Abstract
Monocyte-endothelial adhesion plays an important role in monocyte trafficking and hence is important for immune responses and pathogenesis of inflammatory diseases including atherosclerosis. The cross-talk between different integrins on monocytes may be crucial for a coordinated regulation of the cellular adhesion during the complex process of transendothelial migration. By using monoclonal antibodies and recombinant intercellular adhesion molecule 1 (ICAM-1) to engage lymphocyte function-associated antigen 1 (LFA-1) on monocytic cells, we found that the cellular adhesion to vascular cell adhesion molecule 1 (VCAM-1) mediated by very late antigen 4 (VLA-4) was suppressed after this treatment and the suppression depended on the presence of reactive oxygen species (ROSs). Inhibition of production of ROSs through the use of inhibitor of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, but not inhibitors of mitochondrial electron transport chain or xanthine oxidase, revealed that this suppression on VLA-4-mediated cellular binding was mediated by ROSs produced by phagocyte NADPH oxidase. Activation of phosphoinositol-3 kinase and Akt appears to mediate this NADPH oxidase activation through p47phox phosphorylation and Rac-1 activation. Our results provide a novel pathway in which ROSs play a critical role in integrin cross-talk in monocytes. This signaling pathway may be important for cellular transition from firm arrest to diapedesis during monocyte trafficking. (Blood. 2004;104:4046-4053)
Introduction
Monocyte-endothelial adhesion and monocyte transendothelial migration are important events in the immune surveillance and also in pathogenesis of inflammatory diseases including atherosclerosis. In physiologic conditions, circulating monocytes in various maturation states enter tissue microenvironments and participate in the immune responses through interacting with other leukocytes and producing cytokines and reactive oxygen species (ROSs).1 In response to atherogenic factors, monocytes in the bloodstream attach, adhere, and spread on the luminal surface of vascular endothelium. The cells then migrate across the endothelium and accumulate within the intima and contribute to the formation of atheroma.2,3
A panel of well-coordinated leukocyte adhesion molecules including integrins, selectins, and immunoglobulin (Ig) superfamily molecules is used to mediate the multistep process of leukocyte recruitment to various tissues.4,5 How the adhesion activity of these molecules is regulated has been the subject of intensive investigation in recent years. Monocytes express β1 and β2 integrins including lymphocyte function-associated antigen 1 (LFA-1, αLβ2 integrin) and very late antigen 4 (VLA-4, α4β1 integrin) on their cell surface.6,7 The main ligands of LFA-1 include intercellular adhesion molecule 1 (ICAM-1), ICAM-2, ICAM-3, junctional adhesion molecule 1 (JAM-1), and fibrinogen,8,9 whereas ligands of VLA-4 include vascular cell adhesion molecule 1 (VCAM-1) and fibronectin.10 Binding of integrins to their respective ligands induces outside-in signals that prepare cells for subsequent changes. Outside-in signals by leukocyte integrins involve protein tyrosine phosphorylation, cytoskeleton reorganization, and production of ROSs.11 On the other hand, intracellular events change the adhesiveness of integrins through molecular interactions at the integrin cytoplasmic domains, known as inside-out signals.12,13
Differential regulation of the adhesion activity of integrins through cytokines and cell-cell interaction contribute to flexibility of the cell type-specific leukocyte adhesion in different microenvironments.14,15 Moreover, evidence indicates that on a given cell, one subset of integrin may be regulated by ligation of another,16 hence confers the cell a temporally regulated order of adhesion to different targets. Here, we investigated the interaction between LFA-1 and VLA-4 integrins on monocytes and the potential mechanisms underlying the ligand binding-induced regulation of the integrin adhesion activity.
Materials and methods
Monoclonal antibodies
Mouse monoclonal antibodies (mAbs) against human p47phox, Rac-1, and rabbit polyclonal antibodies for p47phox were purchased from Upstate Biotechnology (Lake Placid, NY). Mouse anti-Akt and antiphospho-Akt mAbs were purchased from Cell Signaling Technology (Beverly, MA). Antiphospho-serine mAb was from Sigma (Saint Louis, MO). Anti-VLA-4 antibody (HP2/1) and horseradish peroxidase (HRP)-conjugated rabbit antimouse IgG were from Immunotech (Marseille, France). Ligand-induced binding site-specific VLA-4 antibody (HUTS-4) was from Chemicon (Temecula, CA). An irrelevant mouse mAb (clone 1-64.1) was a gift from Dr Y. S. Lin (Institute of Microbiology and Immunology, National Cheng-Kung University Medical College, Tainan, Taiwan). Fluorescein isothiocyanate (FITC)-conjugated donkey antihuman IgG was from Bender MedSystems (Burlingame, CA). FITC-conjugated rabbit antimouse IgG was from ICN Biomedical (Aurora, OH).
Cell culture
U937 cells (American Type Culture Collection, Manassas, VA, [ATCC] CRL-1593.2) were cultured in RPMI 1640 medium (Gibco, Grand Island, NY) supplemented with 2 mM l-glutamine, 10% heat-inactivated fetal bovine serum (FBS; Hyclone, Logan, UT), and 40 U/mL penicillin/streptomycin (Gibco).
Recombinant protein-coated beads
Protein G beads (200 μL; Amersham Biosciences, Uppsala, Sweden) were washed twice in phosphate-buffered saline (PBS) and resuspended in adhesion buffer, pH 7. ICAM-1-FC and FC recombinant proteins, which respectively contain extracellular domains of human ICAM-1 and Fc portion of human IgG1 and Fc portion alone,17 were added to final concentrations of 50 to 500 μg/mL. The beads were rotated at 4°C for 14 hours in adhesion buffer and blocked with 0.1% bovine serum albumin (BSA) for 2 hours at room temperature. The beads were then washed twice in adhesion buffer for use in the following adhesion experiments.
Adhesion assay
Cell-to-protein adhesion assays were performed as previously reported.18 Briefly, bottoms of 96-well plates were coated overnight at 4°C with 30 μg/mL recombinant VCAM-1-FC (produced by transfected Chinese hamster ovary [CHO] cells, containing the first 3 N-terminal immunoglobulin domains of human VCAM-1 and the Fc portion of human IgG118 ), fibronectin (2 μg/mL), laminin (2 μg/mL; Gibco), vitronectin (2 μg/mL; Sigma), or FC in a coating buffer (15 mM sodium carbonate, 35 mM sodium bicarbonate; pH 9.6). After washing with PBS, the wells were blocked with 0.1% BSA. U937 cells were labeled with 12.5 μg/mL 2′, 7′-bis-(2-carboxyethyl)-5 (and -6) carboxyfluorescein (BCECF-am; Molecular Probes, Eugene, OR) for 30 minutes at 37°C. After being washed with PBS, 5 × 104 labeled U937 cells were resuspended in 1.2 mL adhesion buffer (137 mM NaCl, 3 mM KCl, 20 mM 4-(2-hydroxyethyl)piperazine-1-ethane sulfonic acid [HEPES, Hyclone], and 1 mM CaCl2, pH 7.4) with or without diphenylene iodonium (DPI), catalase, N-acetyl cysteine (NAC), rotenone, or allopurinol (all purchased from Sigma) for 15 minutes. The cells were then treated with irrelevant antibody, TS1-18, TS1-22, or protein G-ICAM-1-FC or protein G-FC beads for 1 hour at 37°C and incubated in wells at 37°C for 15 minutes. Unbound cells were washed from the plates with adhesion buffer (3 or 4 washes). Bound cells were detected using a fluorescence plate reader (HTS7000 Bioassay Reader; Perkin Elmer, Norwalk, CT) and read as fluorescence units. Cell viability, measured by flow cytometry with the staining of annexin V-FITC (BD PharMingen, Palo Alto, CA), was not changed with LFA-1 ligation in conditions used in the adhesion assays. No cell aggregation or apparent changes in cell morphology were observed with light microscopy or flow cytometric analysis after the LFA-1 ligation (not shown).
Cell surface staining with mAbs
U937 cells were stained with primary antibodies. After 3 washes, these cells were then stained with a FITC-conjugated secondary antibody against mouse immunoglobulin (ICN Biomedical) and analyzed on an Epics XL/MCL flow cytometer (Beckman-Coulter, Miami, FL). The cell staining experiments were performed at 4°C using buffer of PBS (pH 7.4) containing 1% BSA (Sigma).
Detection of ROSs
ROSs were measured using a 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA; Molecular Probes) fluorescent method. H2DCF-DA (5 μM) was added to 6 × 105 U937 cells in adhesion buffer with or without DPI, allopurinol, rotenone (Sigma), LY29002 (Calbiochem, San Diego, CA), Akt inhibitor (Calbiochem), and U0126 (Calbiochem) for 15 minutes, then treated with anti-LFA-1 antibody and incubated at 37°C for 1 hour. The fluorescence was then detected at the indicated time using a fluorescence plate reader at excitation/emission of 475/525 nm (HTS7000 Bioassay Reader; Perkin Elmer).
Immunoprecipitation and Western blot analysis
U937 cells (1 × 107) were incubated in adhesion buffer in the presence or absence of stimuli for the indicated times at 37°C. Where indicated, cells were preincubated with the pharmacologic inhibitor LY294002 (20 μM) for 15 minutes. The reactions were stopped by placing the cells on ice and then centrifuging at 300g for 5 minutes. The cell pellets were lysed with 600 μL lysis buffer (containing Tris-buffered saline [TBS], pH 7.6, 1% Triton X-100 [TX100], 1 mM phenylmethylsulfonyl fluoride [PMSF], and complete protein inhibitor cocktail [Boehringer Mannheim, Mannheim, Germany]) by shaking for 30 minutes at 4°C. The cell lysates were then centrifuged at 17 500g for 15 minutes at 4°C. For immunoprecipitation, the lysates were immediately incubated with polyclonal anti-p47phox antibody (1 μg/L × 107 cells; Upstate Biotechnology) overnight at 4°C. Sepharose-protein G beads (Amersham Biosciences) were added to the lysates (50 μL beads/600 μL lysate), and the mixture was incubated overnight at 4°C with constant rocking. The protein G beads were washed 3 times in lysis buffer and boiled in sodium dodecyl sulfate (SDS) sample buffer (TBS with 10% glycerol, 3% SDS, and 100 mM dithiothreitol [DTT]), and the proteins were resolved on 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE). For Akt Western blotting experiments, lysates prepared from 1 × 106 cells were used for each analysis on 12% SDS-PAGE. For Western blotting analysis, proteins were electroblotted to polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA). The membranes were incubated with 5% nonfat dry milk in TBST (Tween 20 0.2%, NaCl 0.1 M, Tris base, 20 mM, pH 7.6) overnight at 4°C to reduce any nonspecific binding, followed by incubation with the primary antibody, anti-Akt (Cell Signaling Technology) antiphospho-Akt (Cell Signaling Technology), anti-p47phox mAb (Upstate Biotechnology), or antiphospho-serine mAb (Sigma) diluted 1:2000 in 5% nonfat dry milk in TBST. After washing several times with TBST, the membranes were incubated for 1 hour with 0.1 μg/mL HRP-conjugated goat antimouse IgG (ICN Biomedical) in 5% nonfat dry milk in TBST and were washed several times. The blot was then visualized with a chemiluminescent substrate (enhanced chemiluminescence [ECL]; Amersham Biosciences) and then exposed to autoradiography films (Fuji, Tokyo, Japan).
Determination of VLA-4 activation state with recombinant soluble VCAM-1
U937 cells (6 × 105) were resuspended in adhesion buffer. The VCAM-1-FC and FC fusion proteins were added at 10 μg/mL and incubated for 30 minutes at 37°C. After incubation, cells were washed twice in adhesion buffer and resuspended in the same buffer containing FITC-conjugated donkey antihuman IgG at a 1:100 dilution (Bender MedSystems). After a 30-minute incubation at 4°C, cells were washed twice and bound antibody was detected using a flow cytometer (Beckman-Coulter).
Determination of active Rac with the “pull-down” assay
The Escherichia coli-expressing GST protein fused to human PAK was kindly provided by Dr J. G. Collard (Netherlands Cancer Institute, Utrecht, the Netherlands). Bacteria were grown to OD600 = 0.6 at 37°C and then induced with 1 mM isopropylthiogalactoside (IPTG) for 3 hours at 37°C. Bacteria were then pelleted, washed, and resuspended in 1% TX100 containing 1 mM PMSF, 1 mM MgCl2, and 1 mM DTT, and incubated on ice for 30 minutes. The bacteria were then sonicated at 0°C for three 1-minute intervals at a setting of 7 with an Ultrasonic Processor XL (Misonix, Farmingdale, NY). Insoluble material was removed by centrifugation at 15 000g. One hundred microliters of glutathione-S-Sepharose 4B beads (Amersham Pharmacia Biotech) was added to the cleared lysate, incubated for 30 minutes at 37°C, and washed 3 times in 1% TX100. The GST-PAK beads were then added to cleared U937 cells lysates from 1 × 107 cells at 4°C. After 1 hour, the beads were collected by centrifugation and washed 3 times in 25 mM Tris-HCl, pH 7.5, 1% TX100, 100 mM NaCl, and 30 mM MgCl2. The beads were then resuspended in sample buffer and boiled under reducing conditions for 10 minutes. The precipitated proteins and equal amount of cell lysates were resolved with 12% SDS-PAGE and transferred to PVDF membranes. Membranes were incubated with 5% nonfat dry milk in TBST overnight at 4°C to reduce any nonspecific binding and followed by incubation for 1 hour with primary antibody (0.3 μg/mL anti-Rac1 mAb; Upstate Biotechnology). After washing several times with TBST, the membranes were incubated for 1 hour with 0.1 μg/mL HRP-conjugated goat antimouse IgG in 5% nonfat dry milk in TBST and washed several times. The blot was then visualized with a chemiluminescent substrate (ECL; Amersham Biosciences) and then exposed to autoradiography film (Fuji, Tokyo, Japan).
Results
Ligation of LFA-1 on U937 cells decreases cellular binding to VCAM-1
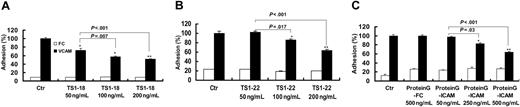
To determine the effects of LFA-1 ligation on the function of β1 integrin-mediated cellular binding, we used a human monocytic cell line, U937, in a static-state adhesion assay. U937 cells were treated with anti-LFA-1 antibodies or a recombinant ICAM-1 immobilized on Sepharose beads and then tested for their ability to bind to VCAM-1 recombinant protein-coated plates after 1 hour of treatment. VCAM-1 binding of U937 cells treated with anti-LFA-1 mAbs, TS 1-18 (Figure 1A) and TS1-22 (Figure 1B), significantly decreased to about 60% of the original level in a dose-responsive manner when compared with cells treated with control antibodies. A similar effect was observed when the natural ligand of LFA-1, ICAM-1, was used to treat the cells. Although treatment of cells with uncoated beads and beads coated with FC protein showed no effects, treatment with ICAM-1-FC-coated beads decreased cellular binding to VCAM-1 to a comparable degree (Figure 1C).
Ligation of LFA-1 on U937 cells decreased cellular binding to VCAM-1. Adhesion assay experiments for U937 cells binding to VCAM-1-FC and FC recombinant proteins were performed in the absence or presence of different concentrations of TS1-18 (A), TS1-22 (B), or immobilized ICAM-1-FC (C). The experiments were performed with 8 replicates and were repeated 3 times with similar results. Data are expressed as mean ± SD and analyzed with 2-tailed Student t test. Results showing significant differences in cellular binding in the presence of LFA-1 ligation treatments in comparison with their respective controls (isotype-matched mAb for panels A and B and immobilized FC for panel C) are marked with ** for P < .005 or * for P < .05. ICAM indicates the ICAM-1-FC recombinant protein in the figures.
Ligation of LFA-1 on U937 cells decreased cellular binding to VCAM-1. Adhesion assay experiments for U937 cells binding to VCAM-1-FC and FC recombinant proteins were performed in the absence or presence of different concentrations of TS1-18 (A), TS1-22 (B), or immobilized ICAM-1-FC (C). The experiments were performed with 8 replicates and were repeated 3 times with similar results. Data are expressed as mean ± SD and analyzed with 2-tailed Student t test. Results showing significant differences in cellular binding in the presence of LFA-1 ligation treatments in comparison with their respective controls (isotype-matched mAb for panels A and B and immobilized FC for panel C) are marked with ** for P < .005 or * for P < .05. ICAM indicates the ICAM-1-FC recombinant protein in the figures.
Ligation of U937 cells to ICAM-1 decreases the expression of VLA-4 with activated conformation
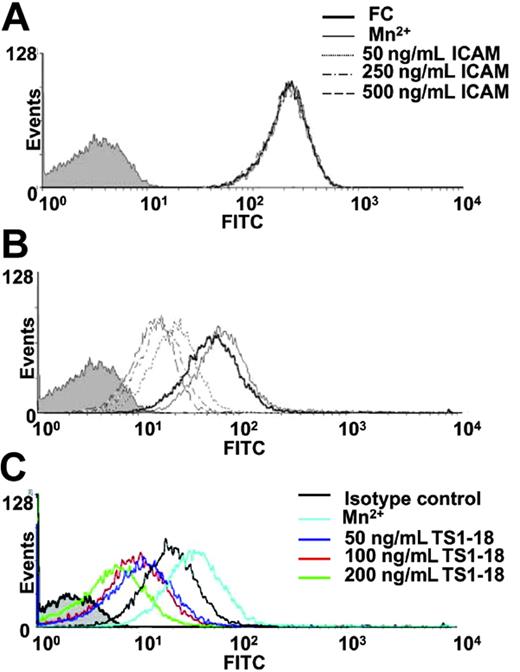
To elucidate the molecular changes that cause the decrease in cellular adhesion to VCAM-1, we treated U937 cells with a recombinant ICAM-1 for 1 hour and then analyzed the expression of total VLA-4 and its ligand-induced activated epitope, which indicates activated VLA-4 integrin on the cell surface. For U937 cells treated or not treated with the recombinant ICAM-1, the expression levels of total VLA-4 integrin were not changed (Figure 2A). However, the level of activated VLA-4 on treated U937 cells decreased with increasing concentrations of ICAM-1 (Figure 2B). We then used flow cytometry to measure the soluble VCAM-1 binding of U937 cells after LFA-1 ligation with TS1-18 mAb. A significant decrease in VCAM-1 binding, which represents suppressed levels of activated VLA-4, was observed in cells stimulated with an increasing concentration of anti-LFA-1 mAb (Figure 2C).
Ligation of LFA-1 on U937 cells decreased the expression of VLA-4 with activated conformation. U937 cells were cultured with plain medium (black profile) or treated with 500 ng/mL, 250 ng/mL, or 50 ng/mL soluble ICAM-1-FC, and 500 ng/mL soluble FC protein or Mn2+ (1 mM) and then stained with (A) a conformation-independent anti-VLA-4 antibody (HP1/2) or (B) a ligand-induced binding site-specific VLA-4 antibody (HUTS-4). U937 cells were then analyzed with flow cytometry. The mean fluorescence intensities (MFIs) of the ligand-induced binding site-specific antibody staining for Mn2+, 500 ng/mL FC, 50, 250, and 500 ng/mL ICAM-1-FC-treated cells are 61.51, 46.81, 19.94, 14.78, and 12.52, respectively. (C) U937 cells were cultured with plain medium (black profile) or treated with 50 ng/mL, 100 ng/mL, or 200 ng/mL TS1-18, and 200 ng/mL isotype-matched control mAb or Mn2+ (1 mM) and then incubated with VCAM-1-FC protein and a FITC-labeled anti-FC antibody. U937 cells were then analyzed with flow cytometry. The MFIs of the VCAM-1-FC protein binding for Mn2+ and 50, 100, or 200 ng/mL TS1-18-treated cells are 34.30, 20.33, 15.62, 9.67, and 8.78, respectively. This experiment was repeated 3 times with similar results.
Ligation of LFA-1 on U937 cells decreased the expression of VLA-4 with activated conformation. U937 cells were cultured with plain medium (black profile) or treated with 500 ng/mL, 250 ng/mL, or 50 ng/mL soluble ICAM-1-FC, and 500 ng/mL soluble FC protein or Mn2+ (1 mM) and then stained with (A) a conformation-independent anti-VLA-4 antibody (HP1/2) or (B) a ligand-induced binding site-specific VLA-4 antibody (HUTS-4). U937 cells were then analyzed with flow cytometry. The mean fluorescence intensities (MFIs) of the ligand-induced binding site-specific antibody staining for Mn2+, 500 ng/mL FC, 50, 250, and 500 ng/mL ICAM-1-FC-treated cells are 61.51, 46.81, 19.94, 14.78, and 12.52, respectively. (C) U937 cells were cultured with plain medium (black profile) or treated with 50 ng/mL, 100 ng/mL, or 200 ng/mL TS1-18, and 200 ng/mL isotype-matched control mAb or Mn2+ (1 mM) and then incubated with VCAM-1-FC protein and a FITC-labeled anti-FC antibody. U937 cells were then analyzed with flow cytometry. The MFIs of the VCAM-1-FC protein binding for Mn2+ and 50, 100, or 200 ng/mL TS1-18-treated cells are 34.30, 20.33, 15.62, 9.67, and 8.78, respectively. This experiment was repeated 3 times with similar results.
LFA-1 ligation-induced decrease in VCAM-1 adhesion is inhibited by antioxidants
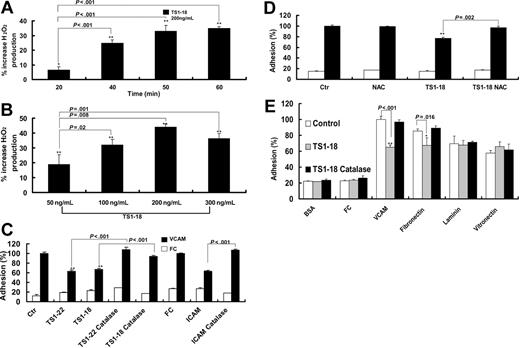
We previously reported that ROSs, including superoxide and hydrogen peroxide, affect integrin-mediated leukocyte adhesion.18 We hence tested whether stimulation of the U937 cells with anti-LFA-1 antibody increased the production of ROSs. After the cells were stimulated with anti-LFA-1 mAb, cellular production of ROSs by U937 cells increased and reached a plateau after 50 minutes of antibody treatment (Figure 3A), whereas treatment with an isotype-matched antibody did not significantly alter cellular production of ROSs. We also tested the production of ROSs in U937 cells after different concentrations of anti-LFA mAb treatment. We found that there was a dose-responsive increase in ROSs in the range of 50 to 300 ng/mL anti-LFA-1 and the production of ROSs plateaued after 200 ng/mL (Figure 3B). We hence used 200 ng/mL anti-LFA-1 for 60 minutes in the following experiments.
LFA-1 ligation induced U937 cells to produce ROSs that may modulate VCAM-1 binding. U937 cells were labeled with H2DCF-DA in the presence or absence of anti-LFA-1 antibody and the production of ROSs was measured by fluorescence intensity. After LFA-1 ligation, the production of ROSs increased in a time-dependent (A) and dose-dependent (B) manner. Adhesion experiments were then performed in the presence or absence of TS1-18, TS1-22, or immobilized ICAM-1-FC in the presence or absence of (C) catalase (0.3 U/μL) or (D) NAC (10 μM). Cellular binding to fibronectin, laminin, or vitronectin in the presence or absence of catalase was compared with the binding to VCAM-1-FC (E). The experiments in panels A-B were performed with 4 replicates and repeated 3 times with similar results. The experiments in panels C-E were performed with 8 replicates and were repeated 3 times with similar results. Data are expressed as mean ± SD and analyzed with 2-tailed Student t test. Results showing significant differences when compared with the baseline fluorescence are marked with ** for P < .005 or * for P < .05.
LFA-1 ligation induced U937 cells to produce ROSs that may modulate VCAM-1 binding. U937 cells were labeled with H2DCF-DA in the presence or absence of anti-LFA-1 antibody and the production of ROSs was measured by fluorescence intensity. After LFA-1 ligation, the production of ROSs increased in a time-dependent (A) and dose-dependent (B) manner. Adhesion experiments were then performed in the presence or absence of TS1-18, TS1-22, or immobilized ICAM-1-FC in the presence or absence of (C) catalase (0.3 U/μL) or (D) NAC (10 μM). Cellular binding to fibronectin, laminin, or vitronectin in the presence or absence of catalase was compared with the binding to VCAM-1-FC (E). The experiments in panels A-B were performed with 4 replicates and repeated 3 times with similar results. The experiments in panels C-E were performed with 8 replicates and were repeated 3 times with similar results. Data are expressed as mean ± SD and analyzed with 2-tailed Student t test. Results showing significant differences when compared with the baseline fluorescence are marked with ** for P < .005 or * for P < .05.
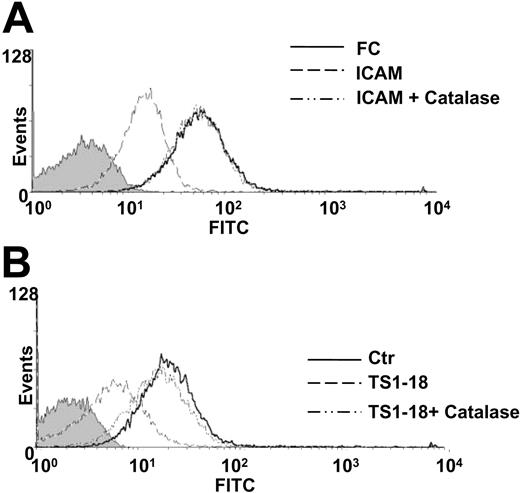
To determine whether this LFA-1 ligation-induced production of ROSs leads to decreased cellular binding to VCAM-1, we tested the effects of catalase on cellular binding after LFA-1 ligation. We found that the decrease of U937 cell binding to VCAM-1 after LFA-1 ligation, either by anti-LFA antibodies or by a recombinant ICAM-1, was reversed by catalase treatment (Figure 3C). A similar reversal of the suppression of cellular binding was observed when the cells were treated with NAC, a scavenger of the hydrogen peroxide (Figure 3D). We performed adhesion experiments to clarify the specificity of the LFA-1-induced suppression of cellular adhesion by using other extracellular matrix protein ligands (fibronectin, laminin, and vitronectin) of monocyte integrins as the fixed proteins. We found that the LFA-1 ligation-induced decrease in cellular binding was found in experiments using VCAM-1 and fibronectin, which are known ligands for VLA-4 integrin, whereas the cellular binding to laminin (ligand for α1β1, α2β1, α3β1, and α6β1 on monocytes) and vitronectin (ligand for αvβ3), was not affected by LFA-1 ligation. Moreover, U937 cell binding to vitronectin and laminin, different from the binding to VCAM-1 and fibronectin, was not affected by the presence of catalase (Figure 3E). These results suggest that LFA-1 ligation-induced suppression of integrin adhesion is limited to VLA-4 integrin. The effects of catalase and NAC on VLA-4-mediated adhesion were also examined with cell surface analysis of activated VLA-4. The presence of catalase restored the expression of activated VLA-4 on U937 cells treated with the recombinant ICAM-1 (Figure 4A) or anti-LFA-1 antibody (Figure 4B) detected with conformation-dependent mAb or soluble recombinant VCAM-1, respectively. These findings indicate that the ROSs produced by leukocytes after LFA-1 ligation are essential for the suppression of cellular binding to VCAM-1.
Catalase reversed the suppressive effects of LFA-1 ligation on VLA-4 activation. The effects of catalase on VLA-4-mediated adhesion were examined with cell surface analysis of activated VLA-4. U937 cells were treated with (A) 500 μg/mL ICAM-1-FC protein and (B) 200 ng/mL TS1-18 antibody in the presence or absence of catalase (0.3 U/μL) and then stained with (A) a ligand-induced binding site-specific VLA-4 antibody (HUTS-4) or (B) the soluble VCAM-1 protein. U937 cells were then analyzed with flow cytometry. The MFIs of the ligand-induced binding site-specific antibody staining for control FC, ICAM-1-FC, and ICAM-1-FC plus catalase-treated cells are 46.81, 12.52, and 46.67 respectively (A). The MFIs of the VCAM-1-FC protein binding for isotype control antibody-treated, TS1-18 plus catalase-treated, and TS1-18-treated cells are 20.33, 8.78, and 15.58, respectively (B). This experiment was repeated twice with similar results.
Catalase reversed the suppressive effects of LFA-1 ligation on VLA-4 activation. The effects of catalase on VLA-4-mediated adhesion were examined with cell surface analysis of activated VLA-4. U937 cells were treated with (A) 500 μg/mL ICAM-1-FC protein and (B) 200 ng/mL TS1-18 antibody in the presence or absence of catalase (0.3 U/μL) and then stained with (A) a ligand-induced binding site-specific VLA-4 antibody (HUTS-4) or (B) the soluble VCAM-1 protein. U937 cells were then analyzed with flow cytometry. The MFIs of the ligand-induced binding site-specific antibody staining for control FC, ICAM-1-FC, and ICAM-1-FC plus catalase-treated cells are 46.81, 12.52, and 46.67 respectively (A). The MFIs of the VCAM-1-FC protein binding for isotype control antibody-treated, TS1-18 plus catalase-treated, and TS1-18-treated cells are 20.33, 8.78, and 15.58, respectively (B). This experiment was repeated twice with similar results.
ROSs produced by NADPH oxidase are modulators of cell adhesion
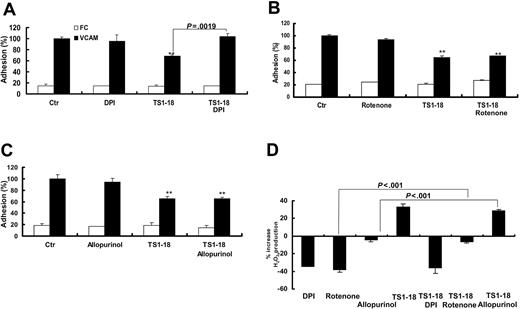
To clarify the origin of the production of ROSs during integrin-mediated cell adhesion, the U937 cells were pretreated with DPI (inhibitor of NADPH oxidase), allopurinol (inhibitor of xanthine oxidase), and rotenone (inhibitor of mitochondria) before seeding them onto VCAM-1-FC-coated wells for adhesion assays. DPI pretreatment of U937 cells reversed the inhibitory effect of LFA-1 ligation on cellular binding to VCAM-1 (Figure 5A). In contrast, pretreatment of U937 cells with rotenone and allopurinol caused no apparent changes on cellular adhesion to VCAM-1 (Figure 5B-C). To directly investigate the change of production of ROSs by U937 cells after inhibitor treatment, we measured the production of ROSs with or without LFA-1 ligation. We found that both DPI and rotenone, but not allopurinol, significantly suppressed levels of ROSs in baseline conditions. After LFA-1 ligation with TS1-18 mAb, DPI-treated U937 cells showed no increase in ROSs, whereas allopurinol- and rotenone-treated cells produced increased levels of ROSs (Figure 5D). These results suggested that increased production of ROSs by NADPH oxidase contributes to the suppression of VCAM-1 binding in U937 cells.
Ligation of LFA-1 decreased cellular adhesion to VCAM-1 through a NADPH oxidase-dependent pathway. U937 cells were labeled with BCECF-am in the absence or presence of (A) DPI (5 μM), (B) rotenone (10 μM) or (C) allopurinol (4 μM) and then treated with anti-LFA-1 mAb TS1-18 and tested for binding to the recombinant VCAM-1-FC and FC. (D) After LFA-1 ligation with TS1-18 mAb, DPI-, allopurinol-, and rotenone-treated U937 cells were measured for their increase in production of ROSs with H2DCFDA fluorescence. The experiments were performed with 8 replicates and were repeated 3 times with similar results. Results showing significant differences in the comparison with negative controls are marked with ** for P < .005 and * for P < .05. Data are expressed as mean ± SD.
Ligation of LFA-1 decreased cellular adhesion to VCAM-1 through a NADPH oxidase-dependent pathway. U937 cells were labeled with BCECF-am in the absence or presence of (A) DPI (5 μM), (B) rotenone (10 μM) or (C) allopurinol (4 μM) and then treated with anti-LFA-1 mAb TS1-18 and tested for binding to the recombinant VCAM-1-FC and FC. (D) After LFA-1 ligation with TS1-18 mAb, DPI-, allopurinol-, and rotenone-treated U937 cells were measured for their increase in production of ROSs with H2DCFDA fluorescence. The experiments were performed with 8 replicates and were repeated 3 times with similar results. Results showing significant differences in the comparison with negative controls are marked with ** for P < .005 and * for P < .05. Data are expressed as mean ± SD.
Inhibition of PI3K activity selectively blocks LFA-1 ligation-induced production of ROSs
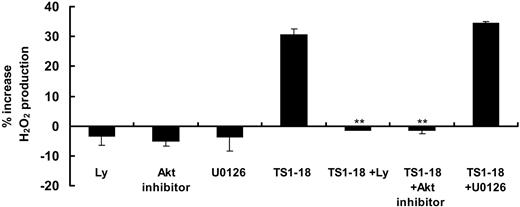
Phosphoinositol-3 kinase (PI3K)-Akt and ERK1/2 are reported to be involved in NADPH oxidase activation by phosphorylating serine residues on p47phox in neutrophils.19-21 To unravel the signaling leading to LFA-1 ligation-stimulated production of ROSs in U937 cells, we investigated the effects of PI3K inhibitor, LY294002, and ERK1/2 inhibitor, U0126, on cellular production of ROSs. U937 cells preincubated with LY294002 or Akt inhibitor did not have increases in production of ROSs induced by ICAM-1 ligation, while U0126 had no significant effects (Figure 6). PI3K-Akt thus may have an essential role in the intracellular signaling leading to NADPH oxidase activation.
Inhibition of PI3K activity selectively blocked LFA-1 ligation-induced production of ROSs. U937 cells (6 × 105 cells for each condition) were preincubated with LY294002 (20 μM), Akt inhibitor, and U0126 (30 μM) for 15 minutes, then labeled with H2DCF-DA and stimulated with anti-LFA-1 antibody. Increase of production of ROSs was measured as increase in fluorescence intensity after 1 hour. These experiments were performed with 4 replicates and were repeated 3 times with similar results. Results showing significance difference in fluorescence intensity in comparison with baseline levels are marked with ** for P < .005 or * for P < .05.
Inhibition of PI3K activity selectively blocked LFA-1 ligation-induced production of ROSs. U937 cells (6 × 105 cells for each condition) were preincubated with LY294002 (20 μM), Akt inhibitor, and U0126 (30 μM) for 15 minutes, then labeled with H2DCF-DA and stimulated with anti-LFA-1 antibody. Increase of production of ROSs was measured as increase in fluorescence intensity after 1 hour. These experiments were performed with 4 replicates and were repeated 3 times with similar results. Results showing significance difference in fluorescence intensity in comparison with baseline levels are marked with ** for P < .005 or * for P < .05.
LFA-1 ligation regulates Akt phosphorylation via a PI3K-dependent pathway
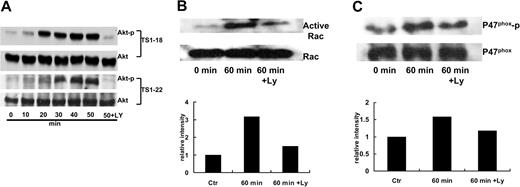
To understand the mechanisms of how LFA-1 ligation induced production of ROSs through PI3K, we examined the effect of LFA-1-binding TS1-18 and TS1-22 mAbs on Akt phosphorylation at 10-minute intervals. Both mAbs induced Akt phosphorylation in a time-dependent manner and plateaued 50 minutes after the stimulation. Preincubation of PI3K inhibitor LY294002 significantly decreased Akt phosphorylation (Figure 7A). These results strongly suggest the involvement of PI3K and Akt in LFA-1 ligation-induced ROS production.
LFA-1 ligation induced Akt phosphorylation, p47 phox phosphorylation, and Rac-1 activation via a PI3K-dependent pathway. (A) U937 cells (1 × 106 cells for each condition) were preincubated with or without LY294002 (20 μM) and stimulated with TS1-18 and TS1-22 for the indicated time periods. The cell lysates were subjected to Western blot analysis using phospho-Akt antibody or Akt antibody to determine the activated and the total amount of the Akt protein. (B) U937 cells were pretreated with or without the PI3K inhibitor LY294002 (20 μM) for 15 minutes and stimulated with TS1-18 for 1 hour. The cell lysates were immunoprecipitated with rabbit anti-p47phox polyclonal antibody. The precipitants were then resolved with SDS-PAGE and subjected to Western blot analysis using antiphospho-serine antibody or a mouse anti-p47phox mAb. (C) After TS1-18 stimulation, cells were lysed and the Rac-1-activated fraction (Rac-1-GTP) was precipitated with GST-PAK beads. Aliquots of lysates were analyzed along with the precipitates by Western blotting using an anti-Rac-1 mAb. These experiments were repeated 3 times with similar results.
LFA-1 ligation induced Akt phosphorylation, p47 phox phosphorylation, and Rac-1 activation via a PI3K-dependent pathway. (A) U937 cells (1 × 106 cells for each condition) were preincubated with or without LY294002 (20 μM) and stimulated with TS1-18 and TS1-22 for the indicated time periods. The cell lysates were subjected to Western blot analysis using phospho-Akt antibody or Akt antibody to determine the activated and the total amount of the Akt protein. (B) U937 cells were pretreated with or without the PI3K inhibitor LY294002 (20 μM) for 15 minutes and stimulated with TS1-18 for 1 hour. The cell lysates were immunoprecipitated with rabbit anti-p47phox polyclonal antibody. The precipitants were then resolved with SDS-PAGE and subjected to Western blot analysis using antiphospho-serine antibody or a mouse anti-p47phox mAb. (C) After TS1-18 stimulation, cells were lysed and the Rac-1-activated fraction (Rac-1-GTP) was precipitated with GST-PAK beads. Aliquots of lysates were analyzed along with the precipitates by Western blotting using an anti-Rac-1 mAb. These experiments were repeated 3 times with similar results.
LFA-1 ligation enhances p47phox phosphorylation and Rac-1 activation via a PI3K-dependent pathway
Phosphorylation of the cytosolic oxidase subunit p47phox is a key event in NADPH oxidase activation. We therefore tested the phosphorylation state of the p47phox after LFA-1 ligation. We found that p47phox phosphorylation increased 60 minutes after TS1-18 stimulation in U937 cells. However, for cells pretreated for 15 minutes with the PI3K inhibitor LY294002, the increase in p47phox phosphorylation was reversed (Figure 7B).
The small guanosine triphosphatase (GTPase) Rac-1 is a major regulator for the NADPH oxidase activity in macrophages.22 We thus explored whether the activity of this small GTPase in U937 monocytic cells is altered following LFA-1 ligation. “Pull-down” assays using the fusion protein GST-PAK, a Rac-binding domain of the PAK effector molecule that only binds the active GTP-bound-Rac, were performed to detect changes in the activity of Rac-1 in U937 cells after LFA-1 ligation. An increase in the activity of Rac-1 is consistently observed 60 minutes following the LFA-1 ligation. Similar to the p47phox phosphorylation, the increase of Rac-1 activation was inhibited by LY294002 pretreatment (Figure 7C). These results suggest that the PI3K pathway participates in the LFA-1-induced phosphorylation of p47phox and Rac-1 activation and leads to the activation of NADPH oxidase.
Discussion
To be recruited to inflammatory tissues, leukocytes express different integrins to adhere to a variety of vascular or tissues ligands.5,23 Integrins are heterodimeric proteins expressed on the cell surface. In addition to acting as “cellular glue,” integrins communicate biochemical or mechanical signals across the plasma membrane to influence cellular functions.13,24 Moreover, integrins may adopt active or inactive conformations to determine their adhesiveness in response to cellular signals.25 When more than one species of integrins are expressed on leukocyte cell surface, cross-talk between these integrins may provide the orderly activation of cellular adhesion molecules necessary for different stages of cellular trafficking.16,26,27 This study was undertaken to examine the functional interaction on monocytic cells between LFA-1 and the VLA-4 integrins when LFA-1 is ligated. We used specific anti-LFA-1 mAbs or a recombinant ICAM-1 protein for ligation of LFA-1 and measured the cellular binding to a recombinant VCAM-1 protein and the conformational changes on VLA-4 integrin on monocytic cells. We found that the ligation of monocytic LFA-1 by ICAM-1 or its specific mAbs leads to a significant decrease in binding of α4β1 to VCAM-1. This inhibitory effect was mediated by a PI3K-dependent pathway that leads to the activation of NADPH oxidase and production of ROSs.
Once in contact with endothelium in postcapillary venules, monocytes begin rolling on the luminal surface of endothelium through a mechanism involving selectins as well as integrins.28 Engagement of VLA-4 by its endothelial ligand, VCAM-1, appears to stimulate subsequent leukocyte migration, which requires LFA-1.29 VLA-4 (α4β1) thus appears to be important in the early steps of tethering, rolling, and firm arrest, whereas LFA-1 (αLβ2) is involved in the process of firm arrest and cell flattening of monocytes.1,30 These monocytes then pass through the border of endothelial cells in a process named diapedesis or transendothelial migration. In the transition from firm arrest to diapedesis, monocytes move from the luminal endothelial cell surface to the cell junctions, where the distribution of integrin ligands is different. A novel molecule, JAM-1, was recently found to be a counterreceptor for LFA-1 and involved in transendothelial migration of T cells and neutrophils.9 This immunoglobulin superfamily adhesion molecule expressed on endothelial cells is redistributed from tight junction to luminal plasma membrane after treatment with proinflammatory cytokines, presumably to guide leukocytes through the transmigration.
During the process of leukocyte transendothelial migration, both endothelial cells and leukocytes need to send signals intracellularly to accommodate this complex cellular interaction involving cellular adhesion and detachment between different cell types. Transmigration induces within the endothelial cells a transient increase in intracellular calcium, which can activate myosin light-chain kinase and facilitate leukocyte passage.31,32 On the leukocyte side, the activation of NADPH oxidase induced by the ligation of LFA-1, which should remain ligated with ICAM-1 and JAM-1 during the transmigration process, may be one of the key signals for the leukocytes to be released from the luminal plasma membrane and continue the migration to the endothelial cell junction.
Phagocyte NADPH oxidase, an enzyme complex made up of membrane-bound gp91phox and p22phox, and cytoplasmic p47phox, p67phox, and p40phox, and the small GTPase Rac1/2, is the major source of ROSs in leukocytes.33 Gene mutations leading to defective production of ROSs in NADPH oxidase cause a severe congenital immunodeficiency named chronic granulomatous disease.34,35 This enzyme has been implicated in the inflammatory process of atherosclerosis in both humans and in animal models.36-38 Recently, polymorphism in NADPH oxidase genes was reported to be among the significant predictors for the risk of myocardial infarction in humans.39 The understanding of the mechanisms regulating NADPH oxidase during monocyte trafficking thus is relevant to the inflammatory pathogenesis of atherosclerosis.
It has been shown that activation of Rac-1/2 and phosphorylation of p47phox are important steps in turning on NADPH oxidase by bringing the cytoplasmic components to the membrane-bound components and form a complete enzyme complex.22,40-42 Recently, Sanchez-Martin et al reported that LFA-1 ligation in T cells induces a transient activation of Rac-1 that is regulated by PI3K/Akt-1.43 That finding is contrary to the result of a previous report showing that integrin-mediated cellular adhesion to extracellular matrix (ECM) proteins transiently suppresses the generation of ROSs due to inhibition of Rac-2 activation in human neutrophils.44 Different from neutrophils, monocytes were reported to use Rac-1, but not Rac-2, as a component of the activated NADPH oxidase.45 In this study, we were not able to detect Rac-2 protein activation in our monocytic cells used in the experiments (data not shown). The regulation of NADPH oxidase after integrin ligation thus may be different in monocytes and neutrophils regarding the regulation by Rac activation. Our results that LFA-1 ligation on monocytic cells leads to significant phosphorylation of p47phox and activation of Rac-1 implicate that these changes are responsible for the increased production of ROSs by NADPH oxides. These results are in accordance with previous reports showing that activation of the NADPH oxidase requires a β2 integrin-induced targeting of the Rac as well as phosphorylated p47phox and p67phox to the plasma membrane in neutrophils.46
Integrin ligation on leukocytes initiates multiple signal transduction pathways through molecular interaction between integrin cytoplasmic domains and intracellular signaling proteins.24 Among the integrin-initiated signaling pathways, Akt and Erk have been reported to mediate the activation of NADPH oxidase through protein phosphorylation in neutrophils.20,21 Recent reports showed that Akt, which is activated by PI3K, phosphorylates the serine residues on p47phox and led to the N-formyl-methyonyl-leucylphenylalanine (fMLP)-induced respiratory burst in neutrophils.20 Our results showing that PI3K inhibition suppresses the LFA-1 ligation-induced NADPH oxidase activation while Erk inhibition did not have apparent effects strongly suggest that PI3K-Akt pathway has a major role in the LFA-1 ligation-induced ROS-mediated signaling in monocytic cells.
The role of ROSs as mediators of cellular signaling has been shown in many cell types.47-49 Our previous investigation demonstrated that granulocyte-generated ROSs modulate eosinophil binding and may affect the development of allergic inflammation.18 Production of ROSs by leukocyte NADPH oxidase has been reported to modulate the expression of adhesion molecules including VCAM-1 and ICAM-1.50,51 Moreover, ROSs are implicated in regulating cell adhesion by affecting protein tyrosine phosphorylation involved in integrin signaling.52 ROSs may also directly affect the redox states and hence the molecular conformations of VLA-4 integrin and lead to changes in their adhesion activity.53 Because the ROSs generated by NADPH oxidase may be released either inside or outside the cells, the ROSs may modify their intracellular or extracellular targets to modulate the cellular adhesion. Based on our finding that catalase, which should act extracellularly in our experiments, reverses the LFA-1-ligation induced VCAM-1 binding (Figure 3C), we surmise that intracellular ROSs diffusing through the plasma member to extracellular space may be essential for regulating VCAM-1 binding. Even though both the mitochondria inhibitor, rotenone, and the NADPH oxidase inhibitor, DPI, reduced the levels of production of ROSs in U937 cells, only DPI, which suppresses the LFA-1-induced increase in ROSs, inhibits cellular binding to VCAM-1 (Figure 5). This suggests that both the absolute concentration and the temporal changes in levels of ROSs may be important for the modulation of integrin-mediated cellular binding in U937 cells. The precise mechanisms through which NADPH oxidase-generated ROSs suppress the activity of VLA-4 in monocytic cells requires further investigation.
In conclusion, the dynamic interaction between integrins on monocytic cells through a bidirectional signaling process may be a key event in determining the transition from firm arrest to diapedesis in leukocyte trafficking. Our finding that ligation of LFA-1 on a monocytic cell line leads to deactivation of VLA-4 in an ROS-dependent manner reveals a novel role of phagocyte NADPH oxidase in monocyte diapedesis. With the critical role of monocytic cell trafficking in antigen presentation and pathogenesis in vascular diseases, our current results not only shed light on a crucial signaling pathway for the leukocyte transendothelial migration but also provide potential therapeutic targets that can be modulated by drugs modifying phagocyte NADPH oxidase activity.54
Prepublished online as Blood First Edition Paper, August 12, 2004; DOI 10.1182/blood-2004-05-1822.
Supported by grants from the National Science Council, Taiwan (C.C.S.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Dr L. B. Klickstein, Dr W. J. Chuang, Dr M. J. Tang, Dr Y. J. Wang, and Dr M. S. Chang for helpful discussions and important reagents.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal