performed for class II alleles (HLA-DRB1, -DQB1) in all patients and as indicated for selected class I loci.
Analysis of chimerism
Hematopoietic chimerism was evaluated on bone marrow cells usually at the first, third, and 12th month after transplantation by restriction fragment length polymorphisms (RFLP) at the AY-29 or YNH24 loci, by conventional cytogenetic analysis by G-banding, or fluorescent in situ hybridization studies in sex-mismatched cases for Y chromosome.15,16 Autologous reconstitution was defined as lack of evidence of donor cell engraftment by cytogenetic and/or molecular analysis, in the absence of molecular or morphologic evidence of disease recurrence.
Results
Regimens
Recipients of FM140 or FM180 had similar age and disease characteristics and a comparable proportion of unrelated donor transplantations (55% and 43%, respectively, P = .4). Outcomes were similar after treatment with the 2 doses of melphalan, and patients receiving FM140 or FM180 were analyzed together.
When compared with patients receiving FM, those treated with FAI were older (61 years vs 54 years, P = .001) and had a higher proportion of patients in remission at the time of treatment (44% vs 16%, P = .006). Furthermore, the proportion of patients in first CR was also significantly higher in the FAI group (28% vs 3%, P = .0008). They also received bone marrow as the source of stem cells for transplantation more often than those treated with FM (85% vs 42%, P < .001) (Table 1).
Engraftment and chimerism
Table 2 summarizes engraftment and chimerism data according to diagnosis and preparative regimen. Neutrophil recovery occurred at a median of 14 days and was not significantly affected by either diagnosis or conditioning regimen. Platelet recovery was faster in patients receiving the less intense regimen of FAI versus FM (17 vs 20 days).
Engraftment and chimerism according to preparative regimen
. | FAI . | FM . | P . |
|---|---|---|---|
| No. of patients | 32 | 62 | NS |
| Median d to ANC 0.5 × 109/L (range) | 14.5 (10-38) | 14 (11-100) | NS |
| Median d to platelet 20 × 109/L (range) | 17 (10-78) | 20 (6-49) | NS |
| Mean % donor cells on d 30 (no. patients) | 79.6 (29) | 95 (50) | .015 |
| % donor cells on d 90 (no. patients) | 66 (24) | 89 (40) | .009 |
| % donor cells on d 360 (no. patients) | 71 (7) | 95 (23) | .08 |
| % autologous reconstitution or graft failure (no. patients) | 19 (6) | 3 (2) | .03 |
. | FAI . | FM . | P . |
|---|---|---|---|
| No. of patients | 32 | 62 | NS |
| Median d to ANC 0.5 × 109/L (range) | 14.5 (10-38) | 14 (11-100) | NS |
| Median d to platelet 20 × 109/L (range) | 17 (10-78) | 20 (6-49) | NS |
| Mean % donor cells on d 30 (no. patients) | 79.6 (29) | 95 (50) | .015 |
| % donor cells on d 90 (no. patients) | 66 (24) | 89 (40) | .009 |
| % donor cells on d 360 (no. patients) | 71 (7) | 95 (23) | .08 |
| % autologous reconstitution or graft failure (no. patients) | 19 (6) | 3 (2) | .03 |
ANC indicates absolute neutrophil count; NS, not significant.
The day +30 mixed chimerism rate was 50% among FAI group survivors and 14% after treatment with FM. The degree of donor cell engraftment was significantly higher in survivors receiving the more intense FM regimens than in those receiving the FAI regimen at all time points analyzed. Likewise, the incidence of autologous reconstitution was also significantly higher among patients receiving the less intensive FAI conditioning.
Relapse, overall survival, and progression-free survival
Thirty-eight patients relapsed, with a median disease-free survival of 95 days. Sixty-three percent of the patients treated with FAI relapsed, while 28% and 30% of the patients treated with FM140 and FM180, respectively, had disease recurrence. Mixed chimerism was associated with relapse in both groups; 71% of patients with detectable recipient DNA or cells on the first assessment (day +30) relapsed, versus 29% of those without it. The relapse rate was identical among patients with mixed chimerism in the FAI and FM groups (71%).
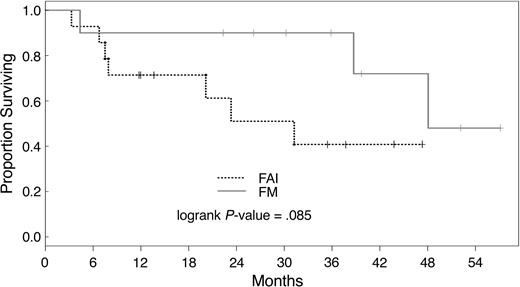
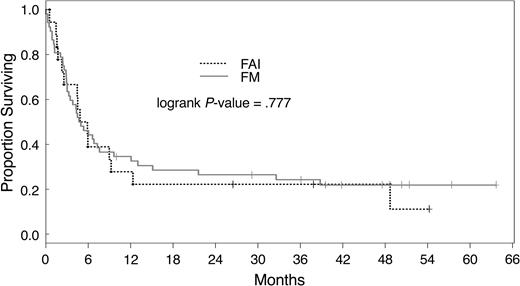
Three-year actuarial PFS was 32% and 19%, respectively, for patients treated with FM and FAI. Median overall survival was 230 days; 29 patients are alive with a median follow-up of 40 months (range, 10 to 64 months). There was no statistically significant difference in actuarial survival after conditioning with the different regimens used here (P = .790). The overall survival and PFS rate at 3 years was 34% and 27%, respectively, for all patients. Patients not in remission had a 3-year overall survival of 23% versus 40% as compared with patients in CR at transplantation (P = .0001). Overall survival by disease status after treatment with FAI and FM is shown in Figures 1 and 2. Actuarial 3-year PFS rates were 20% and 33% for patients with active disease and in CR, respectively (P = .001).
Overall survival of patients in remission at transplantation. Survival of patients in remission at transplantation after conditioning with FM or FAI. FM indicates fludarabine and melphalan; FAI indicates fludarabine, araC, and idarubicin.
Overall survival of patients in remission at transplantation. Survival of patients in remission at transplantation after conditioning with FM or FAI. FM indicates fludarabine and melphalan; FAI indicates fludarabine, araC, and idarubicin.
Overall survival of patients with active disease at transplantation. Overall survival of patients with active disease at transplantation was similar in both treatment groups. FM indicates fludarabine and melphalan; FAI indicates fludarabine, araC, and idarubicin.
Overall survival of patients with active disease at transplantation. Overall survival of patients with active disease at transplantation was similar in both treatment groups. FM indicates fludarabine and melphalan; FAI indicates fludarabine, araC, and idarubicin.
Toxicity, nonrelapse mortality, graft versus host disease, and causes of death
Grade III toxicity (Bearman criteria17 ) occurred in 1 patient in the FAI group (3%), while 18 episodes were documented in patients treated with FM (P = .002, 2-tailed Fisher exact test, for the comparison of grade III-IV toxicity among FAI versus FM). Pulmonary toxicity occurred only after treatment with FM, and all regimen-related deaths occurred within the FM group (n = 6). Table 3 details toxicity data.
Toxicity*by regimen
. | FAI, no. (%) . | . | . | . | FM†, no. (%) . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Toxicity . | Grade I . | Grade II . | Grade III . | Grade IV . | Grade I . | Grade II . | Grade III . | Grade IV† . | ||||||
| Hepatic | ||||||||||||||
| Transaminase elevation | 8 (25) | 6 (19) | 0 (0) | 0 (0) | 31 (50) | 13 (21) | 1 (2) | 0 (0) | ||||||
| Bilirubin elevation | 4 (12) | 1 (3) | 0 (0) | 0 (0) | 9 (15) | 2 (3) | 0 (0) | 0 (0) | ||||||
| Gastrointestinal tract | ||||||||||||||
| Diarrhea | 8 (25) | 0 (0) | 0 (0) | 0 (0) | 26 (42) | 2 (3) | 1 (2) | 0 (0) | ||||||
| Nausea and vomiting | 18 (56) | 2 (6) | 0 (0) | 0 (0) | 44 (71) | 1 (2) | 2 (3) | 0 (0) | ||||||
| Mucositis | 12 (38) | 4 (13) | 0 (0) | 0 (0) | 20 (32) | 7 (11) | 1 (2) | 0 (0) | ||||||
| Pancreatitis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2) | 0 (0) | ||||||
| Urinary tract/kidney | ||||||||||||||
| Creatinine elevation | 10 (31) | 3 (9) | 1 (3) | 0 (0) | 15 (24) | 15 (24) | 2 (3) | 0 (0) | ||||||
| Hemorrhagic cystitis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (3) | 1 (2) | 0 (0) | ||||||
| Neurological | ||||||||||||||
| Central nervous system bleeding | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2) | ||||||
| Torpor | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2) | 0 (0) | ||||||
| Pulmonary | ||||||||||||||
| Pulmonary hemorrhage | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (3) | 0 (0) | 5 (8) | 1 (2) | ||||||
| Pulmonary embolism | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2) | 0 (0) | ||||||
| Pneumonitis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2) | 0 (0) | ||||||
| Pulmonary edema | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2) | ||||||
| Cardiovascular | ||||||||||||||
| Decreased left ventricular ejection fraction | 1 (3) | 1 (3) | 0 (0) | 0 (0) | 2 (3) | 2 (3) | 2 (3) | 2 (3) | ||||||
. | FAI, no. (%) . | . | . | . | FM†, no. (%) . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Toxicity . | Grade I . | Grade II . | Grade III . | Grade IV . | Grade I . | Grade II . | Grade III . | Grade IV† . | ||||||
| Hepatic | ||||||||||||||
| Transaminase elevation | 8 (25) | 6 (19) | 0 (0) | 0 (0) | 31 (50) | 13 (21) | 1 (2) | 0 (0) | ||||||
| Bilirubin elevation | 4 (12) | 1 (3) | 0 (0) | 0 (0) | 9 (15) | 2 (3) | 0 (0) | 0 (0) | ||||||
| Gastrointestinal tract | ||||||||||||||
| Diarrhea | 8 (25) | 0 (0) | 0 (0) | 0 (0) | 26 (42) | 2 (3) | 1 (2) | 0 (0) | ||||||
| Nausea and vomiting | 18 (56) | 2 (6) | 0 (0) | 0 (0) | 44 (71) | 1 (2) | 2 (3) | 0 (0) | ||||||
| Mucositis | 12 (38) | 4 (13) | 0 (0) | 0 (0) | 20 (32) | 7 (11) | 1 (2) | 0 (0) | ||||||
| Pancreatitis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2) | 0 (0) | ||||||
| Urinary tract/kidney | ||||||||||||||
| Creatinine elevation | 10 (31) | 3 (9) | 1 (3) | 0 (0) | 15 (24) | 15 (24) | 2 (3) | 0 (0) | ||||||
| Hemorrhagic cystitis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (3) | 1 (2) | 0 (0) | ||||||
| Neurological | ||||||||||||||
| Central nervous system bleeding | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2) | ||||||
| Torpor | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2) | 0 (0) | ||||||
| Pulmonary | ||||||||||||||
| Pulmonary hemorrhage | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (3) | 0 (0) | 5 (8) | 1 (2) | ||||||
| Pulmonary embolism | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2) | 0 (0) | ||||||
| Pneumonitis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2) | 0 (0) | ||||||
| Pulmonary edema | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2) | ||||||
| Cardiovascular | ||||||||||||||
| Decreased left ventricular ejection fraction | 1 (3) | 1 (3) | 0 (0) | 0 (0) | 2 (3) | 2 (3) | 2 (3) | 2 (3) | ||||||
Bearman criteria17
One patient died of multiorgan failure
The risk of nonrelapse mortality for all patients was 30% at 1 year. Univariate analysis of risk factors for nonrelapse mortality demonstrated that patients receiving the more intense FM regimen as well as patients not in remission at the time of transplantation, recipients of cells from donors other than HLA-identical siblings, and those with poor-risk cytogenetics had a higher risk of nonrelapse mortality than patients without those risk factors (Table 4).
Univariate analysis of nonrelapse mortality
. | No. . | % nonrelapse mortality at 1 y . | P . |
|---|---|---|---|
| Age | |||
| Younger than 55 y | 35 | 31 | |
| 55 y or older | 59 | 23 | NS |
| Donor type | |||
| Matched sibling | 51 | 26 | |
| Other | 43 | 39 | .01 |
| Cytogenetic category | |||
| Intermediate | 38 | 12 | |
| Poor prognosis | 53 | 34 | .005 |
| Stem cell source | |||
| Peripheral blood | 53 | 25 | |
| Bone marrow | 41 | 42 | NS |
| Disease status | |||
| Remission | 24 | 4 | |
| Other | 70 | 34 | .004 |
. | No. . | % nonrelapse mortality at 1 y . | P . |
|---|---|---|---|
| Age | |||
| Younger than 55 y | 35 | 31 | |
| 55 y or older | 59 | 23 | NS |
| Donor type | |||
| Matched sibling | 51 | 26 | |
| Other | 43 | 39 | .01 |
| Cytogenetic category | |||
| Intermediate | 38 | 12 | |
| Poor prognosis | 53 | 34 | .005 |
| Stem cell source | |||
| Peripheral blood | 53 | 25 | |
| Bone marrow | 41 | 42 | NS |
| Disease status | |||
| Remission | 24 | 4 | |
| Other | 70 | 34 | .004 |
NS indicates not significant.
Among HLA-identical sibling transplant recipients, nonrelapse mortality rate was 28% in the FM group and 15% after FAI conditioning. The rate, however, was similar for patients with active disease at the time of treatment (FM, 26%; FAI, 28%).
Grade II-IV and III-IV acute GVHD rates were 36% (n = 31 cases) and 16% (n = 14 cases), respectively. Chronic GVHD was documented in 34% of 68 eligible patients. Patients treated with FAI and FM had a grade II-IV acute GVHD rate of 25% and 39%, respectively, and the acute grade III-IV GVHD rate was 11%, and 19%, respectively. These rates should be interpreted with caution given the proportion of matched unrelated donor (MUD) transplant recipients in the FM group. Recipients of HLA-identical sibling donor transplants had similar incidences of acute grade II-IV (FAI, 19%; FM, 20%) and grade III-IV GVHD (FAI, 4%; FM, 8%) regardless of the treatment regimen. Chronic GVHD rates were 27% and 39% after conditioning with FAI and FM. Patients treated with MUD and HLA-identical sibling APCT had chronic GVHD rates of 53% and 40%, respectively.
The analysis of chimerism and acute GVHD has to consider the fact that the former was generally assessed in a later time point (during the fourth to fifth week after transplantation), after the development of the latter in several cases. Incidence of grade II-IV acute GVHD was 19% among patients with evidence of mixed chimerism on day +30, as compared with 41% for patients who were fully chimeric. The rates of grade III-IV acute GVHD were 5% and 18%, while the chronic GVHD rate was 17% and 55%, respectively, among mixed and complete chimeras, as documented in the initial assessment of donor chimerism (fourth to fifth week).
Twenty-six patients (28%) died during the first 100 days after transplantation: 6 relapsed or did not respond, 10 died of acute GVHD, 2 died due to sepsis, and 2 died of complications of engraftment failure. Regimen-related toxicity was the cause of death in 6 cases, all after treatment with FM (10% regimen-related mortality). The 100-day nonrelapse mortality rate was higher among recipients of FM conditioning (FAI, 13%; FM, 26%; P = .2).
Among patients treated with FAI, acute or chronic GVHD was the cause of death of 9% (n = 3), while disease progression was the cause of death of 41% (n = 13). Those proportions were 26% (n = 16) and 27% (n = 17) among patients treated with FM. Fifty-five percent of the deaths among recipients of MUD transplants were caused by acute or chronic GVHD, while relapse caused another 20%. Among HLA-identical sibling transplant recipients, those proportions were 15% (GVHD related) and 62% (due to relapse), respectively.
Non–relapse-related mortality and relapse-related mortality were considered competing risks for mortality (Figure 3). The 3-year cumulative incidence of non–relapse-related mortality was significantly higher after conditioning with FM than with FAI (P = .036). Conversely, the 3-year cumulative incidence of relapse-related mortality was higher after FAI than after FM (P = .029). The estimates of cumulative incidence of mortality at 3 years are shown in Table 5.
Cumulative incidence of relapse-related and non–relapse-related mortality. The cumulative incidence of relapse-related mortality and the cumulative incidence of non–relapse-related mortality for each treatment group are shown. The P value for comparing FAI and FM with respect to relapse-related mortality is .029. The P value for comparing FAI and FM with respect to non–relapse-related mortality is .036. FAI indicates fludarabine, araC, and idarubicin; FM, fludarabine and melphalan.
Cumulative incidence of relapse-related and non–relapse-related mortality. The cumulative incidence of relapse-related mortality and the cumulative incidence of non–relapse-related mortality for each treatment group are shown. The P value for comparing FAI and FM with respect to relapse-related mortality is .029. The P value for comparing FAI and FM with respect to non–relapse-related mortality is .036. FAI indicates fludarabine, araC, and idarubicin; FM, fludarabine and melphalan.
Estimates of cumulative incidence of mortality at 3 years
. | . | 95% confidence interval . | . | |
|---|---|---|---|---|
| Outcome and treatment . | Cumulative incidence . | Lower bound . | Upper bound . | |
| Relapse-related mortality | ||||
| FAI | 0.534 | 0.343 | 0.725 | |
| FM | 0.260 | 0.149 | 0.371 | |
| Non–relapse-related mortality | ||||
| FAI | 0.156 | 0.028 | 0.285 | |
| FM | 0.392 | 0.267 | 0.517 | |
. | . | 95% confidence interval . | . | |
|---|---|---|---|---|
| Outcome and treatment . | Cumulative incidence . | Lower bound . | Upper bound . | |
| Relapse-related mortality | ||||
| FAI | 0.534 | 0.343 | 0.725 | |
| FM | 0.260 | 0.149 | 0.371 | |
| Non–relapse-related mortality | ||||
| FAI | 0.156 | 0.028 | 0.285 | |
| FM | 0.392 | 0.267 | 0.517 | |
Univariate and multivariate analysis
The following variables were significantly associated with improved survival by univariate analysis: intermediate category cytogenetics (P < .01, hazard ratio [HR] 0.49, 95% confidence interval [CI] 0.29-0.82), disease remission at the time of transplantation (P < .04, HR 2.71, 95% CI 1.07-6.85, for refractory disease at transplantation), achievement or maintenance of remission after transplantation (P < .01, HR 0.25, 95% CI 0.12-0.56), absence of grade II-IV acute GVHD (P = .02, HR 1.88, 95% CI 1.12-3.17, for patients developing grade II-IV acute GVHD), and absence of grade III-IV acute GVHD (P < .01, HR 3.37, 95% CI 1.78-6.37, for patients developing grade III-IV acute GVHD).
PFS was better for subjects who had intermediate-risk cytogenetics (P < .01, HR 0.47, 95% CI 0.29-0.78), who achieved or maintained a CR after transplantation (P = .01, HR 0.29, 95% CI 0.11-0.76), and who did not develop grade II-IV (P = .04, HR 1.73, 95% CI 1.03-2.89, for patients developing grade II-IV acute GVHD) or grade III-IV acute GVHD (P < .01, HR 3.22, 95% CI 1.74-5.96, for patients developing grade III-IV acute GVHD). The association between complete donor chimerism by day 30 after transplantation and longer PFS was of borderline statistical significance (median PFS, 38 versus 16 weeks; P = .06, HR 1.8, 95% CI 0.99-3.27). Development of chronic GVHD was associated with longer overall survival (209 versus 52 weeks for patients with and without chronic GVHD, respectively) and PFS (209 versus 21 weeks), but this association did not reach a statistically significant level (overall survival: P = .99, HR 1.0, 95% CI 0.47-2; PFS: P = .51, HR 0.79, 95% CI 0.4-1.58).
In the multivariate analysis model including patient- and treatment-related variables, relapsed or untreated disease at transplantation was associated with poorer survival. PFS was improved among patients in remission at the time of transplantation, with intermediate-risk cytogenetics, and those who were treated with FM (Tables 6 and 7).
Factors associated with progression-free survival
Patient and treatment-related variables . | No. of patients (no. of events) . | Median survival, wk . | Univariate P . | HR . | 95% CI for HR . | Multivariate P . | HR . | 95% CI for HR . |
|---|---|---|---|---|---|---|---|---|
| Recipient-donor CMV | ||||||||
| Negative-negative | 12 (8) | 52 | — | 1.00 | — | |||
| Other | 82 (64) | 19 | .30 | 1.47 | 0.70-3.07 | |||
| Recipient age, y | .73 | 1.00 | 0.98-1.03 | |||||
| 57 or younger | 47 (35) | 29 | — | 1.00 | — | — | 1.00 | — |
| Older than 57 | 47 (37) | 16 | .45 | 1.20 | 0.75-1.90 | .21 | 0.70 | 0.41-1.21 |
| Donor-recipient sex | ||||||||
| Female-female | 26 (22) | 27 | — | 1.00 | — | |||
| Female-male | 20 (17) | 14 | .63 | 1.17 | 0.62-2.21 | |||
| Male-female | 23 (16) | 13 | .53 | 0.81 | 0.42-1.56 | |||
| Male-male | 25 (17) | 22 | .46 | 0.78 | 0.41-1.49 | |||
| Duration of first CR | ||||||||
| Less than 6 mo | 70 (54) | 19 | — | 1.00 | — | |||
| 6-12 mo | 14 (12) | 12 | .27 | 1.43 | 0.76-2.68 | |||
| More than 12 mo | 10 (6) | 115 | .13 | 0.52 | 0.22-1.22 | |||
| Cytogenetics | ||||||||
| Bad | 53 (45) | 13 | — | 1.00 | — | 1.00 | — | |
| Intermediate | 38 (24) | 56 | <.01 | 0.47 | 0.29-0.78 | .01 | 0.52 | 0.31-0.87 |
| Missing | 3 (2) | 13 | — | — | ||||
| Disease status | ||||||||
| First CR | 11 (9) | 59 | — | 1.00 | — | — | 1.00 | — |
| More than 1 CR | 13 (5) | — | .07 | 0.36 | 0.12-1.07 | .19 | 0.48 | 0.16-1.45 |
| Refractory | 57 (47) | 14 | .34 | 1.42 | 0.69-2.90 | .09 | 2.00 | 0.89-4.49 |
| Untreated | 13 (11) | 13 | .25 | 1.68 | 0.69-4.07 | .03 | 3.22 | 1.10-9.44 |
| Preparative regimen | ||||||||
| FAI | 32 (27) | 16 | — | 1.00 | — | — | 1.00 | — |
| FM | 62 (45) | 21 | .32 | 0.79 | 0.49-1.27 | .01 | 0.42 | 0.21-0.83 |
| Cell type | ||||||||
| Bone marrow | 41 (31) | 21 | — | 1.00 | — | |||
| Peripheral blood | 53 (41) | 19 | .54 | 1.16 | 0.73-1.85 | |||
| Time from diagnosis to transplantation | 94 | NA | .18 | 1.00 | 0.99-1.00 | |||
| ABO mismatch | ||||||||
| No | 56 (42) | 19 | — | 1.00 | — | |||
| Yes | 37 (29) | 21 | .67 | 1.11 | 0.69-1.78 | |||
| Matched sibling | ||||||||
| No | 43 (32) | 19 | — | 1.00 | — | — | 1.00 | — |
| Yes | 51 (40) | 20 | .67 | 1.11 | 0.69-1.76 | .55 | 0.84 | 0.48-1.49 |
Patient and treatment-related variables . | No. of patients (no. of events) . | Median survival, wk . | Univariate P . | HR . | 95% CI for HR . | Multivariate P . | HR . | 95% CI for HR . |
|---|---|---|---|---|---|---|---|---|
| Recipient-donor CMV | ||||||||
| Negative-negative | 12 (8) | 52 | — | 1.00 | — | |||
| Other | 82 (64) | 19 | .30 | 1.47 | 0.70-3.07 | |||
| Recipient age, y | .73 | 1.00 | 0.98-1.03 | |||||
| 57 or younger | 47 (35) | 29 | — | 1.00 | — | — | 1.00 | — |
| Older than 57 | 47 (37) | 16 | .45 | 1.20 | 0.75-1.90 | .21 | 0.70 | 0.41-1.21 |
| Donor-recipient sex | ||||||||
| Female-female | 26 (22) | 27 | — | 1.00 | — | |||
| Female-male | 20 (17) | 14 | .63 | 1.17 | 0.62-2.21 | |||
| Male-female | 23 (16) | 13 | .53 | 0.81 | 0.42-1.56 | |||
| Male-male | 25 (17) | 22 | .46 | 0.78 | 0.41-1.49 | |||
| Duration of first CR | ||||||||
| Less than 6 mo | 70 (54) | 19 | — | 1.00 | — | |||
| 6-12 mo | 14 (12) | 12 | .27 | 1.43 | 0.76-2.68 | |||
| More than 12 mo | 10 (6) | 115 | .13 | 0.52 | 0.22-1.22 | |||
| Cytogenetics | ||||||||
| Bad | 53 (45) | 13 | — | 1.00 | — | 1.00 | — | |
| Intermediate | 38 (24) | 56 | <.01 | 0.47 | 0.29-0.78 | .01 | 0.52 | 0.31-0.87 |
| Missing | 3 (2) | 13 | — | — | ||||
| Disease status | ||||||||
| First CR | 11 (9) | 59 | — | 1.00 | — | — | 1.00 | — |
| More than 1 CR | 13 (5) | — | .07 | 0.36 | 0.12-1.07 | .19 | 0.48 | 0.16-1.45 |
| Refractory | 57 (47) | 14 | .34 | 1.42 | 0.69-2.90 | .09 | 2.00 | 0.89-4.49 |
| Untreated | 13 (11) | 13 | .25 | 1.68 | 0.69-4.07 | .03 | 3.22 | 1.10-9.44 |
| Preparative regimen | ||||||||
| FAI | 32 (27) | 16 | — | 1.00 | — | — | 1.00 | — |
| FM | 62 (45) | 21 | .32 | 0.79 | 0.49-1.27 | .01 | 0.42 | 0.21-0.83 |
| Cell type | ||||||||
| Bone marrow | 41 (31) | 21 | — | 1.00 | — | |||
| Peripheral blood | 53 (41) | 19 | .54 | 1.16 | 0.73-1.85 | |||
| Time from diagnosis to transplantation | 94 | NA | .18 | 1.00 | 0.99-1.00 | |||
| ABO mismatch | ||||||||
| No | 56 (42) | 19 | — | 1.00 | — | |||
| Yes | 37 (29) | 21 | .67 | 1.11 | 0.69-1.78 | |||
| Matched sibling | ||||||||
| No | 43 (32) | 19 | — | 1.00 | — | — | 1.00 | — |
| Yes | 51 (40) | 20 | .67 | 1.11 | 0.69-1.76 | .55 | 0.84 | 0.48-1.49 |
— indicates reference value; NA, not applicable.
Factors associated with overall survival
Patient and treatment-related variables . | No. of patients (no. of deaths) . | Median survival, wk . | Univariate P . | HR . | 95% CI for HR . | Multivariate P . | HR . | 95% CI for HR . |
|---|---|---|---|---|---|---|---|---|
| Recipient-donor CMV | ||||||||
| Negative-negative | 12 (7) | 57 | — | 1.00 | — | |||
| Other | 82 (58) | 30 | .45 | 1.35 | 0.62-2.97 | |||
| Recipient age, y | .58 | 1.01 | 0.98-1.03 | |||||
| 57 or younger | 47 (30) | 42 | — | 1.00 | — | — | 1.00 | — |
| Older than 57 | 47 (35) | 29 | .33 | 1.27 | 0.78-2.07 | .52 | 0.83 | 0.46-1.48 |
| Donor-recipient sex | ||||||||
| Female-female | 26 (19) | 33 | — | 1.00 | — | |||
| Female-male | 20 (16) | 19 | .44 | 1.30 | 0.66-2.55 | |||
| Male-female | 23 (14) | 26 | .65 | 0.85 | 0.42-1.71 | |||
| Male-male | 25 (16) | 52 | .55 | 0.81 | 0.42-1.59 | |||
| Duration of first CR | ||||||||
| Less than 6 mo | 70 (47) | 33 | — | 1.00 | — | |||
| 6-12 mo | 14 (12) | 21 | .14 | 1.62 | 0.86-3.07 | |||
| More than 12 mo | 10 (6) | 169 | .22 | 0.59 | 0.25-1.39 | |||
| Cytogenetics | ||||||||
| Bad | 53 (41) | 20 | — | 1.00 | — | 1.00 | — | |
| Intermediate | 38 (22) | 136 | <.01 | 0.49 | 0.29-0.82 | .08 | 0.61 | 0.36-1.05 |
| Missing | 3 (2) | 13 | — | — | — | — | — | |
| Disease status | ||||||||
| First CR | 11 (5) | 209 | — | 1.00 | — | — | 1.00 | — |
| More than 1 CR | 13 (5) | — | .61 | 0.73 | 0.21-2.51 | .79 | 0.84 | 0.24-3.00 |
| Refractory | 57 (46) | 21 | .04 | 2.71 | 1.07-6.85 | .02 | 3.29 | 1.19-9.15 |
| Untreated | 13 (9) | 26 | .16 | 2.21 | 0.74-6.61 | .04 | 3.85 | 1.08-13.81 |
| Preparative regimen | ||||||||
| FAI | 32 (22) | 40 | — | 1.00 | — | — | 1.00 | — |
| FM | 62 (43) | 30 | .79 | 1.07 | 0.64-1.80 | .06 | 0.48 | 0.23-1.02 |
| Cell type | ||||||||
| Bone marrow | 41 (30) | 32 | — | 1.00 | — | |||
| Peripheral blood | 53 (35) | 34 | .67 | 0.90 | 0.55-1.47 | |||
| Time from diagnosis to transplantation | 96 | NA | .18 | 1.00 | 0.99-1.00 | |||
| ABO mismatch | ||||||||
| No | 56 (35) | 34 | — | 1.00 | — | |||
| Yes | 37 (29) | 30 | .16 | 1.42 | 0.88-2.33 | |||
| Matched sibling | ||||||||
| No | 43 (31) | 23 | — | 1.00 | — | — | 1.00 | — |
| Yes | 51 (34) | 40 | .40 | 0.81 | 0.50-1.32 | .1 | 0.60 | 0.33-1.05 |
Patient and treatment-related variables . | No. of patients (no. of deaths) . | Median survival, wk . | Univariate P . | HR . | 95% CI for HR . | Multivariate P . | HR . | 95% CI for HR . |
|---|---|---|---|---|---|---|---|---|
| Recipient-donor CMV | ||||||||
| Negative-negative | 12 (7) | 57 | — | 1.00 | — | |||
| Other | 82 (58) | 30 | .45 | 1.35 | 0.62-2.97 | |||
| Recipient age, y | .58 | 1.01 | 0.98-1.03 | |||||
| 57 or younger | 47 (30) | 42 | — | 1.00 | — | — | 1.00 | — |
| Older than 57 | 47 (35) | 29 | .33 | 1.27 | 0.78-2.07 | .52 | 0.83 | 0.46-1.48 |
| Donor-recipient sex | ||||||||
| Female-female | 26 (19) | 33 | — | 1.00 | — | |||
| Female-male | 20 (16) | 19 | .44 | 1.30 | 0.66-2.55 | |||
| Male-female | 23 (14) | 26 | .65 | 0.85 | 0.42-1.71 | |||
| Male-male | 25 (16) | 52 | .55 | 0.81 | 0.42-1.59 | |||
| Duration of first CR | ||||||||
| Less than 6 mo | 70 (47) | 33 | — | 1.00 | — | |||
| 6-12 mo | 14 (12) | 21 | .14 | 1.62 | 0.86-3.07 | |||
| More than 12 mo | 10 (6) | 169 | .22 | 0.59 | 0.25-1.39 | |||
| Cytogenetics | ||||||||
| Bad | 53 (41) | 20 | — | 1.00 | — | 1.00 | — | |
| Intermediate | 38 (22) | 136 | <.01 | 0.49 | 0.29-0.82 | .08 | 0.61 | 0.36-1.05 |
| Missing | 3 (2) | 13 | — | — | — | — | — | |
| Disease status | ||||||||
| First CR | 11 (5) | 209 | — | 1.00 | — | — | 1.00 | — |
| More than 1 CR | 13 (5) | — | .61 | 0.73 | 0.21-2.51 | .79 | 0.84 | 0.24-3.00 |
| Refractory | 57 (46) | 21 | .04 | 2.71 | 1.07-6.85 | .02 | 3.29 | 1.19-9.15 |
| Untreated | 13 (9) | 26 | .16 | 2.21 | 0.74-6.61 | .04 | 3.85 | 1.08-13.81 |
| Preparative regimen | ||||||||
| FAI | 32 (22) | 40 | — | 1.00 | — | — | 1.00 | — |
| FM | 62 (43) | 30 | .79 | 1.07 | 0.64-1.80 | .06 | 0.48 | 0.23-1.02 |
| Cell type | ||||||||
| Bone marrow | 41 (30) | 32 | — | 1.00 | — | |||
| Peripheral blood | 53 (35) | 34 | .67 | 0.90 | 0.55-1.47 | |||
| Time from diagnosis to transplantation | 96 | NA | .18 | 1.00 | 0.99-1.00 | |||
| ABO mismatch | ||||||||
| No | 56 (35) | 34 | — | 1.00 | — | |||
| Yes | 37 (29) | 30 | .16 | 1.42 | 0.88-2.33 | |||
| Matched sibling | ||||||||
| No | 43 (31) | 23 | — | 1.00 | — | — | 1.00 | — |
| Yes | 51 (34) | 40 | .40 | 0.81 | 0.50-1.32 | .1 | 0.60 | 0.33-1.05 |
— indicates reference; NA, not applicable.
Discussion
It has been well established that for many malignancies the curative potential of allogeneic transplantation is, in large part, due to the graft versus malignancy (GVM) effect.18 This has led to the development of less toxic, nonmyeloablative, and reducedintensity transplantation regimens that would provide donor cell engraftment and generation of a GVM effect. This approach has allowed treatment of older and debilitated patients who have been considered ineligible for transplantation using ablative regimens.4,6-10
Numerous recent studies have shown that truly nonablative regimens are feasible and can result in durable engraftment and long-term disease control in selected patients. However, dose intensity has been shown to be important for both chronic myelogenous leukemia (CML) and AML in the setting of myeloablative allogeneic transplantation.19-22 Considering that AML appears to be less sensitive to GVM effects than CML, as indicated by lower response rates and shorter remission duration after donor lymphocyte infusions,23,24 cytoreduction from the preparative regimen may be important in achieving durable complete remissions in the context of reduced-intensity or nonablative regimens.
We have studied 2 regimens that produce varying degrees of cytoreduction as pretransplantation conditioning in patients with AML or MDS. This analysis suggests that recurrence is more frequent in patients receiving the truly nonablative regimen as compared with those receiving the reduced-intensity regimen of fludarabine and melphalan. This is particularly striking because imbalances of disease characteristics between the 2 treatment groups would favor the FAI group, with its significantly higher proportion of patients in complete remission at the time of transplantation (44% versus 16%). Furthermore, the FAI group had a higher proportion of HLA-matched sibling donor transplantations (81% versus 40%). Advanced-stage disease and donors other than HLA-identical siblings are 2 factors that contributed to increase the nonrelapse mortality rate among recipients of FM conditioning. Patients in the FM group were younger, but age did not influence survival or PFS in this predominantly older population. The reduced-intensity conditioning also resulted in a higher incidence of mixed chimerism and autologous reconstitution at all time points analyzed. PFS was inferior in the FAI group, and overall survival was similar in both groups.
Several factors likely contribute to the poorer disease control in using FAI preparative regimen. This regimen is less myelosuppressive and likely produced less direct cytoreduction of the leukemia. In addition, there was a higher rate of mixed chimerism in the FAI group that may be associated with a reduced GVM effect. Patients treated with FAI received bone marrow as the source of stem cells more often than recipients of FM did, but stem source did not influence survival or PFS in our analysis. It is also possible that unrelated donor hematopoietic stem cells might be associated with a stronger GVM effect (47% of the patients in the FM cohort received unrelated donor transplants).
These results suggest that for AML and MDS the myelosuppressive intensity of cytotoxic agents is important for disease control as well as establishment of long-term donor chimerism. In the context of late chronic or accelerated-phase CML, our group has documented results that are similar to the observations we described here.25 This is in contrast with the experience in low-grade lymphoma. Khouri et al7,26 recently reported no relapses following allogeneic transplantation using a truly nonmyeloablative conditioning regimen of fludarabine, cyclophosphamide, and rituximab, indicating that marked cytoreduction was not required for patients with this diagnosis as well as in patients with chemosensitive chronic lymphocytic leukemia. Disease “pace” and or increased sensitivity to GVM effects may contribute to these results.
In conclusion, our analysis would suggest that for AML and MDS both cytoreduction of the preparative regimen as well as GVM contribute to disease control after allografting with reduced-intensity or nonablative conditioning regimens. The relative importance of these mechanisms, however, may differ with regimens other than those tested here. Truly nonablative regimens may be effective in minimal disease states or for diseases highly sensitive to GVM effects and may provide a platform for innovative cell therapy approaches that may obviate the need for direct cytoreduction of the malignancy. It is hoped that safer and more effective regimens can be developed to improve the outcome of allogeneic transplantation for AML/MDS.
Prepublished online as Blood First Edition Paper, April 15, 2004; DOI 10.1182/blood-2003-11-3750.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal