Abstract
Erythropoietin (Epo) gene expression is under the control of hypoxia-inducible factor 1 (HIF-1), and is negatively regulated by GATA. Interleukin 1β (IL-1β) and tumor necrosis factor α (TNF-α), which increase the binding activity of GATA and inhibit Epo promoter activity, are increased in patients with anemia of chronic disease (ACD). We previously demonstrated the ability of K-7174 (a GATA-specific inhibitor), when injected intraperitoneally, to improve Epo production that had been inhibited by IL-1β or TNF-α treatment. In the present study, we examined the ability of both K-11706, which inhibits GATA and enhances HIF-1 binding activity, and K-13144, which has no effect on GATA or HIF-1 binding activity, to improve Epo production following inhibition by IL-1β or TNF-α in Hep3B cells in vitro and in an in vivo mouse assay. Oral administration of K-11706 reversed the decreases in hemoglobin and serum Epo concentrations, reticulocyte counts, and numbers of erythroid colony-forming units (CFU-Es) induced by IL-1β or TNF-α. These results raise the possibility of using orally administered K-11706 for treating patients with ACD.
Introduction
Disorders associated with anemia of chronic disease (ACD) are characterized by the production of certain inflammatory cytokines, primarily macrophage-derived, including interleukin 1α (IL-1α), IL-1β, IL-6, transforming growth factor β (TGF-β), and tumor necrosis factor α (TNF-α).1,2 In patients with chronic inflammatory disorders, cellular or humoral factors, including TNF-α and IL-1, may suppress the bone marrow response to erythropoietin (Epo).3,4 Faquin et al5 reported that IL-1 (α or β), TNF-α, and TGF-β inhibit the production of Epo from the hepatoma cell line Hep3B. Jelkmann et al6 reported similar results for IL-1 and TNF-α, but noted no inhibition by TGF-β. In addition, they also reported that IL-1β inhibits Epo production in isolated serum-free perfused kidneys.
Epo gene expression is under the control of hypoxia-inducible factor 1 (HIF-1), positively through a HIF-1 binding site in the Epo enhancer,7 and it is negatively regulated by GATA, which binds to the GATA site in the Epo promoter.8 Thus, drugs that are GATA inhibitors and/or HIF-1 activators might increase the production of Epo, and restore hemoglobin concentrations in patients with ACD. Recently, La Ferla et al9 reported the molecular mechanism of Epo gene inhibition by IL-1β and TNF-α in patients with ACD, showing that treatment with IL-1β or TNF-α increased the DNA binding activities of the transcription factors GATA-2 and nuclear factor (NF)–κB, and inhibited both Epo promoter activity and Epo protein production. Thus, both GATA-2 and NF-κB seem to be involved in the suppression of Epo gene expression in vivo, and may be responsible for the impaired synthesis of Epo in vivo in patients with inflammatory diseases.9 Conversely, NG-monomethyl-l-arginine (L-NMMA), a nitric oxide synthase (NOS) inhibitor, is increased in patients with chronic renal failure.10 We found that L-NMMA inhibits NO and cGMP production, increases GATA mRNA expression and GATA binding activity, and inhibits Epo promoter activity.11 Therefore, a common pathogenesis of ACD and anemia with renal disease appears to be via the stimulation of GATA binding activity by IL-1β, TNF-α, or L-NMMA. The effects of NO on HIF and Epo production are controversial. Under normoxic conditions, Kimura et al12 described stimulating properties of NO donors on HIF-1 activity, which is contrasted by reports showing inhibitory actions of NO in combination with hypoxia.13,14 To explain these discrepancies, Sandau et al15 tested several NO donors with diverse chemical structures and NO-releasing half-lives. They concurred with Sandau et al in showing NO donor–induced HIF-1α protein accumulation.15 Ohigashi et al reported that serum concentrations of Epo in hypoxic polycythemic mice were significantly increased after injections of sodium nitroprusside (an NO donor).16 These results are compatible with our data and strongly suggest that L-NMMA inhibits Epo production via the GATA transcription factor.
In the present study, Hep3B cells were used because of their high expression of Epo during hypoxia.17 Hep3B cells mainly express GATA-2 and a little GATA-3, but not GATA-1.8 Epo transcription is negatively regulated by a subset of GATA transcription factors (GATA-1, -2, and -3), which bind to a GATA site within the minimal promoter.8 Recently, Dame et al18 reported that GATA-4 positively regulates Epo gene expression, is critical for transcription of the Epo gene in hepatocytes, and may contribute to the switch in the site of Epo gene expression from the fetal liver to the adult kidney.
K-7174, a GATA-specific inhibitor that acts through a mechanism independent of NF-κB activity, suppresses the binding activity of GATA-1, -2, and -3 proteins.19 In a previous study, we showed that injection of K-7174 restores the Epo production that has been inhibited by IL-1β, TNF-α, or L-NMMA treatment in Hep3B cells in vitro, and in an in vivo mouse assay.20 In that study, K-7174 was injected intraperitoneally. Here, we examined K-11706, which inhibits GATA binding activity, has a 1000-fold higher affinity than K-7174 (Y.N., S.I., K.M., C.S., N.O., N.S., T.D., T.K., S.T., T.N., and M.Y., unpublished data, November 2001), and enhances HIF-1 binding activity. Our aim was to determine if K-11706 can further improve Epo production following inhibition by IL-1β and TNF-α treatment in Hep3B cells in vitro, and in vivo by oral administration.
Materials and methods
Cell culture
The erythropoietin-producing hepatoma cell line Hep3B was obtained from the American Type Culture Collection (Rockville, MD). Cells were incubated under both 21% (normoxia) and 1% (hypoxia) oxygen for 24 hours as described.21 Aliquots of 3 × 106 Hep3B cells were incubated with 15 U/mL (0.3 ng/mL) recombinant human IL-1β (rhIL-1β; Roche Pharmaceuticals, Mannheim, Germany), 220 U/mL (2.2 ng/mL) recombinant human TNF-α (rhTNF-α; Roche) or 10 nM, 50 nM, or 100 nM K-11706 or K-13144 (Kowa, Tokyo, Japan) under hypoxic conditions (1% O2) for 24 hours. K-13144 (Kowa) was used as a negative control.
Plasmid vectors
We used the reporter plasmid pEPLuc as a basic plasmid construct.22 In this plasmid, both the 126-bp 3′ Epo enhancer and the 144-bp minimal Epo promoter were placed upstream of the firefly luciferase (Luc) gene in pXP2.23 The Epo enhancer corresponds to nucleotides 120 to 245 on the 3′ side of the poly (A) addition site, and the Epo promoter corresponds to nucleotides -118 to +26 relative to the transcription initiation site. This resulting plasmid is referred to as Pwt.24 The enhancer contained an HIF-1 binding site and a steroid receptor response element (SRRE). In the mutant constructs, the HIF-1 binding site in the Epo enhancer was mutated to TAAA (TACG to TAAA) and the GATA sequences in the Epo promoter were mutated to TATA (AGATAAC to ATATAAA) or TTTG (GATA to TTTG). These mutants were termed Pm6, Pm7, and Pm8.
Transfection for promoter assay
Aliquots of 8 × 105 Hep3B cells in tissue-culture plates (28 cm2/well; Becton Dickinson Labware, Franklin Lakes, NJ) were washed with serum-free media. Cells were cotransfected with a mixture containing lipofectin (20 μg/well; Invitrogen, San Diego, CA), DNA constructs (2 μg/well), and β-galactosidase (1 μg/well) as an internal standard, as previously described.23 After transfection, cells were incubated with 15 U/mL rhIL-1β, 220 U/mL rhTNF-α (Roche), or 100 nM K-11706, or K-13144 (Kowa) under normoxic or hypoxic conditions for 24 hours. The hypoxic induction of Luciferase gene expression is presented here as a hypoxia/normoxia ratio.8
DNA binding assay
Nuclear extracts were prepared as described.25 Sense-strand oligonucleotides (wild type for GATA: CAT GCA GAT AAC AGC CCC GAC that reside 30 bp to the Epo 5′ promoter; wild type for HIF-1: GAT CGC CCT ACG TGC TGT CTC AGT CA that reside 30 bp to the Epo gene 3′ enhancer) were end-labeled using T4 polynucleotide kinase (Toyobo, Tokyo, Japan). The DNA binding assay was performed as previously described.25
In vivo mouse assay
ICR strain mice were purchased from Clea Japan (Tokyo, Japan) and used as healthy controls. They were housed in autoclaved metal cages and were given a standard diet (CM; Oriental Yeast, Tokyo, Japan) and tap water ad libitum in an air-conditioned room. Recombinant mouse IL-1β (rmIL-1β; Roche) or recombinant mouse TNF-α (rmTNF-α; Roche) was administered intraperitoneally. Blood samples (0.3 mL) were obtained from the orbital vein on days -2, -1, 0, +3, and +6 for hematologic analyses. Epo was measured using a photometric enzyme-linked immunosorbent assay (ELISA) kit (Roche).
Treatment schedule
ICR mice were divided into 9 groups (A-I) to examine the effects of K-11706 on reversing the inhibition of hemoglobin by IL-1β or TNF-α. The injection schedule for the groups is shown in Table 1. Group A mice were treated with 150 μL polyethylene glycol (PEG; Showa Chemical, Tokyo, Japan) by oral administration on days 0 to 5 as a control. Group B was injected intraperitoneally with 1.67 × 104 U (0.33 μg) rmIL-1β on days 0 to 3. Group C was injected intraperitoneally with 3.33 × 105 U (0.83 μg) rmTNF-α on days 0 to 3. Groups D and E were treated with 3 mg/kg K-11706 or K-13144 by oral tube feeding. Groups F and G were injected intraperitoneally with 1.67 × 104 U rmIL-1β on days 0 to 3, and 3 mg/kg K-11706 or K-13144 was given orally on days 0 to 5. Groups H and I were injected intraperitoneally with 3.33 × 105 U rmTNF-α on days 0 to 3, and 3 mg/kg K-11706 or K-13144 was given orally on days 0 to 5. In a study of the effects of K-11706 or K-13144, IL-1β, and TNF-α on Epo production in vivo, 0.3 mL of blood was removed from the orbital vein of the mice at 0, 12, 24, and 36 hours to increase their Epo concentrations.
Injection schedule for groups of mice used to examine the effects of K-11706 and K-13144 on reversing inhibitions induced by IL-1β and TNFα
. | Injection . | . | Day . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group . | Item . | Amount . | 0 . | 1 . | 2 . | 3 . | 4 . | 5 . | ||||||
| A | PEG | 150 μL | x | x | x | x | x | x | ||||||
| B | IL-1β | 1.67 × 104 U | x | x | x | x | ||||||||
| C | TNF-α | 3.33 × 105 U | x | x | x | x | ||||||||
| D | K-11706 | 3 mg/kg | x | x | x | x | x | x | ||||||
| E | K-13144 | 3 mg/kg | x | x | x | x | x | x | ||||||
| F | IL-1β | 1.67 × 104 U | x | x | x | x | ||||||||
| K-11706 | 3 mg/kg | x | x | x | x | x | x | |||||||
| G | IL-1β | 1.67 × 104 U | x | x | x | x | ||||||||
| K-13144 | 3 mg/kg | x | x | x | x | x | x | |||||||
| H | TNF-α | 3.33 × 105 U | x | x | x | x | ||||||||
| K-11706 | 3 mg/kg | x | x | x | x | x | x | |||||||
| I | TNF-α | 3.33 × 105 U | x | x | x | x | ||||||||
| K-13144 | 3 mg/kg | x | x | x | x | x | x | |||||||
. | Injection . | . | Day . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group . | Item . | Amount . | 0 . | 1 . | 2 . | 3 . | 4 . | 5 . | ||||||
| A | PEG | 150 μL | x | x | x | x | x | x | ||||||
| B | IL-1β | 1.67 × 104 U | x | x | x | x | ||||||||
| C | TNF-α | 3.33 × 105 U | x | x | x | x | ||||||||
| D | K-11706 | 3 mg/kg | x | x | x | x | x | x | ||||||
| E | K-13144 | 3 mg/kg | x | x | x | x | x | x | ||||||
| F | IL-1β | 1.67 × 104 U | x | x | x | x | ||||||||
| K-11706 | 3 mg/kg | x | x | x | x | x | x | |||||||
| G | IL-1β | 1.67 × 104 U | x | x | x | x | ||||||||
| K-13144 | 3 mg/kg | x | x | x | x | x | x | |||||||
| H | TNF-α | 3.33 × 105 U | x | x | x | x | ||||||||
| K-11706 | 3 mg/kg | x | x | x | x | x | x | |||||||
| I | TNF-α | 3.33 × 105 U | x | x | x | x | ||||||||
| K-13144 | 3 mg/kg | x | x | x | x | x | x | |||||||
Hematology
Blood was collected from the retro-orbital plexus into heparin-coated microcapillaries. Hemoglobin concentration was determined colorimetrically (Nihon Kohden, Tokyo, Japan). Reticulocytes were counted on smears of blood that had been stained with methylene blue according to standard procedures. At least 1000 red blood cells were counted for each determination.
Colony assay
Mononucleated cells from bone marrow and spleens harvested on day 4 of the experiments were cultured in 1 mL 0.8% methylcellulose medium containing 30% fetal bovine serum (FBS), 1% bovine serum albumin (BSA), 0.1 mM 2-mercaptoethanol, and 2 mM l-glutamine (Methocult M3231; Stem Cell Technologies, Vancouver, British Columbia, Canada). For the detection of erythroid colony-forming units (CFU-Es), the medium was supplemented with 4 U/mL (40 ng/mL) of recombinant mouse Epo (rmEpo; Roche, Mannheim, Germany) and 100 ng/mL recombinant murine stem cell factor (rmSCF; Peprotech, London, United Kingdom). CFU-E colonies were counted after 3 days of culture. These assays were performed in duplicate and the results are shown as means with plus or minus 1 SD.
Other assays
Luciferase activity was determined in 20-μL aliquots of the cell extract using an Autolumat luminometer (Berthorude, Tokyo, Japan) for 10 seconds. Each measurement of relative light units was corrected by subtraction of the background and standardized to the β-galactosidase internal transfection control activity. Hypoxic inducibility was defined as the ratio of the corrected relative light units of the hypoxic (1% O2) dish to those of the normoxic (21% O2) dish, as described.21 Epo from the Hep3B cells or from mice was measured by ELISA, as described in “In vivo mouse assay.”
Statistical analysis
Student t tests were used to assess the concentration of significance between treatment groups.
Results
The binding activity of GATA is inhibited and HIF-1 is enhanced by K-11706
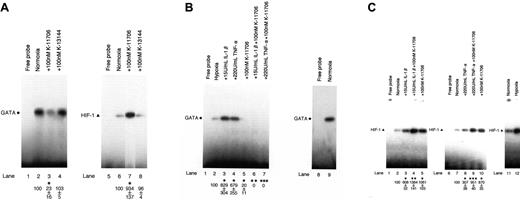
An electrophoretic mobility shift assay (EMSA) revealed the specific binding activity of GATA (Figure 1A, lane 2, left panel, closed circle; data of the competitive assay are not shown). The addition of 100 nM K-11706 inhibited the binding activity of GATA (Figure 1A, lane 3) to 23% ± 16% (measured by densitometric analysis) compared with the control (Figure 1A, lane 2). However, the addition of 100 nM K-13144 did not affect the binding activity of GATA (Figure 1A, lane 4).
Effects of K-11706 and K-13144 on GATA and HIF-1 binding activities. (A) Electrophoretic mobility shift assay (EMSA) was performed using 1.0 μg protein from Hep3B cells under normoxic conditions (lanes 2-4 and 6-8), and incubated with 100 nM K-11706 (lanes 3 and 7) and 100 nM K-13144 (lanes 4 and 8). The closed circle and triangle at the left indicate the positions of the GATA and HIF-1 transcription factors. The autoradiograph is representative of 3 different experiments, using different nuclear extracts, with similar results. Densitometric analyses of the bands expressed relative to the control are indicated by circles or triangles. * indicates significance compared with normoxic control, P < .005. (B) Effect of K-11706 on enhanced expression of GATA induced by IL-1β or TNF-α. EMSA was performed using 1.0 μg protein from Hep3B cells under hypoxic conditions (lanes 2-7), normoxic condition (lane 9), and incubated with 15 U/mL rhIL-1β (lane 3), 220 U/mL rhTNF-α (lane 4), 100 nM K-11706 (lane 5), 15 U/mL rhIL-1β plus 100 nM K-11706 (lane 6), and 220 U/mL rhTNF-α plus 100 nM K-11706 (lane 7) for 24 hours. The closed circle at the left indicates the position of the GATA transcription factors. The autoradiograph is representative of 3 different experiments, using different nuclear extracts, giving similar results. Densitometric analyses of the bands expressed relative to the control are indicated by the circles. * indicates significance compared with hypoxic control, P < .005; **, significance compared with IL-1β, P < .005; ***, significance compared with TNF-α, P < .005. (C) Effects of K-11706 on enhanced expression of HIF-1 induced by IL-1β or TNF-α. EMSA was performed using 1.0 μg protein from Hep3B cells under normoxic conditions (lanes 2-5, 7-11), hypoxic condition (lane 12), and incubated with 15 U/mL rhIL-1β (lane 3), 220 U/mL rhTNF-α (lane 8), 100 nM K-11706 (lanes 5 and 10), 15 U/mL rhIL-1β plus 100 nM K-11706 (lane 4), and 220 U/mL TNF-α plus 100 nM K-11706 (lane 9) for 24 hours. The closed triangle at the left indicates the position of the HIF-1 transcription factor. The autoradiograph is representative of 3 different experiments using different nuclear extracts, giving similar results. Densitometric analyses of the bands expressed relative to the control are indicated by the triangles. * indicates significance compared with normoxic control, P < .005; **, significance compared with IL-1β, P < .005; ***, significance compared with TNF-α, P < .005.
Effects of K-11706 and K-13144 on GATA and HIF-1 binding activities. (A) Electrophoretic mobility shift assay (EMSA) was performed using 1.0 μg protein from Hep3B cells under normoxic conditions (lanes 2-4 and 6-8), and incubated with 100 nM K-11706 (lanes 3 and 7) and 100 nM K-13144 (lanes 4 and 8). The closed circle and triangle at the left indicate the positions of the GATA and HIF-1 transcription factors. The autoradiograph is representative of 3 different experiments, using different nuclear extracts, with similar results. Densitometric analyses of the bands expressed relative to the control are indicated by circles or triangles. * indicates significance compared with normoxic control, P < .005. (B) Effect of K-11706 on enhanced expression of GATA induced by IL-1β or TNF-α. EMSA was performed using 1.0 μg protein from Hep3B cells under hypoxic conditions (lanes 2-7), normoxic condition (lane 9), and incubated with 15 U/mL rhIL-1β (lane 3), 220 U/mL rhTNF-α (lane 4), 100 nM K-11706 (lane 5), 15 U/mL rhIL-1β plus 100 nM K-11706 (lane 6), and 220 U/mL rhTNF-α plus 100 nM K-11706 (lane 7) for 24 hours. The closed circle at the left indicates the position of the GATA transcription factors. The autoradiograph is representative of 3 different experiments, using different nuclear extracts, giving similar results. Densitometric analyses of the bands expressed relative to the control are indicated by the circles. * indicates significance compared with hypoxic control, P < .005; **, significance compared with IL-1β, P < .005; ***, significance compared with TNF-α, P < .005. (C) Effects of K-11706 on enhanced expression of HIF-1 induced by IL-1β or TNF-α. EMSA was performed using 1.0 μg protein from Hep3B cells under normoxic conditions (lanes 2-5, 7-11), hypoxic condition (lane 12), and incubated with 15 U/mL rhIL-1β (lane 3), 220 U/mL rhTNF-α (lane 8), 100 nM K-11706 (lanes 5 and 10), 15 U/mL rhIL-1β plus 100 nM K-11706 (lane 4), and 220 U/mL TNF-α plus 100 nM K-11706 (lane 9) for 24 hours. The closed triangle at the left indicates the position of the HIF-1 transcription factor. The autoradiograph is representative of 3 different experiments using different nuclear extracts, giving similar results. Densitometric analyses of the bands expressed relative to the control are indicated by the triangles. * indicates significance compared with normoxic control, P < .005; **, significance compared with IL-1β, P < .005; ***, significance compared with TNF-α, P < .005.
EMSA also revealed the specific binding activity of HIF-1 (Figure 1A, lane 6, right panel, closed triangle; data of the competitive assay are not shown). In contrast to GATA as a probe, the addition of 100 nM K-11706 enhanced the binding activity of HIF-1 (Figure 1A, lane 7) to 934% ± 137% compared with the control (Figure 1A, lane 6). However, the addition of 100 nM K-13144 did not affect the binding activity of HIF-1 (Figure 1A, lane 8).
The addition of 15 U/mL IL-1β or 220 U/mL TNF-α enhanced the binding activity of GATA (Figure 1B, lanes 3 and 4) to 829% ± 304% or 679% ± 255% compared with the control (Figure 1B, lane 2). These increments of GATA binding activity by IL-1β and TNF-α were inhibited by the addition of 100 nM K-11706 (Figure 1B, lanes 6 and 7) to 0%. Furthermore, the addition of IL-1β or TNF-α enhanced the binding activity of HIF-1 (Figure 1C, lanes 3 and 8) to 608% ± 52% and 307% ± 38% compared with the control (Figure 1C, lanes 2 and 7). However, these stimulations of HIF-1 binding activity by IL-1β or TNF-α were further enhanced by the addition of 100 nM K-11706 (Figure 1C, lanes 4 and 9) to 1384% ± 181% and 951% ± 40% compared with the control (Figure 1C, lanes 2 and 7).
These results suggest that IL-1β and TNF-α enhance GATA and HIF-1 binding activities, whereas K-11706 reduces GATA binding activity and enhances HIF-1 binding activity.
The inhibition of Epo protein production by IL-1β and TNF-α is rescued by K-11706
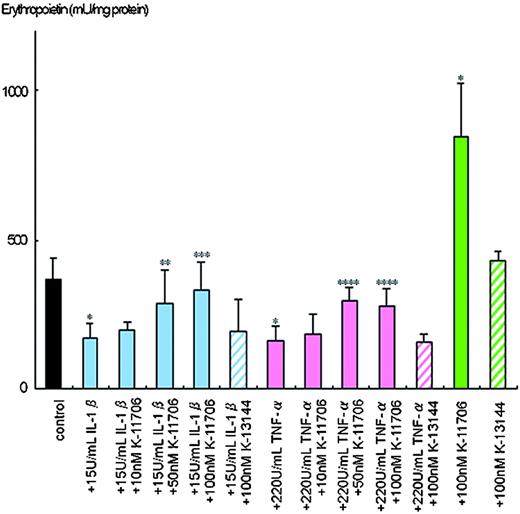
Hypoxia-induced Epo protein production from the Hep3B cells was 370 ± 69 mU/mg protein (Figure 2). However, only 170 ± 51 mU/mg of Epo protein was produced by hypoxia in the presence of 15 U/mL IL-1β, and 162 ± 48 mU/mg Epo protein was produced in the presence of 220 U/mL TNF-α.
Effects of K-11706 and K-13144 on the inhibition of Epo protein production from Hep3B cells by IL-1β and TNF-α. Aliquots of 3 × 106 Hep3B cells were incubated with 15 U/mL rhIL-1β, 220 U/mL rhTNF-α or 10 nM, 50 nM, or 100 nM K-11706 or 100 nM K-13144 under hypoxic conditions (1% O2) for 24 hours. Epo protein was measured by ELISA. Eight separate experiments were performed. Error bars represent 1 SD. * indicates significance compared with control, P < .005; **, significance compared with 15 U/mL IL-1β, P < .025; ***, significance compared with 15 U/mL IL-1β, P < .005; ****, significance compared with 220 U/mL TNF-α, P < .005.
Effects of K-11706 and K-13144 on the inhibition of Epo protein production from Hep3B cells by IL-1β and TNF-α. Aliquots of 3 × 106 Hep3B cells were incubated with 15 U/mL rhIL-1β, 220 U/mL rhTNF-α or 10 nM, 50 nM, or 100 nM K-11706 or 100 nM K-13144 under hypoxic conditions (1% O2) for 24 hours. Epo protein was measured by ELISA. Eight separate experiments were performed. Error bars represent 1 SD. * indicates significance compared with control, P < .005; **, significance compared with 15 U/mL IL-1β, P < .025; ***, significance compared with 15 U/mL IL-1β, P < .005; ****, significance compared with 220 U/mL TNF-α, P < .005.
To elucidate the dose-dependent effects of K-11706 on production of Epo in Hep3B cells, different doses of K-11706 were added to the culture. Addition of 10 nM K-11706 rescued the inhibition of Epo protein production by IL-1β to 199 ± 27 mU/mg protein and that by TNF-α to 186 ± 64 mU/mg protein (Figure 2). Addition of 50 nM or 100 nM K-11706 reversed the inhibition of Epo protein production by IL-1β to 285 ± 112 mU/mg and 335 ± 89 mU/mg protein, and that by TNF-α to 294 ± 47 mU/mg and 276 ± 63 mU/mg protein, respectively (Figure 2). Thus, K-11706 affected Epo production by Hep3B cells in a dose-dependent manner. Furthermore, 100 nM K-11706 alone stimulated Epo production to 846 ± 180 mU/mg protein (Figure 2). However, 100 nM K-13144 alone did not stimulate Epo production (430 ± 29 mU/mg protein). The addition of 100 nM K-13144 did not reverse the inhibitions of IL-1β (197 ± 100 mU/mg protein), or TNF-α (155 ± 31 mU/mg protein; Figure 2).
Inhibition of Epo promoter activity by IL-1β and TNF-α is reversed by K-11706
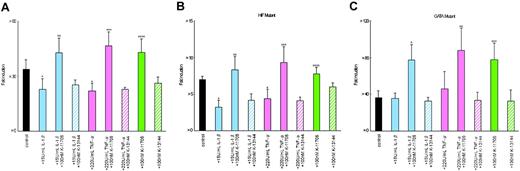
Hypoxic induction of Epo promoter activity from Pwt was 34-fold ± 5-fold (n = 6) times that of normoxic Pwt (Figure 3A). The addition of 15 U/mL IL-1β or 220 U/mL TNF-α inhibited the hypoxic induction of Luc reporter gene expression from Pwt with hypoxia/normoxia ratios of only 23-fold ± 6-fold and 22-fold ± 4-fold times that of normoxic Pwt, respectively (Figure 3A). Thus, the hypoxic induction of Epo gene expression appears to be suppressed by IL-1β or TNF-α through the Epo gene regulatory regions. However, the addition of 100 nM K-11706 induced hypoxic induction from Pwt to 43-fold ± 8-fold times that of normoxic Pwt (Figure 3A). Furthermore, the addition of 100 nM K-11706 to the IL-1β and TNF-α cells rescued hypoxic induction of Luc reporter gene expression from Pwt, with hypoxia/normoxia ratios of 43-fold ± 8-fold and 47-fold ± 8-fold times that of normoxic Pwt, respectively (Figure 3A). In contrast to K-11706, the addition of K-13 144 did not affect the hypoxic induction of Luc reporter gene expression from Pwt, with a hypoxia/normoxia ratio of 26-fold ± 3-fold times that of normoxic Pwt. Furthermore, the addition of 100 nM K-13144 to the IL-1β and TNF-α cells did not affect hypoxic induction of Luc reporter gene expression from Pwt with hypoxia/normoxia ratios of 25-fold ± 3-fold and 23-fold ± 1-fold times that of normoxic Pwt, respectively (Figure 3A). These results suggest that K-11706 rescues the suppression of Epo gene expression by IL-1β or TNF-α through the Epo gene regulatory regions.
Effects of K-11706 and K13144 on Epo promoter/enhancer. (A) Effects of K-11706 and K-13144 on the inhibition of the induction by IL-1β and TNF-α of the wild-type (Pwt) Epo promoter/enhancer with a Luc reporter construct in Hep3B cells. The wild-type (Pwt) Epo promoter/enhancer with a Luc reporter construct was transfected into 8 × 105 Hep3B cells and incubated with 15 U/mL rhIL-1β, 220 U/mL rhTNF-α, or 100 nM K-11706 under normoxic (21% O2) or hypoxic (1% O2) conditions for 24 hours. Hypoxic induction of Luc gene expression is represented here as a hypoxia/normoxia ratio shown as fold induction. Six separate experiments (quadruple samples) were performed. Error bars represent 1 SD. * indicates significance compared with control, P < .005; **, significance compared with 15 U/mL IL-1β, P < .005; ***, significance compared with 220 U/mL TNF-α, P < .005; ****, significance compared with control, P < .025. (B) Effects of K-11706 and K-13144 on the inhibition of the induction by IL-1β and TNF-α of the mutant type (Pm6: TACG-TAAA) in the Epo enhancer with a Luc reporter construct in Hep3B cells. A mutant form (Pm6: TACG-TAAA) of the Epo enhancer with a Luc reporter construct was transfected into 8 × 105 Hep3B cells. Experimental conditions were the same as those described in the legend for panel A. Eight separate experiments (quadruple samples) were performed. Error bars represent 1 SD. * indicates significance compared with control, P < .005; **, significance compared with 15 U/mL IL-1β, P < .005; ***, significance compared with 220 U/mL TNF-α, P < .005; ****, significance compared with control, P < .05. (C) Effects of K-11706 and K-13144 on the inhibition of the induction by IL-1β and TNF-α of the mutant-type (Pm7: AGATAAC-ATATAAA) in the Epo promoter with a Luc reporter construct in Hep3B cells. A mutant form (Pm7: AGATAAC-ATATAAA) of the Epo promoter with Luc reporter construct was transfected into 8 × 105 Hep3B cells. Experimental conditions were the same as those described in the legend for panel A. Five separate experiments (quadruple samples) were performed. Error bars represent 1 SD. * indicates significance compared with 15 U/mL IL-1β, P < .001; **, significance compared with 220 U/mL TNF-α, P < .005; ***, significance compared with control, P < .001.
Effects of K-11706 and K13144 on Epo promoter/enhancer. (A) Effects of K-11706 and K-13144 on the inhibition of the induction by IL-1β and TNF-α of the wild-type (Pwt) Epo promoter/enhancer with a Luc reporter construct in Hep3B cells. The wild-type (Pwt) Epo promoter/enhancer with a Luc reporter construct was transfected into 8 × 105 Hep3B cells and incubated with 15 U/mL rhIL-1β, 220 U/mL rhTNF-α, or 100 nM K-11706 under normoxic (21% O2) or hypoxic (1% O2) conditions for 24 hours. Hypoxic induction of Luc gene expression is represented here as a hypoxia/normoxia ratio shown as fold induction. Six separate experiments (quadruple samples) were performed. Error bars represent 1 SD. * indicates significance compared with control, P < .005; **, significance compared with 15 U/mL IL-1β, P < .005; ***, significance compared with 220 U/mL TNF-α, P < .005; ****, significance compared with control, P < .025. (B) Effects of K-11706 and K-13144 on the inhibition of the induction by IL-1β and TNF-α of the mutant type (Pm6: TACG-TAAA) in the Epo enhancer with a Luc reporter construct in Hep3B cells. A mutant form (Pm6: TACG-TAAA) of the Epo enhancer with a Luc reporter construct was transfected into 8 × 105 Hep3B cells. Experimental conditions were the same as those described in the legend for panel A. Eight separate experiments (quadruple samples) were performed. Error bars represent 1 SD. * indicates significance compared with control, P < .005; **, significance compared with 15 U/mL IL-1β, P < .005; ***, significance compared with 220 U/mL TNF-α, P < .005; ****, significance compared with control, P < .05. (C) Effects of K-11706 and K-13144 on the inhibition of the induction by IL-1β and TNF-α of the mutant-type (Pm7: AGATAAC-ATATAAA) in the Epo promoter with a Luc reporter construct in Hep3B cells. A mutant form (Pm7: AGATAAC-ATATAAA) of the Epo promoter with Luc reporter construct was transfected into 8 × 105 Hep3B cells. Experimental conditions were the same as those described in the legend for panel A. Five separate experiments (quadruple samples) were performed. Error bars represent 1 SD. * indicates significance compared with 15 U/mL IL-1β, P < .001; **, significance compared with 220 U/mL TNF-α, P < .005; ***, significance compared with control, P < .001.
To elucidate the effect of K-11706 via the GATA site in the Epo promoter region and/or HIF-1 binding site in the Epo enhancer, first a mutant form of the HIF-1 binding site in the Epo enhancer (mutated: TACG-TAAA) (Pm6) was transfected into Hep3B cells. Hypoxic induction of Epo promoter activity from Pm6 was 7-fold ± 1-fold times that of normoxic Pm6 (n = 8; Figure 3B). The addition of IL-1β or TNF-α inhibited the hypoxic induction of Luc reporter gene expression from Pm6 to 3-fold ± 1-fold and 4-fold ± 1-fold, respectively (Figure 3B). The addition of K-11706 to control, to +IL-1β, or to +TNF-α induced hypoxic induction by 8-fold ± 1-fold, 9-fold ± 1-fold, and 9-fold ± 2-fold, respectively (Figure 3B). In contrast to K-11706, the addition of K-13144 to control, to +IL-1β,orto +TNF-α did not affect hypoxic induction (6-fold ± 1-fold, 4-fold ± 1-fold, and 4-fold ± 1-fold, respectively; Figure 3B). Thus, K-11706 rescued the inhibition of Epo gene expression by IL-1β and TNF-α through at least the GATA site in the Epo promoter, because K-11706 rescued the inhibition of Epo gene expression by these cytokines even for the HIF-1 mutant promoter.
Second, the mutant form of the GATA site in the Epo promoter (mutated: AGATAAC-ATATAAA; Pm7) was transfected into Hep3B cells. Hypoxic induction from Pm7 was 36-fold ± 7-fold times that of normoxic Pm7 (n = 5; Figure 3C). In contrast to Pwt and Pm6, the addition of IL-1β or TNF-α did not inhibit hypoxic induction (36-fold ± 6-fold and 46-fold ± 19-fold, respectively; Figure 3C). However, the addition of 100 nM K-11706 induced hypoxic induction from Pm7 to 78-fold ± 18-fold times that of normoxic Pm7 (Figure 3C). Furthermore, the addition of 100 nM K-11706 to the IL-1β and TNF-α cells rescued the hypoxic induction of Luc reporter gene expression from Pm7 (77-fold ± 17-fold and 88-fold ± 24-fold, respectively; Figure 3C). In contrast to K-11706, the addition of K-13144 to control, to +IL-1β, or to +TNF-α did not affect hypoxic induction (33-fold ± 12-fold, 33-fold ± 4-fold and 34-fold ± 9-fold, respectively; Figure 3C). These results suggest that IL-1β or TNF-α inhibited Epo gene expression through the GATA site in the Epo promoter, but not through the HIF-1 binding site in the Epo enhancer.
Because a double increment was found with the addition of K-11706 to Pm7, these effects might be caused by TF-IID or other factors. To study this, another mutant of the GATA site in the Epo promoter (mutated: GATA-TTTG; Pm8) was transfected into Hep3B cells. These results were almost identical to the results obtained with Pm7, as shown in Figure 3C (data not shown). Thus, both IL-1β and TNF-α inhibited Epo gene expression through the GATA site in the Epo promoter, and K-11706 appeared to reverse the suppression of Epo gene expression by IL-1β or TNF-α through both the GATA site in the Epo promoter and the HIF-1 binding site in the Epo enhancer.
K-11706 reverses the decrease in hemoglobin induced by IL-1β and TNF-α
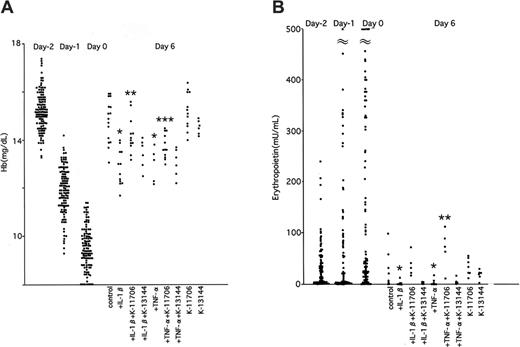
To elucidate the effects of K-11706, IL-1β, and TNF-α on Epo production in vivo, 0.3-mL samples of blood were removed from the orbital vein of mice at 0, 12, 24, and 36 hours to facilitate native Epo protein production. This bleeding decreased the hemoglobin concentrations from 15.2 ± 0.8 g/dL on day -2, to 11.9 ± 1.0 g/dL on day -1, and to 9.4 ± 1.0 g/dL on day 0. ICR mice were divided into 9 groups (A-I; Table 1).
The hemoglobin concentrations of the control group were 13.8 ± 0.6 g/dL on day 3, and 14.8 ± 0.8 g/dL on day 6 (Figure 4A). Injection of IL-1β decreased hemoglobin concentrations on days 3 and 6. Injection of TNF-α also decreased the hemoglobin concentrations on these days. However, oral administration of 3 mg/kg K-11706 significantly reversed this inhibition of hemoglobin production caused by IL-1β or TNF-α on day 6. The hemoglobin concentrations produced by 3 mg/kg K-11706 alone were 13.7 ± 1.0 g/dL on day 3 and 15.2 ± 0.8 g/dL on day 6 (Figure 4A).
Effects of K-11706 or K-13144 on hemoglobin and erythropoietin. (A) Effects of K-11706 or K-13144 on the inhibiton of hemoglobin concentrations induced by IL-1β or TNF-α in ICR mice. ICR mice were treated with 150 μL PEG by oral administration on days 0 to 5 as a control. Mice were intraperitoneally injected with 1.67 × 104 U (0.33 μg) rmIL-1β on days 0 to 3, 3.33 × 105 U (0.83 μg) rmTNF-α on days 0 to 3, and 3 mg/kg K-11706 or K-13144 by oral tube feeding on days 0 to 5 (n = 5-12). In order to study the effects of K-11706 or K-13144, IL-1β and TNF-α on Epo production in vivo, we removed 0.3 mL blood from the orbital vein at 0, 12, 24, and 36 hours to mimic anemia. * indicates significance compared with control, P < .005; **, significance compared with IL-1β, P < .05; ***, significance compared with TNF-α, P < .01. (B) Effects of K-11706 or K-13144 on the inhibition of serum concentrations of Epo induced by IL-1β or TNF-α in ICR mice. Experimental conditions were the same as those described in the legend to panel A (n = 5-9). * indicates significance compared with control, P < .05; **, significance compared with TNF-α, P < .01.
Effects of K-11706 or K-13144 on hemoglobin and erythropoietin. (A) Effects of K-11706 or K-13144 on the inhibiton of hemoglobin concentrations induced by IL-1β or TNF-α in ICR mice. ICR mice were treated with 150 μL PEG by oral administration on days 0 to 5 as a control. Mice were intraperitoneally injected with 1.67 × 104 U (0.33 μg) rmIL-1β on days 0 to 3, 3.33 × 105 U (0.83 μg) rmTNF-α on days 0 to 3, and 3 mg/kg K-11706 or K-13144 by oral tube feeding on days 0 to 5 (n = 5-12). In order to study the effects of K-11706 or K-13144, IL-1β and TNF-α on Epo production in vivo, we removed 0.3 mL blood from the orbital vein at 0, 12, 24, and 36 hours to mimic anemia. * indicates significance compared with control, P < .005; **, significance compared with IL-1β, P < .05; ***, significance compared with TNF-α, P < .01. (B) Effects of K-11706 or K-13144 on the inhibition of serum concentrations of Epo induced by IL-1β or TNF-α in ICR mice. Experimental conditions were the same as those described in the legend to panel A (n = 5-9). * indicates significance compared with control, P < .05; **, significance compared with TNF-α, P < .01.
In contrast, oral administration of K-13144 did not reverse the inhibition of hemoglobin by IL-1β on days 3 and 6. The hemoglobin concentrations produced by 3 mg/kg K-13144 alone were 13.7 ± 0.4 g/dL on day 3 and 14.6 ± 2.4 g/dL on day 6.
K-11706 increases Epo production in mice
The effects of K-11706 on serum Epo concentrations of mice in the above experiments were assayed by ELISA. The mean serum Epo concentration was 16 ± 13 mU/mL (n = 27) on day -2. The concentrations increased to 100 ± 34 mU/mL on day -1 and 197 ± 107 mU/mL on day 0 (Figure 4B). The PEG control mice produced 24 ± 31 mU/mL on day 3 and 25 ± 34 mU/mL on day 6. Compared with the controls, injection of IL-1β significantly decreased Epo production on day 6 (Figure 4B). For the same day, injection of TNF-α also significantly inhibited Epo production (Figure 4B), whereas oral administration of K-11706 significantly rescued the inhibition of Epo production induced by IL-1β and TNF-α (Figure 4B). In contrast to this, oral administration of K-13144 did not reverse the inhibition of Epo production induced by IL-1β and TNF-α on day 6 (Figure 4B).
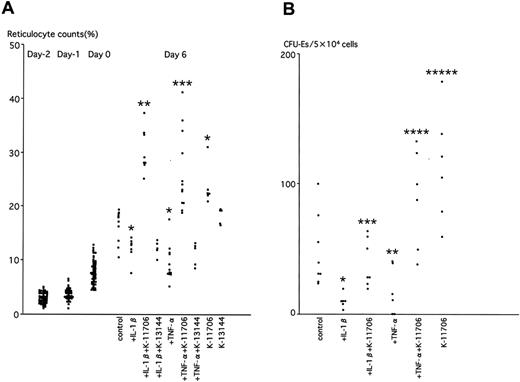
K-11706 reverses the inhibition of reticulocyte counts induced by IL-1β and TNF-α
The effects of K-11706 were measured on reticulocyte counts of mice in the above experiments. The reticulocyte count was 3% ± 1% on day -2. PEG controls showed 32% ± 4% on day 3 and 16% ± 3% on day 6 (Figure 5A). Injection of IL-1β decreased the reticulocyte counts on day 6 compared with the controls (Figure 5A). For the same day, injection of TNF-α also inhibited the reticulocyte counts (Figure 5A), whereas oral administration of K-11706 significantly increased them (Figure 5A). Moreover, K-11706 significantly reversed the decrease in reticulocyte production induced by IL-1β and TNF-α (Figure 5A). In contrast, oral administration of K-13144 did not increase the reticulocyte counts (Figure 5A) and K-13144 did not reverse the decrease in reticulocyte production induced by IL-1β and TNF-α on day 6 (Figure 5A).
Effects of K-11706 or K-13144 on reticulocyte counts and numbers of CFU-Es. (A) Effects of K-11706 or K-13144 on the inhibition of reticulocyte counts induced by IL-1β or TNF-α in ICR mice. Experimental conditions were the same as those described in the legend to Figure 4A (n = 5-12). * indicates significance compared with control, P < .005; **, significance compared with IL-1β, P < .005; ***, significance compared with TNF-α, P < .005. (B) Effects of K-11706 on the inhibition of the numbers of CFU-Es from the bone marrow of ICR mice induced by IL-1β or TNF-α. Experimental conditions were the same as those described in the legend to Figure 4A. Mice were killed on day 4 (n = 6-8). * indicates significance compared with control, P < .005; **, significance compared with control, P < .01; ***, significance compared with IL-1β, P < .005; ****, significance compared with TNF-α, P < .025; *****, significance compared with control, P < .05.
Effects of K-11706 or K-13144 on reticulocyte counts and numbers of CFU-Es. (A) Effects of K-11706 or K-13144 on the inhibition of reticulocyte counts induced by IL-1β or TNF-α in ICR mice. Experimental conditions were the same as those described in the legend to Figure 4A (n = 5-12). * indicates significance compared with control, P < .005; **, significance compared with IL-1β, P < .005; ***, significance compared with TNF-α, P < .005. (B) Effects of K-11706 on the inhibition of the numbers of CFU-Es from the bone marrow of ICR mice induced by IL-1β or TNF-α. Experimental conditions were the same as those described in the legend to Figure 4A. Mice were killed on day 4 (n = 6-8). * indicates significance compared with control, P < .005; **, significance compared with control, P < .01; ***, significance compared with IL-1β, P < .005; ****, significance compared with TNF-α, P < .025; *****, significance compared with control, P < .05.
K-11706 reverses the inhibition of the numbers of CFU-Es from bone marrow induced by IL-1β and TNF-α
The numbers of CFU-Es from bone marrow harvested on day 4 of the above experiments were assayed by colony assay to elucidate the effect of K-11706. The baseline number of CFU-Es from bone marrow was 48 ± 27 per 5 × 104 cells (Figure 5B). Compared with controls, injection of IL-1β or TNF-α decreased the numbers of CFU-Es, whereas oral administration of K-11706 significantly rescued the decrease in the numbers of CFU-Es from bone marrow (Figure 5B) and from spleen (data not shown) caused by IL-1β or TNF-α. Oral administration of K-11706 alone significantly increased the numbers of CFU-Es.
K-11706 increases Epo production and hemoglobin in control mice
K-11706 alone induced Epo protein and promoter activity in an in vitro Hep3B cell assay. However, the effect of K-11706 alone was not clear from the in vivo mice assay using bleeding to reduce hemoglobin concentrations. To elucidate the effect of K-11706 alone in control mice, 3 mg/kg K-11706 was orally administered for days 0 to 5 without bleeding. This increased serum Epo concentrations from 0.1 ± 0.2 mU/mg protein on day 0 to 65 ± 10 mU/mg protein on day 5 (P < .005 compared with controls). Furthermore, 3 mg/kg K-11706 alone increased hemoglobin from 14.0 ± 1.0 g/dL on day 0 to 16.8 ± 0.3 g/dL on day 5 (P < .005). Thus, it is possible that K-11706 might increase both Epo and hemoglobin production, not only by inhibiting GATA, but also by activating HIF-1. This possibility is supported by the transfection studies in Figure 3A-C and is not caused by hemo-concentration (data not shown).
Discussion
The human hepatoma cell lines Hep3B and HepG2 can produce Epo protein in vitro. In particular, the stimulation of Epo production in Hep3B mimics the physiologic response to hypoxia.17 Epo protein production and promoter activity were decreased in Hep3B cells by the addition of IL-1β (15 U/mL) or TNF-α (220 U/mL). These effects were reversed by the addition of 100 nM K-11706, which was only 1% of the concentration of K-7174 used in our previous studies.20 The suppression of promoter activity was not observed in cells transfected with GATA site-mutated constructs (Pm7 and Pm 8). These results suggest that IL-1β and TNF-α inhibited Epo promoter activity through its GATA site.
When Pm7 was transfected into Hep3B cells, the addition of K-7174 (a GATA-specific inhibitor), which did not affect HIF-1 binding activity,20 increased hypoxic induction only 1.2-fold times that of normoxic Pm7.20 However, here the addition of K-11706 increased hypoxic induction 2.1-fold times that of normoxic Pm7. Because Pm7 contains a mutation of GATA to TATA, TF-IID might enhance the Epo promoter activity by K-11706. To this end, Pm8 (GATA to TTTG) was transfected into Hep3B cells, and the addition of K-11706 enhanced the hypoxic induction from Pm8 to 2.0-fold compared with controls, as well as with Pm7. Thus, it is possible that K-11706 alone might enhance Epo promoter activity through stimulating HIF-1 binding activity.
EMSA showed that both IL-1β and TNF-α enhanced GATA binding activity, which is compatible with the results of La Ferla et al,9 whereas the addition of K-11706 decreased this increment in a dose-dependent manner (data not shown). EMSA showed that both IL-1β and TNF-α enhanced HIF-1 binding activity, which is also compatible with the result of Hellwig-Bürgel et al26 and others.27-30 EMSA using HIF-1 as a probe (Figure 1A) showed that K-11706 significantly increased HIF-1 binding activity, even under normoxic conditions. Furthermore, the addition of K-11706 increased HIF-1 binding activity significantly more than that produced by IL-1β or TNF-α alone. This enhancing effect of K-11706 on HIF-1 binding activity suggests that K-11706 alone can significantly increase both Epo protein production and Epo promoter activity. This increment of HIF-1 binding activity was not caused by an increase in HIF-1 protein concentration (data not shown).
To examine whether this reverse in the inhibition of Epo protein and promoter activity by K-11706 is via the GATA and HIF-1, we examined the effect of K-13144, which is an analog of K-11706, but does not affect GATA or HIF-1 binding activity. K-13144 did not reverse the inhibition of Epo protein and promoter activity in an in vitro Hep3B cell assay and in an in vivo mouse assay. The HIF-1 binding site of the Epo gene is found in the enhancer and it is not known if there is a functional HIF-1 binding site in the promoter. Therefore, it is possible that K-11706 increased the Epo protein and promoter activity in Hep3B cells, not only by inhibiting GATA but also by stimulating HIF-1 binding activity.
We also investigated the effect of K-11706 on Epo production in mice with anemia induced by bleeding from the orbital vein and by injection of IL-1β or TNF-α. Usually, the procedures that induce anemia experimentally are the administration of phenylhydrazine, which causes hemolysis, injection of inflammatory cytokines, or bleeding. Compared with controls, hemoglobin concentrations were decreased by bleeding, and further dropped following injections of IL-1β or TNF-α. In contrast to controls, oral administration of K-11706 for 6 days significantly increased hemoglobin concentrations. Intraperitoneal injections of IL-1β or TNF-α decreased Epo production, reticulocyte counts, and the numbers of CD71+/Ter119+ cells (data not shown) and CFU-Es; the administration of K-11706 reversed these inhibitions. These improvements were observed on the fourth day, which seems to be too early to detect any effects of K-11706. However, as in the controls, hemoglobin concentrations were increased on the fourth day, and such quick recovery of hemoglobin concentrations were also observed in other reports.31,32 Moreover, the numbers of CFU-Es and the total numbers of the late erythroid progenitor cell fraction were increased about 100-fold in the spleens of anemic mice.33 We therefore believe this rapid effect of K-11706 is real and reflects enhanced erythropoiesis.
The main mechanism might depend on stimulation of Epo protein production by K-11706. Epo stimulates the proliferation of erythroid progenitor cells, releasing reticulocytes from hematopoietic tissues.34 On the other hand, through expression analysis of the transcription factor genes, GATA-2 expression was down-regulated but GATA-1 was up-regulated during erythroid differentiation, and down-regulation of the GATA-1 gene is needed for erythroid terminal maturation.33 Therefore, direct inhibition of DNA binding activity of GATA-1 or -2 by K-11706 might induce a shortened erythroid differentiation and maturation period, and lead to rapid release of reticulocytes into the peripheral blood.
To avoid an early recovery of red blood cell counts following bleeding even in controls, we used different models of anemia. ICR mice were treated with one mg/kg of methotrexate (MTX) by oral tube feeding for 4 weeks. After 5 weeks, mice were treated with 3 mg/kg of K-11706 twice a day for 3 weeks by oral tube feeding, and control mice were treated with 0.5% hydroxypropylmethylcellulose (HPMC). Three weeks later, after tube feeding of K-11706 or HPMC, mice treated with K-11706 showed 13.3 ± 1.2 g/dL of hemoglobin, although the control group showed 10.9 ± 3.7 g/dL (n = 5, P = .05). Using 15-week-old MRL/Mpj-lpr/lpr mice (a mouse model of severe autoimmune disease),35 3 mg/kg K-11706 was orally administered twice a day for 3 weeks. This group showed 13.8 ± 0.8 g/dL of hemoglobin, although controls showed 11.9 ± 1.6 g/dL (n = 5, P < .005).
Although the mechanisms by which K-11706 inhibits GATA or enhances HIF-1 binding activity remain unknown, these data suggest that K-11706, which inhibits GATA and enhances HIF-1 binding activity, increases Epo promoter activity and Epo protein production, and reverses anemia. As for K-7174, the inhibitory effect of K-11706 on GATA might be via inhibition of GATA acetylation, or through the stimulation of GATA de-acetylation.20 There are 2 lines of evidence suggesting that GATA is irrelevant to the mechanism. First, under conditions in which GATA binding activity is completely eliminated (Figure 1B, lanes 5-7), the Epo levels are identical to the control conditions (Figure 2). Second, in Pm7, K-11706 is fully competent to activate (Figure 3C). Although we think that the binding activity of the transcription factors does not parallel their promoter activity and protein concentrations, further study is needed to clarify the effects of K-11706 on GATA, HIF-1, and other transcription factors and on the balance between GATA and HIF-1. In conclusion, this study raised the possibility that K-11706 might be suitable as a drug for treating patients with ACD by oral administration.
Prepublished online as Blood First Edition Paper, August 24, 2004; DOI 10.1182/blood-2004-04-1631.
Supported by grants-in-aid for scientific research from the Ministry of Education, Science and Culture of Japan, and the Kowa Foundation, Tokyo, Japan. C.S. was supported in this work as a Scholar of the Kanehara Ichiro Foundation in Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank H. Tanaka and H. Iijima for their expert technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal