Abstract
Transferrin receptor 2 (TfR2) plays a critical role in iron homeostasis because patients carrying disabling mutations in the TFR2 gene suffer from hemochromatosis. In this study, iron-responsive regulation of TfR2 at the protein level was examined in vitro and in vivo. HepG2 cell TfR2 protein levels were up-regulated after exposure to holotransferrin (holoTf) in a time- and dose-responsive manner. ApoTf or high-iron treatment with non–Tf-bound iron failed to elicit similar effects, suggesting that TfR2 regulation reflects interactions of the iron-bound ligand. Hepatic TfR2 protein levels also reflected an adaptive response to changing iron status in vivo. Liver TfR2 protein levels were down- and up-regulated in rats fed an iron-deficient and a high-iron diet, respectively. TfR2 was also up-regulated in Hfe-/- mice, an animal model that displays liver iron loading. In contrast, TfR2 levels were reduced in hypotransferrinemic mice despite liver iron overload, supporting the idea that regulation of the receptor is dependent on Tf. This idea is confirmed by up-regulation of TfR2 in β-thalassemic mice, which, like hypotransferrinemic mice, are anemic and incur iron loading, but have functional Tf. Based on these combined results, we hypothesize that TfR2 acts as a sensor of iron status such that receptor levels reflect Tf saturation.
Introduction
Naturally occurring mutations in patients with hemochromatosis have provided great insights into molecular mechanisms that mediate iron homeostasis.1 For example, at least 5 different mutations in transferrin receptor 2 (TfR2) have been discovered in several patients with iron overload, thus defining a critical role for this receptor in maintaining iron balance.2-5 Targeted expression of a premature stop codon (Y245X) orthologous to the human Y250X mutation in mice confirms the importance of TfR2 for iron homeostasis because these animals also accumulate iron deposits.6
As a homolog of transferrin receptor 1 (TfR1), TfR2 is a type II membrane receptor for the serum iron-binding protein transferrin (Tf).7 The cellular internalization of Tf by TfR1 is well characterized and the ubiquitous expression of this receptor suggests that most cells acquire iron via receptor-mediated endocytosis.8 However, although TfR2 can also facilitate uptake of Tf,7 it is likely that this receptor has additional roles in iron metabolism. Unlike TfR1, TfR2 is predominantly expressed in the liver.7,9 TfR2 does not compensate for TfR1 function because TfR1-/- mice die in utero. Furthermore, mice with only one functional TfR1 allele suffer mild tissue iron depletion,10 and disabling mutations in TfR2 result in significant hepatic iron loading.2-6 Finally, although TfR1 associates with HFE, the product of the gene affected in the most frequent form of hereditary hemochromatosis,11,12 in vitro studies have failed to detect interactions between the soluble ectodomains of TfR2 and HFE.13
Consistent with its function in iron import, TfR1 expression responds to changes in iron status; it is down-regulated by high iron conditions and up-regulated on iron depletion. Modulation of TfR1 levels occurs through transcriptional and posttranscriptional regulation. Posttranscriptional regulation is mediated by iron-responsive elements (IREs) present in the 3′ end of the TfR1 transcript. Many studies have characterized the interaction of iron-responsive proteins (IRPs) with IRE-containing transcripts to reveal a highly coordinated pattern of regulation for factors involved in iron metabolism, including TfR1 and the iron storage protein ferritin.8,14 Less is known about transcriptional control, but enhanced TfR1 mRNA synthesis is found under conditions of iron deficiency as well as hypoxia.15-17 In contrast, TfR2 does not appear to be regulated in response to changes in iron status. Its transcript does not contain IREs7 and no change in TfR2 mRNA levels is seen with altered iron status in K562 cells and murine erythroleukemic MEL cells, suggesting that any transcriptional control may be independent of cellular iron levels as well.9,18 Consistent with this observation, K562 cell TfR2 protein levels do not appear to be altered in response to either high iron or iron depletion.18 In vivo studies comparing TfR2 mRNA levels in livers from iron-deficient, iron-loaded, and Hfe knockout mice also show no difference in expression compared to control animals,19 although one study has reported decreased murine TfR2 mRNA levels in response to parenteral iron injection.20 Another study has suggested that intestinal levels of TfR2 mRNA decrease during pregnancy due to iron depletion21 ; however, definitive evidence for iron-responsive regulation of TfR2 is lacking.
As the major circulating serum iron-binding protein, the extent of Tf saturation directly reflects the body's iron status and thus represents a key physiologic parameter of iron homeostasis. Studies using immunofluorescence and flow cytometric analyses have indicated that TfR2 may redistribute when cells are treated with subphysiologic concentrations of fully saturated holotransferrin (holoTf).22,23 Although these investigations suggest that TfR2 localization can be modified by holoTf, how TfR2 is regulated under physiologic conditions and the extent of this regulation in response to the iron-binding capacity of its ligand remain unknown. In this study, we sought to characterize the potential influence of Tf and iron status on TfR2 protein levels. Our results demonstrate that HepG2 cell TfR2 protein, but not mRNA, is up-regulated by holoTf in a time- and dose-dependent manner. Treatment of cells with apoTf or non–Tf-bound iron fails to elicit a similar effect, indicating that TfR2 levels respond to the iron-bound form of ligand independent of changes in cellular iron status. Because the observed regulation of TfR2 at the protein level would be expected to reflect the body's adaptive response to changing iron levels, we further studied hepatic TfR2 protein levels in different dietary and genetic animal models of iron deficiency and overload. These in vivo data confirm that hepatic TfR2 protein levels correlate with changes in Tf saturation, highlighting a role for TfR2 as a sensor of iron status.
Materials and methods
Cell culture and treatment
HepG2 and HEK293 cells were grown in Dulbecco minimal essential medium (DMEM) containing 50 U/mL penicillin, 50 μg/mL streptomycin, and 10% fetal bovine serum (FBS). K562 cells were grown in α-minimal essential medium, supplemented with 50 U/mL penicillin, 50 μg/mL streptomycin, and 7.5% FBS. HeLa cells were grown in DMEM containing 50 U/mL penicillin, 50 μg/mL streptomycin, and 10% FBS, supplemented with 0.3 mg/mL glutamine. Cells were grown to about 40% to 70% confluence prior to addition of holoTf, apoTf, or Fe-nitrilotriacetic acid (Fe-NTA) as described in the figure legends. Fe-NTA (1:4 molar ratio) was prepared as described by Teichmann and Stremmel.24
RNA isolation and Northern analysis
Total RNA was isolated from HepG2 cells using RNAzol B (Tel-Test, Friendswood, TX). Samples were electrophoresed on 0.9% denaturing agarose/formaldehyde gels and transferred to Nytran N membranes (Schleicher & Schuell, Keene, NH). RNA was fixed by UV irradiation and blots were prehybridized for 3 to 7 hours at 42°C in 50% formamide, 10 × Denhardt solution, 10 mM EDTA (ethylenediaminetetraacetic acid), 0.1% sodium dodecyl sulfate (SDS), 750 mM NaCl, 150 mM Tris (tris(hydroxymethyl)aminomethane), 113 mM NaH2PO4, 45 mM Na2HPO4, 4 mM sodium pyrophosphate with 200 μg/mL denatured sonicated salmon sperm DNA. To produce template for synthesis of a 32P-labeled probe by random priming, a 2300 bp XbaI restriction fragment was prepared from pcDNA3-TFR2-FLAG, a plasmid kindly provided by Drs H. Kawabata and H. P. Koeffler (University of California, Los Angeles). For controls, a 2200-bp BamH1/BglII fragment of TfR25 and a commercially available gel purified fragment of mouse β-actin were used (DECAtemplate; Ambion, Austin TX). Blots were hybridized overnight with denatured probes in hybridization solution (10% dextran sulfate, 40% formamide, 8 × Denhardt solution, 8 mM EDTA, 0.08% SDS, 600 mM NaCl, 120 mM Tris, 90 mM NaH2PO4, 36 mM Na2HPO4, 3.2 mM Na4P2O7) with up to 200 μg/mL denatured sonicated salmon sperm DNA. After high stringency washing, radioactivity was detected by phosphorimaging.
Preparation of cell lysates and Western blotting
After experimental treatments outlined in the figure legends, cells were washed twice with phosphate-buffered saline and lysates were collected in NET buffer (150 mM NaCl, 5 mM EDTA, 10 mM Tris, pH 7.4, 1% Triton X-100) containing 1 μg/mL aprotinin, 1 μg/mL leupeptin, 1 μg/mL pepstatin, and 0.174 mg/mL phenylmethylsulfonyl fluoride (PMSF). K562 cells lysates were subjected to an additional postnuclear spin at 14 000g for 5 minutes. Liver tissue from rats or mice was homogenized in 0.05 M Tris, pH 7.4, 0.05% β-mercaptoethanol, and 2% SDS. The homogenate was sonicated for 10 seconds and centrifuged using a Sorvall RP100-AT238 rotor (Kendro Laboratory Products, Asheville, NC) at 88 800 g for 20 minutes at 4°C. Prior to gel electrophoresis, supernatant samples were clarified by centrifugation at 14 000g for 5 minutes. Protein levels were determined using Bradford assay26 for cells or using RCDC assay (Bio-Rad, Hercules, CA) for tissue. After electrophoresis on SDS-polyacrylamide gels, samples were transferred to nitrocellulose for immunoblotting using antihuman TFR1 monoclonal antibody (1:1000, Zymed, San Francisco, CA), monoclonal antihuman TFR2 antibody (1:10 000),27 rabbit antimouse TFR2 (1:1000; Alpha Diagnostics, San Antonio, TX), sheep antihuman ferritin (1:1000; Binding Site, Birmingham, United Kingdom), mouse anti-α-tubulin clone B-5-1-2 (1:10 000, Sigma, St Louis, MO), mouse anti-FLAG antibody (1:500; Sigma), or mouse antiactin antibody (1:1000-1:2500, ICN, Aurora, OH). Secondary labeled goat antimouse antibody (1:10, 000, Pierce, Rockford, IL), rabbit antisheep (1:5000, Pierce), or donkey antirabbit (1:2000, Amersham, Buckinghamshire, United Kingdom) was used to detect immunoreactivity by enhanced chemiluminescence (SuperSignal West Pico Chemi-luminescence Reagent, Pierce). Protein levels were quantified by phosphorimage analysis using Quantity One software (Bio-Rad).
Cell transfection conditions
For transient transfection experiments, HEK293 and HeLa cells were transfected on day 1 with either control (pcDNA3.1) or pcDNA3-TFR2-FLAG using Lipofectamine (Invitrogen, Carlsband, CA) or Effectene (Qiagen, Valencia, CA). On day 2, cells were treated with holo-Tf, such that on day 3, lysates were collected.
Iron-deficient and iron-loaded rats
Male Sprague-Dawley rats (3 weeks old) were obtained from Harlan Sprague-Dawley (Indianapolis, IN) and housed in microisolator cages with free access to water and rodent chow. Rats were divided into 4 groups: an iron-deficient group and an iron-loaded group, with age- and diet-matched control groups for both. Dietary preparations were from Purina Test Diet (Richmond, IN). For iron loading, rats were fed a modified Purina Test Diet no. 5053 prepared with 1% carbonyl iron (10 000 ppm iron). Control rats were fed a matched diet (Purina Test Diet no. 5053) that contained only 210 ppm iron and both cohorts were maintained on their respective diets for 4 weeks. For iron deficiency, rats were fed a modified Purina Test Diet no. 7444 containing 20 to 25 ppm iron, whereas control rats were fed the matched Purina Test Diet no. 5053 containing 210 ppm iron, and both groups were maintained on diet for 3 weeks. Liver tissue for protein analysis was excised after the rats were humanely killed and quickly frozen in liquid nitrogen and stored at -80°C. Animal protocols were approved by the Harvard Medical Area Standing Committee on Animals.
Hfe knockout and hypotransferrinemic mice
Livers from 10-week-old Hfe-/- and Hfe+/+ mice were provided by Dr Nancy Andrews (Harvard Medical School, Boston, MA).10 These animals were of the 129SV/EvTac strain. Livers from 4- to 5-week-old hypotransferrinemic and control mice28 were from Dr Mark Fleming (Harvard Medical School, Boston, MA). Trfhpx/hpx mice were treated with 6 mg transferrin on postnatal days 1, 8, 15, and 22. Livers from 6-week-old male thalassemic Hbbth-1HRI+/+, HbbwtHRI+/+, and HbbwtHRI+/- mice29,30 were obtained from Dr Jane-Jane Chen (Massachusetts Institute of Technology, Cambridge, MA).
Measurement of liver nonheme iron
Hepatic nonheme iron was determined colorimetrically after acid digestion of about 100 mg tissue using the method of Torrance and Bothwell.31
Statistical methods
Reported values are means ± SEM. Comparisons of nonheme iron, hematocrit, and protein levels between control and experimental groups were evaluated using Student unpaired t test and all results discussed in the text were found to be significantly different (P ≤ .05).
Results
HoloTf increases TfR2 protein levels in HepG2 cells
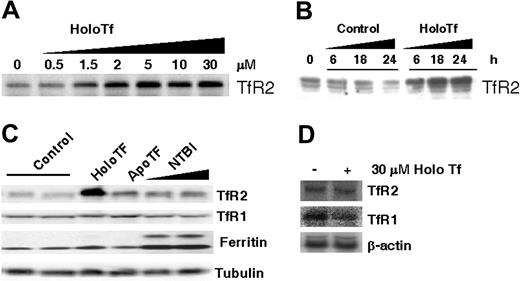
Because TfR2 is predominantly expressed in the liver, we first sought to characterize the regulation of TfR2 in the liver-derived human HepG2 cell line. Previous studies have confirmed expression of TfR2 mRNA and protein in these cells.7,22,27,32 TfR2 protein levels increased in a dose-responsive manner when HepG2 cells were cultured for 24 hours in media supplemented with 0.5 to 30 μM human holoTf (Figure 1A). When cells were exposed to 30 μM holoTf, maximal induction was up to 7-fold above control (n = 5). The half-maximal values measured for this effect (1-5 μM) were consistent with the observations of Johnson and Enns, reported in the accompanying article.33 This dose response to holoTf indicates up-regulation occurs over the physiologic range of Tf saturation.8 Time-course experiments indicated up-regulation of TfR2 could be detected as early as 6 hours after addition of 30 μM holoTf with maximal induction within 18 hours of exposure (Figure 1B).
HoloTf increases HepG2 cell TfR2 protein but not mRNA. HepG2 cells were cultured 24 hours with or without the indicated additions of holoTf, apoTf, or non–Tf-bound iron (NTBI), and cell lysates were prepared for Western blot analysis as detailed in “Materials and methods.” (A) HepG2 cells were treated with indicated concentrations of holoTf, and lysates (120 μg protein) were electrophoresed on a 10% SDS-polyacrylamide gel, transferred to nitrocellulose, and immunoblotted to detect TfR2 protein levels. (B) HepG2 cells were incubated with or without 30 μM holoTf for the indicated times, and TfR2 levels were also determined by Western analysis. (C) HepG2 cells were treated with or without 30 μM holoTf, 30 μM apoTf, 65 μM Fe-NTA, or 100 μM Fe-NTA, and immunoreactivity was determined for TfR2, TfR1, and ferritin protein levels. To control for loading, tubulin levels were also determined. Representative results from one of at least 2 experiments are shown. (D) Total RNA samples were isolated from HepG2 cells incubated with 30 μM holoTf for 24 hours. RNA (56 μg) was electrophoresed on a 0.9% agarose gel and transferred to Nytran N membrane for Northern blotting. The Northern blot was hybridized with 32P-labeled TfR2 probe, then stripped and reprobed for TfR1 and β-actin. Hybridized probes were detected by phosphorimaging (Quantity One software; Bio-Rad).
HoloTf increases HepG2 cell TfR2 protein but not mRNA. HepG2 cells were cultured 24 hours with or without the indicated additions of holoTf, apoTf, or non–Tf-bound iron (NTBI), and cell lysates were prepared for Western blot analysis as detailed in “Materials and methods.” (A) HepG2 cells were treated with indicated concentrations of holoTf, and lysates (120 μg protein) were electrophoresed on a 10% SDS-polyacrylamide gel, transferred to nitrocellulose, and immunoblotted to detect TfR2 protein levels. (B) HepG2 cells were incubated with or without 30 μM holoTf for the indicated times, and TfR2 levels were also determined by Western analysis. (C) HepG2 cells were treated with or without 30 μM holoTf, 30 μM apoTf, 65 μM Fe-NTA, or 100 μM Fe-NTA, and immunoreactivity was determined for TfR2, TfR1, and ferritin protein levels. To control for loading, tubulin levels were also determined. Representative results from one of at least 2 experiments are shown. (D) Total RNA samples were isolated from HepG2 cells incubated with 30 μM holoTf for 24 hours. RNA (56 μg) was electrophoresed on a 0.9% agarose gel and transferred to Nytran N membrane for Northern blotting. The Northern blot was hybridized with 32P-labeled TfR2 probe, then stripped and reprobed for TfR1 and β-actin. Hybridized probes were detected by phosphorimaging (Quantity One software; Bio-Rad).
To examine whether the ligand Tf or its bound iron cargo was responsible for TfR2 up-regulation, HepG2 cells were exposed to either 30 μM holoTf or apoTf. In contrast to the marked increase in TfR2 induced by holoTf, the iron-free ligand did not significantly alter TfR2 protein levels (Figure 1C). To further confirm that receptor regulation reflected actions of the iron-bound ligand, HepG2 cells were also cultured for 24 hours in media supplemented with non–Tf-bound iron. TfR2 protein levels were not influenced by treatment with up to 100 μM Fe-NTA despite the fact that iron loading occurred as indicated by down-regulation of TfR1 and up-regulation of ferritin levels (Figure 1C). The latter observations argue against the idea that TfR2 is regulated by cellular iron and provide further support for a pattern of receptor regulation directly reflecting the iron-binding status of Tf.
Steady-state TfR2 transcript levels are unaffected by holoTf
To determine whether TfR2 transcript levels were up-regulated, HepG2 cells were cultured for 24 hours in media supplemented with or without 30 μM holoTf and total RNA was isolated for Northern analysis. Figure 1D demonstrates that in contrast to the observed induction of TfR2 protein, holoTf did not influence steady-state TfR2 transcript levels in HepG2 cells. Analysis of mRNA levels at earlier times of treatment (12 hours) also showed no difference (data not shown). These observations are consistent with published reports that indicate TfR2 mRNA expression is unaltered in response to iron status in vitro9,18 and in vivo.19
TfR2 regulation by holoTf is context-dependent
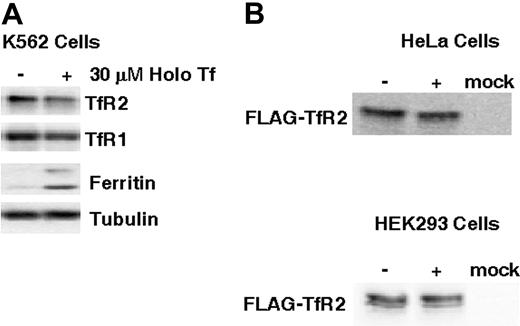
We further investigated the molecular basis for TfR2 regulation by examining the influence of holoTf on receptor levels in K562 cells, which endogenously express TfR2.7,22,27,32 K562 cells were cultured for 24 hours in media supplemented with 30 μM holoTf, and lysates were prepared for Western blot analysis. In contrast to the 5-fold up-regulation of TfR2 protein in HepG2 cells (Figure 1A), no change in K562 cell TfR2 levels was detected in the presence of holoTf, whereas TfR1 was down-regulated as expected (Figure 2A). These results suggest that regulation of TfR2 by holoTf may be specific to cell type. This idea is further supported by the fact that holoTf failed to up-regulate levels of FLAG-tagged TfR2 exogenously expressed in HeLa and HEK293T cells (Figure 2B). Although it is possible that the epitope tag might disrupt appropriate regulation of TfR2 in response to holoTf, previous studies have shown that the N-terminal FLAG-tag does not interfere with receptor function in Tf binding and iron delivery.7,18 Therefore, posttranscriptional regulation of TfR2 may involve untranslated regions of its message lacking in the FLAG construct or it may require liver-specific functions lacking in all 3 cell types studied.
Up-regulation of TfR2 protein levels is context-dependent. (A) K562 cells were incubated with or without 30 μM holoTf for 24 hours. Cell lysates containing 40 μg protein were electrophoresed on 10% SDS-polyacrylamide gels for Western blot analysis of TfR1, TfR2, ferritin, and tubulin levels as described for Figure 1C. (B) HeLa and HEK293T cells were transiently transfected with either pcDNA3.1 (mock) or pcDNA3-TfR2-FLAG. FLAG-TfR2–expressing cells were then treated with or without 30 μM holoTf for 24 hours. Western blot analysis was carried out with 25 μg HeLa cell lysates on 7% SDS-polyacrylamide gels or with 48 μg HEK293 cell lysates on 10% SDS-polyacrylamide gels. Immunoreactivity was detected using anti-TFR2 (HeLa cell lysates) or anti-FLAG (HEK293 cell lysates) antisera as described in “Materials and methods.”
Up-regulation of TfR2 protein levels is context-dependent. (A) K562 cells were incubated with or without 30 μM holoTf for 24 hours. Cell lysates containing 40 μg protein were electrophoresed on 10% SDS-polyacrylamide gels for Western blot analysis of TfR1, TfR2, ferritin, and tubulin levels as described for Figure 1C. (B) HeLa and HEK293T cells were transiently transfected with either pcDNA3.1 (mock) or pcDNA3-TfR2-FLAG. FLAG-TfR2–expressing cells were then treated with or without 30 μM holoTf for 24 hours. Western blot analysis was carried out with 25 μg HeLa cell lysates on 7% SDS-polyacrylamide gels or with 48 μg HEK293 cell lysates on 10% SDS-polyacrylamide gels. Immunoreactivity was detected using anti-TFR2 (HeLa cell lysates) or anti-FLAG (HEK293 cell lysates) antisera as described in “Materials and methods.”
Regulation of TfR2 protein levels in vivo
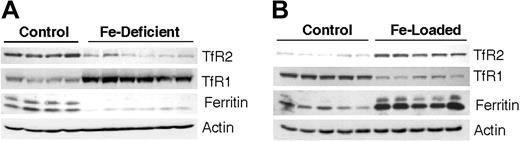
To study regulation of TfR2 in vivo, livers were obtained from iron-deficient and iron-loaded rats. Dietary models included a cohort that was fed a diet with only 20 to 25 ppm iron for 3 weeks. Compared to control rats fed standard chow (210 ppm iron), iron-deficient rats had significantly reduced hepatic nonheme iron (9.6 ± 0.8 μg Fe/g versus 67.9 ± 5.9 μg Fe/g for controls) and lower hematocrit values (25.3% ± 1.6% versus 41.7% ± 0.9% for controls). Western blot analysis (Figure 3) further revealed that TfR1 levels were 2-fold higher in the iron-deficient rats, whereas ferritin levels were reduced to 12% of control levels. These changes are anticipated for iron-deficient rat liver.34-36 Importantly, hepatic TfR2 levels in iron-deficient rats were reduced to 21% of control.
TfR2 protein levels are modulated by iron status in vivo. Rats were made either iron deficient (A) or iron loaded (B) by diet as described in “Materials and methods.” After the rats were humanely killed, lysates were prepared from liver tissue for Western blot analysis. TfR2, TfR1, ferritin, and actin levels are shown for age- and diet-matched control and iron-deficient rats and for age- and diet-matched control and iron-loaded rats. Each lane shows lysates from individual animals for iron-deficient rats (n = 6) versus control rats (n = 4) and for iron-loaded rats (n = 5) versus control rats (n = 5).
TfR2 protein levels are modulated by iron status in vivo. Rats were made either iron deficient (A) or iron loaded (B) by diet as described in “Materials and methods.” After the rats were humanely killed, lysates were prepared from liver tissue for Western blot analysis. TfR2, TfR1, ferritin, and actin levels are shown for age- and diet-matched control and iron-deficient rats and for age- and diet-matched control and iron-loaded rats. Each lane shows lysates from individual animals for iron-deficient rats (n = 6) versus control rats (n = 4) and for iron-loaded rats (n = 5) versus control rats (n = 5).
A second group of rats ate a diet supplemented with or without 1% carbonyl iron (∼10 000 ppm) for 4 weeks to study the influence of iron loading. Rats fed the high-iron diet had 5-fold higher nonheme iron levels (470.1 ± 30 μg Fe/g versus 79.7 ± 4.6 Fe μg/g for the control group). As expected,36-38 TfR1 protein levels in the iron-loaded rats were reduced to only 34% of the control group, whereas ferritin levels were 5-fold greater (Figure 3). The changes observed for the iron-deficient and iron-loaded rats are consistent with the reciprocal regulation of TfR1 and ferritin protein expression due to IRP interactions with 3′ and 5′ IREs, respectively.39 The fact that hepatic TfR2 protein expression in iron-loaded animals was 5-fold greater than the control group further supports the model that this factor is up-regulated by holoTf in vivo, reflecting increased Tf saturation. Combined, these results demonstrate that liver TfR2 expression directly reflects body iron status such that protein levels are reduced by iron deficiency and increased in response to high iron.
TfR2 protein levels in Hfe knockout, hypotransferrinemic, and β-thalassemic mice
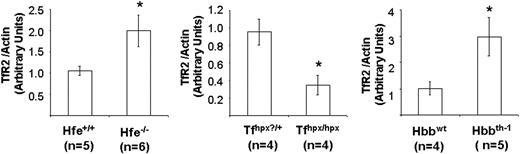
To further explore TfR2 regulation, receptor levels were examined in livers obtained from 3 different murine genetic models that display hepatic iron loading with or without a state of anemia. The effect of Hfe gene deletion on TfR2 protein levels was studied in knockout mice,40 which display hepatic iron loading similar to patients with HFE-associated hemochromatosis.41-43 Nonheme iron levels measured for Hfe-/- mouse livers used in our study were 3-fold higher than Hfe+/+ controls (619.3 ± 81.5 μg Fe/g versus 151.2 ± 10.7 μg Fe/g), whereas TfR2 levels in Hfe-/- mouse livers were doubled (Figure 4). This result is consistent with the observed up-regulation of liver TfR2 in iron-loaded rats (Figure 4) and further indicates that loss of Hfe function does not interfere with iron-responsive regulation of this receptor.
TfR2 protein levels in genetic models of hepatic iron loading. Hepatic tissue was obtained for 3 different mouse models: 10-week-old Hfe-/- mice, a genetic model of human hereditary iron overload due to loss of Hfe function, 4- to 5-week old Trfhpx/hpx mice, which have impaired Tf synthesis, and 6-week-old Hbbth-1 mice, which have defective globin synthesis. Controls for the latter group included 2 wild-type and 2 heterozygous HRI+/- mice that were both Hbbwt; all Hbbth-1 mice were wild type for HRI.29 After Western analysis, TfR2 protein levels were determined by densitometry using Quantity One software (Bio-Rad) to compare expression levels; shown are mean values (± SEM) normalized to actin (loading control). The numbers of mice for each group are noted in the figure. *different from control, P < .05. The increase in TfR2 levels determined for Hfe-/- mice may be an underestimate because a small increase in actin levels was noted for these animals.
TfR2 protein levels in genetic models of hepatic iron loading. Hepatic tissue was obtained for 3 different mouse models: 10-week-old Hfe-/- mice, a genetic model of human hereditary iron overload due to loss of Hfe function, 4- to 5-week old Trfhpx/hpx mice, which have impaired Tf synthesis, and 6-week-old Hbbth-1 mice, which have defective globin synthesis. Controls for the latter group included 2 wild-type and 2 heterozygous HRI+/- mice that were both Hbbwt; all Hbbth-1 mice were wild type for HRI.29 After Western analysis, TfR2 protein levels were determined by densitometry using Quantity One software (Bio-Rad) to compare expression levels; shown are mean values (± SEM) normalized to actin (loading control). The numbers of mice for each group are noted in the figure. *different from control, P < .05. The increase in TfR2 levels determined for Hfe-/- mice may be an underestimate because a small increase in actin levels was noted for these animals.
TfR2 expression was also investigated in hypotransferrinemic (Trfhpx/hpx) mice,28 which carry a splice-site mutation that results in the loss of circulating Tf, rendering these animals anemic due to impaired iron acquisition.44 Because Trfhpx/hpx mice respond to the anemia by absorbing excessive amounts of dietary iron, deposits accumulate in their livers, resulting in severe hepatic iron overload.44,45 In livers used for our study, nonheme iron levels in Trfhpx/hpx mice were 2.6-fold greater than control Trf+/? mice (972.3 ± 67.6 μg Fe/g versus 270.3 ± 72.1 μg Fe/g). Despite hepatic iron loading, TfR2 protein levels in Trfhpx/hpx mouse livers were significantly reduced to only 36% of control (Figure 4). These results argue that increased hepatic TfR2 protein does not simply reflect tissue iron loading, as observed in livers of rats fed a high-iron diet and the Hfe knockout mice. Rather, reduced TfR2 levels in Trfhpx/hpx mice most likely result from either insufficient levels of circulating holoTf or signals generated in response to the anemic state of this genetic model. Both of these possibilities are consistent with reduced hepatic TfR2 in rats made iron deficient by diet.
To further distinguish whether TfR2 responds to low Tf iron saturation or cues generated by anemia, receptor expression was examined in livers from thalassemic (Hbbth-1) mice.30 Hbbth-1 mice have a deletion of the β-major globin gene and suffer from hypochromic, microcytic anemia.30,46 Because of defective globin synthesis, Hbbth-1 mice also incur iron loading but, unlike Trfhpx/hpx mice, these animals have appropriate Tf levels with increased iron saturation.47-49 Consistent with previous reports, hepatic nonheme iron levels in Hbbth-1 livers used in our study were increased (163.5 ± 14.8 μg Fe/g versus 90 ± 8.3 μg Fe/g for controls). Hepatic TfR2 protein levels were also significantly increased, about 3 times control (Figure 4). These observations indicate that Trfhpx/hpx mice most likely fail to up-regulate TfR2 due to loss of Tf function. Combined with the pattern of regulation observed in other dietary and genetic models, our data support the hypothesis that this receptor is modulated in a Tf-dependent manner reflecting the degree of iron saturation.
Discussion
Our in vitro experiments demonstrate that HepG2 cell TfR2 protein levels increase in a dose- and time-dependent manner in response to physiologic levels of holoTf. These data are entirely consistent with the observations reported by Johnson and Enns33 and demonstrate an adaptive response of TfR2 protein levels to reflect changes in the saturation state of iron binding by Tf. Iron-responsive regulation of TfR2 was not observed in previous studies of human erythroleukemic K562 cells and mouse MEL cells.7,9,18 Our results are compatible with these reports because we found that steady-state TfR2 mRNA levels do not respond to holoTf and that protein levels are unaffected in K562 cells. Because holoTf also fails to up-regulate TfR2 exogenously expressed in HeLa or HEK293T cells, it is possible that cell-specific factors are involved in the HepG2 cell response. The idea that TfR2 is regulated in HepG2 cells has been suggested by Deaglio et al.22 In the latter investigation, the authors reported a redistribution of TfR2 following exposure to holoTf but not apoTf. Because these qualitative results were obtained using immunofluorescence microscopy, the actual amounts of receptor were not quantified and it is possible that the apparent recruitment of receptors to the cell surface simply reflects a posttranscriptional increase in protein expression as we report here. Future cell-based studies should explore how increased TfR2 levels in response to holoTf are reflected in membrane trafficking and surface distribution of this receptor.
Our in vivo studies of hepatic TfR2 expression in different dietary and genetic animal models fully support the hypothesis that this receptor is a sensor of Tf saturation. TfR2 expression in rats on high-iron and low-iron diets indicates that protein levels directly respond to changes in Tf saturation (holoTf levels) resulting from iron-loading and iron-deficiency conditions. This regulatory pattern is also supported by TfR2 levels in mouse models of anemia and overload. The increased TfR2 level observed in Hfe-/- mouse livers is consistent with a response increased Tf saturation, which is nearly 100% in Hfe knockout mice.42 Moreover, these results show that Hfe function is not required for up-regulation of TfR2. Because Hfe function appears to be necessary for the appropriate induction of hepcidin in response to iron overload20,50-52 and inflammation,53 we also infer that it is unlikely that this factor is involved in regulation of TfR2 levels. Finally, Hfe interacts with TfR111,12 but not TfR2,13 thus up-regulation of TfR2 in Hfe-/- mice also suggests that Tf signals changes in iron status to alter TfR2 levels independent of its interactions with TfR1. The reduced TfR2 levels in the Trfhpx/hpx mice provide further support for a role for Tf in the regulation of TfR2 because circulating levels of the serum protein in the hypotransferrinemic mouse are only 0.5% to 1% of normal.44 The up-regulated TfR2 protein levels in Hbbth-1 mice also indicate that Tf saturation provides a dominant signal to dictate levels of this receptor. These animals display anemia with concurrent hepatic iron loading but have normal Tf levels with increased saturation.47-49 Thus, we conclude that TfR2 expression directly responds to the level of Tf saturation, integrating information about the storage of iron and the demand for utilization of this nutrient.
Patients with mutations in the TfR2 gene suffer from hemochromatosis and its associated hepatic iron loading,2-5 supporting a key role for this receptor in the maintenance of iron balance. As outlined, we theorize that the adaptive response of TfR2 to iron status reflects a function in iron homeostasis to sense the level of Tf saturation. Although our study identifies that TfR2 protein levels directly correlate with iron status and are regulated in an HFE-independent, Tf-dependent, and hepatocyte-specific manner, the downstream consequences of TfR2 regulation remain to be fully determined. Understanding the regulation and function of TfR2 as well as its relationship with other molecules involved in the regulation of iron metabolism should significantly expand our knowledge of iron homeostasis and possibly provide new therapeutic avenues to treat hemochromatosis.
Prepublished online as Blood First Edition Paper, August 19, 2004; DOI 10.1182/blood-2004-06-2481.
Supported by National Institutes of Health grant P01 DK55495 (Project 3; M.W.R.) and R01 DK56160 (M.W.R.).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors wish to express their gratitude for the great help and assistance from the Andrews, Fleming, and Chen laboratories for providing tissues used in this study. We would also like to acknowledge Drs Mitchell Knutson, Khristy Thompson, and Elizabeth Heilig for their help with nonheme iron and hematocrit measurements. We also thank Drs Caroline Enns and Martha Johnson (Oregon Health Sciences University) for sharing their data in advance of submission of this manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal