Abstract
We report a phase 1 study of pharmacokinetics, dosimetry, toxicity, and response of 131I anti-tenascin chimeric 81C6 for the treatment of lymphoma. Nine patients received a dosimetric dose of 370 MBq (10 mCi). Three patients received an administered activity of 1480 MBq (40 mCi), and 2 developed hematologic toxicity that required stem cell infusion. Six patients received an administered activity of 1110 MBq (30 mCi), and 2 developed toxicity that required stem cell infusion. The clearance of whole-body activity was monoexponential with a mean effective half-life of 110 hours (range, 90-136 hours) and a mean effective whole-body residence time of 159 hours (range, 130-196 hours). There was rapid uptake within the viscera; however, tumor uptake was slower. Activity in normal viscera decreased proportional to the whole body; however, tumor sites presented a slow clearance (T1/2, 86-191 hours). The mean absorbed dose to whole-body was 67 cGy (range, 51-89 hours), whereas the dose to tumor sites was 963 cGy (range, 363-1517 cGy). Despite lack of a “blocking” antibody, 1 of 9 patients attained a complete remission and 1 a partial remission. These data demonstrate this radiopharmaceutical to be an encouraging agent for the treatment of lymphoma particularly if methods to protect the normal viscera are developed.
Introduction
Non-Hodgkin lymphomas (NHLs) represent an array of lymphoproliferative disorders with indolent, as well as rapidly progressive, subtypes. Although many patients with NHL respond to therapy initially, most relapse and often die of progressive disease within 10 years of diagnosis.1,2 New radioimmunotherapy modalities have been developed that target the cellular differentiation antigens CD20 and CD22 by using naked or radiolabeled antibodies.3-8 Two anti-CD20 radiolabeled murine monoclonal antibodies have been approved in the United States for the treatment of relapsed follicular or transformed lymphoma (Zevalin, ie 90Y-ibritumomab tiuxetan, IDEC-Y2B8; IDEC Pharmaceuticals, San Diego, CA; and Bexxar, 131I-tositumomab; Corixa Corp, Seattle, WA). Kaminski et al9 summarized their experience with nonmyeloablative activities of 131I-tositumomab whereby patients received a targeted whole-body dose of 75 cGy. In those studies, the response rate was 71% with 31% achieving complete remission (CR). Moreover, the response rate in low-grade or transformed NHL was 83% in contrast to 41% in intermediate-grade NHL.9 Those studies reveal that targeting lymphoma with radiolabeled antibodies is efficacious, although there is significant room for improvement in complete remission rates and duration of responses, as well as expanding the types of lymphomas to be targeted with these agents. Our group has investigated the changes in the tumor stroma that accompany disease activity in NHL and have noted significant changes in the expression of the extracellular matrix protein tenascin-C, which is limited to the site of lymphoma. Furthermore, the increased stromal expression correlates with increased resistance and disease progression as well. Thus, tenascin-C expression, as in high-grade gliomas,10-12 correlates with the degree of angiogenesis, and the protein is contained in the microvessel membrane and in the membrane-bound reticular stromal network.13 In NHL it has been shown that tenascin-C expression becomes interwoven with tumor cells, where expression correlates with angiogenesis, vessel immaturity, and aggressiveness in B-NHL.13 The enhanced expression of tenascin in diffuse and aggressive lymphomas has prompted the evaluation of radiolabeled therapy directed at this stromal component. Targeting the extracellular matrix in patients with diffuse, large cell lymphomas with radiolabeled monoclonal antibodies may provide a new therapeutic avenue for the treatment of patients who have failed treatments directed at differentiation antigens on tumor cells themselves.
This is the first intravenous use of a radiolabeled antibody directed toward a stromal target, tenascin-C, in patients with lymphoma. Following the standard approach approved by the Food and Drug Administration (FDA) for development of new radiolabeled antibodies, we report the pharmacokinetics, dosimetry, toxicity, and response in 9 patients who did not receive a cold or “blocking” antibody prior to a fixed radiolabeled dose of therapy. The preferential uptake in the tumor and enhanced expression of tenascin in intermediate- and high-grade lymphomas demonstrates the encouraging potential of this approach. Even in this phase 1 trial with no blocking antibody of normal organs, antilymphoma effects were observed. Our results provide the foundation for future trials to improve therapy by blocking nonspecific binding and binding to tenascin-C expressed in normal organs such as the marrow and liver.
Patients, materials, and methods
Patient eligibility
Patients with relapsed or refractory non-Hodgkin lymphoma who had failed at least 1 prior regimen and were not considered eligible for other standard approaches with curative intent were included on this trial. Patients were 18 years of age or older with a minimum white blood cell count of 4 × 109/L, an absolute neutrophil count greater than 1 × 109/L, platelet count greater than 100 × 109/L, and hematocrit greater than 30% before therapy. Aspartate aminotranspherase, alanine aminotranspherase, alkaline phosphatase, and total bilirubin must have been less than 2 times the upper limit of normal, and patients with more than 25% of their liver involved with disease were not eligible. Estimated creatinine clearance was more than 60 mg/mL for all patients, and patients could not have any active obstructive hydronephrosis. Cancer and Leukemia Group B performance status of 3 or less was required with life expectancy of at least 3 months. Patients must have been HIV negative, have no active viral (A, B, or C) or autoimmune hepatitis by screening or history, may not have more than 25% of the marrow involved with lymphoma (on bilateral examination), and not have undergone prior high-dose chemotherapy requiring bone marrow or hematopoietic stem cell support. Furthermore, patients may not have previously received radiation to a maximum dose to any organ, and no more than 25% of the skeleton may have been previously radiated.
Prior to initiation of therapy, patients had hematopoietic stem cells collected as a “backup” in case of prolonged aplasia. Blood work and radiographic studies were carried out to assess patients' disease state and organ function within 2 weeks and 4 weeks of study entry, respectively.
Antibody preparation and iodination
The production of human/mouse chimeric 81C6 (ch81C6) was carried out by combining the murine 81C6 variable region genes with those for the human immunoglobulin G2 (IgG2) constant regions, and the resulting chimeric monoclonal antibody (mAb) was characterized as described elsewhere.14 The ch81C6 mAb was grown in a Mini-Max hollow fiber bioreactor with CD Hybridoma media with no serum or protein additives. Purification was by affinity chromatography over a Sepharose-staphylococcal protein-A column followed by polyethylimine (PEI) ion exchange chromatography. The preparation of each clinical batch followed the FDA Manufacture and Testing Guidelines,15 in which all procedures were under good manufacturing practice (GMP) conditions. Radiolabeling was performed by a modified Iodo-Gen procedure.16 All preparations had an immunoreactivity of more than 75%, with more than 95% of the label eluting as immunoglobulin G on size-exclusion high-pressure liquid chromatography.
Antibody administration
After obtaining informed consent, patients receive thyroid suppression with potassium iodide 2 drops orally and liothyronine 75 μg orally, daily beginning 24 to 48 hours prior to the first dose of therapy and continuing for 1 month following initiation of therapy. The patients received premedications, including diphenhydramine 50 mg intravenously and acetaminophen 650 orally approximately 30 minutes prior to radiolabeled antibody administration, which was delivered intravenously through a peripheral or central venous catheter, with vital signs monitored every 15 minutes for 1 hour after infusion. Following the FDA guidelines for development of new radiolabeled antibodies, patients were given a fixed dose of radiolabel and not dosed by total body dosimetry in this phase 1 trial. Patients were given an initial dosimetric dose of 370 MBq [10 mCi] 131I-labeled ch81C6 mAb on a constant amount of 10 mg monoclonal antibody. Because of slight variations in yield and transfer of radiolabeled protein to injection syringe, the administered amount was ± 2 mg of the antibody. The amount of administered radiation activity may vary ± 10% for the same reason. Patients had gamma camera imaging shortly after infusion and on 5 of the ensuing 7 days. Pharmacokinetic measurements were performed 4 to 6 hours after infusion and on 5 of the next 7 days for each patient, as well. Following this, a therapeutic dose with an escalating dose of 131I-labeled chimeric 81C6 monoclonal antibody was delivered to the patient. Patients were observed daily after infusion for 3 days and then a minimum of weekly until recovery from all toxicity as a result of the therapy. At the completion of recovery from side effects of the radiolabeled antibody, patients had restaging examinations performed, as well as human antimouse antibody (HAMA) assessments.
Quantitative imaging, pharmacokinetics, and dosimetry
Blood samples, serial whole-body gamma camera images, and single photon emission computed tomography (SPECT) were evaluated to estimate absorbed doses to normal organs and identifiable tumor sites. Blood samples were used to measure the activity concentration within the blood pool as a function of time, and they were obtained immediately after administration and approximately 0.5, 1, 5, and 24 hours thereafter. A trichloroacetic acid precipitation was used to assess the protein-associated fraction of 131I in the blood samples. The activity concentration of iodine in blood samples was measured in a calibrated scintillation counter using 2-mL aliquots. The percentage of injected dose in the blood was calculated by comparison with standards prepared from the same administered dose.17 In 3 patients, marrow or nodal core samples were also obtained for direct measurement of activity, allowing a specific activity concentration scale to be developed.
Planar whole-body images were acquired immediately after infusion, between 2 and 4 hours, 24 hours, and between day 4 and day 7. All images were acquired on a dual-head gamma camera system equipped with high-energy general purpose collimators, set at a 15% energy window, and centered on the 131I photo-peak of 364 keV. Moreover, quantitative SPECT imaging was performed by using high-energy collimation to assess the 3-dimensional distribution of 131I-labeled ch81C6 in liver, spleen, lung, kidney, thyroid, and bone marrow of the spine.18 The analysis of whole-body images was performed by using the gamma camera system software to generate regions of interest (ROIs). A set of ROIs for the whole body, liver, spleen, lungs, kidneys, thyroid, discernible tumor sites, and background was defined for each patient and used on all sequential images in the anterior and posterior views to obtain count rates, and the geometric mean was obtained for each ROI. This analysis yielded relative clearance curves for the whole body and organs. Organ and tumor dosimetry was calculated on the basis of standard quantitative SPECT methods for 131I and patient-specific quantitative SPECT-based dosimetry as described elsewhere.18-21
Bone marrow and lymph node needle biopsy samples
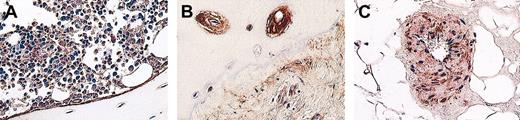
Core needle bone marrow and, when accessible, lymph node biopsy samples were obtained within 48 hours after the dosimetric activity of 370 MBq (10 mCi) was administered. These were approximately 20 mg in mass. Biopsy samples were evaluated for tumor infiltration and morphology, and also they were used to assess the activity concentration and distribution at the small-scale level. Activity measurements in tumor needle biopsy samples were compared with activities computed from quantitative SPECT scans.20 Because of the high uptake of 131I-labeled ch81C6 mAb in bone, bone marrow biopsy samples were used as a reference for bone surface and red marrow dosimetry.22 All bone marrow biopsies were obtained from the posterior iliac crest of patients. Bone marrow dosimetry was carried out by using histologic images stained for tenascin-C that reflect the morphology of bone and activity distribution of tenascin-C (Figure 1). The Monte Carlo transport code EGS4-PRESTA was adapted to assess the small-scale dose coefficients (S values expressed in cGy-g/MBq h) in bone marrow, trabecular bone, and bone surfaces by using histologic images.23 The absorbed dose DT to target T (bone marrow or bone surfaces) from sources in R (represented by tenascin-C expression) is given as DT = S(T←R)ã, where DT is expressed in cGy, ã is the estimated cumulated activity concentration in the bone biopsy sample expressed in MBq-h/g, and (T←R) is the small-scale S-value to target T from sources in R expressed in (cGy g)/(MBq h).
Histologic samples of bone marrow biopsy samples from patients with NHL stained for tenascin-C expression. (A) Tenascin-C expression along bone surfaces in trabecular bone. (B) Tenascin-C expression around Haversian canals and tumor saturated bone marrow, and (C) tenascin-C expression on a singular tumor nodule within bone marrow stroma. These photographs were taken using a Nikon Eclipse TE 300 using a 20× lens with an aperture of 0.5 of the objective lens. Total magnification is 200×. ImageJ software was used to acquire the images, and the camera was a Sony 970MD triple color camera.
Histologic samples of bone marrow biopsy samples from patients with NHL stained for tenascin-C expression. (A) Tenascin-C expression along bone surfaces in trabecular bone. (B) Tenascin-C expression around Haversian canals and tumor saturated bone marrow, and (C) tenascin-C expression on a singular tumor nodule within bone marrow stroma. These photographs were taken using a Nikon Eclipse TE 300 using a 20× lens with an aperture of 0.5 of the objective lens. Total magnification is 200×. ImageJ software was used to acquire the images, and the camera was a Sony 970MD triple color camera.
Toxicity and efficacy
Hematologic toxicity was assessed according to the National Cancer Institute common toxicity criteria (CTC version 3.0; Common Toxicity Criteria, Cancer Therapy Evaluation Program, National Cancer Institute, Bethesda, MD). Toxicity grades were obtained for platelets, neutrophils, and leukocytes. Patients were evaluated on recovery of side effects (approximately 6-12 weeks after infusion) with physical examination, laboratory analyses, and restaging radiographs to assess for degree of response as a secondary end point of this phase 1 trial. Previously published criteria for response were used.24 HAMA titers were obtained after 131I-ch81C6 therapy.
The primary end point of this trial was to estimate the maximum tolerated dose in terms of toxicity of 131I-labeled chimeric 81C6 anti-tenascin antibody delivered intravenously to patients with progressive non-Hodgkin lymphoma. National Cancer Institute Toxicity Criteria, Version 2.0 was followed, with documentation of all toxicities encountered. Dose-limiting toxicity was defined as a grade III or higher nonhematopoietic toxicity, or prolonged cytopenia. Prolonged cytopenia was defined as hematopoietic toxicity consisting of more than 10 days of an absolute neutrophil count (ANC) less than 0.5 × 109 cells/L or the requirement of platelet transfusions for more than 14 days to maintain a count greater than 10 × 109 platelets/L or the need for red cell transfusion to maintain a hematocrit greater than 25%, unless because of the underlying disease documented by the treating physician. The maximum tolerated dose was the dose of radiolabeled antibody that produced 0 of 3 or 2 or less of 6 patients experiencing a dose-limiting toxicity at a specific dose. Dose escalation or deescalation followed the requirements of the FDA for development of new radiolabeled antibodies and was based on total administered activity (millicuries) and not dosimetry. Our Institutional Review Board and the FDA approved the protocol, and a signed informed consent was obtained prior to initiation of therapy.
Results
Imaging
Whole-body gamma camera images showed mainly blood pool activity immediately after administration, which decreased over time but was clearly present up to 200 hours after administration (Figure 2). There was rapid uptake of 131I-labeled ch81C6 mAb in bone marrow on all patients because of the presence of tenascin-C in the extracellular matrix of bone marrow and tumor involment.25,26 Patients with large tumor masses, as shown in Figures 2 and 3, showed tumor retention of 131I-labeled ch81C6 with an effective half-life close to the physical half-life of 131I, demonstrating the long-term retention of ch81C6 mAb; however, in small masses of tumor as determined by computed tomography (CT), tumor contrast was very low because of the prolonged blood activity, and tumor retention was difficult to determine.
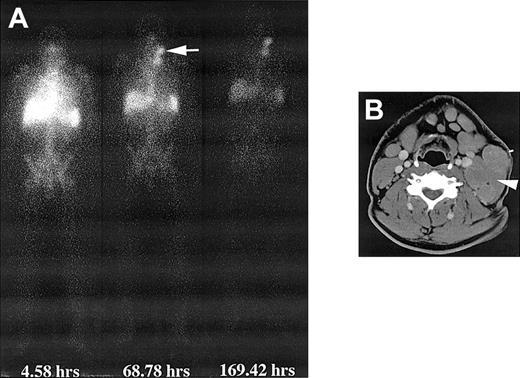
Whole-body and CT images after infusion of 131I-labeled chimeric 81C6 mAb. (A) Whole-body images of a patient obtained at different time points after infusion of 370 MBq 131I-labeled chimeric 81C6 mAb. Note the prolonged uptake in the nodal disease in the neck. (B) CT scan depicting the nodal disease (arrow) that accumulated the radiolabeled antibody. A biopsy sample was obtained from this node 44 hours after dosimetric infusion of 370 MBq, and the measured activity concentration was 70 kBq/g.
Whole-body and CT images after infusion of 131I-labeled chimeric 81C6 mAb. (A) Whole-body images of a patient obtained at different time points after infusion of 370 MBq 131I-labeled chimeric 81C6 mAb. Note the prolonged uptake in the nodal disease in the neck. (B) CT scan depicting the nodal disease (arrow) that accumulated the radiolabeled antibody. A biopsy sample was obtained from this node 44 hours after dosimetric infusion of 370 MBq, and the measured activity concentration was 70 kBq/g.
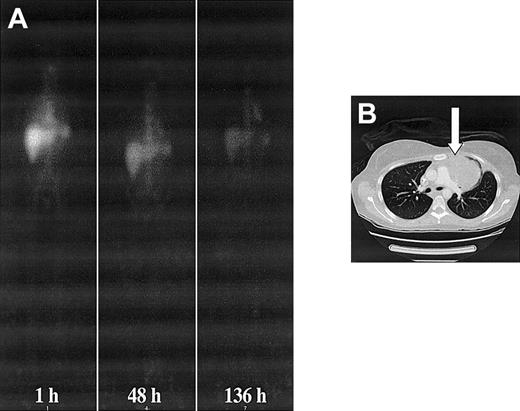
Whole-body and CT images of patient no. 2. (A) Serial anterior whole-body images of patient no. 2 showing increased accumulation of 131I-labeled 81C6 mAb in a large mediastinal lesion. (B) CT image showing the location of the lesion affecting the left lung. A biopsy sample was obtained from this node 17 hours after a dosimetric infusion of 370 MBq. The measured activity concentration was 37 kBq g-1. A supplemental video is available at the Blood website; see the Supplemental Video link at the top of the online article.
Whole-body and CT images of patient no. 2. (A) Serial anterior whole-body images of patient no. 2 showing increased accumulation of 131I-labeled 81C6 mAb in a large mediastinal lesion. (B) CT image showing the location of the lesion affecting the left lung. A biopsy sample was obtained from this node 17 hours after a dosimetric infusion of 370 MBq. The measured activity concentration was 37 kBq g-1. A supplemental video is available at the Blood website; see the Supplemental Video link at the top of the online article.
Pharmacokinetics and dosimetry
The whole-body activity, as determined by whole-body scintigraphy, of 131I-labeled ch81C6 mAb decreased mono-exponentially in all 9 patients with an average effective half-life of 110 hours (range, 90-136 hours). The corresponding average effective residence time of 131I-labeled ch81C6 in the whole body was 159 hours (range, 130-196 hours). The average whole-body dose among all 9 patients was 67 cGy (range, 51-89 cGy). The pharmacokinetics of.131I-labeled ch81C6 in blood was bi-exponential, with an alpha component (fast clearance) of 1.9 hours (range, 0.18-4.4 hours) and a fraction of 0.65 (range, 0.36-0.94), and a beta component (slow clearance) of 70 hours (range, 45-91 hours) and a fraction of 0.35 (range, 0.06-0.64).
The dose-limiting organ was the bone marrow where hematologic toxicity was observed in 2 of 3 patients treated at the 1480 MBq (40 mCi) level. The administered dose was then reduced to 1110 MBq (30 mCi) whereby only 2 of 6 patients developed hematologic toxicity. The activity concentration measured in bone marrow biopsy samples was 31 kBq/g with an activity fraction in bone surfaces (AFBS) of 35% for patient no. 1, 44 kBq/g with an AFBS of 51% for patient no. 2, and 76 kBq/g with an AFBS of 49% for patient no. 3. Using bone marrow histologic samples stained for tenascin-C, we estimated the S-values to red marrow and bone surfaces and corresponding absorbed doses. The range of absorbed doses to red marrow and bone surfaces among all patients varied between 232 and 745 cGy, and 397 and 1135 cGy, respectively. There were few treated people to evaluate for a potential causal relationship between absorbed dose and hematologic toxicity. Absorbed doses to other organs are summarized in Table 1. The organ that received the largest absorbed dose, as expected, was the thyroid.
Patient characteristics, treatment parameters, whole-body pharmacokinetics, and whole-body and organ dosimetry for patients with NHL receiving 131I-chimeric 81C6
. | . | Weight, kg . | Dosimetric activity, MBq . | Therapeutic activity, MBq . | Specific activity, MBq kg-1 . | Whole-body effective half-life, h . | Whole-body residence time, h . | Absorbed dose, cGy* . | . | . | . | . | . | . | . | . | . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient no. . | Sex . | . | . | . | . | . | . | WB . | Thyroid . | RM . | BS . | Blood . | Lungs . | Kidney . | Liver . | Spleen . | Tumor† . | |||||||||
| 1 | M | 101 | 370 | 1480 | 18.3 | 102 | 147 | 58 | 3009 | 329 | 508 | 141 | 223 | 213 | 206 | 567 | 1517 | |||||||||
| 2 | F | 61 | 370 | 1480 | 30.3 | 109 | 158 | 87 | 2155 | 505 | 888 | 247 | 297 | 241 | 306 | 650 | 1258 | |||||||||
| 3 | F | 79 | 370 | 1480 | 23.4 | 95 | 137 | 76 | 2375 | 745 | 1135 | 299 | 264 | 255 | 137 | 450 | — | |||||||||
| 4 | F | 74 | 370 | 1110 | 20.0 | 125 | 180 | 67 | 2419 | 329 | 566 | 192 | 307 | 429 | 195 | 350 | 811 | |||||||||
| 5 | F | 59 | 370 | 1110 | 25.1 | 136 | 196 | 89 | 2056 | 350 | 512 | 240 | 232 | 108 | 133 | 330 | 735 | |||||||||
| 6 | F | 70 | 370 | 1110 | 21.1 | 90 | 130 | 51 | 1096 | 370 | 558 | 137 | 312 | 242 | 149 | 474 | 1198 | |||||||||
| 7 | F | 86 | 370 | 1110 | 17.2 | 114 | 165 | 54 | 1416 | 345 | 543 | 177 | 426 | 319 | 158 | 362 | 682 | |||||||||
| 8 | M | 81 | 370 | 1110 | 18.3 | 121 | 175 | 69 | 2347 | 232 | 397 | 168 | 220 | 247 | 213 | 370 | 1060 | |||||||||
| 9 | M | 97 | 370 | 1110 | 15.3 | 101 | 146 | 53 | 1820 | 380 | 585 | 143 | 310 | 131 | 96 | 344 | 363 | |||||||||
| Average, cGy/MBq | — | — | — | — | — | — | — | 0.04 | 1.29 | 0.25 | 0.51 | 0.12 | 0.18 | 0.15 | 0.11 | 0.27 | 0.60 | |||||||||
| Minimum, cGy/MBq | — | — | — | — | — | — | — | 0.03 | 0.74 | 0.16 | 0.34 | 0.08 | 0.12 | 0.07 | 0.06 | 0.22 | 0.25 | |||||||||
| Maximum, cGy/MBq | — | — | — | — | — | — | — | 0.06 | 1.63 | 0.40 | 0.77 | 0.16 | 0.29 | 0.29 | 0.17 | 0.35 | 0.82 | |||||||||
. | . | Weight, kg . | Dosimetric activity, MBq . | Therapeutic activity, MBq . | Specific activity, MBq kg-1 . | Whole-body effective half-life, h . | Whole-body residence time, h . | Absorbed dose, cGy* . | . | . | . | . | . | . | . | . | . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient no. . | Sex . | . | . | . | . | . | . | WB . | Thyroid . | RM . | BS . | Blood . | Lungs . | Kidney . | Liver . | Spleen . | Tumor† . | |||||||||
| 1 | M | 101 | 370 | 1480 | 18.3 | 102 | 147 | 58 | 3009 | 329 | 508 | 141 | 223 | 213 | 206 | 567 | 1517 | |||||||||
| 2 | F | 61 | 370 | 1480 | 30.3 | 109 | 158 | 87 | 2155 | 505 | 888 | 247 | 297 | 241 | 306 | 650 | 1258 | |||||||||
| 3 | F | 79 | 370 | 1480 | 23.4 | 95 | 137 | 76 | 2375 | 745 | 1135 | 299 | 264 | 255 | 137 | 450 | — | |||||||||
| 4 | F | 74 | 370 | 1110 | 20.0 | 125 | 180 | 67 | 2419 | 329 | 566 | 192 | 307 | 429 | 195 | 350 | 811 | |||||||||
| 5 | F | 59 | 370 | 1110 | 25.1 | 136 | 196 | 89 | 2056 | 350 | 512 | 240 | 232 | 108 | 133 | 330 | 735 | |||||||||
| 6 | F | 70 | 370 | 1110 | 21.1 | 90 | 130 | 51 | 1096 | 370 | 558 | 137 | 312 | 242 | 149 | 474 | 1198 | |||||||||
| 7 | F | 86 | 370 | 1110 | 17.2 | 114 | 165 | 54 | 1416 | 345 | 543 | 177 | 426 | 319 | 158 | 362 | 682 | |||||||||
| 8 | M | 81 | 370 | 1110 | 18.3 | 121 | 175 | 69 | 2347 | 232 | 397 | 168 | 220 | 247 | 213 | 370 | 1060 | |||||||||
| 9 | M | 97 | 370 | 1110 | 15.3 | 101 | 146 | 53 | 1820 | 380 | 585 | 143 | 310 | 131 | 96 | 344 | 363 | |||||||||
| Average, cGy/MBq | — | — | — | — | — | — | — | 0.04 | 1.29 | 0.25 | 0.51 | 0.12 | 0.18 | 0.15 | 0.11 | 0.27 | 0.60 | |||||||||
| Minimum, cGy/MBq | — | — | — | — | — | — | — | 0.03 | 0.74 | 0.16 | 0.34 | 0.08 | 0.12 | 0.07 | 0.06 | 0.22 | 0.25 | |||||||||
| Maximum, cGy/MBq | — | — | — | — | — | — | — | 0.06 | 1.63 | 0.40 | 0.77 | 0.16 | 0.29 | 0.29 | 0.17 | 0.35 | 0.82 | |||||||||
RM indicates red marrow; BS, bone surfaces; WB, whole body; —, not applicable.
Absorbed doses were calculated on the basis of total administered activity (DA + TA).
Estimated absorbed dose to selected discernible radiographic sites.
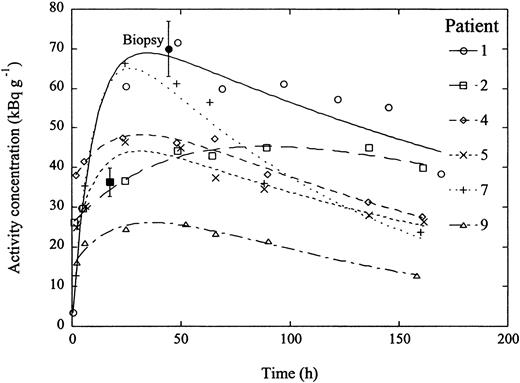
Tumor sites were selected according to discernible radiolabeled uptake from both whole-body planar images and SPECT images. However, many tumor sites that were identified by CT were not discernible in either whole-body images or SPECT because of minimal tumor mass or overlap with normal viscera such as the liver or spleen; therefore, there was a clear heterogeneity in absorbed doses to tumor sites. As an example, Figures 2 and 3 shows the persistence of radiolabeled antibody in the nodal disease of patients no. 1 and 2, respectively, even after 169 hours following infusion whereby the half-life of the clearance phase of the radiolabeled antibody was higher than 150 hours, which was close to that of the physical half-life of 131I, demonstrating good antibody retention. Figure 4 shows similar clearance rates for selected tumor sites in other patients. Among all 9 patients, the average absorbed dose to selected tumor sites was 953 cGy (range, 363-1517 cGy), and the average absorbed dose per administered activity was 0.60 cGy/MBq (range, 0.25-0.82 cGy/MBq).
Pharmacokinetics of 131I-labeled ch81C6 mAb of selected tumor sites from several patients. The initial tumor uptake reached a maximum between 40 and 100 hours among different tumor sites where the half-life of the clearance phase varied between 86 and 191 hours. The average biologic clearance half-life of the mAb in these tumors was estimated at 410 hours, demonstrating the long-term retention of the antibody in tumor sites. The range of absorbed doses varied between 363 and 1517 cGy. The measured activity concentrations from tumor biopsy samples were 70 and 36 kBq/g for patients 1 (•) and 2 (▪), respectively. The error bars indicate the error associated with the biopsy.
Pharmacokinetics of 131I-labeled ch81C6 mAb of selected tumor sites from several patients. The initial tumor uptake reached a maximum between 40 and 100 hours among different tumor sites where the half-life of the clearance phase varied between 86 and 191 hours. The average biologic clearance half-life of the mAb in these tumors was estimated at 410 hours, demonstrating the long-term retention of the antibody in tumor sites. The range of absorbed doses varied between 363 and 1517 cGy. The measured activity concentrations from tumor biopsy samples were 70 and 36 kBq/g for patients 1 (•) and 2 (▪), respectively. The error bars indicate the error associated with the biopsy.
Toxicity
Patients initially received a therapeutic dose of 1480 MBq (40 mCi). However, because of hematopoietic toxicity, this was deescalated in subsequent patients to 1110 MBq (30 mCi) (Table 2). There was no significant nonhematologic toxicity encountered in this study. In the first cohort of patients who received 1480 MBq for the therapeutic dose, the dose per kilogram of patient weight ranged between 18 and 30 MBq/kg. Two of the 3 patients in this cohort had grade 4 white cell, neutrophil, and platelet toxicity. The time to recover from white cells being under 1 × 109/L (grade 4) to more than 3 × 109/L (grade 1) or better was 20 and 12 days for these 2 patients, respectively. Severe platelet suppression was noted with recovery from less than 25 × 109/L (grade 4) to grade 1 or better taking 20 and 30 days for these 2 patients. Both of these patients had stem cells infused to assist recovery. These 2 patients had moderate hemoglobin toxicity, although the 1 patient in this cohort without a dose-limiting toxicity had grade 3 hemoglobin toxicity (65-80 g/L). The subsequent 6 patients were treated with 1110 MBq, corresponding to dose per kilogram of patient weight that ranged between 15 and 20 MBq/kg. All but 1 of these 6 patients experienced at least grade 3 white cell suppression, with a median of 41 days (range, 14-49 days) to recover to a white cell count more than 3 × 109/L in the patients in this cohort, although only 3 of these patients had an absolute neutrophil count less than 0.5 × 109/L with a median time to recover neutrophils of 3.5 days (range, 0-21 days) in this cohort. All patients in this cohort also had grade 3 or 4 thrombocytopenia with a median time to recover to grade 1 or better of 31 days (range, 22-70 days). The median number of days of grade 4 thrombocytopenia in this cohort was 14 days (range, 0-40 days). Patients no. 7 and no. 9 had prolonged thrombocytopenia; however, they were showing recovering within 2 weeks of onset of the white count decreasing under 2 × 109/L and were not considered to have a dose-limiting toxicity (DLT). Two patients in this group had stem cells infused to assist recovery (no. 6 and no. 8). Only one patient in this cohort had severe hemoglobin toxicity as well. Interestingly, the patient with the highest administered activity per unit mass (patient no. 5) in this cohort did not have any severe or prolonged cytopenias. These data demonstrate that the only organ with toxicity from this therapy was the bone marrow.
Hematologic and organ toxicity in patients with NHL treated with 131I-labeled ch81C6 mAb
. | Patient no. . | . | . | . | . | . | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Toxicity . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | ||||||||
| Administered dose, MBq | 1480 | 1480 | 1480 | 1110 | 1110 | 1110 | 1110 | 1110 | 1110 | ||||||||
| Administered dose, MBq/kg | 14.6 | 24.4 | 18.7 | 14.9 | 18.7 | 15.9 | 12.9 | 13.7 | 11.5 | ||||||||
| White cell range, × 109/L (d for recovery to ≤ grade 1) | 5.8-12.2 (0) | 4.7-0.5 (20) | 4.3-0.4 (12) | 4.7-0.5 (21) | 4.1-2.0 (41) | 9-0.9 (41) | 3.2-1.0 (49) | 6.6-0.1 (14) | 5.8-1.1 (49) | ||||||||
| No. d ANC was below 0.5 × 109/L | 0 | 20 | 4 | 7 | 0 | 21 | 0 | 13 | 0 | ||||||||
| Platelet decrease, × 109/L (d for recovery to ≤ grade 1) | 234-12 (35) | 216-10 (20) | 154-5 (30) | 372-7 (28) | 164-47 (22) | 186-6 (70) | 113-8 (28) | 333-8 (33) | 190-8 (40) | ||||||||
| No. d platelets were below 25 × 109/L | 27 | 13 | 18 | 12 | 0 | 40 | 13 | 14 | 28 | ||||||||
| Hemoglobin decrease, g L-1 (no. units red cell transfusions delivered)* | 110-67 (2) | 100-80 (2) | 107-90 (0) | 107-93 (0) | 103-93 (0) | 137-63 (4) | 120-87 (0) | 107-80 (4) | 123-90 (0) | ||||||||
| Required stem cell re-infusion | No | Yes | Yes | No | No | Yes | No | Yes | No | ||||||||
| Pulmonary toxicity | None | None | None | None | None | None | None | None | None | ||||||||
| Hepatic toxicity | None | None | None | None | None | None | None | None | None | ||||||||
| Neural toxicity | None | None | None | None | None | None | None | None | None | ||||||||
| Renal toxicity | None | None | None | None | None | None | None | None | None | ||||||||
| Cardiac toxicity | None | None | None | None | None | None | None | None | None | ||||||||
. | Patient no. . | . | . | . | . | . | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Toxicity . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | ||||||||
| Administered dose, MBq | 1480 | 1480 | 1480 | 1110 | 1110 | 1110 | 1110 | 1110 | 1110 | ||||||||
| Administered dose, MBq/kg | 14.6 | 24.4 | 18.7 | 14.9 | 18.7 | 15.9 | 12.9 | 13.7 | 11.5 | ||||||||
| White cell range, × 109/L (d for recovery to ≤ grade 1) | 5.8-12.2 (0) | 4.7-0.5 (20) | 4.3-0.4 (12) | 4.7-0.5 (21) | 4.1-2.0 (41) | 9-0.9 (41) | 3.2-1.0 (49) | 6.6-0.1 (14) | 5.8-1.1 (49) | ||||||||
| No. d ANC was below 0.5 × 109/L | 0 | 20 | 4 | 7 | 0 | 21 | 0 | 13 | 0 | ||||||||
| Platelet decrease, × 109/L (d for recovery to ≤ grade 1) | 234-12 (35) | 216-10 (20) | 154-5 (30) | 372-7 (28) | 164-47 (22) | 186-6 (70) | 113-8 (28) | 333-8 (33) | 190-8 (40) | ||||||||
| No. d platelets were below 25 × 109/L | 27 | 13 | 18 | 12 | 0 | 40 | 13 | 14 | 28 | ||||||||
| Hemoglobin decrease, g L-1 (no. units red cell transfusions delivered)* | 110-67 (2) | 100-80 (2) | 107-90 (0) | 107-93 (0) | 103-93 (0) | 137-63 (4) | 120-87 (0) | 107-80 (4) | 123-90 (0) | ||||||||
| Required stem cell re-infusion | No | Yes | Yes | No | No | Yes | No | Yes | No | ||||||||
| Pulmonary toxicity | None | None | None | None | None | None | None | None | None | ||||||||
| Hepatic toxicity | None | None | None | None | None | None | None | None | None | ||||||||
| Neural toxicity | None | None | None | None | None | None | None | None | None | ||||||||
| Renal toxicity | None | None | None | None | None | None | None | None | None | ||||||||
| Cardiac toxicity | None | None | None | None | None | None | None | None | None | ||||||||
National Cancer Institute toxicity grading scale for hematologic parameters: white cell toxicity grade 2, 2-3 × 109/L; grade 3, 1-2 × 109/L; grade 4, < 1 × 109/L (growth factor support was not used in this trial). Platelet toxicity grade 2, 50-75 × 109/L; grade 3, 25-50 × 109/L; grade 4, < 25 × 109/L. Hemogl obin toxicity grade 2, 80-100 g/L; grade 3, 65-80 g/L; grade 4, < 65 g/L.
Transfusions delivered with packed red blood cells.
Response
The patients treated on this trial had relapsed disease without other curative options with a median of 3 prior regimens (range, 2-5 prior regimens) and 11 prior cycles (range, 7-14 prior cycles). Seven of the 9 had failed at least 1 course of rituximab therapy. Patients with either low-grade or high-grade NHL were included. Two had small lymphocytic NHL, 1 had mucosa-associated lymphoma, 1 had follicular NHL, 2 had transformed NHL, and 3 had diffuse large cell NHL. Despite the heavy pretreatment and refractory nature of most of the patients as well as no blocking antibody being used, response in this phase 1 study was noted with 1 complete remission and 1 partial remission, and the others had stable disease lasting from 2 to longer than 8 months (Table 3).
Clinical response to 131I-labeled chimeric 81C6 mAb
Patient no. . | Disease . | No. prior regimens . | No. prior cycles . | Response . |
|---|---|---|---|---|
| 1 | CLL/SLL | 3 | 13 | SD |
| 2 | DLCL | 4 | 11 | SD |
| 3 | Transformed NHL | 2 | 7 | SD |
| 4 | MALT lymphoma | 2 | 7 | CRu (6 mo) |
| 5 | Transformed NHL | 4 | 14 | SD |
| 6 | Follicular | 4 | 18 | SD |
| 7 | DLCL | 2 | 8 | SD |
| 8 | DLCL | 5 | 14 | SD |
| 9 | CLL/SLL | 3 | 8 | PR (continues > 8 mo) |
Patient no. . | Disease . | No. prior regimens . | No. prior cycles . | Response . |
|---|---|---|---|---|
| 1 | CLL/SLL | 3 | 13 | SD |
| 2 | DLCL | 4 | 11 | SD |
| 3 | Transformed NHL | 2 | 7 | SD |
| 4 | MALT lymphoma | 2 | 7 | CRu (6 mo) |
| 5 | Transformed NHL | 4 | 14 | SD |
| 6 | Follicular | 4 | 18 | SD |
| 7 | DLCL | 2 | 8 | SD |
| 8 | DLCL | 5 | 14 | SD |
| 9 | CLL/SLL | 3 | 8 | PR (continues > 8 mo) |
CLL indicates chronic lymphocytic leukemia; SLL, small lymphocytic lymphoma; SD, stable disease; DLCL, diffuse large cell lymphoma; MALT, mucosa-associated lymphoid tissue lymphoma; CRu, complete remission unconfirmed; PR, partial remission.
HAMA
Six patients had HAMA testing performed from 33 to 166 days following injection. None of these patients developed HAMA. One patient had a low-titer HAMA noted before therapy, presumably because of her known prior exposure to a murine antibody. This patient had a negative HAMA on retesting 33 days following the therapeutic dose.
Discussion
Available radiolabeled agents approved for follicular (low grade) or transformed lymphoma are directed toward antigens expressed on the cell surface. The CD20 target is often not as highly expressed in the other types of more aggressive diffuse lymphomas or small lymphocytic disease. Further, recent work has identified the tumor stroma as an important supporting network relating to the growth and progression of many types of malignancies.27-29 Recently developed therapies directed at affecting stromal elements, such as vasculogenesis, have been shown to be effective in some tumor types as well.30 It has been reported that stromal changes, such as increased vasculogenesis and expression of extracellular matrix proteins such as tenascin, are increased in lymphoma.13,31 Our group has extended these observations by noting that the change in the stromal components of microvessel density and tenascin expression are limited to the sites of disease in the patient. In addition, the expression of these stromal elements changes over time in correlation with the activity of disease (D.A.R., Martha Wadleigh, Carol J. Wikstrand, Karen P. Mann, Filiz Sen, Bercedis L. Peterson, Alan D. Proia, and D.D.B., unpublished observation, June 2004). These data imply that targeting tumor stroma, rather than the tumor cells directly, may be an advantageous route for therapy. In addition, we and others have shown that the expression of tenascin increases with aggressiveness of NHL subtype.13 Our group has reported use of chimeric 81C6 radiolabeled antibody to tenascin with instillation directly into tumor cavities after resection in those with brain tumors, resulting in improved progression-free survival.17,32,33 This phase 1 trial combines this recognition of the importance of the stromal compartment in promoting progression of lymphoma with intravenous delivery of this same radiolabeled antibody.
We have shown in this report that 131I anti-tenascin antibody, delivered without any blocking antibodies to prevent binding to the liver, spleen, marrow, and nonspecific sites can be safely delivered to patients intravenously. The prolonged half-life of the chimeric antibody used in this study is encouraging for the effective half-life of the agent in attacking tumor; however, it may also remain problematic in terms of damaging normal viscera and the significant hematologic side effects remain dose limiting. Future studies will focus on minimizing the binding in the marrow, allowing increased delivery to the tumor and expansion of the use of this agent to many patients with diffuse disease. Strategies to address this take advantage of the fact that dosimetric studies reveal a distribution consistent with the known low expression of tenascin in normal visceral organs of the lungs, liver, and bone marrow, as well as increased concentration in tumor tissue. Further, pharmacokinetic studies reveal rapid uptake in the liver and marrow and a slower, but enhanced, uptake in selected tumors sites over normal organs. We have previously infused a murine anti-tenascin antibody linked to 123I into patients with escalating doses of antibody (10-100 mg anti-tenascin) linked to the radiolabel. In doing this, we noted a significant decrease in normal viscera uptake of the radiolabel, suggesting a similar approach with the human/mouse chimeric antibody we now use may be efficacious.34 Following these data, future trials of this agent will deliver the unlabeled chimeric antibody intravenously for the prolonged blocking effect of normal viscera prior to the radiolabeled antibody. Alternatively, if this is not effective one could deliver the longer-lived chimeric antibody as unlabeled for the blocking potential and use the shorter-lived murine antibody for the therapeutic dosing to affect a more rapid clearance. Even without a blocking antibody, with the large differential between tumor and normal visceral organ uptake documented in this study, there was approximately 953 cGy radiation delivered to the site of the tumor. We anticipate that by first blocking the areas of binding to normal visceral organs and then proceeding with the therapeutic radiolabeled antibody dose the therapeutic window of this agent may significantly improve by decreasing the marrow uptake and corresponding toxicity and augmenting the uptake of radiolabeled antibody to tumor sites.
The mean whole-body effective half-life of 131I-labeled ch81C6 mAb of 110 hours was similar to that of 131I-rituximab of 88 hours and longer than that of 131I-tositumomab of 56 hours.9 Blood kinetics of 131I-labeled ch81C6 were described by a bi-exponential clearance with a slow-clearance (beta component) half-life of 70 hours, which was lower than that of the whole-body of 110 hours and 36% longer, indicating the large uptake (or sink) observed in spleen, liver, and bone marrow where tenascin-C is ubiquitously expressed.13 Radioactivity was excreted exclusively through the kidneys where approximately 47% of the injected activity was found in urine.
Tumor uptake was well defined in whole-body images in which maximum uptake was observed between 24 and 48 hours. Clearance of 131I-labeled ch81C6 from tumor sites was slow, reflecting closely the physical decay constant of 131I and demonstrating the good tumor retention of ch81C6 mAb. Absorbed doses to preselected tumor sites, as determined by using quantitative imaging, ranged between 353 and 1517 cGy with an average of 953 cGy, which are within the range of absorbed doses estimated for 131I-tositumomab between 37 and 1760 cGy.35 Furthermore, the estimated average absorbed dose to selected tumors sites per unit administered activity among all patients was 0.60 cGy/MBq (range, 0.25-0.82 cGy/MBq), which was higher than that obtained from 131I-tositumomab.35 Comparison of dose factors shows that an absorbed dose of 25 Gy to normal organs other than the thyroid gland and bone marrow would not be exceeded. However, a pretherapeutic determination of the whole-body half-life and residence time of 131I-labeled ch81C6 is necessary for each patient to determine the absorbed dose to these organs and tumor sites in future studies.
This study confirms the safety and potential therapeutic utility of targeting the tumor stroma with 131I-labeled anti-tenascin chimeric 81C6 monoclonal antibody. The high doses to red marrow did not always result in hematologic toxicity in these few patients, although further study is needed to evaluate the true relationship with dose delivered, patient weight, dosimetric calculations, and toxicity. The complete and partial remission noted in some patients treated on this phase 1 study is encouraging and supports further attempts to enhance localization of the radiolabel to the tumor while decreasing normal visceral uptake through the use of unlabeled antibody.
Antistromal radioimmunotherapy has not yet been fully evaluated for patients with lymphoma. Future trials will evaluate methods to minimize normal visceral and nonspecific binding and reescalation of the radiolabeled dose to maximize tumor exposure. Given the known increased expression of tenascin-C in other tumor types, such as lung, breast, and gastrointestinal tumors, the potential applicability of anti-tenascin chimeric 81C6 monoclonal antibody may have a broader therapeutic applicability as well.36-40
Prepublished online as Blood First Edition Paper, April 20, 2004; DOI 10.1182/blood-2003-12-4264.
Supported in part by the Lisa M. Stafford Young Investigator Award (D.A.R.) and by the National Institutes of Health (grants CA11898, CA70164, and S10 RR15697.7).
D.A.R. and G.A. contributed equally to the completion of this study.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the referring physicians and nurses for the care of the patients.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal