Abstract
POEMS syndrome is characterized by peripheral neuropathy (PN), a clonal plasma cell disorder (PCD), organomegaly, endocrinopathy, skin changes, edema, sclerotic bone lesions, and thrombocytosis. Based on the improved response rates observed with peripheral blood stem cell transplantation (PBSCT) in patients with other PCDs, autologous PBSCT may be an attractive treatment option for this syndrome. Sixteen patients with POEMS syndrome have undergone PBSCT at Mayo. Of these patients, 15 had a severe rapidly progressive sensorimotor PN (9 were wheelchair dependent) and 14 were male. Median age was 51 years (range, 19-62 years). The median number of prior therapies was 3 (range, 0-7). From first symptoms and from diagnosis of POEMS the times to transplantation were 42 months and 5 months (ranges, 8-185 months and 2-149 months), respectively. There were 15 patients who had significantly abnormal pretransplant pulmonary function tests. There was one transplant-related death. During the peritransplant period, 5 patients required intubation for respiratory compromise, including one who required intubation during his stem cell mobilization period. Another patient required noninvasive biphasic positive airway pressure throughout his course. Of the 14 evaluable patients, all have had neurologic improvement or stabilization. Other features have improved substantially. PBSCT for POEMS syndrome is effective therapy but may also be associated with significant morbidity. (Blood. 2004;104:3400-3407)
Introduction
POEMS syndrome, also known as osteosclerotic myeloma, Crow-Fukase syndrome, or Takasuki syndrome, is an incompletely understood paraneoplastic syndrome resulting from an underlying plasmaproliferative disorder. More than 95% of patients have monoclonal lambda sclerotic plasmacytoma(s) or bone marrow infiltration. The acronym POEMS refers to the polyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes seen in the majority of patients, but excludes other important characteristics germane to the syndrome including the sclerotic bone lesions, Castleman disease, papilledema, peripheral edema, ascites, pleural effusions, polycythemia, thrombocytosis, fatigue, and clubbing.1,2 Not all features are required to make the diagnosis (Table 1); at a minimum, however, a patient must have: (1) peripheral neuropathy; (2) osteosclerotic myeloma (ie, a clonal plasma cell dyscrasia and at least one sclerotic bone lesion) or Castleman disease; and (3) at least one of the other mentioned features.1 Typically, the overriding manifestation is that of progressive symmetrical sensorimotor neuropathy; other components such as organomegaly, edema, and skin changes are overshadowed by the neuropathy.
Criteria for the diagnosis of POEMS syndrome
. | Symptom/Sign . |
|---|---|
| Major criteria | |
| Polyneuropathy | |
| Monoclonal plasma cell proliferative disorder | |
| Minor criteria | |
| Sclerotic bone lesions* | |
| Castleman disease* | |
| Organomegaly (splenomegaly, hepatomegaly, or lymphadenopathy) | |
| Edema (edema, pleural effusion, or ascites) | |
| Endocrinopathy (adrenal, thyroid,† pituitary, gonadal, parathyroid, pancreatic†) | |
| Skin changes (hyperpigmentation, hypertrichosis, plethora, hemangiomata, white nails) | |
| Papilledema | |
| Known associations‡ | |
| Clubbing | |
| Weight loss | |
| Thrombocytosis | |
| Polycythemia | |
| Hyperhidrosis | |
| Possible associations‡ | |
| Pulmonary hypertension | |
| Restrictive lung disease | |
| Thrombotic diatheses | |
| Arthralgias | |
| Cardiomyopathy (systolic dysfunction) | |
| Fever | |
| Low vitamin B12 values | |
| Diarrhea |
. | Symptom/Sign . |
|---|---|
| Major criteria | |
| Polyneuropathy | |
| Monoclonal plasma cell proliferative disorder | |
| Minor criteria | |
| Sclerotic bone lesions* | |
| Castleman disease* | |
| Organomegaly (splenomegaly, hepatomegaly, or lymphadenopathy) | |
| Edema (edema, pleural effusion, or ascites) | |
| Endocrinopathy (adrenal, thyroid,† pituitary, gonadal, parathyroid, pancreatic†) | |
| Skin changes (hyperpigmentation, hypertrichosis, plethora, hemangiomata, white nails) | |
| Papilledema | |
| Known associations‡ | |
| Clubbing | |
| Weight loss | |
| Thrombocytosis | |
| Polycythemia | |
| Hyperhidrosis | |
| Possible associations‡ | |
| Pulmonary hypertension | |
| Restrictive lung disease | |
| Thrombotic diatheses | |
| Arthralgias | |
| Cardiomyopathy (systolic dysfunction) | |
| Fever | |
| Low vitamin B12 values | |
| Diarrhea |
The research presented in this table was originally published in Dispenzieri A, Kyle RA, Lacy MQ, et al. POEMS syndrome: definitions and long-term outcome. Blood. 2003;101:2496-2506.
Please note that 2 major criteria and at least one minor criterion are required for diagnosis.
Osteosclerotic lesion or Castleman disease is almost always present.
Because of the high prevalence of diabetes mellitus and thyroid abnormalities, this diagnosis alone is not sufficient to meet this minor criterion.
Though the known and possible associations are not required to make a POEMS diagnosis, they lend weight to the diagnosis of the syndrome.
Treatment for POEMS is not standardized. For an isolated plasmacytoma, radiation is the preferred treatment.1 If there are widespread lesions, chemotherapy is indicated. The first report of high-dose chemotherapy with hematopoietic stem cell transplantation was in a 25-year-old female with POEMS; she died of multiorgan failure 63 days after her stem cell transplantation.3 Ours was the first report of a successful peripheral blood stem cell transplantation (PBSCT) in a patient with POEMS.4 Subsequently, there have been an additional 11 patients whose transplantation procedures were examined in the literature.5-9 Herein, we report on our experience with high-dose chemotherapy with peripheral blood stem cell support to 16 patients.
Patients, materials, and methods
Between March 1999 and December 2003, 16 patients with POEMS syndrome underwent PBSCT at the Mayo Clinic. Patients were classified as having POEMS syndrome if they met the criteria described in Table 1. Peripheral blood stem cells were collected using either cyclophosphamide and growth factor (n = 10) or growth factor alone (n = 6). The median number of CD34 cells was 5.4 × 106/kg (range, 3.3 to 9.9). There were 3 patients conditioned using 140 mg/m2 melphalan and 12 received 200 mg/m2 melphalan. One of the patients receiving 200 mg/m2 also received samarium-153 ethylene diamine tetramethylene phosphonate as part of his conditioning regimen,4 and another patient received carmustine, etoposide, cytarabine, and melphalan (BEAM). Standard supportive care with prophylactic antibiotics and growth factor support was provided. Data were retrospectively collected on patients with approval from the Mayo Clinic Institutional Review Board and in accordance with Minnesota and Florida state laws. All patients had given informed consent for chart review. Charts were reviewed by A.D. and A.M.-A.
Hematologic responses were defined according to the International Bone Marrow Transplant Registry/American Bone Marrow Transplant Registry (IBMTR/ABMTR) criteria.10 A complete response is defined as the disappearance of monoclonal protein in the serum and urine as well as fewer than 5% bone marrow plasmacytosis. A partial response includes a 50% reduction in the monoclonal protein in the serum and urine and the absence of any additional evidence of progressive myeloma. A “very good partial response” was also included as a hematologic response category, and included patients who no longer had a measurable monoclonal protein in the serum or urine, but were immunofixation positive (or did not have immunofixation performed to verify complete response).11 A neurologic response was defined both by subjective criteria and objective criteria. We have used the Neuropathy Impairment Score (NIS) and attributes of nerve conduction studies as objective parameters. The NIS provides a numeric value that expresses neurologic impairment from neuropathic deficits. Its elements are based on the Mayo Clinic neurologic examination, and the items provide a measure of severity of muscle weakness (scored as 0 = normal, 1 = 25%, 2 = 50%, 3 = 75% weak, 4 = paralyzed); loss of deep tendon reflexes scored as normal (0), decreased (1), or absent (2), and sensory loss graded as normal (0), diminished (1), or absent (2). The NIS provides an overall balanced numerical score of these 3 types of deficits. We have used this instrument extensively in epidemiologic studies and controlled clinical trials and it has shown a good, reproducible measure of longitudinal change in many neuropathies.12 Nerve conduction studies (NCS) provide specific, quantitative, and reproducible information to characterize nerve function. One of the attributes of NCS, the summated value of compound muscle action potential (CMAP) amplitudes of the tibial, peroneal, and ulnar nerves, provides reliable data about the function of motor axons. Objective criteria for neurologic response included a reduction in the NIS by 10 points and improvement in the summated CMAP amplitude by 2 mV.12
A patient was considered to have an organ response if the liver or spleen size had reduced by at least 2 cm or if lymphadenopathy had resolved. An endocrine response was normalization of biochemical markers of endocrine measurements in the absence of specific replacement therapy, recovery of erectile function, or improvement in gynecomastia. Dermatologic responses included improvement of hyperpigmentation, scleropachia, hemangiomata, or hypertrichosis. A pulmonary response was defined as a 15% improvement in any of the pulmonary function parameters.
Results
Baseline characteristics
Patient characteristics are shown in Table 2 and Table 3. Median age at the time of transplantation was 51 years (range, 20-62 years). All but 1 patient had a severe, rapidly progressive sensorimotor peripheral neuropathy (PN) involving both upper and lower extremities; 9 patients were restricted to wheelchairs. One patient had sensory neuropathy without significant motor involvement. All but 2 patients were male. A monoclonal lambda plasma cell dyscrasia was documented in all patients, and 2 had biopsy-proven Castleman disease. There were 9 patients who had a monoclonal immunoglobulin A (IgA) lambda in their serum and 4 who had an IgG lambda. The other 3 patients had no monoclonal protein documented by immunofixation, but had a biopsy-proven clonal plasmaproliferative disorder, which was lambda by immunohistochemistry. None of the patients had a serum M-spike of more than 10 g/L or urine M-spike of more than 200 mg/24 hours. There were 11 patients who had organomegaly. Endocrinopathy and skin changes were present in 14 patients and 13 patients, respectively. There were 7 patients with thrombocytosis and/or erythrocytosis, and 15 had sclerotic bone lesions (diffuse in 9, solitary lesion in 6). Using the Bardwick criteria, the median number of POEMS features was 4.5 (range, 2-5). Using the Mayo criteria1 —which include the 5 criteria of the Bardwick acronym, but also sclerotic bone lesions, Castleman disease, extravascular volume overload, papilledema, and a monoclonal plasma proliferative disorder rather than Bardwick's “M-protein”—the median number of features was 7 (range, 3-9). There were 3 patients who had had prior cerebrovascular accidents, 1 a history of transient ischemic attacks, and another a splenic vein thrombosis after undergoing diagnostic splenectomy. The median number of therapeutic regimens prior to PBSCT was 3 (range, 0-7); 2 of the patients with an irradiated solitary bone lesion progressed despite external beam radiation. Time from diagnosis of POEMS to transplantation was 4 months (range, 2-149 months).
General patient characteristics (N = 16)
. | Median (range) . |
|---|---|
| Sex, no. male/no. female* | |
| Male | 14 |
| Female | 2 |
| Race, no. white/Hispanic* | |
| White | 15 |
| Hispanic | 1 |
| Age at transplantation, y | 51 (20-63) |
| Time from symptoms to diagnosis, mos | 24 (3-36) |
| Time from diagnosis to PBSCT, mos | 4 (2-149) |
| Prior therapies, no. | 3 (0-7) |
| Immunoglobulin, no. IgA/no. IgG/no. nonsecretory* | |
| IgA | 9 |
| IgG | 4 |
| Nonsecretory | 3 |
| Hemoglobin level, g/dL | 14.4 (11.4-17.4) |
| Platelet count, × 109/L | 425 (162-783) |
| Creatinine level, mg/dL | 1.1 (0.6-1.9) |
| M-spike, g/L | 2 (0-9.4) |
| Serum albumin level, g/L | 37 (27-41) |
| Urine protein, g/day | 0.16 (0.04-1.2) |
| Bone marrow plasma cells, % | 4 (1-10) |
| Bardwick features† | 4.5 (2-5) |
| Mayo features‡ | 7 (3-9) |
. | Median (range) . |
|---|---|
| Sex, no. male/no. female* | |
| Male | 14 |
| Female | 2 |
| Race, no. white/Hispanic* | |
| White | 15 |
| Hispanic | 1 |
| Age at transplantation, y | 51 (20-63) |
| Time from symptoms to diagnosis, mos | 24 (3-36) |
| Time from diagnosis to PBSCT, mos | 4 (2-149) |
| Prior therapies, no. | 3 (0-7) |
| Immunoglobulin, no. IgA/no. IgG/no. nonsecretory* | |
| IgA | 9 |
| IgG | 4 |
| Nonsecretory | 3 |
| Hemoglobin level, g/dL | 14.4 (11.4-17.4) |
| Platelet count, × 109/L | 425 (162-783) |
| Creatinine level, mg/dL | 1.1 (0.6-1.9) |
| M-spike, g/L | 2 (0-9.4) |
| Serum albumin level, g/L | 37 (27-41) |
| Urine protein, g/day | 0.16 (0.04-1.2) |
| Bone marrow plasma cells, % | 4 (1-10) |
| Bardwick features† | 4.5 (2-5) |
| Mayo features‡ | 7 (3-9) |
Values in the sex, race, and immunoglobulin rows are shown as the number of patients in the study cohort with that characteristic.
Bardwick features include peripheral neuropathy, organomegaly, endocrinopathy, monoclonal protein, and skin changes.
Mayo features include Bardwick features as well as volume overload (effusions, edema, ascites), sclerotic bone lesions, Castleman disease, and papilledema; in the Mayo criteria, monoclonal plasmaproliferative disorder is used rather than monoclonal protein.
Patient characteristics by POEMS syndrome features criteria (N = 16)
. | Number of patients (%) . |
|---|---|
| Peripheral neuropathy | 16 (100) |
| Wheelchair confined | 8 (50) |
| Motor | 15 (94) |
| Sensory | 16 (100) |
| Organomegaly | 13 (81) |
| Splenomegaly | 10 (62) |
| Hepatomegaly | 5 (31) |
| Lymphadenopathy | 7 (44) |
| Castleman disease | 2 (12) |
| Endocrinopathy | 14 (87) |
| Skin changes | 13 (81) |
| Sclerotic lesions | |
| None | 1 (6) |
| 1 | 6 (37) |
| More than 1 | 9 (56) |
| Polyclonal plasma cells in iliac crest bone marrow aspiration | 6 (37) |
| Edema/ascites/effusion | 14 (87) |
| Ascites | 6 (37) |
| Effusion | 4 (25) |
| Papilledema | 4 (25) |
| Thrombocytosis/erythrocytosis | 7 (44) |
| Erythrocytosis | 2 (12) |
| Prior thrombotic event | 5 (31) |
. | Number of patients (%) . |
|---|---|
| Peripheral neuropathy | 16 (100) |
| Wheelchair confined | 8 (50) |
| Motor | 15 (94) |
| Sensory | 16 (100) |
| Organomegaly | 13 (81) |
| Splenomegaly | 10 (62) |
| Hepatomegaly | 5 (31) |
| Lymphadenopathy | 7 (44) |
| Castleman disease | 2 (12) |
| Endocrinopathy | 14 (87) |
| Skin changes | 13 (81) |
| Sclerotic lesions | |
| None | 1 (6) |
| 1 | 6 (37) |
| More than 1 | 9 (56) |
| Polyclonal plasma cells in iliac crest bone marrow aspiration | 6 (37) |
| Edema/ascites/effusion | 14 (87) |
| Ascites | 6 (37) |
| Effusion | 4 (25) |
| Papilledema | 4 (25) |
| Thrombocytosis/erythrocytosis | 7 (44) |
| Erythrocytosis | 2 (12) |
| Prior thrombotic event | 5 (31) |
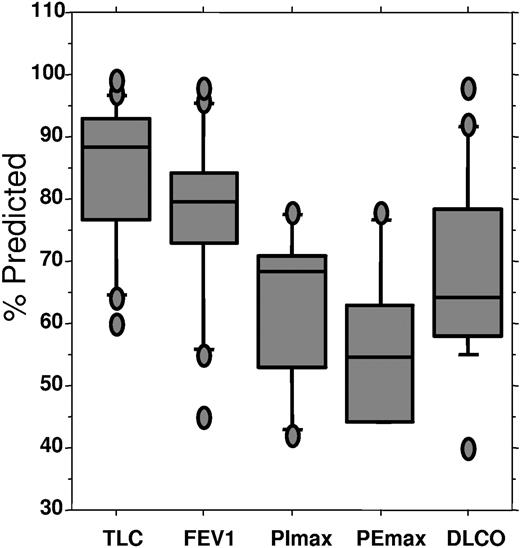
All but 1 patient had significantly abnormal pulmonary function tests before transplantation (Figure 1 and Table 4). As expected, total lung capacity, forced expiratory volume, forced vital capacity, and maximum inspiratory and expiratory pressures were reduced, consistent with neuromuscular disease. More striking was the reduction in diffusing capacity of carbon monoxide in all but 1 patient, with a median diffusing capacity of carbon monoxide of 64% of predicted (range, 40%-98% of predicted).
Pulmonary status before transplantation, percent predicted. TLC indicates total lung capacity; FEV1, forced expiratory volume at one sect; PImax, maximum inspiratory pressure; PEmax, maximal expiratory pressure; DLCO; diffusing capacity of carbon monoxide. Upper bar indicates 90th percentile; top of box, 75th percentile; line through box, median; bottom of box: 25th percentile; lower bar: 10th percentile.
Pulmonary status before transplantation, percent predicted. TLC indicates total lung capacity; FEV1, forced expiratory volume at one sect; PImax, maximum inspiratory pressure; PEmax, maximal expiratory pressure; DLCO; diffusing capacity of carbon monoxide. Upper bar indicates 90th percentile; top of box, 75th percentile; line through box, median; bottom of box: 25th percentile; lower bar: 10th percentile.
Baseline pulmonary function
. | No. . | % expected median (range) . |
|---|---|---|
| VC | 15 | 87 (47-94) |
| TLS | 16 | 88 (60-99) |
| FVC | 16 | 80 (47-94) |
| FEV1 | 16 | 79 (45-98) |
| PI max | 6 | 68 (42-78) |
| PE max | 6 | 54 (44-78) |
| DLCO | 16 | 64 (40-98) |
. | No. . | % expected median (range) . |
|---|---|---|
| VC | 15 | 87 (47-94) |
| TLS | 16 | 88 (60-99) |
| FVC | 16 | 80 (47-94) |
| FEV1 | 16 | 79 (45-98) |
| PI max | 6 | 68 (42-78) |
| PE max | 6 | 54 (44-78) |
| DLCO | 16 | 64 (40-98) |
TLC indicates total lung capacity; FEV1, forced expiratory volume at one sect; PImax, maximum inspiratory pressure; PEmax, maximal expiratory pressure; DLCO; diffusing capacity of carbon monoxide.
Patient outcomes
The transplantation course was associated with significant morbidity for the majority of patients. There were 6 patients admitted into the intensive care unit during their transplantation course: 1 for atrial fibrillation with rapid response and 5 for respiratory insufficiency. There were 5 patients who required intubation and mechanical ventilation: 1 during his cyclophosphamide and growth factor mobilization period. One patient (patient no. 6) required noninvasive biphasic positive airway pressure throughout his transplantation course because of his neurogenic respiratory muscle weakness. There was no difference between patients who did and those who did not require invasive mechanical ventilation with regards to pulmonary function test parameters or presence or absence of any of the 9 POEMS features. All 5 had received 200 mg/m2 melphalan as conditioning.
Median time to neutrophil count 0.5 × 109/L and platelets 20 × 109/L was 14 days with respective ranges of 11 to 29 days and 11 to 107 days. All but 1 patient engrafted. This same patient had an episode of a culture-negative systemic inflammatory response syndrome on day +8, resulting in renal failure requiring dialysis for 2.5 weeks; he died 115 days after his transplantation with a failed graft.
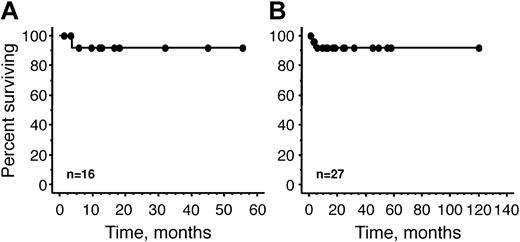
Survival from the time of transplantation of our 16 patients is demonstrated in Figure 2. After a median follow-up of 10.8 months (range, 1.3-55.5 months), none of the patients has shown evidence of progression of either their plasma cell dyscrasia or their paraneoplastic syndrome. One patient developed Philadelphia-positive chronic myelogenous leukemia 17 months after his transplantation. There is no evidence of a plasma cell clone in his bone marrow or in his peripheral blood. Prior to transplantation, his cytogenetics were normal and he had neither organomegaly nor thrombocytosis.
Overall survival. (A) Overall survival of 16 Mayo Clinic patients. (B) Reported world experience, including 16 Mayo Clinic patients and 11 previously reported patients.6-9
Overall survival. (A) Overall survival of 16 Mayo Clinic patients. (B) Reported world experience, including 16 Mayo Clinic patients and 11 previously reported patients.6-9
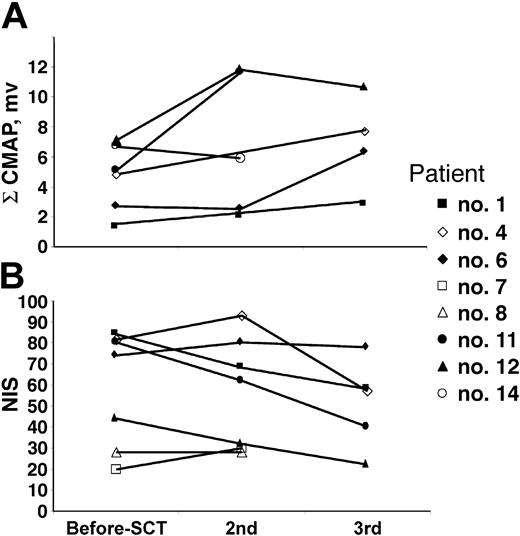
There were 14 patients who were evaluable for response (1 patient died early and was not evaluated for response, 1 patient has not yet returned for his 100-day follow-up). Of the remaining 14 patients (Table 5), all have achieved neurologic improvement or stabilization of neuropathic deficits. All 9 patients who were wheelchair dependent are now either independent of walking aids or using ankle foot orthotics (AFOs) with or without a cane. One patient has moved from using a walker to just an AFO, whereas another has discarded his AFO. An additional 3 patients have improved pain and function. Most patients did not have repeat formal neurologic testing. As shown in Figure 3, only 6 patients had serial nerve conduction studies performed (patient nos. 1, 6, 8, 11, 12, and 14). Of the 5 patients with serial studies performed 1 year or longer after their first nerve conduction study, 4 had significant improvement in their summated CMAPs (patient nos. 6, 8, 11, and 12) and 1 (patient no. 1) showed a trend toward improvement. Patient no. 14 has not shown improvement of summated CMAPs (not unexpected at 3 months of follow-up) but had significant reduction in his dysesthesias and narcotic need. Repeat neuropathy impairment scores (NISs) were documented in 7 patients (patient nos. 1, 4, 6, 7, 8, 11, and 12; Figure 3). Of these patients, 4 had significant improvement (NISs: patient no. 1, 84-58; patient no. 4, 82-57; patient no. 11, 80-40; and patient no. 12, 44-22). NISs for patients no. 6 and no. 8 were stable at 1.3 years and 0.3 years, respectively. At 15 months after transplantation, patient no. 7 had borderline deterioration by his impairment score (NIS 20-30), but nerve conduction studies were not done to confirm. It was thought that the subjective worsening of sensory symptoms was a function of nerve repair since he had objective and subjective improvement of his motor strength in his upper extremities. Moreover, by 24 months, the patient reported a substantial improvement of the paresthesias in his lower extremities, but a repeat NIS was not performed. In patients who had a significant clinical improvement (patient nos. 1, 4, 11, and 12), there was a good correlation with the improvement of summated CMAPs.
Response after PBSCT
. | No. . | Response, % . | Stable, % . | Not evaluated, % . |
|---|---|---|---|---|
| Neurologic | 16 | 87 | 0 | 13 |
| Subjective | 16 | 87 | 0 | 13 |
| Nerve conduction studies at 1 y | 11 | 36 | 9 | 55 |
| Neuropathy impairment score | 16* | 25 | 12 | 56 |
| Hematologic | 16 | 56 | 6 | 37 |
| Nonsecretory | 3 | NA | NA | 100 |
| Immunofixation only | 7 | 43 | 14 | 43 |
| SPEP evaluable | 6 | 100† | 0 | 0 |
| Organ | 11 | 82 | 0 | 18 |
| Hepatomegaly | 5 | 60 | 0 | 40 |
| Splenomegaly | 10 | 50 | 20 | 30 |
| Lymphadenopathy | 7 | 71 | 29 | 0 |
| Extravascular volume overload | 14 | 79 | 0 | 21 |
| Ascites | 6 | 83 | 0 | 17 |
| Effusion | 4 | 100 | 0 | 0 |
| Edema | 14 | 79 | 0 | 21 |
| Dermatologic | 13 | 54 | 15 | 31 |
| Papilledema | 4 | 50 | 0 | 50 |
| Endocrine | 13 | 23 | 8 | 69 |
| Pulmonary | 15 | 20 | 0 | 80 |
. | No. . | Response, % . | Stable, % . | Not evaluated, % . |
|---|---|---|---|---|
| Neurologic | 16 | 87 | 0 | 13 |
| Subjective | 16 | 87 | 0 | 13 |
| Nerve conduction studies at 1 y | 11 | 36 | 9 | 55 |
| Neuropathy impairment score | 16* | 25 | 12 | 56 |
| Hematologic | 16 | 56 | 6 | 37 |
| Nonsecretory | 3 | NA | NA | 100 |
| Immunofixation only | 7 | 43 | 14 | 43 |
| SPEP evaluable | 6 | 100† | 0 | 0 |
| Organ | 11 | 82 | 0 | 18 |
| Hepatomegaly | 5 | 60 | 0 | 40 |
| Splenomegaly | 10 | 50 | 20 | 30 |
| Lymphadenopathy | 7 | 71 | 29 | 0 |
| Extravascular volume overload | 14 | 79 | 0 | 21 |
| Ascites | 6 | 83 | 0 | 17 |
| Effusion | 4 | 100 | 0 | 0 |
| Edema | 14 | 79 | 0 | 21 |
| Dermatologic | 13 | 54 | 15 | 31 |
| Papilledema | 4 | 50 | 0 | 50 |
| Endocrine | 13 | 23 | 8 | 69 |
| Pulmonary | 15 | 20 | 0 | 80 |
Median follow-up was 10.8 months. One patient died before evaluation and another patient has not yet returned for evaluation. SPEP indicates serum protein electrophoresis.
One patient showed borderline worsening at 15 months. At 24 months, the patient reported substantial improvement; no repeat neuropathy impairment score was performed.
Two complete responses, 3 very good partial responses, and 1 minimal response.
Serial formal neuropathy testing. (A) Summated compound muscle action potential (ΣCMAP) amplitudes. (B) Neuropathy impairment score (NIS).
Serial formal neuropathy testing. (A) Summated compound muscle action potential (ΣCMAP) amplitudes. (B) Neuropathy impairment score (NIS).
Determining the hematologic response was complex. Before treatment, none of the patients had “measurable” disease by multiple myeloma criteria, that is, serum M-spike of 10 g/L or urine M-spike of 0.2 g/24 hours.10 There were 3 patients who had nonsecretory disease, only one of whom had clonal plasma cells on routine bone marrow aspirate; the other 2 had clonal plasma cells detected in a computed tomography-directed biopsy of a sclerotic bone lesion. These 3 patients were not evaluable for hematologic response. Of the 6 patients with immunofixation-positive disease, but no quantifiable M-spike, 3 became immunofixation negative (complete response), 1 remained immunofixation positive (stable), and 2 did not have repeat immunofixation studies (very good partial response versus stable). Of the 6 patients with a quantifiable serum M-spike (4 g/L-9.4 g/L), 5 had disappearance of the M-spike on protein electrophoresis. There were 2 patients who achieved a complete response, 1 achieved a very good partial response, and 2 did not have a repeat immunofixation performed to verify a complete remission and were therefore classified as having very good partial responses.
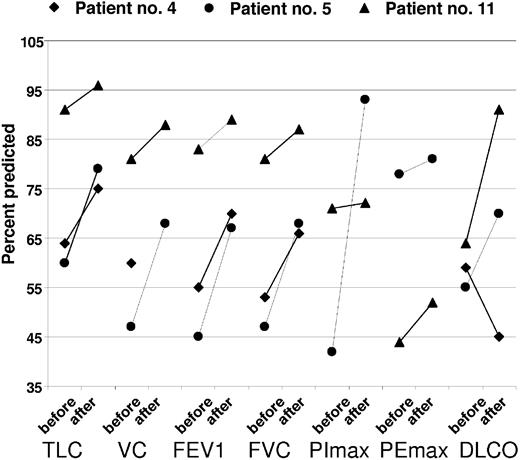
Organomegaly, extravascular volume overload, and skin changes have improved in a majority of affected patients (Table 6). Papilledema was improved in 2 of 4 patients. Nonspecific findings such as fatigue, performance status, and sense of well being also improved. There was limited information collected to characterize endocrine response. One patient regained erectile function and 2 other patients' testosterone levels normalized at 3 months and 17 months, respectively. Of the 3 patients with serial pulmonary function tests performed, there were objective improvements in all (Figure 4). One of the patients, who had spent more than a month in the intensive care unit, had an asymptomatic decline in his DLCO (patient no. 4). Patient no. 5, who required BiPAP through his transplantation and overnight CPAP with supplemental oxygen, now requires neither.
Comparison of patients in our series to those in the published literature
Patient no. (ref. no.) . | Pretransplantation features . | . | . | . | . | . | . | . | . | . | . | Response . | . | . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | Age, y/sex . | Polyneuropathy . | Organomegaly . | Endocrinopathy . | M protein . | Skin changes . | Bone . | Elevated platelets . | Ed/Ef . | Papilledema/Thrombosis . | Rx/TTTd, mo/TTTs, mo . | Follow-up after SCT, mos . | PCD . | Comments . | ||||||||||||
| 1 (4) | 44/M | SM | H | + | + | + | > 3 | − | − | −/− | PE, MP, RT/30/35 | 56 | CR | None | ||||||||||||
| 2 | 63/M | SM | S | + | + | + | > 3 | − | Ed | −/CVA | None/3/22 | 3.8 | Inevaluable | Death | ||||||||||||
| 3 | 20/M | SM | − | − | + | − | 1 | +* | − | −/− | RT, AD/5/8 | 32 | CR | Wheelchair to climbing stairs | ||||||||||||
| 4 | 40/M | SM | − | + | + | + | 0 | + | − | −/− | Pred, IVIG, Hydrea/2/16 | 32 | CR | None | ||||||||||||
| 5 | 43/M | SM | − | + | + | − | > 3 | + | − | −/− | RT, Dex/66/78 | 6 | VGPR | Pain improved | ||||||||||||
| 6 | 59/M | SM | − | + | + | + | 1 | − | Ed | −/− | PE, IVIG, Pred, RT/7/32 | 17 | NE | Wheelchair to AFO and occasional cane | ||||||||||||
| 7 | 53/M | M | SA | + | +, Cas | + | 1 | +* | Ed, Ef, A | −/Splenic vein thrombosis | Pred, Rituxan, Thalidomide, Remicade, CSA, IFN/33/73 | 18 | VGPR | None | ||||||||||||
| 8 | 53/M | SM | S | + | + | + | 1 | + | Ed, A | −/CVA | Pred/2/37 | 10 | CR | Pain and function improved | ||||||||||||
| 9 | 37/M | SM | HSA | + | +, Cas | + | > 3 | − | Ed, A | −/− | Steroids, RT/4/41 | 13 | ND | Wheelchair to basketball and jogging | ||||||||||||
| 10 | 50/F | SM | H | − | + | + | > 3 | + | Ed, Ef, A | −/− | PE, IVIG, Imuran, Pred, oral CTX, Hydrea, IFN/149/185 | 10 | ND | Wheelchair to walker, signing name, using spoon | ||||||||||||
| 11 | 53/M | SM | HS | + | + | + | > 3 | + | Ed, Ef, A | −/CVA | Pred, IVIG/2/37 | 7 | NE | None | ||||||||||||
| 12 | 36/M | SM | S | + | + | − | > 3 | − | Ed | −/− | Pred, IVIG/2/9 | 7 | MR | No more AFO | ||||||||||||
| 13 | 51/M | SM | HSA | + | + | + | 1 | + | Ed | −/− | Pred, IVIG, PE, Imuran, MTX, MMF/3/75 | 3 | CR | None | ||||||||||||
| 14 | 59/M | SM | HS | + | + | + | > 3 | − | −/CVA | None/2/26 | 1 | ND | None | |||||||||||||
| 15 | 60/M | SM | HSA | + | + | + | 1 | − | Ed, A | +/− | RT, Pred/7/67 | 3 | VGPR | Wheelchair to walker | ||||||||||||
| 16 | 50/M | SM | S | + | + | + | > 3 | + | Ed | +/− | CTX, Pred/5/9 | 1 | ND | None | ||||||||||||
| 17 (3) | 25/F | SM | + | + | + | + | > 1 | NR | As | +/− | Pred, RT/−/− | 2 | Inevaluable | Death | ||||||||||||
| 18 (8) | 44/F‡ | M | S | − | + | − | > 1 | − | − | +/− | RT, CSA, Imurel, PE/−/− | 58§ | CR | Improved VEGF levels | ||||||||||||
| 19 (8) | 52/M‡ | S | − | + | + | + | > 1 | − | Ed | †/− | None/−/− | 49§ | CR | Improved VEGF levels | ||||||||||||
| 20 (8) | 50/M∥ | S | HSA | + | + | + | 0 | − | Ed | †/− | CTX/−/− | 32§ | VGPR | None | ||||||||||||
| 21 (8) | 62/M | SM | − | + | + | + | > 1 | − | Ed | †/− | PE, RT/−/− | 12§ | CR | None | ||||||||||||
| 22 (8) | 54/M | S | H | + | + | ±¶ | 1 | − | − | −/− | RT/−/− | 12§ | CR | None | ||||||||||||
| 23 (9) | 58/F | SM | − | + | + | + | > 3 | + | − | −/− | VMCP, MP/−/− | 12 | CR | None | ||||||||||||
| 24 (6) | 43/M | SM | − | + | + | + | 1 | + | − | +/− | PE, CS, RT, AMP, CTX-CS/−/25 | 120 | CR | Improved VEGF levels | ||||||||||||
| 25 (6) | 59/F | S | HSA | − | +♯ | + | 3 | +* | − | −/− | RT, CTX-CS/18/− | 6 | CR | Improved VEGF levels | ||||||||||||
| 26 (7) | 56/M | SM | + | + | + | − | 1 | — | − | −/− | IVIG, CS, RT/−/− | 24 | VGPR | None | ||||||||||||
| 27 (7) | 38/F | SM | + | + | + | + | 1 | — | + | +/− | VAMP/5/− | 6 | CR | None | ||||||||||||
| 28 (5)** | 30 | SM | HA | − | −** | − | > 3 | + | Ed/Ef | −/− | MP, Tam, Ifos/−/24 | 72 | Inevaluable | Not documented POEMS | ||||||||||||
Patient no. (ref. no.) . | Pretransplantation features . | . | . | . | . | . | . | . | . | . | . | Response . | . | . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | Age, y/sex . | Polyneuropathy . | Organomegaly . | Endocrinopathy . | M protein . | Skin changes . | Bone . | Elevated platelets . | Ed/Ef . | Papilledema/Thrombosis . | Rx/TTTd, mo/TTTs, mo . | Follow-up after SCT, mos . | PCD . | Comments . | ||||||||||||
| 1 (4) | 44/M | SM | H | + | + | + | > 3 | − | − | −/− | PE, MP, RT/30/35 | 56 | CR | None | ||||||||||||
| 2 | 63/M | SM | S | + | + | + | > 3 | − | Ed | −/CVA | None/3/22 | 3.8 | Inevaluable | Death | ||||||||||||
| 3 | 20/M | SM | − | − | + | − | 1 | +* | − | −/− | RT, AD/5/8 | 32 | CR | Wheelchair to climbing stairs | ||||||||||||
| 4 | 40/M | SM | − | + | + | + | 0 | + | − | −/− | Pred, IVIG, Hydrea/2/16 | 32 | CR | None | ||||||||||||
| 5 | 43/M | SM | − | + | + | − | > 3 | + | − | −/− | RT, Dex/66/78 | 6 | VGPR | Pain improved | ||||||||||||
| 6 | 59/M | SM | − | + | + | + | 1 | − | Ed | −/− | PE, IVIG, Pred, RT/7/32 | 17 | NE | Wheelchair to AFO and occasional cane | ||||||||||||
| 7 | 53/M | M | SA | + | +, Cas | + | 1 | +* | Ed, Ef, A | −/Splenic vein thrombosis | Pred, Rituxan, Thalidomide, Remicade, CSA, IFN/33/73 | 18 | VGPR | None | ||||||||||||
| 8 | 53/M | SM | S | + | + | + | 1 | + | Ed, A | −/CVA | Pred/2/37 | 10 | CR | Pain and function improved | ||||||||||||
| 9 | 37/M | SM | HSA | + | +, Cas | + | > 3 | − | Ed, A | −/− | Steroids, RT/4/41 | 13 | ND | Wheelchair to basketball and jogging | ||||||||||||
| 10 | 50/F | SM | H | − | + | + | > 3 | + | Ed, Ef, A | −/− | PE, IVIG, Imuran, Pred, oral CTX, Hydrea, IFN/149/185 | 10 | ND | Wheelchair to walker, signing name, using spoon | ||||||||||||
| 11 | 53/M | SM | HS | + | + | + | > 3 | + | Ed, Ef, A | −/CVA | Pred, IVIG/2/37 | 7 | NE | None | ||||||||||||
| 12 | 36/M | SM | S | + | + | − | > 3 | − | Ed | −/− | Pred, IVIG/2/9 | 7 | MR | No more AFO | ||||||||||||
| 13 | 51/M | SM | HSA | + | + | + | 1 | + | Ed | −/− | Pred, IVIG, PE, Imuran, MTX, MMF/3/75 | 3 | CR | None | ||||||||||||
| 14 | 59/M | SM | HS | + | + | + | > 3 | − | −/CVA | None/2/26 | 1 | ND | None | |||||||||||||
| 15 | 60/M | SM | HSA | + | + | + | 1 | − | Ed, A | +/− | RT, Pred/7/67 | 3 | VGPR | Wheelchair to walker | ||||||||||||
| 16 | 50/M | SM | S | + | + | + | > 3 | + | Ed | +/− | CTX, Pred/5/9 | 1 | ND | None | ||||||||||||
| 17 (3) | 25/F | SM | + | + | + | + | > 1 | NR | As | +/− | Pred, RT/−/− | 2 | Inevaluable | Death | ||||||||||||
| 18 (8) | 44/F‡ | M | S | − | + | − | > 1 | − | − | +/− | RT, CSA, Imurel, PE/−/− | 58§ | CR | Improved VEGF levels | ||||||||||||
| 19 (8) | 52/M‡ | S | − | + | + | + | > 1 | − | Ed | †/− | None/−/− | 49§ | CR | Improved VEGF levels | ||||||||||||
| 20 (8) | 50/M∥ | S | HSA | + | + | + | 0 | − | Ed | †/− | CTX/−/− | 32§ | VGPR | None | ||||||||||||
| 21 (8) | 62/M | SM | − | + | + | + | > 1 | − | Ed | †/− | PE, RT/−/− | 12§ | CR | None | ||||||||||||
| 22 (8) | 54/M | S | H | + | + | ±¶ | 1 | − | − | −/− | RT/−/− | 12§ | CR | None | ||||||||||||
| 23 (9) | 58/F | SM | − | + | + | + | > 3 | + | − | −/− | VMCP, MP/−/− | 12 | CR | None | ||||||||||||
| 24 (6) | 43/M | SM | − | + | + | + | 1 | + | − | +/− | PE, CS, RT, AMP, CTX-CS/−/25 | 120 | CR | Improved VEGF levels | ||||||||||||
| 25 (6) | 59/F | S | HSA | − | +♯ | + | 3 | +* | − | −/− | RT, CTX-CS/18/− | 6 | CR | Improved VEGF levels | ||||||||||||
| 26 (7) | 56/M | SM | + | + | + | − | 1 | — | − | −/− | IVIG, CS, RT/−/− | 24 | VGPR | None | ||||||||||||
| 27 (7) | 38/F | SM | + | + | + | + | 1 | — | + | +/− | VAMP/5/− | 6 | CR | None | ||||||||||||
| 28 (5)** | 30 | SM | HA | − | −** | − | > 3 | + | Ed/Ef | −/− | MP, Tam, Ifos/−/24 | 72 | Inevaluable | Not documented POEMS | ||||||||||||
Patient nos. 1 through 16 are from our study cohort; patient nos. 17 through 28 are patients studied in the published literature. Ed indicates edema; Ef, effusion; Rx, treatments; TTTd, time to transplant from diagnosis; TTTs, time to transplant from symptoms; SCT, stem cell transplant; PCD, plasma cell disorder; M, motor; SM, sensorimotor; H, hepatomegaly; +, present; −, absent; PE, plasmapheresis; MP, melphalan and prednisone; RT, radiation therapy; CR, complete response; S, splenomegaly; CVA, cerebrovascular accident; AD, adriamycin and dexamethasone; Pred, prednisone; IVIG, intravenous gammaglobulin; Hydrea, hydroxyurea; Dex, dexamethasone; VGPR, very good partial response; NE, not evaluable; AFO, ankle foot orthotic; SL, splenomegaly and hepatomegaly; Cas, Castleman disease; A, ascites; CSA, cyclosporin; IFN, interferon; LSA, hepatomegaly, splenomegaly, lymphadenopathy; ND, not done; F, female; CTX, cyclophosphamide; HS, hepatomegaly and splenomegaly; MR, minimal response; MTX, methotrexate; MMF, mycophenolate mofetil; VEGF, vascular endothelial cell-derived growth factor; VMCP, vincristine, mephalan, cyclophosphamide, and prednisone; CS, corticosteroids; (V)AMP, vincristine, adriamycin, and methylprednisolone; Tam, tamoxifen; Ifos, ifosfamide.
Erythrocytosis.
There were 2 patients said to have papilledema, but it was not indicated which 2.
Patients received tandem transplantation.
Follow-up calculated from PBSC collection rather than after transplantation. Median follow-up after transplantation for whole group is 36 months.
Patient also had nephropathy.
Though listed by authors, telangectasia not a recognized skin feature of POEMS.
Monoclonal kappa.
Patient with osteosclerotic myeloma (OSM), but unclear basis of diagnosis since no documentation of a plasma cell proliferative disorder by biopsy or immunofixation.
Serial pulmonary function tests before and after transplantation, percent predicted. TLC, total lung capacity; VC, vital capacity; FEV1, forced expiratory volume at 1 sect; FVC; forced vital capacity; PImax, maximum inspiratory pressure; PEmax, maximal expiratory pressure; DLCO; diffusing capacity of carbon monoxide; pre, before transplantation; post, after transplantation.
Serial pulmonary function tests before and after transplantation, percent predicted. TLC, total lung capacity; VC, vital capacity; FEV1, forced expiratory volume at 1 sect; FVC; forced vital capacity; PImax, maximum inspiratory pressure; PEmax, maximal expiratory pressure; DLCO; diffusing capacity of carbon monoxide; pre, before transplantation; post, after transplantation.
Discussion
The use of high-dose chemotherapy with peripheral blood stem cell support for plasma cell disorders is not novel. PBSCT has been used as a therapeutic strategy for patients with myeloma for more than 20 years13,14 and for primary systemic amyloidosis for nearly 10 years.15 Randomized trials have repeatedly demonstrated superior response rates and event-free and overall survival in the case of multiple myeloma.11,16 Several phase 2 trials17,18 and a recent case control study19 suggest that there is a survival benefit in patients with primary systemic amyloidosis undergoing PBSCT. It is therefore a reasonable assumption that this strategy would be beneficial in patients with POEMS—yet another condition driven by an abnormal plasma cell clone.
The first case report of high-dose chemotherapy with hematopoietic stem cell support was by Wong and Wade.3 The patient died of transplant-related complications. Our case report in 2001 was the first published report of a good outcome after PBSCT in a patient with POEMS syndrome (Table 6, patient no. 1).4 Since then, there have been 11 patients reported,6-9 one of whom does not appear to have a bona fide case5 (Table 7). The cumulative experience demonstrates that PBSCT is an effective therapeutic option for patients with POEMS syndrome. All authors reported a universal improvement in neuropathy and other features6-9 with the exception of endocrine traits.7,8 Not only does our series of 16 patients more than double the collective world experience treating POEMS patients with PBSCT, it also elicits several novel points. First, though patients with POEMS achieve dramatic clinical improvement with this therapeutic modality, there can be significant morbidity associated with the procedure. Second, poor pulmonary reserve is an important source of procedure-related morbidity. Third, outcomes and indications must be better defined. This final point is critical since there are few systematic reviews of treatment outcomes1,20,21 and no prospective studies for this disease.
Recommended minimum testing
Test . | Baseline . | Every 3 months . | Yearly . |
|---|---|---|---|
| Neurologic | |||
| Detailed neurologic history (numbness, pain, weakness, balance, orthostasis) and examination (including funduscopic exam) | X | X* | X |
| Electrophysiologic study (nerve conduction studies) | X | X* | X |
| Organomegaly/lymphadenopathy/extravascular volume overload | |||
| Physical exam documenting lymphadenopathy, organomegaly, ascites, pleural effusions, and edema | X | X | X |
| Computed tomography of chest/abdomen/pelvis: lymphadenopathy, organomegaly, ascites, and effusions | X | X† | X† |
| Endocrinopathy | |||
| History regarding menstrual and sexual function | X | X | X |
| Testosterone, estradiol, fasting glucose, glycosylated hemoglobin, thyroid stimulating hormone, and serum cortisol (morning and evening) | X | X† | X† |
| Lutenizing hormone, follicle stimulating hormone, prolactin, adrenocorticotropin hormone, parathyroid hormone, and Cortrosyn stimulation test | X‡ | X†‡ | X†‡ |
| Hematologic | |||
| Serum protein electrophoresis and immunofixation | X | X | X |
| Affected quantitative immunoglobulin | X | X | X |
| Complete blood count (hemoglobin, platelet) | X | X | X |
| 24-hour urine total protein, electrophoresis, and immunofixation | X | X | X |
| Bone marrow aspirate and biopsy (test for kappa/lambda by immunohistochemistry or flow cytometry or PCL1) | X | X | |
| Skin | |||
| History regarding skin pigment, thickening and texture, body hair quantity and texture, color of distal extremities, and development of cherry angiomata | X | X | X |
| Physical examination with attention to skin pigment, thickening and texture, body hair quantity and texture, color of distal extremities, and development of cherry angiomata | X | X | X |
| Sclerotic bone lesions | |||
| Skeletal radiographs | X | X | |
| Computed tomography of bones | X§ | ||
| Positron emission tomography | X‡ | X‡ | |
| Bone scan | X‡ | X‡ | |
| Pulmonary function | |||
| Pulmonary function tests including TLC, VC, FEV1, FVC, maximum inspiratory and expiratory pressures, and diffusing capacity of carbon monoxide | X | X† | X† |
| Echocardiography to assess right ventricular systolic and pulmonary artery pressures | X | X† | X† |
Test . | Baseline . | Every 3 months . | Yearly . |
|---|---|---|---|
| Neurologic | |||
| Detailed neurologic history (numbness, pain, weakness, balance, orthostasis) and examination (including funduscopic exam) | X | X* | X |
| Electrophysiologic study (nerve conduction studies) | X | X* | X |
| Organomegaly/lymphadenopathy/extravascular volume overload | |||
| Physical exam documenting lymphadenopathy, organomegaly, ascites, pleural effusions, and edema | X | X | X |
| Computed tomography of chest/abdomen/pelvis: lymphadenopathy, organomegaly, ascites, and effusions | X | X† | X† |
| Endocrinopathy | |||
| History regarding menstrual and sexual function | X | X | X |
| Testosterone, estradiol, fasting glucose, glycosylated hemoglobin, thyroid stimulating hormone, and serum cortisol (morning and evening) | X | X† | X† |
| Lutenizing hormone, follicle stimulating hormone, prolactin, adrenocorticotropin hormone, parathyroid hormone, and Cortrosyn stimulation test | X‡ | X†‡ | X†‡ |
| Hematologic | |||
| Serum protein electrophoresis and immunofixation | X | X | X |
| Affected quantitative immunoglobulin | X | X | X |
| Complete blood count (hemoglobin, platelet) | X | X | X |
| 24-hour urine total protein, electrophoresis, and immunofixation | X | X | X |
| Bone marrow aspirate and biopsy (test for kappa/lambda by immunohistochemistry or flow cytometry or PCL1) | X | X | |
| Skin | |||
| History regarding skin pigment, thickening and texture, body hair quantity and texture, color of distal extremities, and development of cherry angiomata | X | X | X |
| Physical examination with attention to skin pigment, thickening and texture, body hair quantity and texture, color of distal extremities, and development of cherry angiomata | X | X | X |
| Sclerotic bone lesions | |||
| Skeletal radiographs | X | X | |
| Computed tomography of bones | X§ | ||
| Positron emission tomography | X‡ | X‡ | |
| Bone scan | X‡ | X‡ | |
| Pulmonary function | |||
| Pulmonary function tests including TLC, VC, FEV1, FVC, maximum inspiratory and expiratory pressures, and diffusing capacity of carbon monoxide | X | X† | X† |
| Echocardiography to assess right ventricular systolic and pulmonary artery pressures | X | X† | X† |
TLC indicates total lung capacity; VC, vital capacity; FEV1, forced expiratory volume at 1 sect; FVC; forced vital capacity.
At 6 months and then yearly.
Only if affected.
Utility not yet clearly defined.
As clinically indicated.
Effectiveness of peripheral blood stem cell transplantation for patients with POEMS
Our data demonstrate that the peripheral neuropathy improves after PBSCT over a period of months to years. Because this study is retrospective, formal serial neurologic evaluations, including neurology impairment scores and nerve conduction studies, were not done systematically in all patients. There was, however, a dramatic improvement in patients with regards to their functional ability. This improvement is gradual, and continued to improve at 2 to 3 years after PBSCT. Organ responses occurred in at least 82% of patients with organomegaly or lymphadenopathy and in at least 79% of patients with manifestations of extravascular volume overload. Other less disabling features such as dermatologic and endocrine abnormalities were much harder to assess in the context of a retrospective study, but there were improvements in at least 54% and 23% of patients, respectively. Respiratory function improved in the 3 patients with serial pulmonary function tests. Others have reported “improvement” in features such as plasma cell burden, neuropathy, ascites, organomegaly, vascular-derived endothelial growth factor levels, and skin changes.6-9 The clinical response observed in all of our evaluable patients compares favorably with our prior experience with conventional melphalan and prednisone (55% response rate) or prednisone alone (20% response rate).1
Morbidity and mortality associated with peripheral blood stem cell transplantation for patients with POEMS
Although Jaccard et al8 suggest in their report of 5 patients with POEMS who underwent transplantation that there may not be higher risk than PBSCT in patients with classic multiple myeloma, we differ with this opinion based on our own experience and a review of the literature. In our study cohort, 37% of our patients spent time in the intensive care unit and 37% required mechanical ventilation. Although only one of our patients died (6.2% mortality rate), if the published experience of POEMS patients who have undergone transplantation is pooled, the mortality figure is 2 of 27, or 7.4%. These numbers appear higher than the 2% transplant-related mortality observed in patients with multiple myeloma11 but lower than the 14% transplant-related mortality observed in patients with primary systemic amyloidosis.17
Defining outcomes and indications for peripheral blood stem cell transplantation
The outcomes of our study and those of others are largely descriptive. Determining response in patients with POEMS syndrome is challenging for 2 reasons. First, POEMS is a rare disease and prospective studies with comprehensive serial testing and data collection are lacking; second, POEMS is a multisystem disease, with no defined response criteria. Most patients do not have a quantifiable M protein, as is the case in myeloma. Without clear measurements of response, making evidence-based therapeutic recommendations for patients is not possible. Moreover, the following important questions cannot be answered. Which aspects of the syndrome improve? How long do these improvements last? How does PBSCT fit into the treatment plan for individual patients? Which patients are at excess risk of complication of PBSCT? Although the high rates of clinical improvement observed to date are compelling, the long-term risks of PBSCT in these patients are not yet known. The first-line therapy for a POEMS patient with a solitary sclerotic plasmacytoma in the absence of clonal plasma cells on blind iliac crest aspirate has yet to be defined, but we would recommend tumoricidal external beam irradiation to the affected area.
How then can the state of the art be improved for patients with POEMS? More uniform study of these patients with attention toward the underlying pathogenesis of the disease would be a major advance. Table 7 is a recommended test schedule useful for collecting pertinent clinical data. Although the challenge of defining response is formidable in this patient cohort, response criteria have been established for primary systemic amyloidosis22,23 as well as for multisystem diseases like systemic lupus erythematosus24,25 and scleroderma.26 Until such criteria are defined and validated for the POEMS syndrome, it will be difficult to differentiate between a lack of therapeutic efficacy and a lack of sensitivity in the outcome measures used. This difficulty will be further compounded in patients with POEMS by the delayed response of the dominant feature of the syndrome, that is, the peripheral neuropathy.
In summary, PBSCT for POEMS syndrome results in clinical improvement in a majority of patients and should be considered as a therapeutic option in these patients though durability of benefit has not yet been defined. Prompt and accurate diagnosis of this syndrome is essential, and consideration of referral to specialized treatment centers is recommended. Delays in the diagnosis are associated with increased morbidity for the patient. Care must be taken in differentiating the POEMS syndrome from a monoclonal gammopathy of undetermined significance (MGUS)-associated neuropathy in order to avoid putting this latter population at unnecessary risk for excess morbidity and mortality.
Prepublished online as Blood First Edition Paper, July 27, 2004; DOI 10.1182/blood-2004-05-2046.
Supported in part by CA 62242 (R.A.K) from the National Cancer Institute.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank other members of the Dysproteinemia and Blood and Marrow Transplant groups for their excellent care of the patients.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal