Abstract
Despite posttransplantation immunosuppressive therapy, acute graft-versus-host disease (GVHD) remains a major cause of sickness and death. Tumor necrosis factor-α (TNF-α) is implicated in the pathophysiology of GVHD at several steps in the process. Infliximab is a genetically constructed immunoglobulin G1 (IgG1) murine–human chimeric monoclonal antibody that binds the soluble subunit and the membrane-bound precursor of TNF-α, blocking its interaction with receptors and causing lysis of cells that produce TNF-α. In this study we retrospectively evaluated 134 patients who had steroid-refractory acute GVHD. Of these, 21 who received infliximab as a single agent were analyzed. The overall response rate was 67% (n = 14), and 13 patients (62%) experienced complete response (CR). Five patients (24%) did not respond, and 2 (10%) had progressive GVHD. None had a toxic reaction to infliximab. Ten patients (48%) had 18 fungal infections, including Aspergillus species in 7 and Candida species in 10. Seventeen patients (81%) had bacterial infections, including 32 gram-positive and 8 gram-negative infections. Viral infections, primarily cytomegalovirus reactivation, occurred in 14 patients (67%). The Kaplan-Meier estimate of overall survival was 38%. In conclusion, infliximab was well tolerated and active for the treatment of steroid-resistant acute GVHD, particularly with gastrointestinal tract involvement. Survival after steroid-resistant acute GVHD continues to be problematic. The possibility of excessive fungal and other infections must be explored further.
Introduction
Acute graft-versus-host disease (GVHD) remains a major cause of sickness and death. It develops in approximately 30% of recipients of allogeneic blood stem cell or bone marrow transplants from human leukocyte antigen (HLA)–identical related or unrelated donors and from HLA-nonidentical related donors.1-5
The pathophysiology of acute GVHD is best described as a triphasic phenomenon.6,7 The initial phase involves the development of an inflammatory milieu resulting from damage in host tissues induced by the conditioning regimen. Damaged tissues secrete inflammatory cytokines, including interleukin-1 (IL-1), tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ). In the second phase, recipient antigen-presenting cells trigger the activation of donor-derived T cells, which expand and differentiate into effector cells.8,9 In the third phase, the effector phase, activated donor T cells mediate cytotoxicity against target host cells through Fas-Fas ligand interactions, perforin-granzyme B, and cytokine production, including that of TNF-α.2,10 This cascade of events leads to the tissue damage characteristic of acute GVHD.
TNF-α is produced primarily by monocytes and macrophages and secondarily by T-lymphocytes and natural killer cells.11-13 TNF-α has been implicated in the pathophysiology of GVHD at several steps in the process, including the induction of apoptosis in target tissues through the TNF-α receptor; the activation of macrophages, neutrophils, eosinophils, B cells, and T cells; the production of additional inflammatory cytokines (IL-1, IL-6, IL-10, IL-12, and TNF-α itself); the increased expression of human leukocyte antigen; and the facilitation of T-lymphocyte lysis.14,15 High levels of TNF-α have been implicated in an increased incidence of GVHD in recipients of bone marrow transplants.16,17
Infliximab is a genetically constructed IgG1 murine-human chimeric monoclonal antibody that binds the soluble subunit and the membrane-bound precursor of TNF-α.18 Infliximab inhibits a broad range of biologic activities of TNF-α by blocking interaction with its receptors, and it may cause lysis of cells that produce TNF-α.18 The drug has been used successfully for the treatment of a range of autoimmune and inflammatory diseases, including Crohn disease, rheumatoid arthritis, psoriasis, and spondyloarthropathy.19-25 Three trials involving 14 patients with acute GVHD treated with infliximab have been reported.26-28 All patients had advanced steroid-resistant acute GVHD and received infliximab at 10 mg/kg weekly for a median of 4 doses. Significant responses were seen in several patients with severe gastrointestinal (GI) disease, and the drug was not associated with any major toxicity. However, it is difficult to assess the relative value of infliximab in the treatment of steroid-resistant acute GVHD given the small number of patients in these studies and the use of concomitant immunosuppressive therapy. We evaluate here the efficacy and toxicity in the largest series of patients with advanced and steroid-resistant GVHD treated with infliximab.
Patients, materials, and methods
Patient population
We retrospectively evaluated 298 patients who underwent allogeneic transplantation and acquired acute GVHD at the University of Texas M. D. Anderson Cancer Center between March 1999 and June 2001. The patients had received allogeneic, bone marrow, or peripheral stem cell grafts from related or unrelated donors for the treatment of hematologic malignancies. GVHD prophylaxis for all patients consisted of tacrolimus and minidose methotrexate (5 mg/m2 on days 1, 3, and 6, plus an additional dose on day 11 for patients receiving bone marrow transplants). Patients in whom grade 2 or greater acute GVHD developed were treated with 2 mg/kg per day corticosteroids, primarily methylprednisolone (MP), in divided doses.
One hundred thirty-four patients had steroid-refractory acute GVHD according to the definition presented here. Initially, infliximab was used empirically in patients with steroid-resistant GI involvement based on its activity in inflammatory bowel disease; responses in diarrhea volume and skin GVHD were noted.14 Eighty-three patients with steroid-resistant GVHD were treated with infliximab at the discretion of their attending physicians while a prospective, controlled trial was being organized. Most received infliximab in combination with additional immunosuppressive agents for advanced GVHD and are not considered further. Twenty-one patients received infliximab added as a single agent to tacrolimus and corticosteroids for the initial treatment of steroid-resistant acute GVHD; these patients were analyzed for this study.
Patients were classified as having steroid-resistant acute GVHD if any of the following occurred: (1) no change in the stage of skin GVHD after 1 week of 2 mg/kg per day or more methylprednisolone; (2) progression of acute GVHD (ie, increase in disease stage by at least 1) of skin GVHD or lack of response of visceral (liver, GI) GVHD despite treatment with 2 mg/kg per day or more methylprednisolone for at least 72 hours; (3) progression of visceral GVHD despite treatment with 2 mg/kg per day or more methylprednisolone for at least 48 hours; or (4) visceral GVHD progressing to stage 4 after 24 hours of 2 mg/kg per day or more methylprednisolone.
All patients provided written informed consent for hematopoietic stem cell transplantation under studies approved by the institutional review board at the University of Texas MD Anderson Cancer Center. GVHD-related data were prospectively collected for every recipient of an allogeneic transplant. In addition, the University of Texas MD Anderson Cancer Center institutional review board approved this retrospective analysis of infliximab therapy for acute GVHD.
GVHD assessment
Acute GVHD was diagnosed within 90 days of allogeneic hematopoietic transplantation or donor lymphocyte infusion. Staging and grading of acute GVHD were scored according to modified Glucksberg criteria.29 Chronic GVHD was evaluated and was classified as progressing or relapsing.
Treatment
Patients in whom acute GVHD developed continued tacrolimus treatment, maintained blood levels between 5 and 15 ng/mL, and received methylprednisolone 2 mg/kg per day in divided doses. Patients in this analysis in whom steroid-resistant GVHD developed received 10 mg/kg infliximab once weekly for at least 4 doses (median, 4; range, 2-9); additional doses were allowed for partial responders. Tacrolimus was continued, and 2 mg/kg per day methylprednisolone was initially continued and then was tapered as tolerated when significant improvement was achieved (at least a partial response [PR]).
Evaluation of response
Responses were assessed for each involved organ. Complete response (CR) and PR were assessed 7 days after the initiation of infliximab. CR signified the resolution of all manifestations of acute GVHD. PR signified a decrease in organ stage by 1. Progressive disease (PD) signified an increase of 1 in organ stage and was evaluated 48 hours (GI, liver) or 72 hours (skin) after the initiation of infliximab. Patients were considered to have no response (NR) in the absence of CR, PR, or PD 7 days after the initiation of infliximab for skin GVHD or 72 hours after its initiation for visceral GVHD.
Overall response integrated the responses in skin, gut, and liver. CR signified resolution of GVHD in all evaluable organs. PR meant any improvement in at least 1 evaluable organ without the deterioration of others. PD indicated deterioration in at least 1 evaluable organ without improvement of the others. NR was the absence of any change or was any situation other than CR, PR, or PD.
Statistical considerations
Differences in the proportion of response according to the organs involved were assessed by the χ2 and Fisher exact tests.30 Actuarial survival after transplantation was evaluated by the Kaplan-Meier method.31 Predictors of response were evaluated by univariate analysis using logistic regression. Predictors of survival after treatment with infliximab were evaluated by univariate analysis using the Cox proportional hazards model.32 Evaluating predictors of response and survival was limited to univariate analysis because of the sample size.
Results
Patient and disease characteristics
Patient characteristics are summarized in Tables 1 and 2. Most (n = 14; 67%) patients had grade 2 GVHD with lower GI tract involvement. Four patients (19%) had grade 3-4 GVHD at the time of infliximab treatment. One patient had clinical evidence of acute GVHD on day 122 after transplantation but received a donor lymphocyte infusion 20 days before the diagnosis of GVHD and was included in the study. All 21 patients had steroid-resistant GVHD and were receiving methylprednisolone and tacrolimus at the time infliximab was initiated. Infliximab was started at a median of 27 days (range, 4-59 days) after the diagnosis of GVHD and 25 days (range, 1-59 days) after the initiation of methylprednisolone. One of these patients received infliximab 1 day after the initiation of 2 mg/kg per day steroids for GVHD of the GI tract and the liver that progressed to grade 3 disease. Most (n = 15; 71%) patients received 4 doses of 10 mg/kg infliximab (median, 4 doses; range, 2-9 doses).
Patient characteristics
Characteristic . | No. (%)* . |
|---|---|
| Median age, y (range) | 47 (19-64) |
| Sex | |
| Male | 14 (67) |
| Female | 7 (33) |
| Stem cell source | |
| PBSC | 11 (52) |
| BM | 10 (48) |
| Donor source | |
| HLA-matched, related | 11 (52) |
| HLA-mismatched, related | 2 (10) |
| HLA-matched, unrelated | 8 (38) |
| Diagnosis | |
| MDS/AML | 6 (29) |
| CML/myelofibrosis | 4 (19) |
| Non-Hodgkin lymphoma | 6 (29) |
| ALL/CLL | 4 (19) |
| Myeloma | 1 (4) |
| Acute GVHD grade | |
| 1 | 3 (14) |
| 2 | 14 (67) |
| 3-4 | 4 (19) |
| Organ involvement, n (%) | |
| Skin | 9 (43) |
| GI tract | 12 (57) |
| Liver | 3 (14) |
| Median no. infliximab doses (range) | 4 (2-9) |
| Median time to infliximab from onset of GVHD, d (range) | 27 (4-59) |
| Median time from methylprednisolone to infliximab, d (range) | 25 (1-59) |
Characteristic . | No. (%)* . |
|---|---|
| Median age, y (range) | 47 (19-64) |
| Sex | |
| Male | 14 (67) |
| Female | 7 (33) |
| Stem cell source | |
| PBSC | 11 (52) |
| BM | 10 (48) |
| Donor source | |
| HLA-matched, related | 11 (52) |
| HLA-mismatched, related | 2 (10) |
| HLA-matched, unrelated | 8 (38) |
| Diagnosis | |
| MDS/AML | 6 (29) |
| CML/myelofibrosis | 4 (19) |
| Non-Hodgkin lymphoma | 6 (29) |
| ALL/CLL | 4 (19) |
| Myeloma | 1 (4) |
| Acute GVHD grade | |
| 1 | 3 (14) |
| 2 | 14 (67) |
| 3-4 | 4 (19) |
| Organ involvement, n (%) | |
| Skin | 9 (43) |
| GI tract | 12 (57) |
| Liver | 3 (14) |
| Median no. infliximab doses (range) | 4 (2-9) |
| Median time to infliximab from onset of GVHD, d (range) | 27 (4-59) |
| Median time from methylprednisolone to infliximab, d (range) | 25 (1-59) |
ALL indicates acute lymphocytic leukemia; AML, acute myelogenous leukemia; BM, bone marrow; CLL, chronic lymphocytic leukemia; CML, chronic myelogenous leukemia; MDS, myelodysplastic syndrome; PBSC, peripheral blood stem cells.
All data are numbers, with percentages shown in parentheses, unless otherwise indicated.
Summary of outcomes
Patient no. . | Donor . | Age, y . | Time to GVHD, d . | Skin stage . | Gl stage . | Liver stage . | Days to infliximab* . | Response to infliximab . | Death . | Cause . | Days to last follow-up† . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 Ag MM rel | 37 | 28 | 3 | 0 | 0 | 41 | CR | Yes | GVHD | 83 |
| 2 | 1 Ag MM rel | 62 | 41 | 1 | 1 | 0 | 53 | CR | Yes | Relapse | 165 |
| 3 | HLA unrel | 25 | 8 | 3 | 0 | 0 | 48 | CR | No | — | 682 |
| 4 | HLA unrel | 60 | 11 | 2 | 0 | 0 | 31 | CR | No | — | 298 |
| 5 | HLA unrel | 36 | 12 | 3 | 0 | 0 | 42 | PR | Yes | GVHD | 120 |
| 6 | HLA unrel | 45 | 16 | 0 | 1 | 0 | 7 | PD | Yes | GVHD | 178 |
| 7 | HLA unrel | 64 | 20 | 0 | 1 | 0 | 11 | CR | No | — | 640 |
| 8 | HLA unrel | 19 | 32 | 3 | 0 | 0 | 7 | NR | Yes | Relapse | 115 |
| 9 | HLA unrel | 62 | 36 | 0 | 3 | 0 | 37 | CR | Yes | GVHD | 19 |
| 10 | HLA unrel | 45 | 40 | 1 | 1 | 0 | 14 | CR | No | — | 806 |
| 11 | HLA sib | 55 | 18 | 1 | 0 | 0 | 45 | NR | Yes | GVHD | 50 |
| 12 | HLA sib | 62 | 19 | 0 | 2 | 0 | 18 | NR | Yes | Pneumonia | 231 |
| 13 | HLA sib | 63 | 20 | 0 | 1 | 0 | 5 | CR | Yes | GVHD | 257 |
| 14 | HLA sib | 40 | 23 | 0 | 0 | 1 | 41 | CR | Yes | GVHD | 144 |
| 15 | HLA sib | 47 | 30 | 0 | 3 | 1 | 4 | PD | Yes | GVHD | 99 |
| 16 | HLA sib | 27 | 31 | 0 | 4 | 0 | 6 | CR | No | — | 352 |
| 17 | HLA sib | 53 | 34 | 1 | 0 | 0 | 59 | CR | No | — | 390 |
| 18 | HLA sib | 53 | 40 | 0 | 1 | 0 | 7 | CR | No | — | 588 |
| 19 | HLA sib | 55 | 57 | 0 | 1 | 0 | 27 | CR | Yes | GVHD | 130 |
| 20 | HLA sib | 47 | 91 | 0 | 1 | 0 | 4 | NR | No | — | 861 |
| 21 | HLA sib | 29 | 122‡ | 0 | 0 | 1 | 47 | NR | Yes | Relapse | 161 |
Patient no. . | Donor . | Age, y . | Time to GVHD, d . | Skin stage . | Gl stage . | Liver stage . | Days to infliximab* . | Response to infliximab . | Death . | Cause . | Days to last follow-up† . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 Ag MM rel | 37 | 28 | 3 | 0 | 0 | 41 | CR | Yes | GVHD | 83 |
| 2 | 1 Ag MM rel | 62 | 41 | 1 | 1 | 0 | 53 | CR | Yes | Relapse | 165 |
| 3 | HLA unrel | 25 | 8 | 3 | 0 | 0 | 48 | CR | No | — | 682 |
| 4 | HLA unrel | 60 | 11 | 2 | 0 | 0 | 31 | CR | No | — | 298 |
| 5 | HLA unrel | 36 | 12 | 3 | 0 | 0 | 42 | PR | Yes | GVHD | 120 |
| 6 | HLA unrel | 45 | 16 | 0 | 1 | 0 | 7 | PD | Yes | GVHD | 178 |
| 7 | HLA unrel | 64 | 20 | 0 | 1 | 0 | 11 | CR | No | — | 640 |
| 8 | HLA unrel | 19 | 32 | 3 | 0 | 0 | 7 | NR | Yes | Relapse | 115 |
| 9 | HLA unrel | 62 | 36 | 0 | 3 | 0 | 37 | CR | Yes | GVHD | 19 |
| 10 | HLA unrel | 45 | 40 | 1 | 1 | 0 | 14 | CR | No | — | 806 |
| 11 | HLA sib | 55 | 18 | 1 | 0 | 0 | 45 | NR | Yes | GVHD | 50 |
| 12 | HLA sib | 62 | 19 | 0 | 2 | 0 | 18 | NR | Yes | Pneumonia | 231 |
| 13 | HLA sib | 63 | 20 | 0 | 1 | 0 | 5 | CR | Yes | GVHD | 257 |
| 14 | HLA sib | 40 | 23 | 0 | 0 | 1 | 41 | CR | Yes | GVHD | 144 |
| 15 | HLA sib | 47 | 30 | 0 | 3 | 1 | 4 | PD | Yes | GVHD | 99 |
| 16 | HLA sib | 27 | 31 | 0 | 4 | 0 | 6 | CR | No | — | 352 |
| 17 | HLA sib | 53 | 34 | 1 | 0 | 0 | 59 | CR | No | — | 390 |
| 18 | HLA sib | 53 | 40 | 0 | 1 | 0 | 7 | CR | No | — | 588 |
| 19 | HLA sib | 55 | 57 | 0 | 1 | 0 | 27 | CR | Yes | GVHD | 130 |
| 20 | HLA sib | 47 | 91 | 0 | 1 | 0 | 4 | NR | No | — | 861 |
| 21 | HLA sib | 29 | 122‡ | 0 | 0 | 1 | 47 | NR | Yes | Relapse | 161 |
1 Ag MM rel indicates 1 antigen-mismatched, related; HLA sib, HLA-matched sibling; and HLA unrel, HLA-matched, unrelated.
Days from diagnosis of GVHD to infliximab.
Days from initiation of infliximab to last follow-up.
Patient received a donor lymphocyte infusion 20 days before clinical manifestations of acute GVHD.
Response to treatment with infliximab
The overall response rate was 67% (n = 14), and most (62%, n = 13) of these patients experienced CR. Five patients (24%) did not respond, and 2 (10%) experienced GVHD progression. Of the 2 patients treated after 1 and 3 days of corticosteroids, 1 achieved CR and 1 achieved NR.
Duration of response was measured from the time of the first infliximab treatment to the day of progression of disease or initiation of additional immunotherapy. Response duration exceeded 14 days in all 14 patients with CR or PR, and it exceeded 30 days in 11 of these patients. Ten patients who achieved CR after infliximab did not require further immunosuppressive therapy. The remaining 3 patients subsequently required salvage immunosuppressive therapy after remissions lasting 17 (n = 2) and 35 (n = 1) days.
All patients who had NR (n = 5), PD (n = 2), or PR (n = 1) required from 1 to 4 lines of salvage immunosuppressive therapy, including antithymocyte globulin, daclizumab, extracorporeal photopheresis, mycophenolate mofetil, keratinocyte growth factor, and sirolimus.
Responses by organ site are shown in Table 3. The best responses were seen in the 12 patients with GI GVHD—8 CRs and 1 PR. Of 10 patients with cutaneous acute GVHD, 6 achieved CR, and 1 achieved PR. Of 4 patients with liver involvement, 1 had CR, and 3 had NR.
Responses to infliximab by organ
Response . | Skin, no. (%) . | Gut, no. (%) . | Liver, no. (%) . |
|---|---|---|---|
| CR | 6 (60) | 8 (67) | 1 (25) |
| PR | 1 (10) | 1 (8) | 0 (0) |
| NR/PD | 3 (30) | 3 (25) | 3 (75) |
Response . | Skin, no. (%) . | Gut, no. (%) . | Liver, no. (%) . |
|---|---|---|---|
| CR | 6 (60) | 8 (67) | 1 (25) |
| PR | 1 (10) | 1 (8) | 0 (0) |
| NR/PD | 3 (30) | 3 (25) | 3 (75) |
Age, sex, HLA compatibility (related vs unrelated transplantation), and overall grade (grade 2 vs 3-4) were evaluated as prognostic factors for response to infliximab. None of these factors reached statistical significance (Table 4). Among patients who responded to infliximab with CR or PR, chronic GVHD developed in 93% (n = 13).
Univariate analysis for response after infliximab: distribution of responses according to demographic and clinical characteristics
Characteristic . | No. . | No. CR/PR . | % . | P . |
|---|---|---|---|---|
| Age | .5 | |||
| 47 y and younger | 10 | 6 | 60 | |
| Older than 47 y | 11 | 8 | 73 | |
| Sex | .5 | |||
| Male | 14 | 10 | 71 | |
| Female | 7 | 4 | 57 | |
| Acute GVHD grade | .4 | |||
| 2 | 14 | 10 | 71 | |
| 3-4 | 4 | 2 | 50 | |
| Donor type | .2 | |||
| HLA-matched sibling | 11 | 6 | 55 | |
| MM rel/MUD | 10 | 8 | 80 |
Characteristic . | No. . | No. CR/PR . | % . | P . |
|---|---|---|---|---|
| Age | .5 | |||
| 47 y and younger | 10 | 6 | 60 | |
| Older than 47 y | 11 | 8 | 73 | |
| Sex | .5 | |||
| Male | 14 | 10 | 71 | |
| Female | 7 | 4 | 57 | |
| Acute GVHD grade | .4 | |||
| 2 | 14 | 10 | 71 | |
| 3-4 | 4 | 2 | 50 | |
| Donor type | .2 | |||
| HLA-matched sibling | 11 | 6 | 55 | |
| MM rel/MUD | 10 | 8 | 80 |
MM rel/MUD indicates mismatched related/matched-unrelated donor.
Toxicity and infections
No patients had infusion, allergic, or other toxic reactions to infliximab. Infections are summarized in Table 5. Ten patients (48%) had 18 fungal infections, including Aspergillus (n = 7) and Candida species (n = 11). Seventeen patients (81%) had 45 bacterial infections, including gram-positive bacteria (n = 32; 80%) and Gram-negative rods (n = 8; 98%). Almost half of these bacterial infections (n = 18; 40%) were bacteremias, and the rest included urinary tract infections (n = 12; 27%), respiratory tract infections (n = 9; 20%), and Clostridium difficile (n = 4) or enterococcal infections (n = 2) in the stool. Twenty-four viral infections were identified in 14 patients (67%); most of these were secondary to cytomegalovirus (CMV) (n = 16; 67%) and respiratory viruses (n = 5; 21%), including respiratory syncytial virus, influenza, and parainfluenza. Except for 1 urinary tract infection, all CMV infections involved viral reactivation in blood.
Infections after infliximab
Type of infection . | No. patients (%) . | No. positive cultures (%) . |
|---|---|---|
| Bacterial | 17 (81) | 45 (52) |
| Gram-positive | 11 (52) | 32 (37) |
| Gram-negative | 5 (24) | 8 (9) |
| Others | 4 (19) | 5 (6) |
| Fungal | 10 (48) | 18 (21) |
| Aspergillus spp | 6 (29) | 7 (8) |
| Candida glabrata | 5 (24) | 5 (6) |
| Candida spp | 4 (19) | 6 (7) |
| Viral | 14 (67) | 24 (28) |
| Cytomegalovirus | 11 (52) | 16 (18) |
| Respiratory viruses | 5 (24) | 5 (6) |
| Others | 3 (14) | 3 (3) |
| Total | 21 (100) | 87 (100) |
Type of infection . | No. patients (%) . | No. positive cultures (%) . |
|---|---|---|
| Bacterial | 17 (81) | 45 (52) |
| Gram-positive | 11 (52) | 32 (37) |
| Gram-negative | 5 (24) | 8 (9) |
| Others | 4 (19) | 5 (6) |
| Fungal | 10 (48) | 18 (21) |
| Aspergillus spp | 6 (29) | 7 (8) |
| Candida glabrata | 5 (24) | 5 (6) |
| Candida spp | 4 (19) | 6 (7) |
| Viral | 14 (67) | 24 (28) |
| Cytomegalovirus | 11 (52) | 16 (18) |
| Respiratory viruses | 5 (24) | 5 (6) |
| Others | 3 (14) | 3 (3) |
| Total | 21 (100) | 87 (100) |
Long-term outcomes
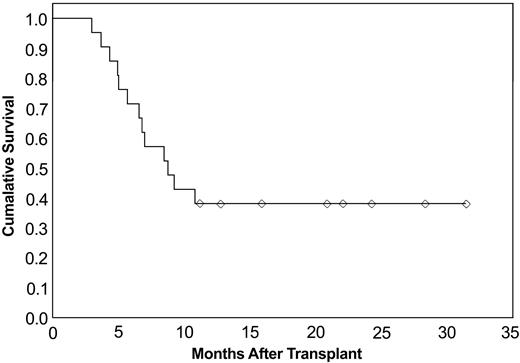
Eight patients remained alive at a median follow-up of 21 months (range, 11-31 months). Chronic GVHD developed in 19 patients (90%) in the group. All 8 surviving patients have chronic GVHD. Thirteen of 21 patients died; overall survival for all patients from the time of transplantation was 38%, as shown in Figure 1. Median survival since transplantation was 8.7 months (95% confidence interval [CI], 30%-71%). When predictors of survival were analyzed, no significant association with survival was observed for grade 3-4 acute GVHD, age, HLA compatibility, or response to infliximab (CR/PR) (Table 6).
Cumulative survival since transplantation. Overall survival of patients receiving infliximab for steroid-refractory acute GVHD (n = 21).
Cumulative survival since transplantation. Overall survival of patients receiving infliximab for steroid-refractory acute GVHD (n = 21).
Univariate analysis of predictors of survival since initiation of treatment with infliximab
. | No. . | No. alive . | No. HR . | 95% CI* . | P* . |
|---|---|---|---|---|---|
| Response | 0.1-1.2 | .1 | |||
| CR/PR | 14 | 7 | 0.4 | ||
| Other | 7 | 1 | 1.0 | ||
| Age, y | 0.2-1.8 | .4 | |||
| 47 and younger | 10 | 3 | 1.0 | ||
| Older than 47 | 11 | 5 | 0.6 | ||
| Sex | 0.4-3.0 | .8 | |||
| Male | 14 | 6 | 1.0 | ||
| Female | 7 | 2 | 1.1 | ||
| Acute GVH grade | 0.5-6.6 | .4 | |||
| 1-2 | 17 | 7 | 1.0 | ||
| 3-4 | 4 | 1 | 1.8 | ||
| Donor type | 0.3-2.6 | .8 | |||
| HLA-matched sibling | 11 | 4 | 0.9 | ||
| MM rel/MUD | 10 | 4 | 1.0 |
. | No. . | No. alive . | No. HR . | 95% CI* . | P* . |
|---|---|---|---|---|---|
| Response | 0.1-1.2 | .1 | |||
| CR/PR | 14 | 7 | 0.4 | ||
| Other | 7 | 1 | 1.0 | ||
| Age, y | 0.2-1.8 | .4 | |||
| 47 and younger | 10 | 3 | 1.0 | ||
| Older than 47 | 11 | 5 | 0.6 | ||
| Sex | 0.4-3.0 | .8 | |||
| Male | 14 | 6 | 1.0 | ||
| Female | 7 | 2 | 1.1 | ||
| Acute GVH grade | 0.5-6.6 | .4 | |||
| 1-2 | 17 | 7 | 1.0 | ||
| 3-4 | 4 | 1 | 1.8 | ||
| Donor type | 0.3-2.6 | .8 | |||
| HLA-matched sibling | 11 | 4 | 0.9 | ||
| MM rel/MUD | 10 | 4 | 1.0 |
MM rel/MUD indicates mismatched related/matched-unrelated donor.
95% Cl and P values refer to HR.
Acute (n = 1) and chronic (n = 8) GVHD were the main causes of death (n = 9; 69%), followed by recurrence of malignancy (n = 3; 23%) and pneumonia (n = 1; 8%) of unknown cause without active GVHD. Outcomes are summarized in Table 2.
Discussion
The initial management of acute GVHD usually consists of adding corticosteroids while continuing prophylaxis with tacrolimus or cyclosporine. Prednisone or methylprednisolone 1 to 2 mg/kg per day is generally accepted as the standard of care.2,3,33 Adding other immunosuppressant drugs to steroids does not improve the treatment outcome and may increase the risk for infectious complications. Approximately 40% of patients with acute GVHD respond to corticosteroid therapy, but most patients have persistent manifestations or progressive GVHD.34 Steroid-resistant acute GVHD is extremely difficult to manage and is associated with death in more than 70% of patients.35-38
There is no consensus regarding the best therapy for steroid-resistant GVHD. Several immunosuppressive drugs, including inhibitors of T-cell activation and IL-2 gene expression,39-42 inhibitors of the IL-2 receptor,43-46 anti–T-lymphocyte heteroantisera, and monoclonal antibodies against CD2, CD3, CD5, and CD14747-50 have been evaluated in clinical trials. Although a fraction of patients respond, none of these treatments have improved survival. Death results predominantly from infection, direct manifestation of GVHD, or recurrence of the underlying malignancy.
Infliximab was initially used to treat GI GVHD because of its activity in inflammatory bowel disease. We and others previously reported responses to infliximab, especially in patients with GVHD of the GI tract.14,26-28 The current analysis comprises 21 patients who received infliximab as a single agent added to corticosteroid and tacrolimus therapy for steroid-resistant GVHD. Fifty-seven percent of patients had gastrointestinal involvement, and only 19% had grade 3-4 GVHD. Prognostic factors analyzed in this study, including age, sex, HLA compatibility (related vs unrelated donor), and overall grade (grade 2 vs grade 3-4) did not seem to have any impact on response or survival. However, given the number of patients, we cannot rule out that the study is underpowered to show statistical significance.
The 67% response rate to infliximab, primarily CR, was encouraging. Patients who achieved CR (n = 13) experienced persistent benefits from therapy, and only 3 of them required any further immunosuppressive therapy. The highest rates of response were observed in GI and skin acute GVHD. Infliximab was well tolerated, and no adverse reactions were observed during or after infusions.
Patients with steroid-resistant acute GVHD are severely immunocompromised and are subject to a high rate of opportunistic infections. Marty et al51 reported an increased risk for non-Candida invasive fungal infections with the use of infliximab, reporting that a non-Candida invasive fungal infection developed in 5 (45%) of 11 patients who had received infliximab compared with 5 (12%) of 42 who had not. Only 11 patients in that report received infliximab, and most were treated with additional immunosuppressants. In our series, Aspergillus infections (n = 7) developed in a lower proportion of infliximab-treated patients (n = 6; 29%), and they required at least 1 additional line of salvage immunosuppressive therapy because they achieved PR or NR with infliximab. The incidence of Aspergillus infection in our series of patients with steroid-resistant acute GVHD who were treated with infliximab is lower than that reported by Marty et al51 but is marginally higher than the 15% to 20% incidence reported in other series of patients after allogeneic transplantation with and without GVHD.52-54 In each of these reports, it is difficult to determine how infliximab, GVHD itself, additional immunosuppressive drugs, and other demographic and clinical factors affected the risk for fungal and other opportunistic infections. Intensive antifungal prophylactic or preemptive strategies are warranted.
This study has several limitations. It is a retrospective analysis of patients with steroid-resistant GVHD who were treated with infliximab as a single agent at the discretion of their attending physicians while a prospective, controlled study of infliximab was being organized. This might have introduced a selection bias, and the observed response rate might not be representative of all steroid-refractory patients. A prospective, controlled study is necessary for definitive evaluation of infliximab.
TNF-α blockade with infliximab was well tolerated and was active for the treatment of steroid-resistant acute GVHD, particularly when the GI tract was involved. The overall response rate of 67% is promising and warrants further investigation, particularly for patients with GI involvement. These responses were generally complete, and many persisted over time. However, survival after steroid-resistant acute GVHD continues to be a problem. The possibility of excessive fungal or other infections must be explored further. Infliximab's role in the treatment of acute GVHD should be definitively evaluated in controlled clinical trials.
Prepublished online as Blood First Edition Paper, April 6, 2004; DOI 10.1182/blood-2003-12-4241.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal