Abstract
HIV-1-associated thrombocytopenia (HIV-1-ITP) is a common complication of HIV-1 infection, frequently caused by increased peripheral platelet destruction mediated by antiplatelet antibodies (Abs) and/or platelet-bound immune complexes. Little is known about the specificity of the antiplatelet Abs at a molecular level. Here, we used immunoglobulin G (IgG) phage-display libraries generated from 3 HIV-1-ITP patients to isolate a large panel of human monoclonal antiplatelet Abs by selection on unfixed platelets. The platelet antigen recognized by all the cloned Abs was identified to be the talin head domain (talin-H), a cleavage product of talin that can be generated by platelet activation or HIV-1 protease. Talin-H was found in HIV-1-ITP-circulating immune complexes, and antitalin Abs were detected in HIV-1-ITP sera but not in controls. The cloned anti-talin-H IgGs were highly somatically mutated, indicative of an antigen-driven, affinity-matured response. These findings suggest that talin-H Ab may be a marker of HIV-1-ITP elicited due to exposure of immunodominant epitopes on talin-H as a result of a disease-related process. Abs to talin-H and related immune complexes (ICs) may contribute to HIV-1-ITP. (Blood. 2004;104:4054-4062)
Introduction
HIV-1-associated thrombocytopenia (HIV-1-ITP) is a common complication of HIV-1 infection1,2 and sometimes one of the first clinical signs.3 In individuals where HIV-1-ITP develops in the early stage of HIV-1 infection prior to the development of AIDS, the low platelet counts are often caused by increased peripheral destruction due to sequestration of antibody (Ab)-coated platelets via Fc receptor- or complement-mediated phagocytosis in the spleen4,5 and/or complement-independent, peroxide-induced, Ab-mediated platelet lysis.6 Impaired platelet production due to direct effects of HIV-1 infection on megakaryocyte production and platelet release from the bone marrow also plays an important role and is thought to contribute to the platelet depletion in the later stages of HIV-1 infection. Reduction of the viral load by highly active antiretroviral therapy (HART) therapy has reduced the problem of decreased platelet production and lowered the frequency of patients with HIV-1-ITP.7 Clinically, immune-mediated HIV-1-ITP is indistinguishable from classic autoimmune thrombocytopenia purpura (ATP); however, the characteristics of the Abs involved in the peripheral platelet destruction in HIV-1-ITP are thought to be distinctly different. HIV-1-ITP patients exhibit significantly higher levels of circulating immune complexes (ICs) and platelet-associated immunoglobulin G (IgG), IgM, and complement proteins C3 and C4 than patients with classic ATP.8 The ICs have been proposed to contain anti-idiotypic Abs, complement factors C3 and C4, and platelet surface membrane fragments, including surface proteins such as the β3 integrin glycoprotein IIIa (GPIIIa).6,9 The Ab specificities in the ICs have been reported to include anti-HIV-1 gp120, anti-CD4, anti-GPIb/IX, and anti-GPIIb/GPIIIa, particularlyAbs to an immunodominant epitope (amino acids [aa's] 49-66) within GPIIIa.9,10 However, these reports were based on characterization of whole or affinity-purified serum and since HIV-1 sera generally have elevated levels of polyreactive antibodies of both IgM and IgG subclasses,11,12 normal serologic analysis can be difficult to interpret. Thus, analysis of the Ab specificities involved in HIV-1-ITP at a molecular level by generating monoclonal Abs is desirable.
In this study, we used phage-display repertoire cloning to analyze the antiplatelet IgG response in HIV-1-ITP and showed that the cytoplasmic talin head domain (talin-H) is an immunodominant epitope, whereas Abs to platelet surface proteins were not identified. Talin cross-links actin filaments and cytoplasmic tails of beta integrins such as gpIIIa at focal adhesion points and plays a key role in the regulation of integrin activation13-15 and virologic synapse formation.16,17 Talin can be cleaved during platelet activation and by HIV-1 protease,18 and, following platelet fragmentation, talin-H may become part of platelet surface membrane pieces through binding to the cytoplasmic tail of GPIIIa, thus becoming exposed to the immune system. In HIV-1-ITP patients, talin-H/anti-talin-H Ab containing ICs may deposit on intact platelets leading to their depletion.
Patients, materials, and methods
ELISA analysis
Sera from HIV-1-ITP patients and healthy individuals, human fragment, antigen binding (Fab) fragments, and murine mAbs were analyzed by enzyme-linked immunosorbent assay (ELISA) for binding to platelets immobilized to modified capture P (MCP) microtiter wells (Immucor, Norcross, GA). Briefly, platelet-rich plasma (PRP) was prepared from acid-citrate-dextrose anticoagulated blood from healthy individuals by centrifugation at 130g for 15 minutes at room temperature. Washed platelets were prepared from the PRP by centrifugation and resuspended in Tyrode buffer according to the method of Samis et al.19 Washed platelets were immobilized to MCP microtiter wells by centrifugation (240g, 5 minutes, 20°C), and the wells were washed with 1% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) and blocked with 3% BSA in PBS for 1 hour. Serum samples were spun at 10 000g for 5 minutes, and the serum/Fab preparations were tested in serial dilutions followed by washing. For the serum studies, bound IgG was detected with an alkaline phosphatase (AP)-labeled F(ab′)2 goat anti-human IgG Fc Ab (Jackson, West Grove, PA), whereas the human Fab's were detected with an AP-labeled F(ab′)2 goat anti-human IgG F(ab′)2 Ab (Jackson), both diluted 1:1000 in PBS. Bound Ab was visualized with nitrophenol substrate (Sigma, St Louis, MO) and measured at 405 nm. All incubations were performed at room temperature to avoid platelet activation. A human serum containing high titers against the platelet alloantigen human platelet antigen 1a (HPA-1a) and a serum containing no platelet Abs were used as controls. In addition, the anti-HIV-1 gp120 Fab's 12.9 and b12 were also used as negative controls. All ELISAs were performed twice in doublets. Serum reactivity against the immunodominant peptide GPIIIa-49-66 (CAPESIEFPVSEARVLED) was measured in ELISA by coating 200 ng peptide in 0.1 M sodium bicarbonate (pH 9.5) overnight at 4°C and against the major platelet glycoproteins GPIIb-IIIa, GPIa-IIa, and GPIb-IX in a commercially available PakAuto antigen capture ELISA (GTI, Brookfield, WI). Following blocking with 3% BSA, sera diluted in 1% BSA were incubated with the peptide or the PakAuto antigen ELISA plates (Costar, Cambridge, MA) for 2 hours at 37°C and bound Ab was detected as described above. Serum from patients with HIV-1-ITP (n = 7), ATP (n = 3), alloimmune thrombocytopenia (n = 3), and healthy individuals (n = 4) was also tested in triplicates for binding to purified human talin (0.5 μg/mL). Testing for significant differences between means was done with Mann-Whitney U test. Talin was purified from outdated human platelet concentrates to greater than 95% purity, using the procedure described by Collier and Wang.20 For evaluation of Fab specificity, microtiter plates (Costar, Cambridge, MA) were coated with appropriate antigens at a concentration of 2 μg/mL in PBS overnight and blocked with 2% dry milk in PBS for 1 hour at 37°C. The following antigens were used for the ELISA: platelet actin purified from human platelets (Cytoskeleton, Denver, CO), muscle actin purified from rabbit skeletal muscle (Cytoskeleton), tetanus toxoid (Calbiochem, La Jolla, CA), BSA (Pierce, Rockford, IL), ovalbumin (Pierce), transferrin (Sigma), hemagglutinin (Sigma), enolase 1 (Sigma). The murine glutathione S-transferase (GST)-talin fusion proteins GST-talin 1-435 and GST-talin 36-124514,21 were kindly provided by Dr David A. Calderwood (The Scripps Research Institute, La Jolla, CA). Abs were incubated with test antigen for 2 hours at 37°C and bound Abs were detected as described above.
Library construction and Ab selection
Bone marrow from 3 HIV-1-ITP patients with high serum antiplatelet reactivity designated D, L, and F were used for IgG (λ/κ) phage-display library construction using the pComb3 or pComb3H M13 surface display system as previously described.22-24 Ab library selection was performed as previously described with minor modifications.23,24 Protocol and the use of all human samples were approved by the Institutional Review Board of the Scripps Research Institute. Libraries were selected against platelets immobilized to MCP microtiter wells and against platelets in suspension. Platelets were isolated and immobilized to MCP microtiter wells as described for platelet ELISA in the previous section. For the library selection against platelets in suspension, 0.5 mL platelets (100 × 109/L) were washed and resuspended in 1 mL 3% BSA in PBS. Phage library (1011 plaque-forming units [pfu's]) was added to the mixture and incubated on an end-over-end mixer for 2 hours at room temperature. Unbound phage was removed by washing 5 × with 1% BSA in PBS and bound phage was eluted by 0.1 M glycine-HCl buffer, pH 2.2. Phagemid DNA was prepared from the fifth and sixth panning round for both selection procedures, and the gene III fragment was removed by NheI-SpeI digestion followed by religation and transformation of XL1-Blue cells to produce clones secreting soluble Fab fragments. Fab Abs were purified from bacterial supernatants as previously described with minor modifications.23,25
Dot blot analysis
Bacterial Fab supernatants were also analyzed for binding to platelets in a dot blot format. Briefly, 5 μL washed platelets (20 000/μL) were adsorbed to a methanol-activated polyvinylidene difluoride membrane (PVDF) membrane (Immobilon-P; Millipore, Bedford, MA) for 30 minutes without drying out the blot, followed by blocking for 2 hours with 2% dry milk-PBS/0.05% Tween 20. After blocking, 5 μL Fab supernatant or control serum was applied and incubated for 30 minutes at room temperature, followed by washing 3 × in PBS/0.05% Tween 20. Bound Ab was detected with a F(ab′)2 goat anti-human IgG F(ab′)2 AP-labeled Ab (Jackson) diluted 1:2000 in PBS, and the blot was developed with a Sigma Fast BCIP/NBT (5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium) substrate (Sigma).
Antigen identification
Platelets from healthy individuals were lysed in a PBS lysis buffer containing 1% Nonidet P-40 (NP40; Sigma), 3 mM EDTA (ethylenediaminetetraacetic acid), 10 mM benzamidine (Sigma), and 100 μg/mL soybean inhibitor (Roche, Indianapolis, IN), pH 7.4, on ice for 1 hour. Cell debris was pelleted by centrifugation and the lysate passed through 0.2-μm filters. Platelet lysate was separated by size exclusion chromatography by loading 6 mg onto a S200 gel filtration column (Amersham-Pharmacia, Piscataway, NJ) and eluting protein with PBS at a flow rate of 0.5 mL/min, collecting 0.5-mL fractions. Fractions were mixed with 4 × sodium dodecyl sulfate (SDS) sample buffer and boiled for 5 minutes, loaded on a 10% Tris·HCL Ready-Gel (Bio-Rad, Hercules, CA), and separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE). Sets of identical samples were run on one gel and, following electrophoresis, the gel was divided into identical parts, one of which was silver-stained with a SilverSNAP staining kit (Pierce), whereas the others were electroblotted to a methanol-activated PVDF membrane (Millipore). The blots were blocked overnight at 37°C in PBS containing 0.5% gelatin and 0.05% Tween 20. Membranes were incubated with purified Fab's (2 μg/mL) diluted in blocking buffer or a murine talin-H Ab TA205 (5 μg/mL; Serotech, Raleigh, NC) diluted in blocking buffer for 2 hours at room temperature followed by washing 4 × in PBS/0.05% Tween 20. Bound Fab's were detected with a horseradish peroxidase (HRP)-labeled F(ab′)2 goat anti-human IgG F(ab′)2 Ab (Jackson) diluted 1:5000 in blocking buffer, and bound mouse mAb was detected with an HRP-labeled rabbit anti-mouse IgG F(ab′)2 Ab with minimal cross-reactivity to human serum proteins (Jackson) diluted 1:5000 in blocking buffer and visualized with a chemiluminescent substrate (Supersignal; WestPico; Pierce). The Western blot of Fab D4 and Fab b12 Fab was compared with the silver-stained gel, and the protein bands corresponding to specific antigen were bound by D4 excised form the silver-stained gel, destained, and subjected to an in-gel trypsin digest26 before identification by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry analysis (MALDI-TOF).
Immunoprecipitation
Talin-enriched platelet fractions were pooled and 200 μL (0.6 mg/mL) was used for immunoprecipitation with 250 μL Fab supernatants (5 μg/mL) at 4°C for 1 hour on an end-over-end rotator. Formed ICs were captured with 50 μL goat anti-human IgG F(ab′)2 Ab (Jackson) conjugated to protein G-Sepharose (Amersham-Pharmacia) by incubation for 1 hour at 4°C. Sepharose beads were washed 3 × with PBS, and bound proteins were eluted by boiling for 5 minutes with 6 × SDS sample buffer and resolved by SDS-PAGE and immunoblotting, as described in the section above, with the murine talin-H Ab TA205 (Serotech). Fab b12 supernatant was included as a negative control Ab.
Preparation and analysis of circulating ICs
Circulating ICs were isolated from the serum of HIV-1-ITP patients or healthy HIV-1-negative controls by polyethylene glycol (PEG) precipitation.27 In brief, 4.75 mL of 0.1 M sodium borate, pH 3.4, was added to 0.25 mL serum and precipitated with 5 mL of 7% PEG (molecular weight [Mw] 20 000) for 18 hours at 4°C. PEG-ICs were isolated by centrifugation at 2500g at 4°C for 15 minutes and subsequently washed with 7% PEG. Precipitated ICs were dissolved in PBS/0.1% azide and stored at 4°C. Five micrograms of PEG-ICs was analyzed by SDS-PAGE on 4% to 12% gradient gels (Bio-Rad) and visualized by Coomassie staining and immunoblotting. Immunoblots were blocked with 5% dry milk in PBS/0.05% Tween 20, and talin antigen was detected by a primary murine talin-H Ab TA205 (5 μg/mL; Serotech) diluted in blocking buffer and a secondary HRP-labeled rabbit anti-mouse IgG F(ab′)2 Ab with minimal cross-reactivity to human serum proteins (Jackson) diluted 1:20 000 in blocking buffer. Unfractionated platelet lysate was used as a positive control, and control staining of immunoblots without the primary Ab was included.
Results
Cloning of antiplatelet IgG Abs from phage-display libraries generated from HIV-1-ITP patients
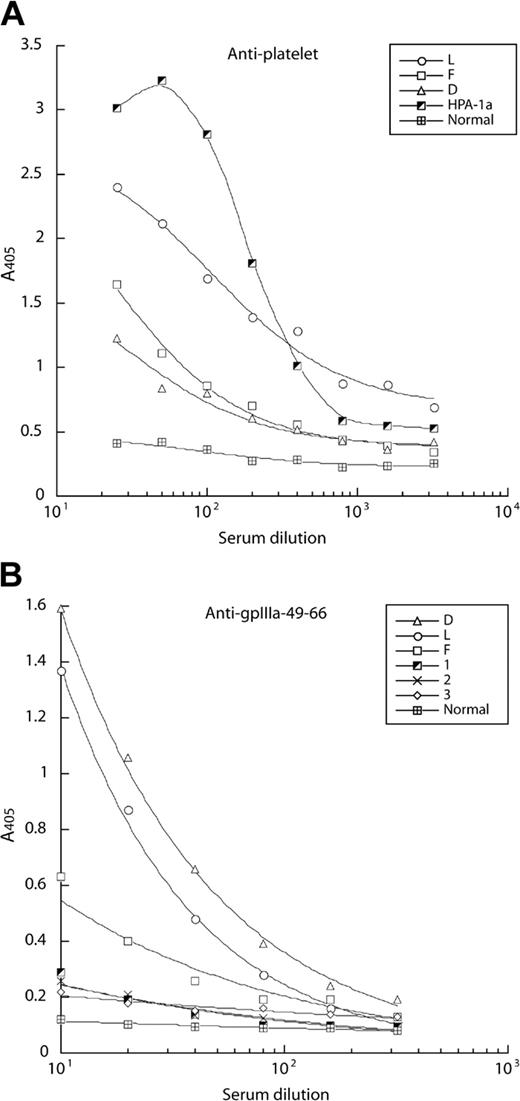
To characterize the specificity of the antiplatelet Ab response in patients with HIV-1-ITP, sera from a panel of such patients were tested for binding to platelets immobilized in chemically modified MCP microtiter wells, using an AP-labeled Fab fragment of a goat Ab against human IgG Fc as secondary Ab. Three patients designated D, L, and F exhibited high serum titers to platelets when compared with a high-titered anti-HPA-1a serum (positive control) and a serum containing no platelet Abs (negative control; Figure 1A). Patient L, who exhibited the highest antiplatelet Ab titer, had severe thrombocytopenia requiring therapeutic splenectomy. Serum from these 3 patients, but not sera from 3 HIV-1-seropositive individuals without HIV-1-ITP, also bound efficiently to a peptide derived from GPIIIa (GPIIIa-49-66), previously reported as an immunodominant epitope targeted by Abs in HIV-1-ITP patients10 (Figure 1B). In contrast, the 3 HIV-1-ITP sera exhibited no reactivity against the major platelet glycoproteins, including GPIIb/IIIa, GPIa/IIa, and GPIb/IX, as measured by ELISA (data not shown).
Sera from HIV-1-ITP patients contain IgG Abs against platelets and an immunodominant GPIIIa-49-66 peptide as measured by ELISA. Serial dilutions of sera from HIV-1-ITP patients (D, L, and F) were tested for binding to platelets immobilized on MCP microtiter plates (A) and to a GPIIIa-49-66 peptide immobilized on ELISA plates (B). (A) In the antiplatelet assay, a high-titer serum to the platelet alloantigen HPA-1a was used as a positive control, whereas a normal serum containing no platelet Abs was used to measure background level. (B) In the GPIIIa-49-66 peptide ELISA, serum from 3 HIV-1 patients without HIV-1-ITP (1-3) and one healthy donor (Normal) were included. The data presented in panels A and B are the median of 2 separate experiments. A405 indicates an optical density of 405 nm.
Sera from HIV-1-ITP patients contain IgG Abs against platelets and an immunodominant GPIIIa-49-66 peptide as measured by ELISA. Serial dilutions of sera from HIV-1-ITP patients (D, L, and F) were tested for binding to platelets immobilized on MCP microtiter plates (A) and to a GPIIIa-49-66 peptide immobilized on ELISA plates (B). (A) In the antiplatelet assay, a high-titer serum to the platelet alloantigen HPA-1a was used as a positive control, whereas a normal serum containing no platelet Abs was used to measure background level. (B) In the GPIIIa-49-66 peptide ELISA, serum from 3 HIV-1 patients without HIV-1-ITP (1-3) and one healthy donor (Normal) were included. The data presented in panels A and B are the median of 2 separate experiments. A405 indicates an optical density of 405 nm.
In an effort to identify and characterize the antiplatelet Ab specificities of these HIV-1-ITP patients at a molecular level, IgG1/κ/λ Ab libraries of approximately 1 × 107 members expressed on the surface of filamentous phage were constructed from each of patients D, L, and F. As starting material for the Ab display libraries, total RNA isolated from bone marrow obtained concomitantly with the serum samples was used, since it has been shown that bone marrow is a major repository for plasma cells producing the Abs found in serum.
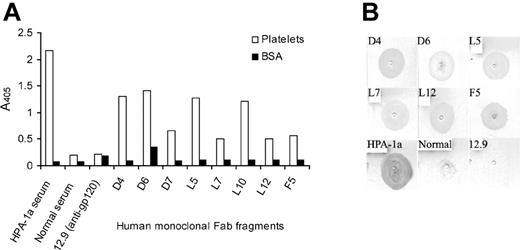
To isolate antiplatelet-specific human Fab's, each library was selected separately against freshly isolated unfixed platelets in suspension or immobilized on MCP capture microtiter wells. After 6 rounds of biopanning, phagemid DNA was isolated and treated with Nhe1-Spe1 endonuclease to remove the gene III fragment, allowing the expression of soluble Fab protein. Forty Fab clones from each of the library pannings were analyzed by ELISA for binding to immobilized platelets (Figure 2A). Twenty-five clones tested positive against platelets and did not react with BSA used as negative control. Sequencing of the DNA encoding the heavy-chain variable region of the platelet-reactive Fab's revealed that 8 clones were unique, whereas the remaining sequences were repeats. Four Fab's were retrieved by selection against platelets immobilized to MCP microtiter (Fab's D4, D6, and D7 from library D and Fab F5 from library F), whereas 4 other Fab's (L5, L7, L10, and L12, all from library L) were retrieved by selection against platelets in suspension. Platelet binding of the isolated Fab's was confirmed by binding the individual Fab's to platelets immobilized to a PVDF membrane in a dot blot format (Figure 2B). No binding was observed in this assay with the serum lacking antiplatelet Abs or an anti-gp120 control Fab 12.9. Fab D7 and L10 were not tested in this assay. The 8 platelet-reactive Fab's were also tested for binding to the major platelet glycoproteins, including GPIIb/IIIa, GPIa/IIa, and GPIb/IX, in ELISA, and none exhibited reactivity with these antigens (data not shown).
Binding of human IgG Fab's to purified platelets. Fab supernatants were tested for binding to purified platelets immobilized on (A) MCP microtiter wells or (B) PVDF membranes. BSA was included in the ELISA to evaluate for nonspecific binding. A high-titer platelet alloantigen HPA-1a serum was used as a positive control, whereas a serum containing no platelet Abs and the anti-HIV-1 gp120 Fab 12.9 were used as negative controls. The data presented are the median of 2 separate experiments. Images were scanned using Adobe Photoshop.
Binding of human IgG Fab's to purified platelets. Fab supernatants were tested for binding to purified platelets immobilized on (A) MCP microtiter wells or (B) PVDF membranes. BSA was included in the ELISA to evaluate for nonspecific binding. A high-titer platelet alloantigen HPA-1a serum was used as a positive control, whereas a serum containing no platelet Abs and the anti-HIV-1 gp120 Fab 12.9 were used as negative controls. The data presented are the median of 2 separate experiments. Images were scanned using Adobe Photoshop.
Identification of antigen recognized by the panel of antiplatelet Abs
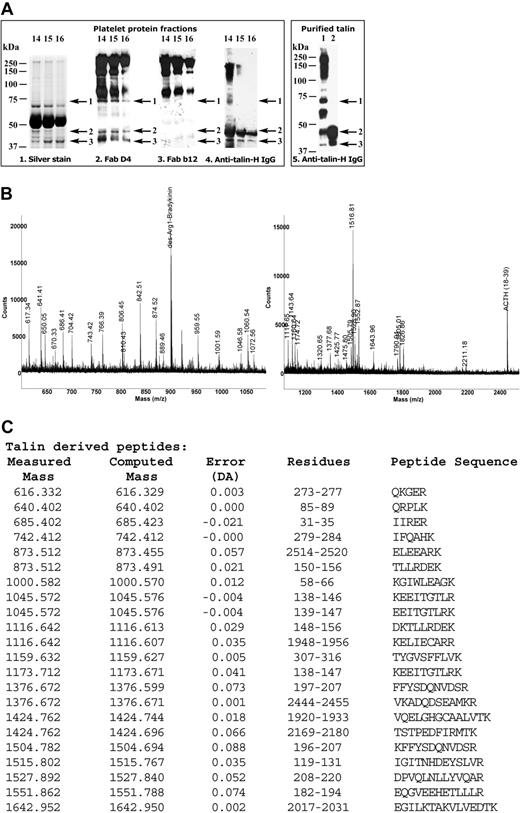
To identify the target antigen(s) recognized by the selected antiplatelet Fab's, a lysate was generated from pooled normal platelets and fractionated by size exclusion chromatography using a Sephadex 200 column. Next, each protein fraction was separated by SDS-PAGE and tested for binding to selected antiplatelet Fab's by Western blotting. Since normal platelets contain large amounts of IgG acquired from the surrounding serum by pinocytosis,28 immunoblotting of platelet lysate with secondary HRP-labeled anti-human IgG F(ab′)2 Ab alone resulted in staining of broad protein bands at 200 to 250 kDa, 150 kDa, and 90 kDa. Detection of proteins by the antiplatelet Fab's in these molecular weight regions was therefore not possible. However, comparison of the antiplatelet Fab-stained blots and those stained with irrelevant human Fab's as primary Ab, as well as those in which the primary Ab was omitted, allowed identification of protein bands specifically stained by the antiplatelet Fab's. Immunoblots stained with purified Fab D4 revealed reactivity with 3 protein bands at 70, 47, and 42 kDa (protein bands 1-3 respectively; Figure 3Aii), whereas no binding to those bands was observed with the control Ab, an anti-HIV-1 gp120 Fab b12 (Figure 3Aiii). The major platelet protein bands as seen on the silver-stained gel of the separated platelet fractions run in parallel (Figure 3Ai) were not recognized by Fab D4 (Figure 3Aii). To identify the antigens recognized in the Western blot, the protein band from the silver-stained gel corresponding to 47-kDa band stained with Fab D4 was excised and examined by limiting proteolysis and MALDI-TOF (Figure 3B). The mass spectrum data were entered into the ProFound protein database29 to search for the best possible match using a Bayesian algorithm.30 With the high estimated Z values of 1.89 and 2.17 (> 99% probability), respectively, the 47-kDa band was shown to contain both talin head domain (talin-H) and enolase 1. Sixteen tryptic talin peptides were identified and sequenced, which were well spread out and covered greater than 50% of the total sequence of the head domain of talin (amino acids 1-433; Table 1). The 2 protein bands corresponding to the stained 42-kDa and 70-kDa bands were also examined by MALDI-TOF; the 42-kDa band was identified as the talin-binding protein actin (estimated Z = 2.38), whereas no protein was identified from the analysis of the 70-kDa band.
Identification of the platelet protein recognized by the cloned human antiplatelet Abs by Western blotting and mass spectrometry. (A) Platelet lysate was fractionated by size exclusion chromatography, and fractions 14, 15, and 16 were separated by SDS-PAGE. The gel was divided and either silver-stained (Ai) or analyzed by Western blotting with Fab D4 (Aii; 2μg/mL), the negative control anti-gp120 Fab b12 (Aiii; 2 μg/mL), or murine antitalin IgG Ab TA205 (Aiv; 5 μg/mL). Arrows 1 to 3 indicate the positions of the protein bands (70, 47, and 42 kDa) specifically recognized by Fab D4 in Western blotting and present on silver-stained gel. Protein bands 1 to 3 were separately excised from the silver-stained gel, destained, and analyzed by matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF), identifying the talin head domain as the antigen recognized by antiplatelet Fab D4. Western blots of highly purified talin digested by calpain II (Av, lane 2) or not (Av, lane 1) stained with murine anti-human talin-H mAb TA205 (Av) were used as a reference. (B) Mass spectrum obtained from the MALDI-TOF analysis of the peptides derived from the 47-kDa protein band (band 2). (C) Talin-derived peptides. DA indicates Dalton.
Identification of the platelet protein recognized by the cloned human antiplatelet Abs by Western blotting and mass spectrometry. (A) Platelet lysate was fractionated by size exclusion chromatography, and fractions 14, 15, and 16 were separated by SDS-PAGE. The gel was divided and either silver-stained (Ai) or analyzed by Western blotting with Fab D4 (Aii; 2μg/mL), the negative control anti-gp120 Fab b12 (Aiii; 2 μg/mL), or murine antitalin IgG Ab TA205 (Aiv; 5 μg/mL). Arrows 1 to 3 indicate the positions of the protein bands (70, 47, and 42 kDa) specifically recognized by Fab D4 in Western blotting and present on silver-stained gel. Protein bands 1 to 3 were separately excised from the silver-stained gel, destained, and analyzed by matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF), identifying the talin head domain as the antigen recognized by antiplatelet Fab D4. Western blots of highly purified talin digested by calpain II (Av, lane 2) or not (Av, lane 1) stained with murine anti-human talin-H mAb TA205 (Av) were used as a reference. (B) Mass spectrum obtained from the MALDI-TOF analysis of the peptides derived from the 47-kDa protein band (band 2). (C) Talin-derived peptides. DA indicates Dalton.
Comparison of the nucleotide and amino acid sequences of the platelet reactive Fab antibody heavy chains with the closest germ line
. | . | . | . | R/S ratio (replacement-silent) . | . | |
|---|---|---|---|---|---|---|
| Clone . | Closest germ line . | Amino acid homology, % . | Nucleotide homology, % . | CDR . | FR . | |
| D4 | VH4-31 | 69 | 82 | 5.0 (10:2) | 1.9 (19:10) | |
| D6 | VH1-2 | 82 | 89 | > 8.0 (8:0) | 1.6 (8:5) | |
| D7 | VH1-69 | 87 | 94 | 4.0 (4:1) | 8.0 (8:1) | |
| L5 | VH1-8 | 76 | 89 | 7.0 (7:1) | 3.75 (15:4) | |
| L7 | VH5-51 | 83 | 91 | 1.5 (3:2) | 2.6 (13:5) | |
| L10 | VH1-8 | 91 | 96 | > 2.0 (2:0) | 2.0 (6:3) | |
| L12 | VH4-31 | 76 | 88 | 11.0 (11:1) | 2.0 (12:6) | |
| F5 | VH1-69 | 62 | 77 | 4.33 (13:3) | 3.0 (24:8) | |
. | . | . | . | R/S ratio (replacement-silent) . | . | |
|---|---|---|---|---|---|---|
| Clone . | Closest germ line . | Amino acid homology, % . | Nucleotide homology, % . | CDR . | FR . | |
| D4 | VH4-31 | 69 | 82 | 5.0 (10:2) | 1.9 (19:10) | |
| D6 | VH1-2 | 82 | 89 | > 8.0 (8:0) | 1.6 (8:5) | |
| D7 | VH1-69 | 87 | 94 | 4.0 (4:1) | 8.0 (8:1) | |
| L5 | VH1-8 | 76 | 89 | 7.0 (7:1) | 3.75 (15:4) | |
| L7 | VH5-51 | 83 | 91 | 1.5 (3:2) | 2.6 (13:5) | |
| L10 | VH1-8 | 91 | 96 | > 2.0 (2:0) | 2.0 (6:3) | |
| L12 | VH4-31 | 76 | 88 | 11.0 (11:1) | 2.0 (12:6) | |
| F5 | VH1-69 | 62 | 77 | 4.33 (13:3) | 3.0 (24:8) | |
R/S ratio indicates replacement-to-silent mutation ratio.
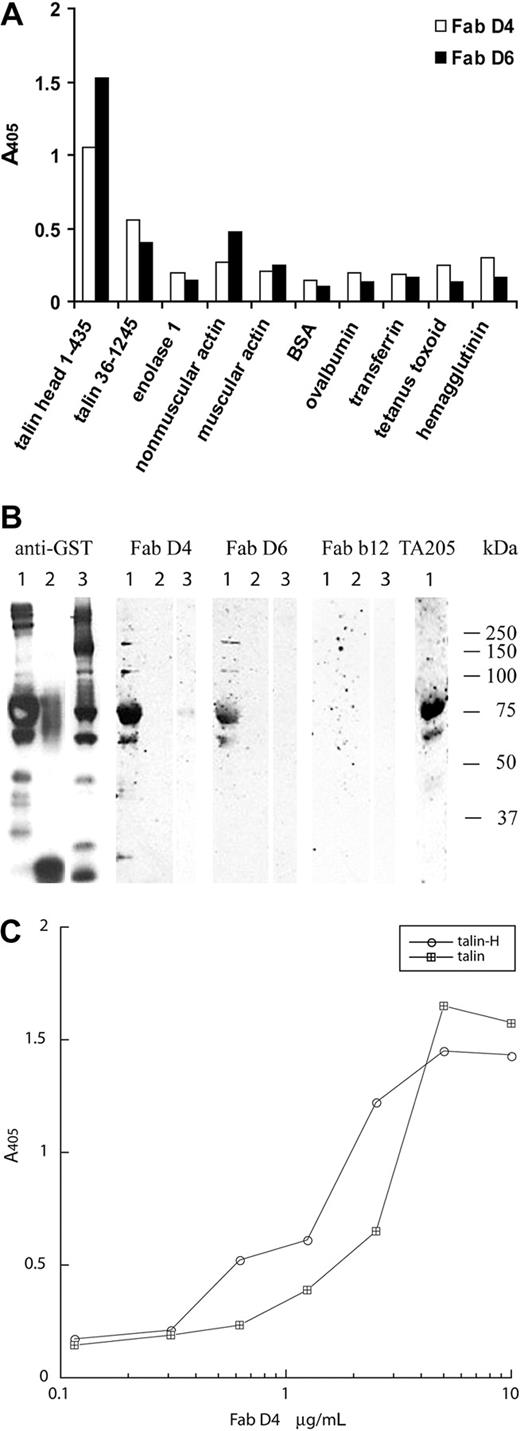
To confirm the specificity of the antiplatelet Abs, purified Fab's D4 and D6 were analyzed by ELISA for binding to purified enolase 1, actin, and 2 recombinant murine GST-talin fusion proteins along with a panel of control antigens (Figure 4A). Fab D4 and D6 specifically bound to the recombinant talin-H 1-435 construct, whereas only weak binding was observed to the recombinant talin 36-1245 construct. Weak or no binding of Fab's D4 and D6 to enolase 1, muscular or nonmuscular actin, or the other control antigens was observed. In the same setup, 2 anti-gp120 control Fab's, 12.9 and b12, exhibited no reactivity with the recombinant talin-H 1-435 or the recombinant talin 36-1245 constructs, demonstrating that the binding of Fab's D4 and D6 was not simply due to stickiness of the talin protein. To confirm that talin-H was the antigen specifically recognized by the antiplatelet Fab's, Western blots of the recombinant GST-talin constructs were stained with Fab's D4 and D6 (Figure 4B). Both of the Fab's bound to the GST-talin-H 1-435 fusion protein (Figure 4B; 70 kDa lane 1) but not to a GST-irrelevant peptide control protein (Figure 4B lane 2) or the GST-talin C-terminal part (36-1245; Figure 4B lane 3). The murine mAb TA205, specific for talin-H, also recognized the 70-kDa band, whereas no binding to any of the GST fusion proteins was observed with the control anti-HIV-1 gp120 Fab b12, demonstrating the specific interaction of the other Abs with GST-talin-H 1-435. In addition, ELISA titration experiments showed that Fab D4 bound with approximately the same affinity (108 M-1) to recombinant GST-talin-H and human talin purified from platelets (Figure 4C). The other antitalin Fab's exhibited similar affinities for recombinant talin-H and purified human talin (data not shown).
The cloned antiplatelet IgGs bind specific to talin-H. (A) Fab D4 and D6 were tested for binding to murine GST-talin-H (1-435), murine GST-talin (36-1245), and a panel of other antigens by ELISA. (B) Western blot analysis of purified Fab D4 and D6 to GST-talin fusion proteins. GST constructs were visualized with an HRP-conjugated murine anti-GST Ab. Anti-gp120 Fab b12 was used as a negative control Ab, whereas the murine anti-human talin-H mAb TA205 was used as a positive control antibody. Lane 1, GST-talin 1-435; lane 2, GST-irrelevant peptide; lane 3, GST-talin 36-1245. (C) Titration of Fab D4 against purified human talin and recombinant GST-talin-H.
The cloned antiplatelet IgGs bind specific to talin-H. (A) Fab D4 and D6 were tested for binding to murine GST-talin-H (1-435), murine GST-talin (36-1245), and a panel of other antigens by ELISA. (B) Western blot analysis of purified Fab D4 and D6 to GST-talin fusion proteins. GST constructs were visualized with an HRP-conjugated murine anti-GST Ab. Anti-gp120 Fab b12 was used as a negative control Ab, whereas the murine anti-human talin-H mAb TA205 was used as a positive control antibody. Lane 1, GST-talin 1-435; lane 2, GST-irrelevant peptide; lane 3, GST-talin 36-1245. (C) Titration of Fab D4 against purified human talin and recombinant GST-talin-H.
Why Fab D4 recognized 3 protein bands at 70, 47, and 42 kDa on blots of fractionated platelet lysate whereas only talin was identified in the 47-kDa band by MALDI-TOF analysis remained to be determined. To address this, blots of the fractionated plate lysate were stained with the murine anti-talin-H IgG mAb TA205, demonstrating that this antitalin Ab as well as Fab D4 recognized the protein bands at 70, 47, and 42 kDa (Figure 3Aiv). In addition, mAb TA205 stained the 70-, 47-, and 42-kDa bands in Western blots of talin purified to greater than 95% purity (Figure 3Av lane 1), demonstrating that all 3 bands contained talin fragments. When the highly pure talin preparation was digested by calpain II, which cleaves talin between amino acids 433 and 434,14,31 the 70-kDa band recognized by the antitalin Abs was proteolytically degraded, whereas an increased amount of the N-terminal 47-kDa talin head domain was observed (Figure 3Av lane 2). Other dominant proteins in the 42- and 70-kDa bands analyzed by MALDI-TOF may explain why talin was not identified in these bands.
The cloned human Fab's from all 3 HIV-1-ITP patients recognize the head domain of human talin
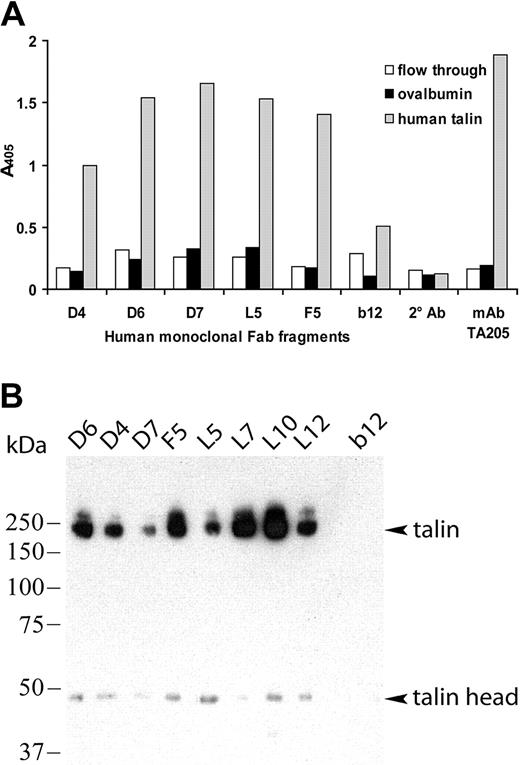
To determine whether the other cloned antiplatelet Fab's also recognized talin-H, human talin was purified from platelet concentrates to greater than 95% purity.20 The purified human talin, platelet lysate depleted for talin (flow through from the talin purification), and ovalbumin were incubated with each Fab (5 μg/mL) in ELISA (Figure 5A). Fab's D4, D6, D7, L5, and F5 bound efficiently and specifically to purified human talin comparable to that of the murine anti-talin-H control Ab TA205. In contrast, 2 anti-gp120 control Fab's, 12.9 and b12, in the same assay did not react with the purified human talin preparation, demonstrating that the binding of the antiplatelet Fab's was due to specific epitope/paratope interaction and not simply stickiness of the talin protein. Fab's L7, L10, and L12 did not bind human talin-H in this setup but did immunoprecipitate human talin-H from a platelet lysate as described in the next paragraph.
All the cloned antiplatelet IgGs bind specifically to purified human talin and immunoprecipitate talin from platelet lysates. (A) Fab's were tested by ELISA for binding to talin purified from human platelets, the irrelevant antigen ovalbumin (OVA, control), and the flow-through preparation from the talin purification containing the talin-depleted platelet lysate. The anti-gp120 Fab b12 and secondary Ab alone were included as negative controls. (B) Fab's (5 μg) were incubated with platelet protein fractions enriched for talin, followed by capture of the ICs with goat anti-human IgG F(ab′)2 protein G-Sepharose. The immunoprecipitated proteins were separated by SDS-PAGE, blotted, and stained with a murine anti-talin-H mAb, TA205. Bands corresponding to intact talin (235 kDa) and talin-H domain (47 kDa) are identified. No talin staining was observed following immunoprecipitation using the anti-HIV-1 gp120 Fab b12 (negative control).
All the cloned antiplatelet IgGs bind specifically to purified human talin and immunoprecipitate talin from platelet lysates. (A) Fab's were tested by ELISA for binding to talin purified from human platelets, the irrelevant antigen ovalbumin (OVA, control), and the flow-through preparation from the talin purification containing the talin-depleted platelet lysate. The anti-gp120 Fab b12 and secondary Ab alone were included as negative controls. (B) Fab's (5 μg) were incubated with platelet protein fractions enriched for talin, followed by capture of the ICs with goat anti-human IgG F(ab′)2 protein G-Sepharose. The immunoprecipitated proteins were separated by SDS-PAGE, blotted, and stained with a murine anti-talin-H mAb, TA205. Bands corresponding to intact talin (235 kDa) and talin-H domain (47 kDa) are identified. No talin staining was observed following immunoprecipitation using the anti-HIV-1 gp120 Fab b12 (negative control).
To further confirm that the cloned antiplatelet Abs were directed against talin-H, the 8 Fab's (5 μg/mL) were tested for their ability to immunoprecipitate talin from platelet lysates (Figure 5B). A talin-enriched platelet fraction from the Sephadex 200 size exclusion chromatography, containing multiple other proteins, was mixed with each Fab. Talin/antitalin Fab complexes were captured with goat anti-human IgG F(ab′)2 Ab-coated protein G-Sepharose and analyzed by SDS-PAGE and Western blotting. The murine anti-human talin-H mAb TA205 was used in the Western blots to detect potential immunoprecipitated talin protein. All 8 cloned antiplatelet Fab's were able to precipitate proteins with molecular masses of 235 and 47 kDa corresponding to intact talin and the talin head domain. No talin bands were observed in the lanes where anti-HIV-1 gp120 Fab b12 (control) was used to immunoprecipitate proteins in the platelet lysates.
The cloned IgG Abs to talin-H result from an antigen-driven response
We next examined whether human antitalin Abs evolved as a result of an antigen-driven response. The variable heavy (VH) and light (VL) chain genes of the 8 IgG-derived anti-talin-H Fab fragments were sequenced and compared with the closest immunoglobulin germ line sequences in the GenBank32 and EMBL33 databases (Figure 6). The Ab heavy chain is the major contributor to antigen binding in many instances, and thus detailed analysis focused on this chain. All the variable heavy-chain region genes of the antitalin IgG Fab's were highly mutated, with nucleotide and amino acid homologies in the range of 62% to 91% (average 78%) and 77% to 96% (average 88%) to the closest germ line, respectively (Table 1), characteristic of an affinity-matured Ab response.34,35 Furthermore, the variable heavy-chain genes exhibited a high replacement (R) to silent (S) mutation ratio (R/S ratio) for the complementarity determining regions (CDRs; CDR1 and 2) compared with the framework regions (FRs; FR1, 2, and 3), supporting the supposition that these IgG Abs evolved as a result of an antigen-driven, affinity-maturated response (Table 1).
Comparison of the deduced amino acid sequence of VH and VL domains from the platelet-binding Fab's to the closest germ line sequence. (A) VH sequences. (B) VL sequences. Amino acid identity within a group is indicated by dots.
Comparison of the deduced amino acid sequence of VH and VL domains from the platelet-binding Fab's to the closest germ line sequence. (A) VH sequences. (B) VL sequences. Amino acid identity within a group is indicated by dots.
Circulating ICs from patients with HIV-1-ITP contain talin-H
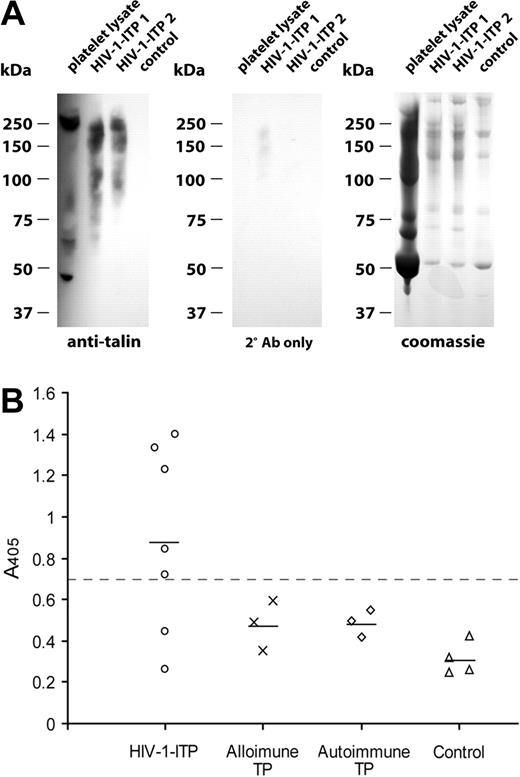
Previous studies have shown that patients with HIV-1-ITP contain elevated levels of circulating ICs and that platelet surface membrane fragments, including GPIIIa, are segregated within these ICs.6 To evaluate whether HIV-1-ITP ICs also contained talin-H, ICs were isolated by PEG precipitation of serum from 5 patients with HIV-1-ITP or 2 healthy controls. Immunoblotting and staining of the ICs with a mouse anti-talin-H Ab revealed talin degradation products within the circulating ICs of HIV-1-ITP patients, whereas a similar amount of IC (5 μg) isolated from healthy controls contained no talin antigen (Figure 7A). No staining was observed with secondary Ab alone.
PEG-precipitated ICs from serum of patients with HIV-1-ITP contain talin degradation products, and sera from HIV-1-ITP patients contained anti-talin IgG. (A) Circulating ICs from HIV-1-ITP patients and healthy HIV-1-negative controls were PEG precipitated and analyzed by SDS-PAGE and Coomassie staining or immunoblotting. Talin degradation products in platelet lysate or PEG-ICs were visualized with a murine anti-talin-H mAb, TA205, and a secondary HRP-labeled rabbit anti-mouse IgG F(ab′)2 antibody with minimal cross-reactivity to human serum proteins. No staining was detected with PEG-ICs of healthy controls or with the secondary anti-mouse IgG F(ab′)2 alone. (B) Sera, diluted 1:100 in PBS, from patients with HIV-1-ITP, alloimmune thrombocytopenia (TP), autoimmune thrombocytopenia, and healthy donors were tested for binding to purified talin by ELISA. Bound IgG was detected with an AP-conjugated F(ab′)2 goat antihuman IgG-Fc. Samples with A405 values of more than 2 SD above the mean of alloimmune thrombocytopenia patient values (> 0.7, dashed line) were considered positive. Depicted points are the mean of triplicate determinations.
PEG-precipitated ICs from serum of patients with HIV-1-ITP contain talin degradation products, and sera from HIV-1-ITP patients contained anti-talin IgG. (A) Circulating ICs from HIV-1-ITP patients and healthy HIV-1-negative controls were PEG precipitated and analyzed by SDS-PAGE and Coomassie staining or immunoblotting. Talin degradation products in platelet lysate or PEG-ICs were visualized with a murine anti-talin-H mAb, TA205, and a secondary HRP-labeled rabbit anti-mouse IgG F(ab′)2 antibody with minimal cross-reactivity to human serum proteins. No staining was detected with PEG-ICs of healthy controls or with the secondary anti-mouse IgG F(ab′)2 alone. (B) Sera, diluted 1:100 in PBS, from patients with HIV-1-ITP, alloimmune thrombocytopenia (TP), autoimmune thrombocytopenia, and healthy donors were tested for binding to purified talin by ELISA. Bound IgG was detected with an AP-conjugated F(ab′)2 goat antihuman IgG-Fc. Samples with A405 values of more than 2 SD above the mean of alloimmune thrombocytopenia patient values (> 0.7, dashed line) were considered positive. Depicted points are the mean of triplicate determinations.
Serum from patients with HIV-1-ITP contain antitalin IgG
To investigate the specificity of talin antibodies for HIV-1-ITP, a panel of HIV-1-ITP sera and controls was titered for binding to purified human talin in ELISA. Figure 7B shows the binding data for sera diluted 1:100 from 7 patients with HIV-1-ITP, 3 with ATP, 3 with alloimmune thrombocytopenia, and 4 healthy donors. A strong correlation between HIV-1-ITP and antitalin IgG antibodies was observed, as 5 of the 7 sera from HIV-1-ITP patients and none of the control sera were considered positive. Positivity was defined as an optical density 405 nm (OD405) value more than 2 standard deviations above the mean alloimmune thrombocytopenia patient group value (> 0.7). The mean level of antitalin Abs in the HIV-1-ITP patient group (0.889 ± 0.43) was significantly higher than that of the alloimmune thrombocytopenia group (0.481 ± 0.11; P < .02), the ATP patient group (0.486 ± 0.06; P < .02), and the healthy control group (0.315 ± 0.07; P < .001). An AP-conjugated F(ab′)2 fragment of a goat anti-human IgG Fc-specific antibody was used as secondary antibody to avoid problems of rheumatoid factor in the serum samples.
Discussion
Antibodies play a principal role in immune-based HIV-1-ITP, and previous studies using serum from HIV-1-ITP patients demonstrated that antiplatelet IgG and IgM antibodies directed against GPIIIa, GPIIb, and GPIb/IX, as well as anti-HIV-1 gp120 antibodies, are found in the circulation where they form ICs.6,9,10 However, since serum from HIV-1 patients usually contains large amounts of polyreactive Abs,11,12 determination of the Ab specificities involved in HIV-1-ITP disease using serum is difficult. In this study, we used phage-display technology to characterize the antiplatelet Ab response in HIV-1-ITP patients at the molecular level and mass spectrometry to identify the corresponding antigen. Bone marrow of 3 HIV-1-ITP patients was used as immune source for antibody library construction, since previous studies have shown that bone marrow is a major repository for plasma cells that produce the Abs found in serum36 and that bone marrow-derived IgG phage libraries broadly reflect the donor serum Ab repertoire.37
Our study revealed that talin-H is a major antigenic target of antiplatelet Abs in HIV-1-ITP and a panel of human monoclonal talin-H-specific Abs was isolated from IgG Ab libraries of 3 HIV-1-ITP patients. The question remains why an IgG response to talin-H is elicited in several patients with HIV-1-ITP. Talin is normally not very immunogenic, and antitalin Abs have not been described in other diseases or in healthy subjects, distinguishing our study from previous reports on Abs to intracellular platelet-associated antigens.38-40 In these studies, the Abs were found in both ATP patients and healthy individuals and exhibited features characteristic of natural Abs.41,42 The antitalin IgGs cloned in our study were of high affinity and showed evidence of extensive somatic mutation with high replacement-to-silent mutation ratio in the CDR regions, indicating that the antibodies were the result of a matured immune response to talin-H. Presumably, B cells do not normally encounter talin, unless it is exposed after platelet fragmentation. However, additional processes may be required to initiate the observed immune response in HIV-1-ITP, such as insufficient clearance of fragmented platelets and/or immune complex formation.
Clearance of Ab-coated5 or immune complex-coated43-45 platelets through Fcγ-mediated endocytosis by phagocytotic cells in the reticular endothelial system may be one of the major mechanisms of immune-mediated platelet depletion in HIV-1-ITP. In particular, the low-affinity FcγRIII on macrophages may play an important role in the clearance of autoantibody-platelet immune complexes as shown in a murine model of ATP.46 More recently, purified anti-GPIIIa-49-66 Abs from HIV-1-ITP patients have been shown to cause platelet fragmentation by catalyzing hydrogen peroxide generation in a complement-independent manner.6 This mechanism leads to fragmentation of platelet membranes and exposure of intracellular antigens. Talin and other cytoskeletal proteins are specifically cleaved by HIV-1 proteases,18 which may make the platelets more susceptible to fragmentation and may generate talin neoepitopes that, when released, could drive an Ab response. Additionally, during platelet activation, talin is also cleaved between amino acids 433 and 434 by various proteases like calpain II and thrombin, producing the 47-kDa N-terminal head and the 200-kDa C-terminal rod domains.14,31 Consequently, following platelet fragmentation mediated by Abs against platelet surface antigens or other mechanisms, talin-H may be released in tight association with its binding partner, the beta 3 integrin GPIIIa, and other platelet surface membrane proteins. These surface membranes, or soluble proteins thereof, form ICs. In agreement with this, we found talin degradation products within circulating ICs of HIV-1-ITP patients, and other investigators similarly observed platelet membrane receptors segregated within circulating ICs of HIV-1-ITP patients.6,10 These circulating ICs are likely ingested by antigen-presenting cells like macrophages or dendritic cells, resulting in presentation of talin-derived neoantigens in the context of major histocompatibility complex (MHC) class II to potential autoreactive CD4+ T cells. This could drive the activation of autoreactive CD4+ TH2 cells, especially if the professional antigen-presenting cells themselves are activated due to local inflammation caused by, for example, deposition of ICs or microbial stimuli. Such activated autoreactive CD4+ helper T cells type 2 (TH2) cells may again activate B cells recognizing cryptic talin epitopes exposed on released platelet degradation products. The generated talin Abs in the HIV-1-ITP patients, therefore, likely evolved as a result of an immune reaction against previously fragmented platelet products and epitope spreading.
It was surprising that the dominant specificity of the HIV-1-ITP antibody response was an intracellular antigen and not a cell surface protein, since the antibody library selection was performed on freshly isolated platelets. However, because unfixed platelets were used for Ab selection and screening, it is possible that fragmentation or perturbations of the cell membrane arose in a fraction of the platelets either during platelet isolation or the subsequent ELISA and panning procedures. Such perturbations may explain the exposure of intracellular antigens like talin on washed platelets. Similar observations have been described by Fujisawa et al,47 who found that Abs against the cytosolic domain of GPIIIa could be adsorbed from ATP sera by washed platelets. Another possibility is that talin-H becomes exposed on the surface of platelets under specific conditions: For example, platelet activation by thrombin has been shown to expose several cytoskeleton proteins on the surface of platelets.48 However, preliminary fluorescence-activated cell sorter (FACS) analysis of thrombin-activated fixed platelets has not shown evidence of talin exposure on the surface of activated platelets (data not shown).
The investigated serum from the HIV-1-ITP patients showed reactivity with the immunodominant GPIIIa-49-66 peptide but no reactivity against the intact platelet protein complexes GPIIb/IIIa, GPIa/IIa, and GPIb/IX. In agreement, library selection against the intact platelet protein complexes GPIIb/IIIa, GPIa/IIa, and GPIb/IX yielded no positive clones, whereas library selection against the GPIIIa-49-66 peptide yielded several positive clones. Nevertheless, none of the selected clones were monospecific for the GPIIIa-49-66 peptide but also reacted with a variety of other test antigens, suggesting that the serum binding to the GPIIIa-49-66 peptide resulted from polyreactive rather that monospecific Abs. However, the possibility exists that the monospecific anti-GPIIIa-49-66 Ab phage fragmented the platelets, thus preventing elution of these Ab phages from washed platelets.
In conclusion, our data indicate that the combination of antibody phage library selection and mass spectrometry analysis is an efficient proteomic method to identify novel disease-associated antigens. The results indicate that talin-H is an immunodominant epitope for affinity-matured, antigen-driven IgG Abs in HIV-1-ITP and likely a consequence of the perturbed state of increased platelet fragmentation. The abundance of anti-talin-H Abs in the repertoire and their presence in ICs that may bind to intact platelets and cause their clearance suggests that anti-talin-H may perpetuate platelet depletion in HIV-1-ITP.
Prepublished online as Blood First Edition Paper, August 17, 2004; DOI 10.1182/blood-2004-01-0386.
Supported by National Institutes of Health (NIH) grant HL63651 and the Danish Research Council.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank M. Schaller and M. K. Occhipinti for technical and editorial assistance and D. A. Calderwood, M. H. Ginsberg, D. R. Burton, and A. N. Theofilopoulos for fruitful discussions.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal