Abstract
The CD150 receptor is expressed on activated T and B lymphocytes, dendritic cells, and monocytes. A TxYxxV/I motif in the CD150 cytoplasmic tail can bind different SH2-containing molecules, including tyrosine and inositol phosphatases, Src family kinases, and adaptor molecules. To analyze CD150-initiated signal transduction pathways, we used DT40 B-cell sublines deficient in these molecules. CD150 ligation on DT40 transfectants induced the extracellular signal-regulated kinase (ERK) pathway, which required SH2-containing inositol phosphatase (SHIP) but not SH2 domain protein 1A (SH2D1A). CD150-mediated Akt phosphorylation required Syk and SH2D1A, was negatively regulated by Lyn and Btk, but was SHIP independent. Lyn directly phosphorylated Y327 in CD150, but the Akt pathway did not depend on CD150 tyrosine phosphorylation and CD150-SHP-2 association. Analysis of CD150 and SH2D1A expression in non-Hodgkin and Hodgkin lymphomas revealed stages of B-cell differentiation where these molecules are expressed alone or coexpressed. Signaling studies in Hodgkin disease cell lines showed that CD150 is linked to the ERK and Akt pathways in neoplastic B cells. Our data support the hypothesis that CD150 and SH2D1A are coexpressed during a narrow window of B-cell maturation and SH2D1A may be involved in regulation of B-cell differentiation via switching of CD150-mediated signaling pathways. (Blood. 2004;104:4063-4070)
Introduction
The CD150 (IPO-3, SLAM) cell surface receptor is expressed on activated T and B lymphocytes, dendritic cells, and monocytes. CD150 is a member of the CD2 subfamily of the immunoglobulin (Ig) superfamily of receptors and shares homology with members of this subfamily such as CD2, CD48, CD58, CD229, CD244, BLAME, SF2001, NTB-A/SF2000, CD84, and CS1/CRACC.1-5 CD150 has diverse functions including costimulation of T and B cells, augmentation of T-cell cytotoxicity and CD95-mediated apoptosis, and also regulation of proliferation and differentiation of B cells.1,2,6-8 Moreover, a number of morbilliviruses, including the measles virus, use CD150 as a receptor for cellular entry.9,10
The apparently opposing functions of CD150 are linked to the unique structure of its cytoplasmic tail, which contains a paired immunoreceptor tyrosine-based switch motif (ITSM) TxYxxV/I.3,11 This switch motif is involved directly and/or indirectly in binding different signal transduction molecules, including the protein tyrosine phosphatase SHP-2, the inositol phosphatase SHIP, the Src-family kinases Fyn, Lyn, and Fgr, and the adaptor molecules SH2D1A and EAT-2.7,12-14 Since SH2D1A regulates binding of different sets of SH2-containing molecules to the CD150 cytoplasmic tail,11,13,15-17 it may switch the signal transduction pathways initiated via CD150.3
SH2D1A is encoded by a gene that is altered in most patients with X-linked lymphoproliferative disorder (XLP), as well as in a subset of patients with B-cell non-Hodgkin lymphoma (NHL), common variable immunodeficiency syndrome, and familial hemophagocytic lymphohistiocytosis.12,14,18,19
SH2D1A was found in T lymphocytes,18,20 natural killer (NK) cells,21,22 and in a small subpopulation of tonsillar B cells.11 In contrast to its limited expression in primary human B cells, some Burkitt lymphoma cell lines with germinal center phenotype, Hodgkin disease (HD) cell lines, and a few B lymphoblastoid cell lines express high level of SH2D1A mRNA and protein.11,23,24 Since the malignant cells in NHL and HD represent normal B-cell counterparts arrested at different stages of differentiation,25,26 we hypothesize that SH2D1A may be expressed during a restricted period of B-cell maturation, where it might function to coordinate the intracellular signaling pathways required for cell survival, proliferation, and/or differentiation.
Despite our knowledge about CD150 interaction with molecules that are involved in intracellular signaling, very little is known about the mechanisms that regulate CD150-mediated signal transduction in normal or transformed B lymphocytes. To further address this question we used the DT40 B-cell line model system. Here we showed that in B cells, CD150 is linked to ERK and Akt pathways, and signals elicited by CD150 may differ depending on concurrent SH2D1A expression. To determine whether SH2D1A and CD150 may cooperate during B-cell development, we analyzed CD150 and SH2D1A expression in malignant B cells in situ, including NHL and HD specimens. Taken together, our data suggest that CD150 and SH2D1A are coexpressed at the narrow window of B-cell maturation and that SH2D1A could be involved in regulation of B-cell differentiation via switching of CD150-mediated signaling pathways.
Materials and methods
Antibodies and reagents
Rabbit antisera against SHP-2, actin, and p38 MAPK were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Phospho-Akt (Ser473) and phospho-ERK1/2 (Thr202/Tyr204) antisera were from Cell Signaling Technology (Beverly, MA). A rabbit polyclonal antiserum recognizing SH2D1A (DSHP) was described earlier.18 For cross-linking of B-cell antigen receptors (BCRs) on DT40 cells we used the anti-chicken IgM monoclonal antibody (mAb) M4 (IgM)27 and, for CD150 ligation, the mAb IPO-3 (IgG1).1 Biotinylated antiphosphotyrosine mAb 4G10 was from Upstate Biotechnology (Lake Placid, NY). Glutathione-agarose was purchased from Sigma (St Louis, MO), and protein A- and protein G-sepharose were from Pharmacia (Piscatway, NJ).
Plasmid constructs
Forward and reverse primers with the appropriate restriction sites for in-frame cloning into the expression vectors were used to amplify cDNAs encoding human CD150 (kindly provided by Dr G. Aversa, DNAX) and SH2D1A18 by polymerase chain reaction (PCR) with pfu polymerase (Stratagene, La Jolla, CA). CD150 was cloned into the pApuro expression vector,27 and SH2D1A was cloned into the pcDNA3 vector (Invitrogen, Carlsbad, CA). The GST-fusion protein constructs of the cytoplasmic tail of CD150 (GST-CD150ct) with point mutations in tyrosines were prepared and expressed in the bacterial strains XL2-Blue or XLI-BlueMRF' (Stratagene) for fusion protein production as previously described.7 All constructs were verified by DNA sequence analysis.
Reverse-transcriptase PCR
The cDNA sequences used to design the primers were based on the published SH2D1A,20 mCD150, sCD150, and tCD150 sequences.2 mRNA was isolated from cell lines using TRIzol reagent (GibcoBRL) according to manufacturer's instructions. Two different reactions were performed for each RNA sample. One reaction was performed with antisense primer for mCD150 and sCD150 isoforms 5′-GCTCTCTGGAAGTGTCACACTAGC-3′ (nucleotide positions 1230-1254) and antisense primer for SH2D1A (Table 1). Second reaction was performed with antisense primer for tCD150 isoform (Table 1). Into each reaction tube, antisense primer for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was added. First-strand cDNA was synthesized at 37°C by using reverse transcriptase (MBI Fermentas, Lithuania). Obtained cDNAs were amplified by PCR using Taq DNA Polymerase (MBI Fermentas), and the sense and antisense primers indicated below (Table 1). The PCR products were resolved in agarose gels and visualized as ultraviolet fluorescence after staining with ethidium bromide.
Primers for PCR of GAPDH, mCD150, sCD150, tCD150, SH2D1A
Product . | Sense primer 5′→ 3′ . | Antisense primer 5′→ 3′ . | Product size . |
|---|---|---|---|
| GAPDH | TGAAGGTCGGAGTCAACGGATTTGGT65-90 | CATGTGGGCCATGAGGTCCACCAC1024-1047 | 982 bp |
| mCD150 | GGGGATACGGGAAAGCAGGAAGGAG448-472 | AACAGCCCAGCATACACTGCCCATG843-867 | 419 bp |
| sCD150 | GGGGATACGGGAAAGCAGGAAGGAG448-472 | TTCGTTTTACCTGAGGGGTCTG822-833, 924-933 | 386 bp |
| tCD150 | TTGAGAAGAAGAGGTAAAACG911-931 | GAATAAGTCCGAAGTCTGATGATGAGTGTC998-1027 | 117 bp |
| SH2D1A | GACGCAGTGGCTGTGTAT303-320 | TCATGGGGCTTTCATTTCAGGCAGACATCAGG660-686 | 383 bp |
Product . | Sense primer 5′→ 3′ . | Antisense primer 5′→ 3′ . | Product size . |
|---|---|---|---|
| GAPDH | TGAAGGTCGGAGTCAACGGATTTGGT65-90 | CATGTGGGCCATGAGGTCCACCAC1024-1047 | 982 bp |
| mCD150 | GGGGATACGGGAAAGCAGGAAGGAG448-472 | AACAGCCCAGCATACACTGCCCATG843-867 | 419 bp |
| sCD150 | GGGGATACGGGAAAGCAGGAAGGAG448-472 | TTCGTTTTACCTGAGGGGTCTG822-833, 924-933 | 386 bp |
| tCD150 | TTGAGAAGAAGAGGTAAAACG911-931 | GAATAAGTCCGAAGTCTGATGATGAGTGTC998-1027 | 117 bp |
| SH2D1A | GACGCAGTGGCTGTGTAT303-320 | TCATGGGGCTTTCATTTCAGGCAGACATCAGG660-686 | 383 bp |
Cell lines
The chicken B-cell line DT40 and DT40 sublines deficient in Lyn, Syk, Btk, SHIP, or SHP-2,27,28 kindly provided by Dr Tomohiro Kurosaki, were maintained in RPMI 1640 medium with 10% fetal calf serum (FCS), 1% chicken serum, 50 μM 2-mercaptoethanol, 2 mM l-glutamine, and antibiotics. Transfections of DT40 cells with linearized constructs were carried out using electroporation at 360 V and 960 μF (BioRad Genepulser II, 0.4 cm cuvette). CD150 transfectants were selected in 0.5 μg/mL puromycin, and SH2D1A transfectants in neomycin/G418 (2 mg/mL). Expression of transfected cDNA was confirmed by cell surface staining (CD150) or by reverse transcriptase-polymerase chain reaction (RT-PCR) and Western blot analysis (SH2D1A). HD cell lines of B-cell origin L1236 (kindly provided by Prof Eva Klein, Karolinska Institute, Stockholm, Sweden), KM-H2, and L428 (obtained from the German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany) were maintained in RPMI 1640 medium containing 20% FCS, 50 μM 2-mercaptoethanol, 2 mM l-glutamine, and antibiotics.
Cell staining and stimulation
To measure cell surface expression of CD150, DT40 cell sublines were incubated with CD150 mAb followed by fluorescein isothiocyanate (FITC)-labeled goat F(ab')2 anti-mouse immunoglobulin (Biosource International, Camarillo, CA), and analyzed using a FACScan flow cytometer (Becton Dickinson, San Jose, CA). For cell surface receptor ligation DT40 cells were pelleted, resuspended at 107/mL in fully supplemented RPMI-1640 media, and equilibrated at 37°C for 10 to 15 minutes. Then the cells were stimulated using anti-IgM mAb (final concentration, 5 μg/mL) to cross-link the BCR and/or with anti-CD150 mAb (final concentration, 10 μg/mL) to ligate CD150. Stimulations were stopped at various time points by diluting of cell suspensions into more than 10 volumes of ice-cold phosphate-buffered saline (PBS) with 0.1% NaN3. Cells were pelleted and washed with ice-cold PBS before lysis in 0.5% NP-40 lysis buffer.
Biochemical methods
Cell lysis, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), Western blotting, surface biotinylation, and in vitro kinase assays were performed as described.7 Western blotting was performed with a Renaissance Western blot chemiluminescence reagent (NEN Life Science Products, Boston, MA).
Immunohistochemistry
Immunohistochemical analyses were performed on sections of tonsils obtained by routine tonsillectomy (7 cases) and on lymph node biopsies obtained from patients with non-Hodgkin lymphoma (29 cases) and Hodgkin disease (14 cases). Lymphomas were classified according to the World Health Organization (WHO) classification.29 Verification of the diagnosis of non-Hodgkin lymphoma and Hodgkin disease was performed based on a combination of morphologic, immunophenotypic, and clinical characteristics. To visualize CD150 expression, frozen sections were stained with CD150 mAb IPO-3 (10 μg/mL) followed by the dextran polymeric conjugate (EnVision) technique (DakoCytomation, DK). SH2D1A expression was studied on paraffin-embedded sections using anti-SH2D1A serum followed by standard PAP (peroxidase anti-peroxidase) or EnVision staining. CD3 and CD20 visualizations with specific rabbit antibodies (DakoCytomation, DK) on serial paraffin-embedded sections were used for identification of SH2D1A-positive cells. For double-staining of paraffin sections we used anti-SH2D1A rabbit serum and anti-CD3 (PC3/188A) mouse monoclonal antibody (DakoCytomation) followed by goat anti-rabbit serum conjugated with alkaline phosphatase and goat anti-mouse serum conjugated with peroxidase (Santa Cruz Biotechnology). Immunohistochemical reactions on peroxidase were developed with diaminobenzidine tetrahydrochloride (DAB) substrate (DAKO) and on alkaline phosphatase, with Fast Red substrate (Sigma, Germany). Sections were examined on an Opton Axiophot (Oberkochen, Germany) microscope using Opton Plan-Neofluor oil objective lenses (10 × /0.30, 40 × /0.75, and 100 × /1.30). Optical images were obtained with standard conditions of illumination and exposure on the Opton Axiophot 33 mm camera.
Results
Regulation of CD150-mediated signal transduction pathways
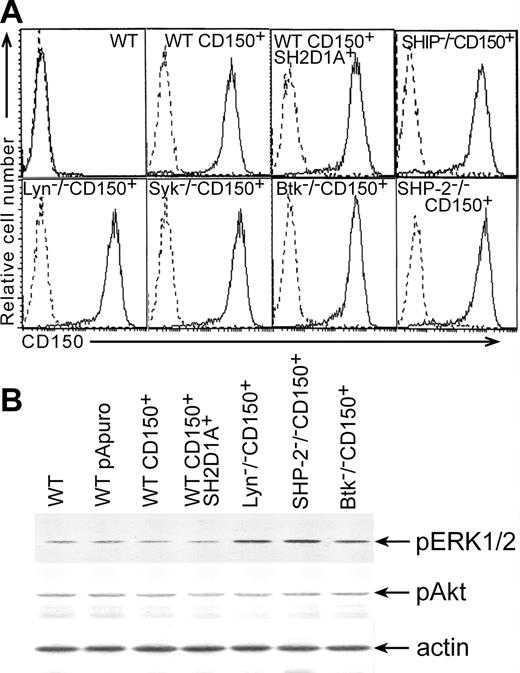
To elucidate the signal transduction pathways linked to CD150, we developed a model system using the chicken B-cell line DT40. Chicken homologs of human SH2D1A and CD150 were not found in different chicken nucleotide databases including Bursal Est database http://swallow.gsf.de/DT40/dt40Est.html. Antibodies that recognize human CD150 and SH2D1A did not show any cross-reactivity with DT40 sublines (Figure 1A; Figure 2A, lower panel). Moreover, semiquantative RT-PCR with primers to human SH2D1A did not reveal any specific product (data not shown). Therefore, DT40 sublines were transfected with plasmids encoding full-length CD150 (CD150+) or CD150 plus SH2D1A (CD150+SH2D1A+). Stable transfectants had comparable levels of CD150 expression and SH2D1A coexpression did not alter the expression of CD150 (Figure 1A).
Levels of CD150, pERK1/2, and pAkt expressed in DT40 transfectants. (A) Cell surface expression of CD150 on DT40 transfectants. DT40 cells were transfected with CD150 alone or together with SH2D1A. Stable transfectants were stained with the anti-CD150 mAb IPO-3 or with isotype-matched mAb (dotted histograms) and evaluated by flow cytometry analysis. (B) Basal levels of ERK1/2 and Akt phosphorylation in DT40 transfectants. Transfection of CD150 with or without SH2D1A into DT40 cell line did not induce changes in the basal levels of ERK1/2 and Akt phosphorylation compared to WT DT40 cells and WT DT40 cells transfected with empty vector. Western blot analyses of cell lysates. Western blot with anti-actin serum served as a control for equal protein loading (bottom panel).
Levels of CD150, pERK1/2, and pAkt expressed in DT40 transfectants. (A) Cell surface expression of CD150 on DT40 transfectants. DT40 cells were transfected with CD150 alone or together with SH2D1A. Stable transfectants were stained with the anti-CD150 mAb IPO-3 or with isotype-matched mAb (dotted histograms) and evaluated by flow cytometry analysis. (B) Basal levels of ERK1/2 and Akt phosphorylation in DT40 transfectants. Transfection of CD150 with or without SH2D1A into DT40 cell line did not induce changes in the basal levels of ERK1/2 and Akt phosphorylation compared to WT DT40 cells and WT DT40 cells transfected with empty vector. Western blot analyses of cell lysates. Western blot with anti-actin serum served as a control for equal protein loading (bottom panel).
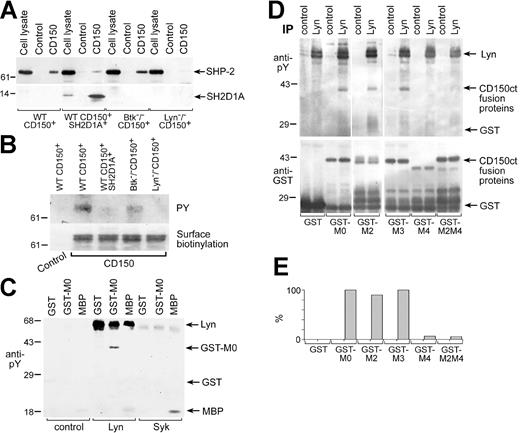
Lyn is involved in CD150 tyrosine phosphorylation. CD150-SHP-2 association in DT40 transfectants depends on Lyn. Anti-SHP-2 (A, top panel), anti-SH2D1A (lower panel), and antiphosphotyrosine (B, top panel) Western blots on CD150 immunoprecipitated with anti-CD150 mAb. The binding of SH2D1A to CD150 reduced the association of CD150 with SHP-2. The CD150-SHP-2 association was BTK-independent, completely depended on Lyn expression and CD150 tyrosine phosphorylation. Amount of CD150 in immunoprecipitates was controlled by surface biotinylation of CD150 (B, bottom panel). One of 5 experiments. (C) Lyn directly phosphorylates CD150. Lyn and Syk tyrosine kinases were immunoprecipitated from cell line MP-1, and cold in vitro kinase assays followed by antiphosphotyrosine Western blot were performed. GST fusion protein of CD150ct (GST-CD150ct) and MBP served as a substrate in in vitro kinase assays. One of 3 experiments. (D) Lyn predominately phosphorylates Y327 in CD150ct (MU). Cold in vitro kinase assays on Lyn immunoprecipitates were performed using the following substrates: GST and GST-CD150ct with point mutations Y281F (M2), Y307F (M3), Y327 (M4), and Y281/327F (M2M4). Antiphosphotyrosine Western blot (top panel) and anti-GST Western blot (bottom panel). Concentrations of GST-proteins based on anti-GST blot were taken into account for densitometry analysis of anti-phosphotyrosine Western blot (E). One of 3 experiments.
Lyn is involved in CD150 tyrosine phosphorylation. CD150-SHP-2 association in DT40 transfectants depends on Lyn. Anti-SHP-2 (A, top panel), anti-SH2D1A (lower panel), and antiphosphotyrosine (B, top panel) Western blots on CD150 immunoprecipitated with anti-CD150 mAb. The binding of SH2D1A to CD150 reduced the association of CD150 with SHP-2. The CD150-SHP-2 association was BTK-independent, completely depended on Lyn expression and CD150 tyrosine phosphorylation. Amount of CD150 in immunoprecipitates was controlled by surface biotinylation of CD150 (B, bottom panel). One of 5 experiments. (C) Lyn directly phosphorylates CD150. Lyn and Syk tyrosine kinases were immunoprecipitated from cell line MP-1, and cold in vitro kinase assays followed by antiphosphotyrosine Western blot were performed. GST fusion protein of CD150ct (GST-CD150ct) and MBP served as a substrate in in vitro kinase assays. One of 3 experiments. (D) Lyn predominately phosphorylates Y327 in CD150ct (MU). Cold in vitro kinase assays on Lyn immunoprecipitates were performed using the following substrates: GST and GST-CD150ct with point mutations Y281F (M2), Y307F (M3), Y327 (M4), and Y281/327F (M2M4). Antiphosphotyrosine Western blot (top panel) and anti-GST Western blot (bottom panel). Concentrations of GST-proteins based on anti-GST blot were taken into account for densitometry analysis of anti-phosphotyrosine Western blot (E). One of 3 experiments.
CD150 may be involved in homotypic adhesion and may act as a self ligand with low affinity for itself.30 A number of morbilliviruses, including the measles virus, use CD150 as a receptor for interfering with intracellular signaling and for infection of cells.9,31 Previously, we found that CD150 ligation with mAb on human B-cell lines and DT40 transfectants resulted in Akt and ERK1/2 phosphorylation.3,7 After transfection of DT40 cells with CD150, the cells formed large clusters in vitro, consistent with a possible CD150-mediated interactions between the transfected cells. In a mouse T-cell line, cotransfection of CD150 and SH2D1A resulted in tyrosine phosphorylation of some proteins, possibly due to self engagement of CD150.13 To determine whether CD150-mediated homotypic adhesion could transmit the signals that activate ERK1/2 and Akt pathways in DT40 transfectants, we examined basal levels of ERK1/2 and Akt phosphorylation. Both CD150+ and CD150+SH2D1A+ DT40 transfectants demonstrated the same basal levels of ERK1/2 and Akt phosphorylation as DT40 WT and DT40 WT transfected with empty pApuro and pApuro/pcDNA3 vectors (Figure 1B). Thus, we found no evidence that simply transfecting CD150 into DT40 cells altered these signaling pathways.
To find out how the CD150 receptor is linked to ERK and Akt activation, we analyzed DT40 cells and sublines deficient in Lyn, Syk, Btk, SHIP, or SHP-2 transfected with CD150 alone or together with the adaptor protein SH2D1A. Ligation of CD150 on WT CD150+ transfectants resulted in weak ERK1/2 activation, as measured by phosphorylation of Thr202/Tyr204 (Figure 3A). We examined ERK phosphorylation up to 3 hours after stimulation and found kinase activation only within first 30 minutes. At the same time we did not detect any significant changes in ERK phosphorylation in unstimulated cells or cells incubated with isotype-specific (IgG1) MOPC 21 myeloma protein (data not shown). CD150-initiated ERK1 phosphorylation was not altered significantly by the presence of SH2D1A (Figure 3A).
CD150-initiated ERK1/2 phosphorylation was not affected by SH2D1A cotransfection but was dependent on SHIP. (A) Lyn-/- CD150+, Btk-/- CD150+, and SHP-2-/- CD150+ cells had elevated basal levels of ERK phosphorylation, and ERK phosphorylation did not exceed control levels after CD150 ligation. (B) WT DT40 cells and DT40 cells transfected with empty pApuro or pApuro/pcDNA3 vectors served as negative controls. Each sample contained lysate from 106 cells. Equal loading was monitored in all experiments by anti-p38 MAPK kinase or anti-actin Western blot (control panels). One of 5 experiments.
CD150-initiated ERK1/2 phosphorylation was not affected by SH2D1A cotransfection but was dependent on SHIP. (A) Lyn-/- CD150+, Btk-/- CD150+, and SHP-2-/- CD150+ cells had elevated basal levels of ERK phosphorylation, and ERK phosphorylation did not exceed control levels after CD150 ligation. (B) WT DT40 cells and DT40 cells transfected with empty pApuro or pApuro/pcDNA3 vectors served as negative controls. Each sample contained lysate from 106 cells. Equal loading was monitored in all experiments by anti-p38 MAPK kinase or anti-actin Western blot (control panels). One of 5 experiments.
To dissect the molecular requirements for CD150 signaling, we next examined CD150+ DT40 cell sublines that lacked key signaling molecules such as Lyn, Syk, Btk, SHIP, or SHP-2. The Lyn-/- CD150+, Btk-/- CD150+, and SHP-2-/- CD150+ cells had elevated background levels of ERK phosphorylation, and after CD150 ligation ERK phosphorylation did not exceed control levels (Figure 3B). Neither BCR nor CD150 cross-linking on Syk-/- CD150+ cells resulted in ERK phosphorylation (Figure 3A). Unlike BCR-induced ERK activation, CD150-mediated ERK phosphorylation was SHIP dependent (Figure 3A). We did not find any difference in the mode of ERK phosphorylation in SHIP-/- cell sublines that expressed CD150 or coexpress CD150 and SH2D1A. Thus, CD150-induced ERK phosphorylation requires SHIP and Syk but not SH2D1A. Lyn, Btk, and SHP-2 appear to be involved in the regulation of constitutive levels of ERK1/2-phosphorylation.
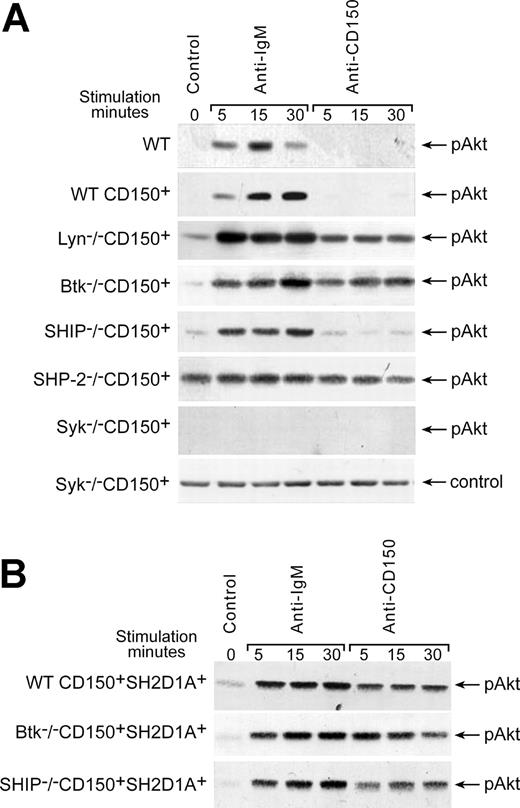
In contrast to BCR ligation, cross-linking of CD150 on DT40 WT CD150+ transfectants did not induce Akt phosphorylation on S473 (Figure 4A). However, in the absence of either Lyn or Btk, CD150 ligation could induce Akt phosphorylation (Figure 4A), suggesting that Lyn and Btk are both involved in the negative regulation of CD150-mediated Akt-phosphorylation. As expected, both BCR- and CD150-induced Akt phosphorylation was completely abrogated in Syk-deficient cells. However, SHIP deficiency did not affect the level of either BCR- or CD150-mediated Akt-phosphorylation (Figure 4A). Several studies suggest that SHP-2 is an important negative regulator of the PI3-kinase pathway.32,33 A constitutive high level of Akt phosphorylation in the SHP-2-/- CD150+ cells (Figure 4A) supports this hypothesis.
Regulation of CD150-mediated Akt activation. (A) Cross-linking of CD150 on WT CD150+ DT40 transfectants did not induce Akt phosphorylation on S473. Lyn and Btk were involved in negative regulation of CD150-mediated Akt pathway. SHP-2-/- DT40 transfectant showed constitutive high level of pAkt phosphorylation, but neither BCR nor CD150 ligation on Syk-deficient cells was able to trigger the Akt pathway. (B) Cotransfection of CD150 and SH2D1A resulted in rapid CD150-mediated activation of Akt that was SHIP-independent. Wild-type DT40 transfected with empty pApuro or pApuro/pcDNA3 vectors served as negative controls. Western blot analysis on cell lysates. Each sample contained lysate from 106 cells. Equal loading was monitored by anti-p38 MAPK kinase or anti-actin Western blot in all experiments (control panels). One of 5 experiments.
Regulation of CD150-mediated Akt activation. (A) Cross-linking of CD150 on WT CD150+ DT40 transfectants did not induce Akt phosphorylation on S473. Lyn and Btk were involved in negative regulation of CD150-mediated Akt pathway. SHP-2-/- DT40 transfectant showed constitutive high level of pAkt phosphorylation, but neither BCR nor CD150 ligation on Syk-deficient cells was able to trigger the Akt pathway. (B) Cotransfection of CD150 and SH2D1A resulted in rapid CD150-mediated activation of Akt that was SHIP-independent. Wild-type DT40 transfected with empty pApuro or pApuro/pcDNA3 vectors served as negative controls. Western blot analysis on cell lysates. Each sample contained lysate from 106 cells. Equal loading was monitored by anti-p38 MAPK kinase or anti-actin Western blot in all experiments (control panels). One of 5 experiments.
Further studies revealed that SH2D1A is involved in the positive regulation of CD150-mediated Akt activation. DT40 cells expressing both CD150 and SH2D1A (WT CD150+SH2D1A+) showed rapid CD150-mediated phosphorylation of Akt (Figure 4B). Coligation of the BCR with CD150 did not show synergistic effect of these receptors on Akt phosphorylation, which remained on the level of BCR ligation alone (data not shown). Moreover, neither Btk nor SHIP deficiencies affected CD150-mediated Akt phosphorylation in SH2D1A transfectants (Figure 4B). The detailed kinetics studies showed that CD150 initiated Akt phosphorylation reached the maximum at 5 minutes, stayed practically on the same level up to 30 minutes, and in 45 minutes dropped to the initial level. SH2D1A cotransfection in Lyn-, Btk-, and SHIP-deficient cell sublines did not affect this kinetics (data not shown).
It has been hypothesized that one function of SH2D1A is to regulate the binding of SHP-2 to ITSM motif within the cytoplasmic tail of CD150.11,12,34 Thus, we tested whether cotransfection of SH2D1A affects SHP-2 binding to CD150. Consistent with the proposed model, the high level of SHP-2 binding to CD150 in WT CD150+ cells was greatly diminished by concomitant SH2D1A expression (Figure 2A). Lyn- and Btk-deficient sublines, like SH2D1A-expressing cells, showed CD150-mediated phosphorylation of Akt (Figure 4B). Therefore, we tested whether these sublines also had reduced levels of SHP-2 associated with CD150. In Lyn-/- CD150+ cells, SHP-2 did not coprecipitate with CD150 (Figure 2A). However, in Btk-/- CD150+ cells (Figure 2A) and also in Syk-/- CD150+ cells (data not shown) the level of SHP-2 binding to CD150 was comparable to that in wild-type DT40 cells. The level of SHP-2 in CD150 immunoprecipitates correlated with CD150 tyrosine phosphorylation (Figure 2B, upper panel). Next we addressed the question as to which tyrosine kinase most likely phosphorylates CD150 in B cells. Lyn is the only one Src family kinase expressed in DT40 cells.35 To check if Lyn can directly phosphorylate CD150 in B cells, we used a “cold” in vitro kinase assay followed by antiphosphotyrosine Western blotting (Figure 2C). A nonphosphorylated form of GST fusion protein of the CD150 cytoplasmic tail (GST-MO) and MBP served as substrates for the tyrosine kinases Lyn, Syk, and Btk. We used the B-cell line MP-1 as a source of constitutively active kinases. Only Lyn specifically phosphorylated GST-CD150ct (Figure 2C). Syk (Figure 2C) and Btk (data not shown) were not able to use CD150ct as a substrate but could phosphorylate MBP. To determine which tyrosines within CD150ct are preferably phosphorylated by Lyn, we performed in vitro kinase assays of Lyn kinase with GST-MO or GST-CD150ct mutants containing substitutions of tyrosines (Y) with the nonphosphorylatable amino acids phenylalanine (F) at positions 281(GST-M2), 307 (GST-M3), 327 (GST-M4), and 281/327 (GST-M2M4) (Figure 2D). These assays revealed that Lyn mainly phosphorylates Y327 in the CD150ct. Taken together, these data suggest that Lyn is responsible for CD150 tyrosine phosphorylation in DT40 B cells and this phosphorylation is required for SHP-2 recruitment.
CD150 and SH2D1A expression in lymphomas
B-cell malignancies are considered to arise from normal lymphocytes at different stages of B-cell differentiation, and often these malignant cells retain the genetic program of their normal B-cell counterparts.25,36 To find the stages of B-cell differentiation where SH2D1A could be expressed in B cells and also coexpressed with CD150, we studied lymphomas of B-cell origin in parallel with tonsils and reactive lymph nodes. Immunohistochemistry approach revealed CD150 and SH2D1A expression directly in the tumor cells.
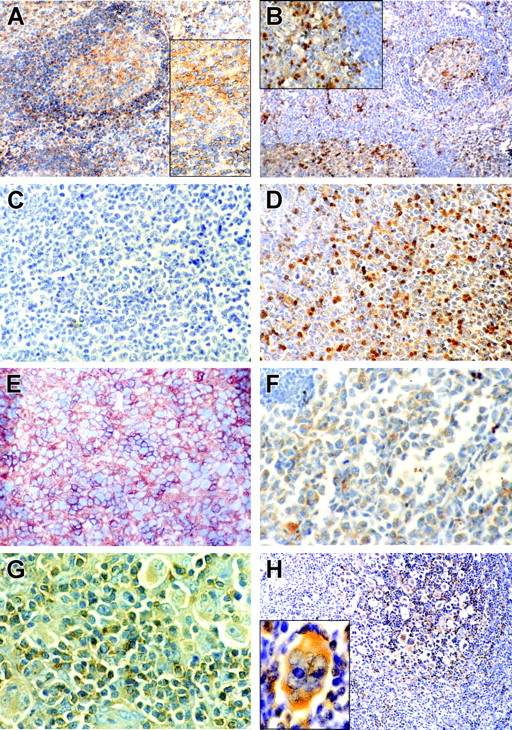
In reactive lymph nodes and tonsils CD150 was localized in the cytoplasm and on the surface of germinal center (GC) cells and also on the surface of the mantle zone B cells (Figure 5A). In GCs the number of SH2D1A+cells usually exceeded the number of CD150+ cells, while only few scattered SH2D1A+ cells were found in the mantle zone (Figure 5B). On the other hand, the interfollicular zone contained both CD150+ and SH2D1A+ cells (Figure 5A-B).
CD150 and SH2D1A expression in reactive tonsils and primary lymphoma cells. Immunohistochemical staining of CD150 (left panels: A, C, E, G) and SH2D1A (right panels: B, D, F, H,) on reactive tonsils (A, B), lymph node sections from patients with GC-DLBCL (C, D), ABC-DLBCL (E, F), and HD (G, H). CD150 and SH2D1A were coexpressed in malignant cells in ABC-DLBCL and HD. Original magnifications: 100 × (A, B, H), 400 × (C-F, and insets in A and B), 1000 × (G and H inset). H inset was magnified with Photoshop software (Adobe Systems, San Jose, CA). DAB staining shows specific reactions in brown.
CD150 and SH2D1A expression in reactive tonsils and primary lymphoma cells. Immunohistochemical staining of CD150 (left panels: A, C, E, G) and SH2D1A (right panels: B, D, F, H,) on reactive tonsils (A, B), lymph node sections from patients with GC-DLBCL (C, D), ABC-DLBCL (E, F), and HD (G, H). CD150 and SH2D1A were coexpressed in malignant cells in ABC-DLBCL and HD. Original magnifications: 100 × (A, B, H), 400 × (C-F, and insets in A and B), 1000 × (G and H inset). H inset was magnified with Photoshop software (Adobe Systems, San Jose, CA). DAB staining shows specific reactions in brown.
Malignant cells in small lymphocytic lymphoma of B-cell origin (7 cases: IgM+(weak), CD5+, CD10-, CD19+, CD20+, CD22+, CD23+) and sporadic Burkitt lymphoma (3 cases: IgM+, CD5-, CD10-, CD19+, CD20+, CD22+) did not express neither CD150 nor SH2D1A. Mantle cell lymphoma (2 cases: IgM+, CD5+, CD10-, CD19+, CD20+, CD22+) also was SH2D1A negative, but neoplastic cells expressed low levels of CD150 (data not shown).
Gene expression profiling has revealed 2 distinct DLBCL subtypes: germinal center (GC-DLBCL) and activated B cell (ABC-DLBCL).37,38 Patients with GC-DLBCL have better survival than those with ABC-DLBCL.37,38 All studied 12 cases of DLBCL were SH2D1A positive (Figure 5D, F). These include 9 cases of GC-DLBCL (IgM+/-, CD5+/-, CD10+/-, CD19+, CD20+, CD22+) and 3 cases with ABC-DLBCL (IgM+, CD5-, CD10-, CD19-, CD20-, CD22+/-, CD30+/-, CD38-/+) phenotype. However, only ABC-DLBCL and not GC-DLBCL expressed CD150 (Figure 5C, E). Coexpression of CD150 with SH2D1A was also found in Hodgkin and Reed-Sternberg cells (HRS) in both classical and lymphocyte predominant types of HD (14 cases) (Figure 5G, H). Malignant cells in most differentiated lymphoplasmacytic lymphoma (2 cases: cyIgM+, CD5-, CD10-, CD19+, CD20+, CD22+) did not express either CD150 or SH2D1A (data not shown).
To confirm SH2D1A expression in malignant cells of B-cell origin, we performed CD20, CD3, and SH2D1A detection on serial paraffin-embedded tissue sections of DLBCL (Figure 6). Most cells on DLBCL sections were CD20+ (Figure 6A, E), and only scattered CD3+ T cells were detected (Figure 6B, F). SH2D1A was expressed in cytoplasm of large neoplastic B cells (Figure 6C, G), however, CD20+ small B lymphocytes were SH2D1A-negative (Figure 6G, lower left corner). Moreover, double staining of CD3 and SH2D1A also confirmed presence of SH2D1A in majority of cells that were CD3- (Figure 6D, H). Taken together, our immunohistochemical data showed that malignant B cells can express CD150 and/or SH2D1A. SH2D1A is expressed in DLBCL and furthermore it is coexpressed with CD150 in ABC-DLBCL and HRS cells. Coexpression of CD150 and SH2D1A in ABC-DLBCL and HRS cells once more confirms a similar molecular expression profile of these 2 malignancies that was shown by large-scale gene expression profiling.39
CD20, CD3, and SH2D1A expression on serial sections of DLBCL. Immunohistochemical staining of CD20 (A, E), CD3 (B, F), and SH2D1A (C, G) on paraffin-embedded sections. DAB staining shows specific reactions in brown. (D, H) Double-staining of CD3 (DAB substrate in brown) and SH2D1A (Fast Red substrate in red). GC-DLBCL (A-D) and ABC-DLBCL (E-H). Original magnifications: 400 × (A-G), 1000 × (H).
CD20, CD3, and SH2D1A expression on serial sections of DLBCL. Immunohistochemical staining of CD20 (A, E), CD3 (B, F), and SH2D1A (C, G) on paraffin-embedded sections. DAB staining shows specific reactions in brown. (D, H) Double-staining of CD3 (DAB substrate in brown) and SH2D1A (Fast Red substrate in red). GC-DLBCL (A-D) and ABC-DLBCL (E-H). Original magnifications: 400 × (A-G), 1000 × (H).
CD150 is linked to ERK1/2 and Akt pathways in HD cell lines
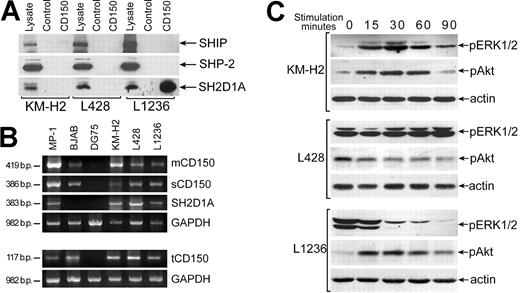
Since CD150 and SH2D1A are coexpressed in malignant HRS in Hodgkin disease (Figure 5G, H), and in HD cell lines,24 we used HD B-cell lines to further define signal transduction pathways initiated via CD150 in the presence of SH2D1A. Our immunofluorescence and flow cytometry studies of HD cell lines confirmed the data of Kis et al24 that showed the comparable levels of CD150 in these cell lines (data not shown). Coprecipitation experiments revealed association CD150-SH2D1A only in the L1236 cell line and not in KM-H2 and L428 lines (Figure 7A). However, SH2D1A was detected in all 3 HD cell lines based on both WB of cell lysates and RT-PCR analysis (Figure 7A, B). The lack of CD150-SH2D1A association was not due to the absence of a full transmembrane form of CD150, since RT-PCR analysis showed that all 3 CD150 isoforms, including mCD150, sCD150, and tCD150, are expressed in these lines (Figure 7B). Coprecipitation experiments showed that neither SHIP nor SHP-2 were associated with CD150 in these HD cell lines (Figure 7A), even though they were previously detected with CD150 in human T and B cell.11-13
CD150 is linked to the ERK1/2 and Akt pathways in HD cell lines. (A) SH2D1A coprecipitated with CD150 in the L1236 cell line but did not associate with CD150 in the KM-H2 and L428 HD cell lines (bottom panel). Anti-SH2D1A Western blot on CD150 immunoprecipitates. SHIP and SHP-2 did not associate with CD150 in HD cell lines (top and middle panels). Western blot with anti-SHP-2 and anti-SHIP. One of 3 experiments. (B) All CD150 splice forms (mCD150, sCD150, and tCD150) and SH2D1A were expressed in HD cell lines. RT-PCR analysis. MP-1 lymphoblastoid cell line, BJAB, and DG75 Burkitt lymphoma cell lines served as positive and negative controls. (C) Cross-linking of CD150 on HD cell lines affects the level of ERK1/2 and Akt phosphorylation. Anti-pERK1/2 and anti-pAkt Western blot analyses on cell lysates. Western blot with antiactin controlled for equal loading (bottom panels).
CD150 is linked to the ERK1/2 and Akt pathways in HD cell lines. (A) SH2D1A coprecipitated with CD150 in the L1236 cell line but did not associate with CD150 in the KM-H2 and L428 HD cell lines (bottom panel). Anti-SH2D1A Western blot on CD150 immunoprecipitates. SHIP and SHP-2 did not associate with CD150 in HD cell lines (top and middle panels). Western blot with anti-SHP-2 and anti-SHIP. One of 3 experiments. (B) All CD150 splice forms (mCD150, sCD150, and tCD150) and SH2D1A were expressed in HD cell lines. RT-PCR analysis. MP-1 lymphoblastoid cell line, BJAB, and DG75 Burkitt lymphoma cell lines served as positive and negative controls. (C) Cross-linking of CD150 on HD cell lines affects the level of ERK1/2 and Akt phosphorylation. Anti-pERK1/2 and anti-pAkt Western blot analyses on cell lysates. Western blot with antiactin controlled for equal loading (bottom panels).
In KM-H2 and L1236 cell lines CD150 ligation activates the Akt pathway (Figure 7C), inducing a rapid and transient Akt phosphorylation. In contrast, in L428 cells, where the constitutive level of phosphorylated Akt was high, CD150 cross-linking resulted in dephosphorylation of Akt (Figure 7C). In KM-H2 cells signaling via CD150 also activated ERK1/2 (Figure 7C, upper panel). L1236 cell line showed a high basal level of ERK1/2 phosphorylation and CD150 ligation resulted in rapid ERK1/2 dephosphorylation (Figure 7C, lower panel). At the same time CD150 ligation on L428 cell line did not affect the basal level of ERK1/2 phosphorylation (Figure 7C, middle panel). These results demonstrate that in malignant B cells expressing SH2D1A, CD150 is linked to both the ERK1/2 and Akt pathways. Studied HD cell lines originate from patients with classical type of HD: KM-H2 and L1236—from mixed cellularity subtype and L428—from nodular sclerosis subtype. Generally, nodular sclerosis HD has better prognosis than mixed cellularity variant.40 Interestingly that CD150 ligation on L428 cells (nodular sclerosis) down-regulated Akt phosphorylation and did not affect ERK pathway. Possibly CD150 signaling may contribute to apoptosis resistance and survival of HRS cells and therefore play an important role in HD pathogenesis.
Discussion
The TxYxxV/I motif in the CD150 cytoplasmic tail can bind different SH2-containing molecules, including tyrosine and inositol phosphatases, Src-family kinases, and adaptor molecules. However, little is known about CD150-mediated signal transduction pathways and their regulation. Using the DT40 cell line model system, we found that CD150-induced activation of the ERK signaling pathway is not dependent on expression of SH2D1A but does require both Syk and SHIP. Our results are consistent with the finding that the MEK1/2 inhibitor U0126 abolished CD150-induced cytotoxicity of T cells.8 Moreover, the recently cloned member of CD2 subfamily, the CRACC receptor (CS1), mediates NK cell activation via an SH2D1A-independent ERK-mediated pathway.41
CD150 also can trigger activation of the Akt pathway.7 However, unlike ERK activation, Syk and SH2D1A are required for CD150-initiated Akt phosphorylation, but SHIP is not (Figure 4). We also found that CD150 ligation induced Akt phosphorylation in Lyn-/- or Btk-/- DT40 cells, suggesting negative regulation of CD150 signaling by Lyn and Btk. Lyn has been previously shown to negatively regulate BCR-mediated Akt phosphorylation in both DT40 B cells and mouse B cells.42 Since in Lyn-/- cell line CD150 was not phosphorylated and did not coprecipitate with SHP-2 (Figure 2), CD150-mediated Akt phosphorylation did not depend on either CD150 tyrosine phosphorylation or SHP-2 association with CD150.
CD150-initiated Akt phosphorylation was also found in SH2D1A+ HD cell lines KM-H2 and L1236 (Figure 7C), however this activation did not depend on CD150 association with SH2D1A (Figure 7A). Moreover, in a cell line L428 with a high basal level of Akt phosphorylation, CD150 ligation resulted in dephosphorylation of Akt (Figure 7C). Possibly, difference in CD150 signaling in these cell lines reflects their origin and CD150 contribution in HD pathogenesis. Thus, nodular sclerosis HD subtype (L428) has better prognosis than mixed cellularity subtype (KM-H2 and L1236).40 HD is characterized by loss of B-lineage specific gene expression program owing to global transcriptional deregulation. Nevertheless, HRS cells proliferate and even escape the apoptotic death.43-45 One testable possibility is that CD150-mediated signaling may be involved in the regulation of HRS cell survival program.
Our studies of CD150 and SH2D1A expression in B-cell lymphoma cells revealed stages of B-cell differentiation where these molecules are expressed. SH2D1A was found in GC-DLBCL, and more mature cells in ABC-DLBCL coexpressed SH2D1A together with CD150. Based on these findings, we hypothesize that SH2D1A is expressed at an earlier stage of B-cell differentiation than CD150, and CD150 is up-regulated later, especially after cell activation. This is also in line with CD150 and SH2D1A coexpression in HRS cells that have activated phenotype and derive from pre-apoptotic crippled germinal center B cells. SH2D1A was found in some Burkitt lymphoma cell lines with GC phenotype and was down-regulated in BL cell lines with activated (immunoblastic) phenotype that express CD150.23 We did not detect SH2D1A or CD150 in Burkitt lymphoma in situ. Since expression of these molecules in Burkitt lymphoma could depend on presence of EBV, this question needs further investigation.
In summary, a number of studies using human B lymphoblastoid cell lines,1,7,11 Burkitt lymphoma and HD cell lines,23,24 tonsillar B cells,11 or B-cell lymphomas (Figures 5, 6, 7) have demonstrated that CD150 and SH2D1A can be coexpressed or expressed individually in both normal and malignant B cells. In view of the fact that SH2D1A regulates CD150 association with different SH2-containing molecules and CD150-mediated signal transduction pathways, differential expression of SH2D1A and CD150 during B-cell maturation could contribute to B-cell developmental program and pathogenesis of human lymphomas of B-cell origin.
Prepublished online as Blood First Edition Paper, August 17, 2004; DOI 10.1182/blood-2004-04-1273.
Supported by Howard Hughes Medical Institute grant 76195-548101, International Association (INTAS) grant 011-2883 (S.P.S.), US Civilian Research and Development foundation grants UB2-531 and UB2-2443-KV-02 (S.P.S.), and National Institutes of Health grant AI44250 (E.A.C).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to thank Professor Eva Klein for providing HD cell line L1236 and Dr Tomohiro Kurosaki for DT40 cell sublines.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal