Abstract
We report the outcomes after reduced-intensity conditioning allogeneic stem cell transplantation (RIT) for non-Hodgkin lymphoma (NHL) in 88 patients (low-grade NHL [LG-NHL], n = 41; high-grade NHL [HG-NHL], n = 37; mantle cell lymphoma [MCL], n = 10). Thirty-seven patients had previously received autografts, and 21 were in complete remission (CR) at transplantation. Conditioning therapy consisted of alemtuzumab, fludarabine, and melphalan. Sixty-five patients received peripheral blood stem cells (PBSCs) from HLA-identical siblings, and 23 received bone marrow (BM) from matched unrelated donors. Prophylaxis for graft-versus-host disease (GVHD) consisted of cyclosporin A. Grade III-IV acute GVHD developed in 4 patients, and chronic GVHD developed in 6 patients. With a median follow-up of 36 months (range, 18-60 months), the actuarial overall survival (OS) rates at 3 years were 34% for HG-NHL, 60% for MCL, and 73% for LG-NHL (P < .001). The 100-day and 3-year transplant-related mortality (TRM) rates for patients with LG-NHL were 2% and 11%, respectively, and were better (P = .01) than they were for patients with HG-NHL (27% and 38%, respectively). The actuarial current progression-free survival (PFS) rate at 3 years, including the rate for patients who achieved remission after donor lymphocyte infusion (DLI) for progression, was 65% for LG-NHL, 50% for MCL, and 34% for HG-NHL (P = .002). Twenty-one patients underwent DLI for matched related donor (MD)-persistent disease or relapse, and 15 underwent DLI for mixed hematopoietic chimerism. Patients who experienced relapses of LG-NHL and chronic lymphocytic leukemia (CLL) achieved excellent PFS with extremely low TRM and GVHD, even when matched related donors were unavailable. (Blood. 2004;104:3865-3871)
Introduction
Treatment options for patients with non-Hodgkin lymphoma (NHL) are many and varied. For those with relapsed or refractory disease, outcomes after conventional and high-dose chemotherapy remain poor.1-3 Conventional allogeneic stem cell transplantation has been shown to have curative potential in the management of indolent and aggressive NHL, but because of the high transplant-related mortality (TRM) rate, improvement in overall survival (OS) has been insignificant.4-6
Recent reports have confirmed the feasibility of reduced-intensity conditioning allogeneic stem cell transplantation (RIT) as a treatment option for many older patients with NHL.7-11 However, there are few published data on longer-term follow-up and outcomes after RIT and on subsequent donor lymphocyte infusion (DLI) for relapsed and refractory NHL.
Durable engraftment of allogeneic cells and persistence of the graft-versus-lymphoma (GVL) effect is particularly important when considering the value of RIT in the management of indolent NHL with a long natural history.12 The use of alemtuzumab (anti-CD52 humanized monoclonal antibody) to deplete recipient and incoming donor T cells as part of RIT has provided for durable engraftment in sibling and unrelated donor transplantation while significantly reducing the risk for acute and chronic graft-versus-host disease (GVHD).13-15 As with myeloablative T cell-depleted allografts, immune reconstitution is delayed after the administration of alemtuzumab,16 potentially impairing the GVL effect early after transplantation. Long-term progression-free survival (PFS) may be achieved with the use of adjuvant DLI from 6 months after transplantation for patients with persistent recipient hematopoiesis, persistent disease, or relapsed disease.17
Here we report the RIT outcomes for 88 patients with NHL treated with the same conditioning regimen at 10 transplantation centers throughout the United Kingdom after a median follow-up of 36 months (range, 18-60 months).
Patients, materials, and methods
Eligibility criteria
Patients with non-Hodgkin lymphoma (NHL) from 10 centers within the United Kingdom were studied. Ethics committee approval was obtained at each participating center, and patients gave written informed consent before participation. Patients with NHL were eligible if they were 18 to 75 years of age and had an HLA-identical sibling donor or an HLA-compatible unrelated donor as determined by serologic typing for HLA A/B and molecular typing for HLA C/DR/DQ. Unrelated donor selection was performed according to published criteria.18 Unrelated donors gave written consent through the current accepted procedures of the relevant registry. Patients were excluded if life expectancy was less than 8 weeks, left ventricular ejection fraction was less than 40%, creatinine clearance was less than 30 mL/min, bilirubin level was greater than 34 μM, or liver transaminases were more than 3 times the upper limit of normal. Data were analyzed as of December 2003.
Patient characteristics
Eighty-eight consecutive patients were enrolled from June 1997 until June 2002. Sixty-six were men and 22 were women. Age range was 18 to 73 years (median, 48 years). Forty-one patients had indolent/low-grade NHL (LG-NHL) (follicular NHL, n = 29; chronic lymphocytic leukemia [CLL], n = 9; and lymphoplasmacytoid NHL, n = 3), 37 patients had aggressive/high-grade NHL (HG-NHL) (diffuse large B-cell NHL, n = 22; transformed LG-NHL, n = 11; peripheral T-cell NHL, n = 4), and the remaining 10 patients had mantle cell lymphoma (MCL).
Thirty-seven (42%) patients had previously received autografts, including 51% of those with HG-NHL, 40% of those with MCL, and 37% with LG-NHL. A median of 4 lines (range, 2-6 lines) of previous treatment had been given to patients with HG-NHL before referral for RIT, and a median of 3 lines (range, 1-6 lines) had been given for patients with LG-NHL and MCL. Twenty-one patients were in complete remission (CR) at the time of RIT, 57 were in chemosensitive partial remission (PR), and 10 had refractory or progressive disease.
Of the 88 donor-recipient pairs, 54 (61%) were at risk for cytomegalovirus (CMV) reactivation, whereby either the donor or the recipient was CMV seropositive. Twenty-three (26%) patients underwent matched unrelated donor (MUD) RIT, of which 6 were mismatched for 1 or more HLA class I or class II alleles. Patient and transplant characteristics are summarized in Table 1.
Patient and transplant characteristics
Characteristics . | No. . |
|---|---|
| Total patients | 88 |
| Men/women | 66/22 |
| Median age at transplantation, y (range) | 48 (18-73) |
| Histology at diagnosis | |
| Indolent/LG-NHL | 41 |
| Follicular NHL | 29 |
| Lymphopiasmacytoid NHL | 3 |
| CLL/prolymphocytic leukemia | 9 |
| MCL | 10 |
| Aggressive/HG-NHL | 37 |
| Peripheral T-cell NHL | 4 |
| Transformed low-grade NHL | 11 |
| Diffuse large B-cell lymphoma | 22 |
| Previous chemotherapy regimens, median (range) | 3 (1-6) |
| Previous autograft (%) | |
| Total | 37 (42) |
| LG-NHL | 15 (37) |
| MCL | 4 (40) |
| HG-NHL | 19 (51) |
| Disease status at transplantation | |
| LG-NHL | |
| CR | 11 |
| Stable PR | 29 |
| Ref/prog disease | 1 |
| MCL | |
| CR | 4 |
| Stable PR | 5 |
| Ref/prog disease | 1 |
| HG-NHL | |
| CR | 6 |
| Stable PR | 23 |
| Ref/prog disease | 8 |
| Donor | |
| HLA-identical related | 63 |
| 1 antigen-mismatch related | 2 |
| Matched unrelated | 17 |
| 1 antigen-mismatch unrelated | 6 |
| Stem cell source | |
| PBSC | 63 |
| PBSC + BM | 1 |
| BM | 24 |
| CMV serostatus, donor/recipient | |
| Negative/negative | 34 |
| Negative/positive | 19 |
| Positive/negative | 10 |
| Positive/positive | 25 |
Characteristics . | No. . |
|---|---|
| Total patients | 88 |
| Men/women | 66/22 |
| Median age at transplantation, y (range) | 48 (18-73) |
| Histology at diagnosis | |
| Indolent/LG-NHL | 41 |
| Follicular NHL | 29 |
| Lymphopiasmacytoid NHL | 3 |
| CLL/prolymphocytic leukemia | 9 |
| MCL | 10 |
| Aggressive/HG-NHL | 37 |
| Peripheral T-cell NHL | 4 |
| Transformed low-grade NHL | 11 |
| Diffuse large B-cell lymphoma | 22 |
| Previous chemotherapy regimens, median (range) | 3 (1-6) |
| Previous autograft (%) | |
| Total | 37 (42) |
| LG-NHL | 15 (37) |
| MCL | 4 (40) |
| HG-NHL | 19 (51) |
| Disease status at transplantation | |
| LG-NHL | |
| CR | 11 |
| Stable PR | 29 |
| Ref/prog disease | 1 |
| MCL | |
| CR | 4 |
| Stable PR | 5 |
| Ref/prog disease | 1 |
| HG-NHL | |
| CR | 6 |
| Stable PR | 23 |
| Ref/prog disease | 8 |
| Donor | |
| HLA-identical related | 63 |
| 1 antigen-mismatch related | 2 |
| Matched unrelated | 17 |
| 1 antigen-mismatch unrelated | 6 |
| Stem cell source | |
| PBSC | 63 |
| PBSC + BM | 1 |
| BM | 24 |
| CMV serostatus, donor/recipient | |
| Negative/negative | 34 |
| Negative/positive | 19 |
| Positive/negative | 10 |
| Positive/positive | 25 |
Prog/ref disease indicates progressive or refractory disease.
Conditioning regimen
All patients received the same conditioning regimen: 20 mg/d alemtuzumab (CAMPATH-1H) by intravenous infusion over 8 hours on days -8 to -4; 30 mg/m2 fludarabine by intravenous infusion over 30 minutes on days -7 to -3; and 140 mg/m2 melphalan by intravenous infusion over 30 minutes on day -2. Of the 65 recipients of sibling/matched related RIT, 63 received unmanipulated peripheral blood stem cells (PBSCs), 1 received PBSCs and unmanipulated marrow (BM), and 1 received BM. All 23 recipients of MUD RIT received BM from matched unrelated donors.
Stem cell and bone marrow collection
Sibling donors received 10 μg/kg granulocyte-colony-stimulating factor (G-CSF) subcutaneously once daily on days -4 to 0. Leukapheresis was performed on days 0 and 1 using conventional techniques for PBSC collection. Additional BM was harvested from 1 sibling donor after less than 2 × 106/kg CD34+ cells were mobilized peripherally and for another sibling donor who was a carrier of hepatitis B virus. BM was harvested under general anesthesia from unrelated donors according to donor registry protocols. All recipients received more than 2 × 106/kg CD34+ cells. Unmanipulated mobilized PBSCs or BM was infused on day 0 or days 0 and 1.
Supportive care and infection prophylaxis
Infection prophylaxis was similar for all patients. Antiviral prophylaxis consisted of acyclovir, as described previously.13 Cytomegalovirus (CMV) reactivation was managed variously, depending on institutional policy. Antiviral and Pneumocystis carinii prophylaxis were continued until the absolute CD4+ T-cell count was greater than 200 × 106/L. Fungal prophylaxis consisted of intravenous itraconazole or fluconazole until discharge. Febrile neutropenia was treated with broad-spectrum antibiotics according to institutional guidelines. All blood products were irradiated to 25 Gy and, for CMV-seronegative transplant recipients only, were derived from screened CMV-negative blood donors. Hemoglobin concentrations and platelet counts were maintained above 9 g/dL and 10 × 109/L, respectively.
GVHD prophylaxis and grading
Cyclosporin A (CSA) administration was initiated on day -1 at a dose of 3 mg/kg per day. Intravenous CSA was converted to oral CSA when the patient could tolerate medications by mouth, and it was continued for 2 to 3 months. In the absence of GVHD, CSA was tapered over a period of 4 to 8 weeks. Patients who survived 100 days were evaluable for chronic GVHD. Acute and chronic GVHD was graded according to consensus criteria.19
Schedule for donor leukocyte infusion
Two broad categories of patients were eligible for DLI. Patients who had relapses or showed evidence of progressive disease were given therapeutic DLI. Patients with persistent disease, minimal residual disease, or mixed hematopoietic chimerism at 6 months or more after transplantation were candidates for preemptive DLI. Patients were excluded from DLI if they had active GVHD or other contraindications to the receipt of DLI. The schedule for DLI administration varied between centers. Either an escalated regimen was used starting with 1 × 106 CD3+ T cells per kilogram, with dose escalation every 3 months and an absence of GVHD, until a response was seen, or repeat infusion was given with low-dose DLI.
Follow-up
Patients had regular follow-ups at 3-month intervals after transplantation to assess disease response and remission status. Evaluations varied according to the underlying diagnosis and BM aspiration or biopsy, cytogenetic or molecular evaluation, multiparameter fluorescence-activated cell sorter (FACS) analysis, computed tomography, and positron emission tomography.
Chimerism
Chimerism studies were performed by means of fluorescence in situ hybridization for X and Y chromosomes or by microsatellite polymerase chain reaction (PCR). DNA was prepared from pretransplantation recipient blood and donor blood. After transplantation, either unfractionated buffy coat or granulocyte, T-cell, and B-cell preparations were obtained from peripheral blood, as previously described.20 PCR of highly polymorphic short tandem repeat (STR) units was performed, as previously described in detail.13 Complete donor chimerism was defined by lack of detection of a previously determined recipient-specific peak on STR PCR, and mixed chimerism was defined by detection of donor- and recipient-specific peaks.
Study end points
Data were analyzed as of December 2003. Major study end points were overall survival, disease-free survival, and response to DLI, in addition to shorter-term end points of engraftment, TRM, and incidence/severity of GVHD. DLI patients were evaluable for GVHD if they survived at least 100 days after the first infusion or had clinical signs of GVHD before that time. Complete and partial responses, stable PR, relapse, and progressive/refractory disease were determined by standard disease-specific criteria, as defined by the National Cancer Institute (NCI).21,22 Additional definitions were persistent disease after transplantation, disease bulk equal to or less than that immediately before transplantation, minimal residual disease (MRD), and CLL or follicular lymphoma (FL) cells detected by multiparameter FACS analysis in the absence of morphologic evidence of disease or symptoms.
Toxicity was graded according to standard criteria.23 An infective complication was defined as any infection that occurred after day 21 and that required continued or new hospital admission or referral. CMV reactivation was defined as 2 consecutive peripheral blood PCR-positive results. Neutrophil recovery was defined as a neutrophil count exceeding 0.5 × 109/L for at least 3 consecutive days, and platelet recovery was defined as an untransfused platelet count greater than 20 × 109/L for 7 consecutive days.
Statistical methods
Actuarial curves were estimated according to the Kaplan-Meier method. Patient-, disease-, and transplant-related variables were studied for associations with outcomes by univariate analysis using the Wilcoxon test and by multivariate analysis using Cox proportional hazards regression. In all statistical evaluations, P less than or equal to .05 was considered significant. Overall survival was measured from transplantation until death from any cause. Patients still alive at the time of analysis were censored at the last follow-up date. PFS was measured from transplantation until progression or death from any cause. TRM was determined from the date of transplantation until death related to transplantation. Patients who died of nontransplant-related causes were censored at the time of death.
Results
Toxicity
All patients in the study were assessable for toxicity. Nineteen patients died of causes not related to relapse. The most common cause was multiorgan failure (MOF) secondary to sepsis (n = 9) at a median of 42 days after transplantation (range, 18-364 days). Other causes of death were predominantly infective or infection related: CMV disease (n = 2); Epstein-Barr virus-posttransplantation lymphoproliferative disorder (EBV-PTLD) (n = 2); disseminated adenovirus (n = 1); sepsis with concurrent posttransplantation GVHD (n = 1); sepsis (without GVHD) after DLI for mixed chimerism (n = 1); MOF with peripheral neuropathy of uncertain cause (n = 1); and demyelinating neuropathies or Guillain-Barré-like syndromes (n = 2).
There were no cases of veno-occlusive disease, and no patients had grade 3 or 4 mucositis. Only 2 deaths were directly attributable to GVHD. Both these patients contracted GVHD after DLI for progressive disease.
The actuarial probability of nonrelapse mortality for the LG-NHL group at 100 days was 2%, and at 3 years it was 11% (95% CI, 0%-19%). Significantly higher nonrelapse mortality rates were observed in the HG-NHL group, with actuarial probability of nonrelapse mortality at 100 days of 27% and at 3 years of 38% (95% CI, 19%-48%; P = .01, Wilcoxon test), as shown in Figure 1. Nonrelapse mortality rates observed in the MCL group were 20% at 100 days and at 3 years.
Results of multivariate analysis confirm that nonrelapse deaths are significantly associated with HG-NHL (relative risk, 2.3; P = .02) and not with disease status at transplantation, previous autograft, unrelated donors, or age older than 45 years (Table 2).
Results of multivariate analysis
Outcome . | Variable . | Relative risk . | Confidence interval . | P . |
|---|---|---|---|---|
| NRM | HG-NHL | 2.3 | 1.2-5.2 | .02 |
| Relapse | CR at transplantation | 0.3 | 0.1-0.6 | .0001 |
| Relapse | < 45 y | 0.6 | 0.4-0.9 | .01 |
| PFS | HG-NHL | 1.4 | 0.9-2.3 | .05 |
| PFS | CR at transplantation | 0.4 | 0.2-0.7 | .005 |
| PFS | < 45 y | 0.7 | 0.5-1.0 | .05 |
Outcome . | Variable . | Relative risk . | Confidence interval . | P . |
|---|---|---|---|---|
| NRM | HG-NHL | 2.3 | 1.2-5.2 | .02 |
| Relapse | CR at transplantation | 0.3 | 0.1-0.6 | .0001 |
| Relapse | < 45 y | 0.6 | 0.4-0.9 | .01 |
| PFS | HG-NHL | 1.4 | 0.9-2.3 | .05 |
| PFS | CR at transplantation | 0.4 | 0.2-0.7 | .005 |
| PFS | < 45 y | 0.7 | 0.5-1.0 | .05 |
Only statistically significant variables are shown.
Engraftment
Of the 88 patients, 85 (97%) had sustained neutrophil recovery exceeding 0.5 × 109/L for 3 consecutive days, with a median time to engraftment of 13 days (range, 8-23 days). Two patients died before achieving neutrophil engraftment at 20 and 23 days, respectively. In another patient, donor cells failed to engraft with full recipient hematopoiesis from day 13. Median time to achieve transfusion-independent platelet counts greater than 20 × 109/L for 7 consecutive days was 17 days (range, 3-96 days). Three patients had not achieved platelet independence by death or last follow up. After initial early engraftment, 3 patients rejected the graft (2 patients received autologous cells at day 26 and day 60, respectively), including 1 late rejection at 946 days.
Chimerism
Chimerism studies were performed in 69 patients who were evaluable beyond 30 days after transplantation. Lineage-specific chimerism studies were performed in 43 patients, and whole-blood chimerism studies were available in 26 patients. Chimerism studies were not performed in 19 patients (early death, n = 11; lack of informative primers, n = 4; not performed, n = 4).
Of the 69 patients tested, 36 had full donor hematopoiesis at first chimerism analysis after transplantation (1-3 months after transplantation), 19 were mixed chimeras (both donor and recipient cells present), and 2 had full recipient hematopoiesis. Results were unavailable from 5 patients and are pending from 7 patients.
Patients with persistent mixed hematopoietic chimerism 6 months after transplantation were eligible for DLI on the basis of mixed chimerism alone. Mixed chimerism was defined as the detection of recipient-specific and donor-specific DNA peaks on STR-PCR. In mixing experiments, the sensitivity of such analyses was shown to vary between 2% and 5%. Of the 21 eligible patients, 15 (71%) received DLI; DLI was not being administered to those with active GVHD or with other contraindications to the procedure. Conversion to full donor hematopoiesis was observed in 8 (53%) patients at a median of 14 weeks after DLI (range, 9-26 weeks). Six patients maintained mixed hematopoietic chimera status at the time of last assessment, despite DLI (follow-up chimerism data were not yet available for 1 patient).
Graft-versus-host disease
The incidence and severity of GVHD after transplantation (excluding post-DLI GVHD) was low, in keeping with previously published data using this conditioning protocol.13,14,16,24 Sixty-three (72%) patients did not develop acute GVHD (aGVHD), 13 (15%) developed grade I aGVHD, and another 13 (15%) developed grades II to IV aGVHD, of which only 4 (4.5%) patients developed grades III and IV GVHD. The incidence of chronic GVHD (cGVHD) was also low, with a total of 6 (6.8%) patients developing limited (n = 2) or extensive (n = 4) cGVHD.
Thirty-six patients received DLI after transplantation, 15 for mixed chimerism and 21 for relapse, progression, persistent disease, or MRD. They received a median of 2 doses (range, 1-3 doses) and a maximum dose of 5 × 107 T cells/kg at a median of 242 days after transplantation (range, 131-971 days). Ten (28%) patients developed GVHD after DLI (1 grade II acute, 2 grade IV acute, 4 chronic limited, 3 chronic extensive). Nine of these had sibling donors, and 1 had an unrelated donor. Both patients with grade IV aGVHD died.
Disease response and relapse
Seventy-four (84%) patients were evaluable for disease response (14 early TRM). Overall, at 6 months after transplantation, 42 were in CR (57%), 17 were in stable PR (23%), and 15 had progressive disease (20%).
After 6 months, ongoing disease responses were dependent on disease status at the time of transplantation. Of those who underwent transplantation while in CR (n = 21; 6 HG-NHL, 4 MCL, 11 LG-NHL), 13 patients (3 HG-NHL, 3 MCL, 7 LG-NHL) were in continued CR at a median of 38 months after transplantation (range, 21-58 months).
Most patients underwent transplantation while in PR (n = 57; 23 HG-NHL, 5 MCL, 29 LG-NHL). Of these, 26 were in stable PR (n = 9; 3 HG-NHL, 6 LG-NHL) or had achieved CR (n = 17; 5 HG-NHL, 1 MCL, 10 LG-NHL) at a median of 37 months (range, 18-60 months) after transplantation. Six patients (3 HG-NHL, 3 LG-NHL) had disease progression by 6 months after transplantation, and all subsequently died of progressive disease at a median of 10 months (range, 4-25 months).
Ten patients underwent transplantation with refractory or progressive disease (8 HG-NHL, 1 MCL, 1 LG-NHL). Only 2 of these patients showed evidence of disease control. One patient (HG-NHL) was in CR 31 months after transplantation following a response to DLI. The other patient (LG-NHL) achieved PR at 6 months after transplantation but subsequently died of viral encephalitis after DLI for mixed chimerism.
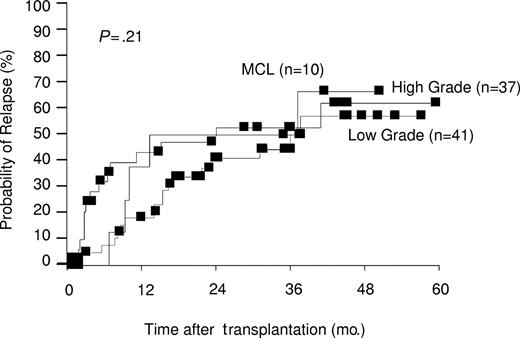
Figure 2 shows the estimated cumulative relapse rates in all patients. At 3 years after transplantation, actuarial relapse rates of 50% for the MCL group, 52% for the HG-NHL group, and 44% for the LG-NHL group were observed (P = .21). Of the 18 patients censored beyond 3 years, 4 had relapses (1 MCL, 1 HG-NHL, 2 LG-NHL). Multivariate analysis confirmed disease status at transplantation (P = .0001) and age older than 45 years (P = .01) to predict for subsequent relapse, whereas disease grade, presence of GVHD, and donor type were not significant (Table 2).
Estimated probability of relapse by disease grade. Three-year actuarial relapse rates were 50% for MCL, 52% for high grade, and 44% for low grade.
Estimated probability of relapse by disease grade. Three-year actuarial relapse rates were 50% for MCL, 52% for high grade, and 44% for low grade.
Disease response to DLI
Excluding those patients who received DLI for mixed hematopoietic chimerism or PTLD, 21 patients received DLI—3 for minimal residual disease (MRD), 1 for persistent disease, and 17 for disease progression or relapse. The median maximum dose of DLI given was 1.15 × 107 T cells/kg (range, 106-108 T cells/kg) after a median of 2 escalating doses (range, 1-5 doses) per patient.
All 3 patients receiving DLI for MRD had LG-NHL. Within this group, 2 responses and 1 transient response were observed. One patient received DLI for persistent disease and responded. Seventeen patients (HG-NHL n = 8; MCL n = 2; LG-NHL n = 7) received DLI for disease progression or relapse. In the HG-NHL group, 2 responses and 5 no responses were observed. The remaining patient was not restaged at the time of follow-up. One of the 2 patients with MCL responded to DLI. Of the 7 patients treated with DLI with LG-NHL, 4 had responses, 2 had no responses, and 1 was not evaluable because of concurrent local radiotherapy. Responses are summarized in Table 3.
Disease response to DLI
. | MRD . | Persistent disease . | Progression or relapse . |
|---|---|---|---|
| No. patients receiving DLI | 3 | 1 | 17 |
| Histology, no. | |||
| HG-NHL | 0 | 1 | 8 |
| MCL | 0 | 0 | 2 |
| LG-NHL | 3 | 0 | 7 |
| Donor source, no. | |||
| Sibling | 3 | 1 | 15 |
| MUD | 0 | 0 | 2 |
| Maximum dose, T cells/kg (range) | 5 × 107 (3-5 × 107) | 6 × 107 (—) | 108 (106-108) |
| No. doses, median (range) | 4 (3-5) | 3 (—) | 2 (1-4) |
| Response, no. | |||
| PR or CR | |||
| Transient | |||
| HG | 0 | 1 | 2 |
| MCL | 0 | 0 | 1 |
| LG | 2 | 0 | 4 |
| None | |||
| HG | 0 | — | 5 |
| MCL | 0 | — | 1 |
| LG | 1 | — | 2 |
| Not evaluable | |||
| HG | — | — | 1 |
| MCL | — | — | 0 |
| LG | — | — | 1 |
. | MRD . | Persistent disease . | Progression or relapse . |
|---|---|---|---|
| No. patients receiving DLI | 3 | 1 | 17 |
| Histology, no. | |||
| HG-NHL | 0 | 1 | 8 |
| MCL | 0 | 0 | 2 |
| LG-NHL | 3 | 0 | 7 |
| Donor source, no. | |||
| Sibling | 3 | 1 | 15 |
| MUD | 0 | 0 | 2 |
| Maximum dose, T cells/kg (range) | 5 × 107 (3-5 × 107) | 6 × 107 (—) | 108 (106-108) |
| No. doses, median (range) | 4 (3-5) | 3 (—) | 2 (1-4) |
| Response, no. | |||
| PR or CR | |||
| Transient | |||
| HG | 0 | 1 | 2 |
| MCL | 0 | 0 | 1 |
| LG | 2 | 0 | 4 |
| None | |||
| HG | 0 | — | 5 |
| MCL | 0 | — | 1 |
| LG | 1 | — | 2 |
| Not evaluable | |||
| HG | — | — | 1 |
| MCL | — | — | 0 |
| LG | — | — | 1 |
— indicates not applicable.
CMV reactivation
Of the 54 at-risk donor-recipient pairs, CMV reactivation was observed in 34 (63%) patients, 2 of whom had CMV disease (CMV colitis and CMV retinitis). CMV reactivation data were unavailable for 5 patients. No deaths were directly attributable to CMV disease alone.
Survival analyses
Median follow-up time of the patients is 36 months (range, 18-60 months). Kaplan-Meier estimated probabilities of OS and current PFS for all 88 patients are shown in Figures 3 and 4. The current progression-free survival curve includes patients who have reentered CR or achieved stable PR after DLI for persistent or relapsed disease.
Kaplan-Meier plots of estimated OS. (A) Estimated OS by disease grade. Three-year actuarial overall survival rates were 73% for low grade, 60% for MCL, and 34% for high grade. (B) Estimated OS by disease status at transplantation (LG-NHL only). Three-year actuarial overall survival rates were 81% for complete remission, 72% for partial remission, and 0% for refractory disease.
Kaplan-Meier plots of estimated OS. (A) Estimated OS by disease grade. Three-year actuarial overall survival rates were 73% for low grade, 60% for MCL, and 34% for high grade. (B) Estimated OS by disease status at transplantation (LG-NHL only). Three-year actuarial overall survival rates were 81% for complete remission, 72% for partial remission, and 0% for refractory disease.
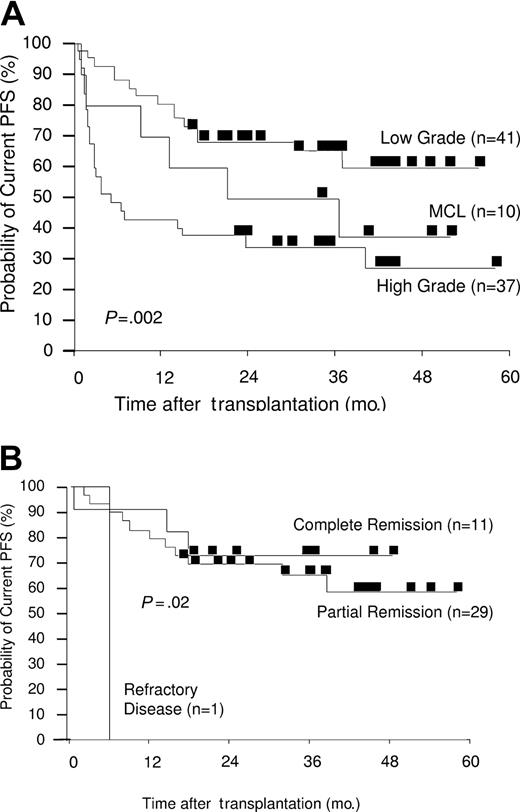
Kaplan-Meier plots of estimated current PFS. (A) Estimated PFS by disease grade. Three-year actuarial current PFS rates were 65% for low grade, 50% for MCL, and 34% for high grade. (B) Estimated PFS by disease status at transplantation (LG-NHL only). Three-year actuarial current PFS rates were 73% for complete remission, 65% for partial remission, and 0% for refractory disease.
Kaplan-Meier plots of estimated current PFS. (A) Estimated PFS by disease grade. Three-year actuarial current PFS rates were 65% for low grade, 50% for MCL, and 34% for high grade. (B) Estimated PFS by disease status at transplantation (LG-NHL only). Three-year actuarial current PFS rates were 73% for complete remission, 65% for partial remission, and 0% for refractory disease.
The actuarial OS rate at 3 years for all patients was 55% (73% for LG-NHL, 60% for MCL, 34% for HG-NHL, respectively; P < .001) (Figure 3A). Disease status at the time of transplantation (CR, PR, or progressive disease) affected the outcome for LG-NHL (P = .003). In the HG-NHL group, the effect of disease status at transplantation on OS did not reach statistical significance (P = .5).
LG-NHL patients who underwent transplantation while in CR (n = 11) had an actuarial OS rate of 81% at 3 years, and those who underwent transplantation in chemosensitive PR (n = 29) have an actuarial OS rate of 72% (P = .003) (Figure 3B). The patient with chemoresistant LG-NHL died of disease progression 6 months after transplantation. Patients with HG-NHL who underwent transplantation while in CR (n = 6) had an actuarial OS rate of 50% at 3 years, while those who underwent transplantation while in chemosensitive PR (n = 23) or with refractory disease (n = 8) fared worse, with OS rates of 37% at 3 years and 13% at 2.6 years, respectively (P = .5) (data not shown). Despite the small number of patients in the MCL group, disease status at transplantation significantly affected outcome (P = .004), with OS rates of 100% and 40% at 3 years for those who underwent transplantation in CR (n = 4) and PR (n = 5), respectively. The patient with refractory disease died early after transplantation (day 35) of causes not related to relapse (data not shown).
Actuarial OS rates at 3 years were 78% for LG-NHL patients with matched related donors and 56% for those with unrelated donors (P = .09) (data not shown).
The actuarial current PFS rates at 3 years, including for patients who achieved remission after DLI for progressive or persistent disease, were significantly superior for those with LG-NHL (65%) than for those with MCL (50%) or HG-NHL (34%) (P = .002) (Figure 4A).
Again, disease status at transplantation affected outcome. The actuarial current PFS rate at 3 years for patients with LG-NHL who underwent transplantation while in CR was 73%. Patients who underwent transplantation while in chemosensitive PR achieved a current PFS rate of 65% at 3 years (P = .02) (Figure 4B). The effect of disease status at transplantation on the current PFS rate in the MCL and HG-NHL groups did not reach statistical significance. Actuarial current PFS rates at 3 years were 50% for HG-NHL patients who underwent transplantation while in CR (n = 6) and 37% for those in chemosensitive PR (n = 23); for those who underwent transplantation with refractory disease (n = 8), only 1 patient was free of disease progression at 2.5 years (P = .5) (data not shown). For the 10 patients with MCL who underwent transplantation while in CR (n = 4) and PR (n = 5), actuarial PFS at 3 years after transplantation were 60% and 50%, respectively (P = .01) (data not shown).
The current PFS rate at 3 years is 71% for LG-NHL patients with matched related donors and 44% for those with unrelated donors (P = .04) (data not shown). Multivariate analysis (Table 2) of factors predicting for PFS confirmed disease grade (P = .05), disease status at transplantation (P = .005), and age (P = .05) to be statistically significant. Estimated actual PFS rates (not incorporating responses to subsequent DLI and, therefore, representing disease responses to the initial transplantation procedure only) at 3 years were 49% for LG-NHL, 30% for HG-NHL, and 40% for MCL (P = .004).
Discussion
Adding alemtuzumab to a fludarabine- and melphalan-containing RIT regimen dramatically reduces the risk for aGVHD and cGVHD in sibling and unrelated donor allografts.13,14 We have demonstrated that this regimen is well tolerated in older patients (median age, 48 years) who are heavily pretreated, including in 42% of patients who previously received autografts. The 100-day and 3-year non-relapse mortality (NRM) rates for all patients were 14% and 22%, respectively. However, patients undergoing RIT for LG-NHL had significantly lower 100-day and 3-year NRM rates (2% and 11%, respectively) than those undergoing RIT for HG-NHL (27% and 38%, respectively) or for MCL (20% at 100 days and 3 years) (P = .01). The NRM rate for patients with LG-NHL was comparable to that observed after autologous transplantation,25 increasing to 14% (100-day and 3-year) after previous autograft (data not shown). Despite 51% of patients with aggressive histology having previously undergone autograft, this factor did not predict for higher NRM on multivariate or univariate (data not shown) analysis. Therefore, the significantly higher NRM rate observed in the HG-NHL group appears to be related to the underlying disease itself or to the intensity of chemotherapy before allograft. There were no significant differences in the number of previous therapies received by this group of patients.
This regimen is more immunosuppressive and myelosuppressive than a number of other RIT regimens currently used, and it results in high levels of durable engraftment. However, the depletion of graft T cells can result in higher incidences of mixed chimerism, and, if the T-cell compartment is not fully donor, the GVL effect may be impaired, as has been observed in studies on CML.20 Sixty-three percent of patients with available chimerism analyses achieved full donor chimerism at initial testing after transplantation. Of the patients requiring DLI for persistent mixed chimerism at 6 months after transplantation and beyond, 53% converted to full donor hematopoiesis after a median of 14 weeks after DLI. Therefore, most patients achieved full donor hematopoiesis either spontaneously or after DLI. There are few published data on the effect of chimerism during DLI for persistent or relapsed disease. Peggs et al17 recently reported chimerism and disease responses to DLI after the same conditioning regimen and included 10 patients with NHL. In his cohort of 27 patients with myeloma, Hodgkin lymphoma, or NHL, 17 had DLI-responsive disease, and, of these, 15 had converted to multilineage full donor chimerism. However, of the 11 nonresponders, 8 had full donor chimerism. These data, from a small number of patients, suggest that though achieving full donor chimerism is desirable, it may not be sufficient for a disease response after DLI.
Actual PFS and current PFS were estimated to demonstrate the efficacy of DLI in maintaining disease responses after RIT. Because LG-NHL is an indolent disease characterized by multiple relapses, usually with progressively shorter time periods in remission, ongoing current PFS is a marker of disease control. The T-depleted RIT regimen described here relies on subsequent DLI for the achievement of maximal disease responses.
We demonstrate good disease responses in the LG-NHL group, despite low levels of GVHD. Considering the LG-NHL group alone (n = 41; 11 in CR, 29 patients in chemosensitive PR, 1 patient with refractory disease), the influence of disease status at transplantation on OS and current PFS was significant (P = .003 and P = .02, respectively). For patients who underwent transplantation during CR at 3 years, the actuarial current PFS rate was as high as 73%, and it was an encouraging 65% for those who underwent transplantation during PR. These figures represent excellent ongoing disease control in a group of patients for whom a median of 3 lines (range, 1-6) of previous therapy failed; this includes high-dose therapy in 37% of patients.
When performing RIT with enhanced antilymphoma conditioning regimens, such as BEAM-Campath (BCNU; etoposide, cytosine arabinoside, melphalan, and alemtuzumab), disease status at transplantation was not observed to affect outcome.15 With shorter follow-up times, Faulkner et al15 observed an event-free survival rate of 69% and an OS rate of 74% at 2 years for a group of 51 patients with low-grade lymphoproliferative disease and Hodgkin lymphoma using predominantly matched related donors. However, when incorporating higher doses of in vivo alemtuzumab in the conditioning regimen, as shown here, disease status at transplantation significantly affected outcome with respect to relapse risk, PFS, and OS, and disease control required the administration of DLI.
Because of the low GVHD rates, this regimen enables good outcomes from transplantation even without matched related donors. Patients with LG-NHL achieved 3-year OS rates of 78% (sibling donor) and 56% (MUD) (P = .09) and 3-year current PFS rates of 71% (sibling donor) and 44% (MUD) (P = .04). We observed no effect on outcome with the presence or absence of GVHD.
Like others, we have observed significantly poorer outcomes for those with transformed LG-NHL, MCL, and HG-NHL.8,26 To achieve prolonged control of disease, patients with intermediate or high-grade disease must be in chemosensitive PR or CR at the time of RIT.
Surprisingly, no statistically significant difference in relapse rates after transplantation was observed between patients with different underlying disease histology. Multivariate analysis identified age and disease status at transplantation as significant risk factors in predicting relapse, independent of histology in this cohort of patients. These findings may relate to the observation that most patients with LG-NHL were heavily pretreated, experienced multiple relapses, and underwent transplantation late in their disease course. It is clear that performing transplantation while patients are in CR results in excellent PFS, despite extensive T-cell depletion. If patients with LG-NHL undergo transplantation earlier in their disease course, it is possible that relapse rates will be lower than those observed in this study.
For patients with relapsed or progressive disease after transplantation, disease control can be achieved with escalated DLI beginning 6 months after transplantation. Forty-eight percent of patients receiving DLI for persistent or progressive disease after transplantation achieved durable PR or CR, without concurrent additional therapy. At present, the number of patients who have received DLI remains small. Response rates between those with LG-NHL and those with HG-NHL have not been significantly different, despite the marked difference in PFS between the 2 groups, suggesting a GVL effect for patients with low-grade disease.
In summary, patients with relapsed CLL and LG-NHL achieve good rates of PFS with extremely low TRM and GVHD, even when matched related donors are unavailable. For patients with advanced LG-NHL, median survival time is only 5 years after first disease recurrence27 when they are treated with salvage chemotherapy. We have shown that relapse after transplantation continues in both groups and is predicted by disease status at the time of transplantation. Relapse rate was independent of underlying histology in the cohort studied, with small numbers of patients in each histologic subgroup. Excluding patients with refractory or progressive disease at the time of transplantation, differences in outcome were primarily attributed to differences in TRM. With this T cell-depleting conditioning regimen, responses to subsequent DLI are required to achieve overall disease control, similar to that observed by others using non-T cell-depleting regimens. Despite this, our results demonstrate a similar outcome after allogeneic transplantation in patients at poor risk, but even longer follow-up is required to establish whether the plateau observed in PFS can be maintained to result in prolonged disease control and possibly in cure. The optimal dose of alemtuzumab is being assessed in phase 1/2 studies.
These encouraging results indicate that a prospective, randomized, control trial of RIT and second-line therapy should be considered for younger patients with progressive low-grade disease after initial therapy.
Prepublished online as Blood First Edition Paper, August 10, 2004; DOI 10.1182/blood-2004-03-1105.
Supported by The Elephant Trust for Cancer Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal