Abstract
The bleeding diathesis associated with hereditary factor XI (fXI) deficiency is prevalent in Ashkenazi Jews, in whom the disorder appears to be an autosomal recessive condition. The homodimeric structure of fXI implies that the product of a single mutant allele could confer disease in a dominant manner through formation of heterodimers with wild-type polypeptide. We studied 2 unrelated patients with fXI levels less than 20% of normal and family histories indicating dominant disease transmission. Both are heterozygous for single amino acid substitutions in the fXI catalytic domain (Gly400Val and Trp569Ser). Neither mutant is secreted by transfected fibroblasts. In cotransfection experiments with a wild-type fXI construct, constructs with mutations common in Ashkenazi Jews (Glu117Stop and Phe283Leu) and a variant with a severe defect in dimer formation (fXI-Gly350Glu) have little effect on wild-type fXI secretion. In contrast, cotransfection with fXI-Gly400Val or fXI-Trp569Ser reduces wild-type secretion about 50%, consistent with a dominant negative effect. Immunoprecipitation of cell lysates confirmed that fXI-Gly400Val forms intracellular dimers. The data support a model in which nonsecretable mutant fXI polypeptides trap wild-type polypeptides within cells through heterodimer formation, resulting in lower plasma fXI levels than in heterozygotes for mutations that cause autosomal recessive fXI deficiency.
Introduction
Coagulation factor XI (fXI) is the zymogen of a plasma protease (fXIa) that contributes to hemostasis through activation of factor IX.1 Hereditary fXI deficiency is an autosomal disorder characterized by trauma or surgery-induced hemorrhage and only rarely by “spontaneous” bleeding into soft tissues or joints.2,3 The condition is prevalent in Ashkenazi Jews, in whom the heterozygote frequency may be as high as 10%.4,5 Two mutations, Glu117Stop and Phe283Leu, account for most of the abnormal alleles in this population.2,5 Reports on inheritance patterns for fXI deficiency have been conflicting. First described by Rosenthal and coworkers in 1953 as plasma thromboplastin antecedent (PTA) deficiency,6 fXI deficiency was initially considered an autosomal dominant disorder with variable expressivity based on clinical symptoms and family histories.7-9 However, Rapaport et al observed that PTA deficiency exists as major (homozygous) and minor (heterozygous) variants and is best described as following an incompletely recessive or “intermediate” mode of inheritance, with few symptoms occurring in heterozygotes.10 These conclusions, based on measurements of plasma fXI activity primarily in Jewish kindreds, demonstrated distinct ranges for major (fXI level up to 20% of normal) and minor (fXI 30%-65% of normal) PTA deficiency. Subsequent studies supported this work4,11 and led to the general opinion that fXI deficiency in Jewish patients is a recessive disorder.12
Not all cases of fXI deficiency are consistent with a simple autosomal recessive model. Ragni and colleagues studied 25 fXI-deficient kindreds, half of whom were of non-Jewish ancestry, and noted poor correlation between plasma fXI levels and bleeding symptoms.13 Indeed, it was not possible to distinguish severely deficient patients (presumed homozygotes) from carriers (presumed heterozygotes) based on symptoms in this study. Poor correlation between fXI levels and bleeding symptoms has, in fact, been noted in Jewish patients by several groups.11,13,14 Furthermore, there are families in whom transmission from a single affected parent to child appears to be consistent with a dominant form of transmission.8,14-16 While several factors may account for bleeding in these families, it seems likely that the nature of the underlying mutation contributes in some cases. Saito et al made the interesting observation that non-Jewish patients with homozygous and heterozygous fXI deficiency have fXI activity and antigen levels that tend to be lower than their respective Jewish counterparts.17 This is consistent with mutations in non-Jewish families having different effects from those associated with deficiency in Jewish patients.
FXI has structural features that distinguish it from other coagulation proteases. The fXI polypeptide comprises an N-terminal noncatalytic region containing 4 repeats called apple domains (A1 to A4, from the N-terminus) and a C-terminal trypsin-like catalytic domain.18,19 The mature fXI molecule is a dimer composed of 2 of these polypeptides connected by a single disulfide bond,18-20 with the A4 domain playing a critical role in dimer formation.19,21 The functional importance of the dimeric structure is not completely understood; however, it appears to be a prerequisite for normal secretion from cells.22 Hypothetically, a mutation in one fXI gene could produce dominant disease through secretion of mutant homodimers as well as heterodimers with wild-type fXI polypeptide. This mechanism has been described in type II von Willebrand disease23 and some dysfibrinogenemias,24 2 other disorders of multimeric proteins. However, the premise that this occurs in fXI deficiency is not supported by the observation that cross-reactive material–positive (CRM+) variants of fXI are very rare.14,17,25 Alternatively, a dominant inheritance pattern might be caused by mutant fXI polypeptides forming nonsecretable heterodimers within hepatocytes, effectively trapping a substantial portion of wild-type fXI polypeptide within the cell. Such a mechanism has been demonstrated for a CRM-negative mutation responsible for severe type I von Willebrand disease26,27 and could explain non-Jewish families with unusually low fXI levels in heterozygotes.
In this report we describe 2 patients with severe deficiencies of plasma fXI activity and antigen (less than 20%), heterozygosity for a fXI gene mutation, and family histories suggestive of direct transmission from a single affected parent to an affected child (dominant transmission). We demonstrate through cotransfection experiments that the mutations involved cause decreased secretion of wild-type fXI from transfected cells, consistent with a dominant negative effect on wild-type fXI secretion through intracellular heterodimer formation.
Materials and methods
Identification of mutations in the fXI gene
Genomic DNA was isolated from peripheral blood collected into EDTA (ethylenediaminetetraacetic acid) using a QIAamp DNA blood kit (Qiagen, Valencia, CA). Exons of the fXI gene were amplified by PCR as described,15 size fractionated on 2% NuSieve agarose gels (FMC BioProducts, Rockland, ME), and purified with a QIAEX II gel extraction kit (Qiagen). Dideoxyfingerprinting (ddF) was performed by the method of Martincic et al.15,28 Primers used for polymerase chain reactions (PCRs) were end-labeled with γ-32P–deoxyadenosine triphosphate (γ-32P–dATP) and T4 polynucleotide kinase. Reactions (8.0 μL volume) contained 20 mM Tris (tris(hydroxymethyl)aminomethane)–HCl (pH 8.4), 50 mM KCl, 2 mM MgCl2,20 μM of each deoxyribonucleoside triphosphate (dNTP), 100 μM dideoxy–guanosine triphosphate (dideoxy-GTP), 1 pmol 32P-labeled primer, 0.25 units of Taq DNA Polymerase, and 5 to 15 ng of PCR fragment. Reactions were carried out on a Perkin-Elmer (Norwalk, CT) model 460 DNA Thermal Cycler using the following cycling parameters: denaturation at 94° C for 45 seconds, annealing at 60° C for 30 seconds, and extension at 72° C for 1 minute for 30 cycles. Reactions were mixed with 16 μL loading buffer (98% formamide, 10 mM EDTA) and subjected to electrophoresis at 30 W for 3 hours on a 0.5 × MDE nonreducing gel (FMC BioProducts) in 0.5 × Tris–boric acid–EDTA buffer. Gels were dried and exposed to x-ray film overnight. DNA sequences of PCR fragments with ddF patterns that differed from controls were determined using a Thermo Sequenase Radio-labeled Terminator Cycle Sequencing kit (Amersham Pharmacia, Piscataway, NJ).
Factor XI expression constructs
Expression constructs for human wild-type fXI and fXI with the A2 domain replaced with the prekallikrein (PK) A2 domain (fXI/PKA2) in vector pJVCMV have been reported.29,30 Point mutations were introduced into the wild-type fXI cDNA using a Chameleon Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA). Sequences of oligonucleotides used for mutagenesis are as follows: Gly400Val, 5′-GTTTCCAATGATGGAGACTCCACACAGGTGTCTCTG-3′; Trp569Ser, 5′-CTACCAGATGCGAGACCTCATTG-3′; Glu117Stop, 5′-CTTTCTTGGCATTATTGAGCACTC-3′; Phe283Leu, 5′-CTTCTCCCAAGAGATCAGTGTC-3′; and Gly350Glu, 5′-GGAGGCATCTCTGAATACACATTAAGG-3′. Mutations were confirmed by DNA sequencing using the Thermo Sequenase kit (Amersham Pharmacia).
FXI expression in transient transfections
Human embryonic kidney 293 fibroblasts (American Type Culture Collection [ATCC CRL 1573], Rockville, MD) were grown in Dulbecco modified Eagle medium (DMEM) supplemented with sodium pyruvate, l-glutamine, 5% (vol/vol) fetal bovine serum (FBS), and penicillin/streptomycin in a humidified 5% CO2 atmosphere at 37° C. Transient transfections were performed in 6-well Falcon culture plates (Becton Dickinson, Franklin Lakes, NJ) using SuperFect transfection reagent (Qiagen). Transfections contained 80 ng (single transfections) or 160 ng (cotransfections) fXI expression constructs and contained empty pJVCMV vector to bring the total DNA for each transfection to 2 μg. Transfections also included 40 ng of a construct for expression of Renilla luciferase (pRL-cytomegalovirus [pRL-CMV]; Promega, Madison, WI) to control for transfection efficiency. Sixty-seven hours after transfection, samples of culture media were collected and stored at –20° C pending enzyme-linked immunosorbent assay (ELISA). Cell lysates were prepared using a Dual Luciferase Reporter kit (Promega), and luciferase activity was measured on a Monolight 2010 luminometer (Analytical Luminescence Laboratory, San Diego, CA). Cell lysates were stored at –20° C pending ELISA.
Enzyme-linked immunosorbent assays (ELISAs) for fXI
FXI in culture media and cell lysates was measured by ELISA using a goat polyclonal antihuman fXI capture antibody and the same antibody conjugated to horseradish peroxidase (HRP) for detection (Affinity Biologicals, Hamilton, ON, Canada) according to the supplier's recommendations. All assays were performed in 96-well Immulon 4 HBX flat-bottom microtiter plates (ThermoLabSystems, Franklin, MA). For transfections with the fXI/PKA2 construct, the capture antibody was either a mixture of goat antihuman fXI and anti-PK polyclonal antibodies (Affinity Biologicals) or the murine antihuman fXI monoclonal antibody 1A6.29 Detection antibody for fXI/PKA2 transfections was a mixture of HRP-conjugated goat polyclonal antibodies against fXI and PK. After the final wash, 100 μL substrate solution (8.8 mM H2O2, 3 mM o-Phenylenediamine [OPD; Sigma, St Louis, MO], 25 mM citric acid, 97 mM Na2HPO4; pH 5.0) was added to each well. Reactions were stopped after 10 minutes with 50 μLof 2.5 M H2SO4, and absorbance at 495 nm was measured on a Thermomax Microplate Reader (Molecular Devices, Sunnyvale, CA). Each sample was measured in triplicate and the mean taken as the value for that transfection. Concentrations of fXI were corrected for variation in transfection efficiency as determined by Renilla luciferase signal and are reported as relative fXI level, with the mean for wild-type transfections arbitrarily assigned a value of 100%.
Intracellular fXI dimer formation studied by metabolic labeling
An expression construct consisting of the human fXI cDNA in vector pZEM229R has been described.22 Site-directed mutagenesis to introduce the Phe283Leu, Gly350Glu, or Gly400Val substitutions in this construct used primers 5′-GACACTGATCTCTTGGGAGAA-3′, 5′-GGAGGCATCTCTGAATACACATTAAGG-3′, and 5′-CTGTGTGGAGTCTCCATCATT-3′, respectively. Thymidine kinase–deficient baby hamster kidney (BHK) cells (ATCC; CRL 10314) growing in 150-mm plates in DMEM, 5% fetal calf serum (FCS), and penicillin/streptomycin (PCN/strep) were transfected with 30 μg of construct by calcium phosphate precipitation.22 After 24 hours, medium was replaced with fresh medium containing 1 μmol methotrexate for 7 to 10 days. Methotrexate-resistant clones were grown to confluence in 100-mm dishes.
Metabolic labeling studies were performed as described by Meijers et al.22 Clones expressing fXI or control were grown in 6-well culture plates in cysteine-free minimum essential media and 50 to 100 μCi/mL (1.85-3.7 MBq/mL) [35S]–l-cysteine (Amersham Pharmacia) in a 5% CO2 atmosphere at 37° C for 24 hours. Cells were washed twice with phosphate-buffered saline (PBS) and then lysed with RIPA (10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid] [pH 7.5], 50 mM NaCl, 5 mM EDTA, 0.3 M sucrose, 1% Triton X-100, 0.5% deoxycholate, 0.2% sodium dodecyl sulfate [SDS]) for 1 minute. Lysates were precleared for 1 hour at 4° C with 10 μL of a 1:1 suspension of goat antimouse immunoglobulin G (IgG) coupled to agarose, followed by centrifugation. Antihuman factor XI monoclonal IgG XI-522 (1 μL) was added to supernatants, followed by incubation on ice for 1 hour. The 1:1 suspension of goat antimouse IgG coupled to agarose (10 μL) was added, and incubation was continued for 2 hours at 4° C. Agarose was washed 3 times with 50 mM Tris-HCl (pH 7.4), 50 mM NaCl, and 0.1% Nonidet P-40 (NP-40) and once with 50 mM Tris-HCl (pH 7.4), 50 mM NaCl, followed by elution for 1 hour at 37° C with 50 μL buffer containing 5% SDS. Eluted material was size fractionated on a 7.5% polyacrylamide SDS gel, followed by drying and autoradiography.
Results
FXI-deficient patients
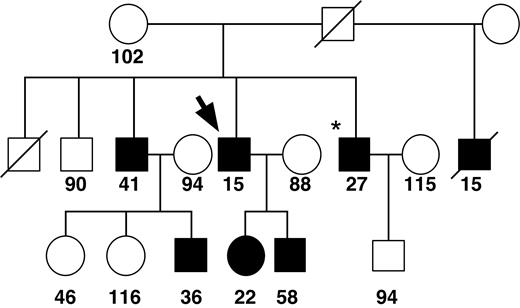
Patient no. 1 is a 53-year-old man of Italian and Czechoslovakian heritage, with a history of excessive bleeding after surgery and tooth extraction. His plasma fXI activity has been measured at 15% of normal on several occasions, with comparably decreased fXI antigen. This patient's family was originally reported by Litz and colleagues in 1988 (Figure 1).16 Several family members experienced excessive postoperative bleeding or gingival/nose bleeds associated with fXI activities of 15% to 58% of normal.16 The patient's 2 children have abnormal bleeding (fXI levels 22% and 58%), while their mother is asymptomatic, with normal fXI activity.16 Analysis of the patient's fXI genes revealed heterozygosity for a G>T transition at base pair 1296 (cDNA sequence18 ) in exon 11, resulting in valine substituting for the wild-type glycine at amino acid 400 (Gly400Val). No other changes were identified. FXI-Gly400Val was previously identified in a Japanese patient and named fXI-Nagoya III (Rei Asakai, personal written communication, August 2003). Subsequently, we identified a Chinese patient with frequent gingival bleeding and severe fXI deficiency (activity less than 2% of normal) who is homozygous for Gly400Val. Her parents, who do not have abnormal bleeding, are first cousins and have plasma fXI activities of 27% to 35% of normal. Her son is heterozygous for Gly400Val and has excessive gingival bleeding.
Family pedigree for patient 1 (fXI-Gly400Val). The bold arrow indicates patient no. 1, while the asterisk indicates the original proband from the study by Litz et al.16 Symbols filled in black represent family members with bleeding symptoms. Plasma fXI activity levels are indicated below each symbol.16 A diagonal slash indicates that the person is deceased. The figure is modified from Litz et al16 and reprinted by permission of Wiley-Liss Inc, a subsidiary of John Wiley & Sons Inc.
Family pedigree for patient 1 (fXI-Gly400Val). The bold arrow indicates patient no. 1, while the asterisk indicates the original proband from the study by Litz et al.16 Symbols filled in black represent family members with bleeding symptoms. Plasma fXI activity levels are indicated below each symbol.16 A diagonal slash indicates that the person is deceased. The figure is modified from Litz et al16 and reprinted by permission of Wiley-Liss Inc, a subsidiary of John Wiley & Sons Inc.
Patient no. 2 is a 49-year-old man of German descent with a history of numerous episodes of epistaxis, some requiring plasma infusions or cautery. He has required surgery on his shoulder and knee for trauma-induced intramusclar/intrarticular bleeding. His plasma fXI activity is 10% to 20% of normal. The patient's father and paternal grandfather had similar problems with frequent epistaxis, and the father was diagnosed with fXI deficiency (activity 25% to 30% of normal). The plasma fXI antigen of patient no. 2 and his father match plasma activity levels. FXI gene analysis for both individuals revealed a G>C transition in exon 15 at base pair 1804 (cDNA sequence18 ), resulting in serine substituting for the wild-type tryptophan residue at position 569 (Trp569Ser). No other changes were noted in the fXI genes of either subject.
Transient transfections of fXI in 293 cells
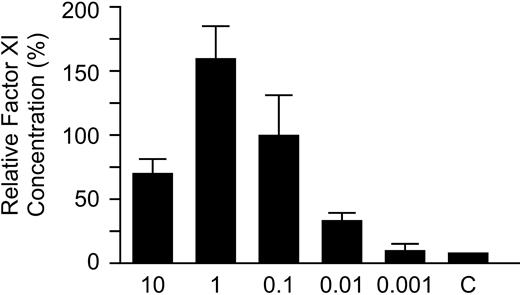
Recombinant wild-type fXI and fXI mutants were expressed in 293 fibroblasts using a vector (pJVCMV) containing a CMV promoter.29,30 This system expresses and secretes wild-type fXI that is functionally similar to plasma-derived fXI.29,30 Initially, it was necessary to determine the optimal concentration of expression constructs for transfections so as not to exceed the system's capacity to produce fXI. Transfections with logarithmically increasing amounts of wild-type fXI construct were performed, and fXI in the media was measured (Figure 2). FXI concentration rises with increasing concentrations of construct up to 1 μg per well. Based on the results, subsequent single transfections were carried out with 0.08 μg and cotransfections with 0.16 μg of construct.
Effect of wild-type fXI construct amount on fXI expression. The 293 fibroblasts were transfected with different amounts of wild-type fXI/pJVCMV expression construct as described in “Materials and methods.” For concentrations between 0.001 and 1 μg the total amount of DNA transfected was brought up to 2 μg with empty pJVCMV vector. Results are relative concentrations of fXI in media 67 hours after transfection compared with the mean for the 0.1 μg transfection, which was assigned a value of 100%. Results are means and standard deviations for 3 separate transfections, each tested in triplicate by ELISA. All results are corrected for transfection efficiency using a Renilla luciferase assay.
Effect of wild-type fXI construct amount on fXI expression. The 293 fibroblasts were transfected with different amounts of wild-type fXI/pJVCMV expression construct as described in “Materials and methods.” For concentrations between 0.001 and 1 μg the total amount of DNA transfected was brought up to 2 μg with empty pJVCMV vector. Results are relative concentrations of fXI in media 67 hours after transfection compared with the mean for the 0.1 μg transfection, which was assigned a value of 100%. Results are means and standard deviations for 3 separate transfections, each tested in triplicate by ELISA. All results are corrected for transfection efficiency using a Renilla luciferase assay.
Results of transfections for wild-type and mutant fXI constructs are shown in Figure 3. Figure 3A shows the relative concentrations of fXI in media compared with wild-type fXI, while Figure 3B shows intracellular protein. The Glu117Stop mutation generates signals for extracellular and intracellular protein (1.3% ± 0.9% and 1.0% ± 0.2%, respectively) that are indistinguishable from the empty vector control (2.5% ± 4.2% and 1.0% ± 0.1%, respectively). This is expected, because the mutation results in early termination of translation. The Phe283Leu mutation causes a moderate decrease in protein secretion into the media (61.5% ± 10.8% of wild-type) compared with wild-type fXI and is associated with a somewhat higher intracellular protein concentration (130% ± 21.8% of wild-type fXI). Meijers et al previously showed that Phe283Leu, which is located in the fXI A4 domain, partially interferes with dimer formation, resulting in accumulation of monomeric protein within the cell.22 Analysis of protein secreted into the medium of 293 cells stably expressing fXI-Phe283Leu revealed that the secreted protein is dimeric (data not shown). The amount of fXI-Phe283Leu in medium in our experiments is significantly greater than reported with baby hamster kidney (BHK) cells.22 The reason for this is not clear but may relate to differences in the 293 and BHK lines or the high levels of expression obtained with the pJVCMV vector. We also tested fXI-Gly350Glu (fXI-Nagoya II), which has a more profound defect in dimer formation than fXI-Phe283Leu.22 FXI-Gly350Glu is detectable in cell lysates (95.5% ± 14.5%) but not in media (2.6% ± 0.6%). FXI-Gly400Val is barely detectable in media of transfected cells above negative control (5.3% ± 3.1% and 2.5% ± 4.2%, respectively), while fXI-Trp569Ser (0.5% ± 0.1%) is not detectable in media. FXI-Gly400Val and fXI-Trp569Ser are present within cells (53.5% ± 22.2% and 14.2% ± 25.9%, respectively) but at reduced levels compared with wild-type fXI, suggesting the proteins are degraded more rapidly than wild-type fXI or are synthesized at lower rates.
Expression of wild-type and mutant fXI constructs. The 293 cells were transfected with 0.08 μg fXI/pJVCMV expression construct. (A) Relative concentrations of protein in media 67 hours after transfection compared with the mean for wild-type fXI, which was assigned a value of 100%. (B) Relative concentrations of intracellular fXI, again compared with wild-type fXI. Results are means and standard deviations for 9 separate transfections, each tested in triplicate by ELISA. Results are corrected for transfection efficiency using a Renilla luciferase assay. Lanes are as follows: (1) Wild-type fXI; (2) fXI-Glu117Stop; (3) fXI-Phe283Leu; (4) fXI-Gly350Glu; (5) fXI-Gly400Val; (6) fXI-Trp569Ser; and (7) pJVCMV without cDNA.
Expression of wild-type and mutant fXI constructs. The 293 cells were transfected with 0.08 μg fXI/pJVCMV expression construct. (A) Relative concentrations of protein in media 67 hours after transfection compared with the mean for wild-type fXI, which was assigned a value of 100%. (B) Relative concentrations of intracellular fXI, again compared with wild-type fXI. Results are means and standard deviations for 9 separate transfections, each tested in triplicate by ELISA. Results are corrected for transfection efficiency using a Renilla luciferase assay. Lanes are as follows: (1) Wild-type fXI; (2) fXI-Glu117Stop; (3) fXI-Phe283Leu; (4) fXI-Gly350Glu; (5) fXI-Gly400Val; (6) fXI-Trp569Ser; and (7) pJVCMV without cDNA.
Intracellular dimer formation studied with metabolic labeling
BHK cell clones stably expressing wild type, Phe283Leu, Gly350Glu, and Gly400Val fXI were labeled with [35S]-cysteine. Intracellular fXI was immunoprecipitated from cell lysates, size fractionated by SDS–polyacrylamide gel electrophoresis (SDS-PAGE), and autoradiographed. Wild-type fXI (Figure 4, lane 2) is present in cells largely as disulfide bond–linked dimer, with a small amount of single chain protein that represents either monomer or noncovalently associated dimer. The A4 domain mutants fXI-Phe283Leu and fXI-Gly350Glu (Figure 4, lanes 3-4) have relatively greater amounts of monomer, consistent with the previously proposed defects in dimer formation.22 In the case of fXI-Gly350Glu, the defect appears to be complete. In contrast, Gly400Val (Figure 4, lane 5) is a dimer, and poor release into media in transfection experiments cannot be attributed to a problem with dimerization. The data indicate fXI-Gly400Val is likely to form heterodimers with wild-type fXI polypeptide in heterozygotes and in cotransfection experiments. The molecular mass of fXI-Gly400Val appears to be slightly smaller than wild-type fXI. This may be due to differences in posttranslational modification or altered tertiary structure. Northern blot analysis (not shown) demonstrated that wild-type, Phe283Leu, Gly350Glu, and Gly400Val fXI constructs generated similar amounts of mRNA, indicating differences in transcription do not account for differences in protein level in media.
Metabolic labeling studies for intracellular fXI. BHK cells were transfected with pZEM229R expression vector22 containing the following cDNAs: (1) none; (2) wild-type fXI; (3) fXI-Phe283Leu; (4) fXI-Gly350Glu; or (5) fXI-Gly400Val. Stable transfectants for each construct were grown in media containing [35S]-cysteine for 24 hours and then lysed by radioimmunoprecipitation assay (RIPA) buffer treatment. Immunoprecipitates were prepared from cell lysates and then size fractionated by SDS-PAGE, followed by autoradiography. The positions of disulfide bond–linked dimeric fXI (dimer) and nondisulfide bond–linked fXI (monomer) are shown on the right. Positions of molecular mass standards are shown on the left in kilodaltons.
Metabolic labeling studies for intracellular fXI. BHK cells were transfected with pZEM229R expression vector22 containing the following cDNAs: (1) none; (2) wild-type fXI; (3) fXI-Phe283Leu; (4) fXI-Gly350Glu; or (5) fXI-Gly400Val. Stable transfectants for each construct were grown in media containing [35S]-cysteine for 24 hours and then lysed by radioimmunoprecipitation assay (RIPA) buffer treatment. Immunoprecipitates were prepared from cell lysates and then size fractionated by SDS-PAGE, followed by autoradiography. The positions of disulfide bond–linked dimeric fXI (dimer) and nondisulfide bond–linked fXI (monomer) are shown on the right. Positions of molecular mass standards are shown on the left in kilodaltons.
Cotransfection experiments
Results of cotransfection experiments with wild-type and mutant fXI constructs are shown in Figures 5A (secreted protein) and 5B (intracellular protein). The mean level for wild-type fXI cotransfected with empty vector was assigned a value of 100% (secreted protein, 100% ± 24.7%; intracellular protein, 100% ± 19.0%). Cotransfection of wild-type fXI with an additional equal amount of wild-type construct resulted in moderate increases in secreted and intracellular fXI (121.1% ± 12.8% and 144.8% ± 18.1%, respectively), consistent with data shown in Figure 2. Cotransfection of wild-type fXI with fXI-Glu117Stop, fXI-Phe283Leu, or fXI-Gly350Glu (Figure 5A, lanes 3-5) had relatively modest effects on protein secretion (91.7% ± 15.6%, 98.7% ± 8.2%, and 81.6% ± 8.9%, respectively). For fXI-Phe283Leu and fXI-Gly350Glu there is an associated increase in intracellular protein (160% ± 30.7% and 186.2% ± 15.7%, respectively). While not directly assessed, this probably reflects a combination of normal accumulation of wild-type fXI as well as nonsecretable mutant polypeptides in monomeric form.
Cotransfections with wild-type and mutant fXI expression constructs. The 293 cells were cotransfected with 0.08 μg wild-type fXI construct and 0.08 μg pJVCMV (lane 1), 0.08 μg additional wild-type fXI construct (lane 2), or 0.08 μg mutant fXI constructs (lanes 3-7). Cells transfected with 0.16 μg pJVCMV without cDNA insert (lane 8) were the negative control. Shown are relative concentrations of protein in media (A) and cell lysates (B) 67 hours after transfection, compared with the means for wild-type fXI (lane 1), which were assigned a value of 100%. Results are means and standard deviations for 9 separate transfections, each tested in triplicate by ELISA. Results are corrected for transfection efficiency using a Renilla luciferase assay. Lanes are as follows: (1) Wild-type (WT)–fXI + pJVCMV; (2) WT-fXI + WT-fXI; (3) WT-fXI + fXI-Glu117Stop; (4) WT-fXI + fXI-Phe283Leu; (5) WT-fXI + fXI-Gly350Glu; (6) WT-fXI + fXI-Gly400Val; (7) WT-fXI + fXI-Trp569Ser; and (8) pJVCMV control.
Cotransfections with wild-type and mutant fXI expression constructs. The 293 cells were cotransfected with 0.08 μg wild-type fXI construct and 0.08 μg pJVCMV (lane 1), 0.08 μg additional wild-type fXI construct (lane 2), or 0.08 μg mutant fXI constructs (lanes 3-7). Cells transfected with 0.16 μg pJVCMV without cDNA insert (lane 8) were the negative control. Shown are relative concentrations of protein in media (A) and cell lysates (B) 67 hours after transfection, compared with the means for wild-type fXI (lane 1), which were assigned a value of 100%. Results are means and standard deviations for 9 separate transfections, each tested in triplicate by ELISA. Results are corrected for transfection efficiency using a Renilla luciferase assay. Lanes are as follows: (1) Wild-type (WT)–fXI + pJVCMV; (2) WT-fXI + WT-fXI; (3) WT-fXI + fXI-Glu117Stop; (4) WT-fXI + fXI-Phe283Leu; (5) WT-fXI + fXI-Gly350Glu; (6) WT-fXI + fXI-Gly400Val; (7) WT-fXI + fXI-Trp569Ser; and (8) pJVCMV control.
Cotransfections of wild-type fXI with fXI-Gly400Val or fXI-Trp569Ser result in about 50% reductions in secreted antigen (50.1% ± 7.7% and 50.2% ± 7.3%, respectively). In contrast with results for fXI-Phe283Leu and fXI-Gly350Glu, cotransfection with fXI-Gly400Val or fXI-Trp569Ser is not associated with an appreciable increase in intracellular protein (123% ± 20.7% and 93.2% ± 20.0%, respectively; Figure 5B, lanes 6 and 7). The intracellular material could be a mixture of wild-type and mutant homodimers and heterodimers. Alternatively, given that levels of intracellular protein are similar to wild-type control, this could represent primarily wild-type homodimer.
Cotransfections of mutant fXI constructs with fXI/PKA2
As shown in Figure 3A, fXI-Gly400Val and fXI-Trp569Ser are secreted poorly from transfected cells. However, in cotransfection experiments, it is possible that wild-type fXI polypeptide forms heterodimers with fXI-Gly400Val or fXI-Trp569Ser that are secreted. To assess this possibility it is necessary to be able to distinguish mutant from wild-type polypeptide. We previously prepared recombinant fXI in which the A2 domain is replaced with the A2 domain from PK (fXI/PKA2).29 FXI/PKA2 is functionally similar to wild-type fXI. We also characterized a monoclonal antibody (1A6) that recognizes an epitope on fXI A2 but not fXI/PKA2 29 ; 1A6 does not require dimeric fXI to recognize its epitope29 and recognizes fXI on Western blots under nonreducing29 and reducing conditions (data not shown). We conducted cotransfection experiments as described in the previous section (“Cotransfection experiments”) but with the wild-type fXI construct replaced by fXI/PKA2. Two types of ELISA were performed using different capture antibodies. Polyclonal anti-fXI/anti-PK capture antibodies will recognize both mutant (fXI-Gly400Val or fXI-Trp569Ser) and normal (fXI/PKA2) polypeptides. The 1A6 capture antibody will recognize mutant polypeptides because they have a fXI A2 domain but will not recognize the fXI/PKA2 polypeptide. A positive signal in the 1A6 ELISA, therefore, indicates the presence of fXI-Gly400Val or fXI-Trp569Ser in the media.
Figure 6A shows results for control transfections with wild-type and fXI/PKA2 using the 2 ELISA systems. The concentration of fXI/PKA2 in conditioned media is less than wild-type protein (27.4% ± 3.2% and 100% ± 8.1%, respectively) and, as expected, fXI/PKA2 is not recognized by 1A6 antibody. The lower signal for fXI/PKA2 is not due to differences in recognition by the ELISA antibodies, because calibrations with control curves using purified fXI and fXI/PKA2 gave similar results (data not shown). Results of cotransfections with mutant fXI constructs are shown in Figure 6B. Consistent with experiments with wild-type fXI (Figure 5A), cotransfection of fXI/PKA2 with fXI-Gly400Val or fXI-Trp569Ser results in substantially lower levels of antigen in the media (27.3% ± 9% and 31.1% ± 2.6% of control, respectively) as measured by polyclonal capture antibody–based ELISA. Signals with the 1A6 ELISA are indistinguishable from background for cotransfections, indicating that signals in the polyclonal-based ELISA are due to fXI/PKA2 homodimer and not to heterodimers containing fXI-Gly400Val or fXI-Trp569Ser. From this, we can conclude that if fXI-Gly400Val or fXI-Trp569Ser polypeptides are forming heterodimers with wild-type fXI, the heterodimers are not secreted.
Cotransfections with fXI/PKA2. (A) Comparison of wild-type (WT) and fXI/PKA2 expression. The 293 cells were transfected with the following combinations of expression constructs: (1) 0.08 μg WT-fXI; (2) 0.08 μg fXI/PKA2; (3) 0.16 μg WT-fXI; (4) 0.08 μg fXI/PKA2 + 0.08 μg WT-fXI; and (5) 0.16 μg pJVCMV. Shown are relative concentrations of protein in media 67 hours after transfection compared with the mean for wild-type fXI (assigned a value of 100%). Results are means and standard deviations for 6 separate transfections, each tested in triplicate by ELISA and corrected for transfection efficiency by Renilla luciferase assay. Capture antibody was polyclonal (▪) or monoclonal 1A6 (□) anti-fXI antibody; 1A6 recognizes the fXI A2 domain and will not recognize fXI/PKA2. (B) Cotransfection of fXI/PKA2 with mutant fXI constructs. Transient transfections of 293 cells were performed with 0.08 μg fXI/PKA2 expression construct and 0.08 μg of (1) pJVCMV; (2) fXI-Gly400Val; and (3) fXI-Trp569Ser. Lane 4 represents control transfection with 0.16 μg pJVCMV. Shown are relative concentrations of protein in media 67 hours after transfection compared with the mean for fXI/PKA2, which was assigned a value of 100%. Results are means and standard deviations for 6 separate transfections, each tested in triplicate by ELISA and corrected for transfection efficiency by Renilla luciferase assay. Capture antibody was polyclonal (▪) or monoclonal 1A6 (□) anti-fXI antibody.
Cotransfections with fXI/PKA2. (A) Comparison of wild-type (WT) and fXI/PKA2 expression. The 293 cells were transfected with the following combinations of expression constructs: (1) 0.08 μg WT-fXI; (2) 0.08 μg fXI/PKA2; (3) 0.16 μg WT-fXI; (4) 0.08 μg fXI/PKA2 + 0.08 μg WT-fXI; and (5) 0.16 μg pJVCMV. Shown are relative concentrations of protein in media 67 hours after transfection compared with the mean for wild-type fXI (assigned a value of 100%). Results are means and standard deviations for 6 separate transfections, each tested in triplicate by ELISA and corrected for transfection efficiency by Renilla luciferase assay. Capture antibody was polyclonal (▪) or monoclonal 1A6 (□) anti-fXI antibody; 1A6 recognizes the fXI A2 domain and will not recognize fXI/PKA2. (B) Cotransfection of fXI/PKA2 with mutant fXI constructs. Transient transfections of 293 cells were performed with 0.08 μg fXI/PKA2 expression construct and 0.08 μg of (1) pJVCMV; (2) fXI-Gly400Val; and (3) fXI-Trp569Ser. Lane 4 represents control transfection with 0.16 μg pJVCMV. Shown are relative concentrations of protein in media 67 hours after transfection compared with the mean for fXI/PKA2, which was assigned a value of 100%. Results are means and standard deviations for 6 separate transfections, each tested in triplicate by ELISA and corrected for transfection efficiency by Renilla luciferase assay. Capture antibody was polyclonal (▪) or monoclonal 1A6 (□) anti-fXI antibody.
Discussion
The relatively mild and highly variable bleeding diathesis caused by fXI deficiency makes it difficult to generalize regarding inheritance patterns for this disorder. While it is the opinion of some investigators that fXI deficiency in Ashkenazi Jews follows an autosomal recessive mode of transmission, several studies have documented poor correlation between plasma fXI levels and bleeding symptoms,13,14,31,32 with some reporting no clinically distinguishing characteristics between heterozygotes and homozygotes.11,13,14 It is almost certain that varying criteria for abnormal bleeding, along with coinheritance of other bleeding risk factors, contribute to this situation. However, it is likely that the nature of the genetic abnormality responsible for the deficiency state also contributes to the pattern of disease transmission.
The fXI protein has structural features that are unique among coagulation proteases. The mature protein is a homodimer linked by noncovalent and covalent disulfide interactions primarily involving the A4 domain.18-21 Given this multimeric structure, we can predict that fXI deficiency may follow different inheritance patterns. Mutations that interfere with transcription that prevent synthesis of the fXI polypeptide or that result in an abnormal protein that cannot dimerize might be expected to behave in an autosomal recessive manner. Mutations that result in synthesis of dysfunctional polypeptides that form dimers may produce a dominant type of disorder through the generation of dysfunctional heterodimers with wild-type fXI polypeptide. This mechanism for autosomal dominant disease has been well characterized for the multimeric coagulation proteins von Willebrand factor23 and fibrinogen24 ; however, there are few examples of CRM-positive proteins in fXI deficiency (discussed in the final paragraph).
Alternatively, a dominant negative effect could be produced by mutant fXI polypeptides forming nonsecretable heterodimers with wild-type fXI polypeptide. Hypothetically, in a heterozygote, if wild-type and mutant fXI polypeptides are synthesized in equal amounts and form dimers equally well, wild-type homodimer (secreted), heterodimer (not secreted), and mutant homodimer (not secreted) would be produced in a 1:2:1 ratio. The result would be, on average, a plasma level of fXI activity and antigen about 25% of normal, compared with the expected 50% for a mutation not exerting a dominant negative effect. A similar process has been demonstrated for a variant of von Willebrand factor (Cys1149Arg) that causes type I disease with high penetrance26,27 and has been proposed as a generally applicable mechanism to explain CRM-negative autosomal dominant disorders of other multimeric proteins,27 including hypofibrinogenemia,33-36 and collagen abnormalities responsible for osteogenesis imperfecta37 and hypochondrogenesis.38
Congenital fXI deficiency is most prevalent in Ashkenazi Jews, in whom the carrier frequency is estimated at 5% to 11%.4,5 More than 90% of abnormal fXI alleles in this population are due to 2 point mutations of approximately equal prevalence, Glu117Stop and Phe283Leu.2,5 Glu117Stop (also called the type II mutation) truncates the fXI polypeptide within the A2 domain, resulting in a protein lacking domains for dimer formation and a catalytic domain.39 In the present study, ELISA signals from cell lysates for transfections with Glu117Stop expression construct are indistinguishable from the control, suggesting that any truncated polypeptide formed is rapidly degraded. FXI genes with the Glu117Stop are therefore essentially null alleles. A homozygote for Glu117Stop does not have measurable plasma fXI antigen (D.G., unpublished observation, July 2003), and heterozygosity for Glu117Stop should not affect secretion of wild-type fXI from the normal allele. Phe283Leu (type III mutation) is located in the A4 domain, an area critical for normal dimer formation.21,29,40 Phe283Leu causes a partial defect in dimerization, resulting in retention of monomeric fXI within cells and reduced fXI secretion.22 Homozygotes for Phe283Leu have plasma fXI levels about 10% of normal,2 and the fXI-Phe283Leu that is secreted is dimeric and functions normally.22 It would be expected, then, that homozygosity for Phe283Leu would not have a deleterious effect on secretion of fXI from the normal allele, because it would dimerize poorly with wild-type polypeptide, and heterodimers that do form are secreted and function normally. The cotransfection experiments in the present study support this premise. Seminal work by Asakai et al demonstrated that heterozygotes for Glu117Stop or Phe283Leu have plasma fXI levels of 52% ± 18% and 67% ± 24% of normal, respectively,2 consistent with a recessive mechanisms of inheritance in which the mutant allele has little or no effect on expression of the wild-type allele. Furthermore, fXI-Gly350Glu (fXI-Nagoya II), with a more profound defect in dimerization than fXI-Phe283Leu, would also not be expected to influence secretion of wild-type protein appreciably, as was observed in our cotransfection experiments.
Initially, the case for autosomal dominant inheritance in fXI deficiency was based on family studies in which a bleeding predisposition appeared to be passed from one affected parent to a child7-9 rather than on measurements of plasma activity. Cases of apparent autosomal dominant transmission in Jewish families, given the high carrier rate in this population, are explainable by one parent having severe (homozygous) disease while the other is an asymptomatic carrier (heterozyote).4 Subsequent studies measuring plasma activity levels, primarily in Jewish patients, demonstrated distinct ranges for homozygotes and heterozygotes.10,14 However, families where inheritance is difficult to explain by a simple recessive mechanism have been reported.8,14-16 The 2 patients described in this paper have novel mutations in the catalytic domain of the fXI protein outside of domains involved in protein dimerization. These patients have fXI activity and antigen levels that fall into the severely deficient range, heterozygosity for fXI gene mutations, and family histories consistent with dominant transmission (with variable expression). Results of cotransfection experiments support the premise that fXI with a Gly400Val or Trp569Ser mutation can exert a negative effect on secretion of wild-type fXI. In vivo, this may lead to plasma fXI levels low enough to cause symptoms. It is not clear if these cases are atypical or represent a common mechanism in fXI deficiency. To date, more than 80 distinct mutations have been identified in the fXI genes of patients with low plasma fXI activity. While most are not completely characterized, it appears that only 3 (Ser248Asn,15 Pro520Leu,41 and Gly555Glu42 ) are associated with circulating dysfunctional (CRM+) fXI. One could hypothesize that any fXI mutation that produces a nonsecretable fXI polypeptide that retains the ability to form a dimer could exert a negative effect on expression of the normal fXI allele. Therefore, this mechanism may be operating in additional cases of fXI deficiency and could explain the observation that fXI activity and antigen levels in non-Jewish patients tend to be lower than in Jewish patients.14,17
Prepublished online as Blood First Edition Paper, March 16, 2004; DOI 10.1182/blood-2003-10-3530.
Supported by grant HL58837 (D.G.) from the National Heart, Lung, and Blood Institute. D.G. is an Established Investigator of the American Heart Association.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Drs Earl Davie and Dominic Chung for review of the manuscript and Jean McClure for expert graphics work and manuscript preparation.


![Figure 4. Metabolic labeling studies for intracellular fXI. BHK cells were transfected with pZEM229R expression vector22 containing the following cDNAs: (1) none; (2) wild-type fXI; (3) fXI-Phe283Leu; (4) fXI-Gly350Glu; or (5) fXI-Gly400Val. Stable transfectants for each construct were grown in media containing [35S]-cysteine for 24 hours and then lysed by radioimmunoprecipitation assay (RIPA) buffer treatment. Immunoprecipitates were prepared from cell lysates and then size fractionated by SDS-PAGE, followed by autoradiography. The positions of disulfide bond–linked dimeric fXI (dimer) and nondisulfide bond–linked fXI (monomer) are shown on the right. Positions of molecular mass standards are shown on the left in kilodaltons.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/1/10.1182_blood-2003-10-3530/6/m_zh80130463310004.jpeg?Expires=1765900296&Signature=Lg2fwBYxUf7TA3tYDEHolCF9AwF1IZEKfhsZeSVEKV4LWlvt24sW5ZMDY5s~HRQoT5XuBFigBb9C50NIx-zEghCylLyrhyBaOm4RLh2JeFKdfWDO6JSYQy-kBpxc9RWN3g31KGYWYlDJoT4J1fF1I5rSwSQeXMJsgOOvcu68WqMmG9fGmFlmiqLLmvUjfDZ4nI51CPX0eRenYZEfm03oC9EYvlzoCKt~wbrfIdlQnFlMg97yEc3x2Qls1scb-FZXke~Pq~sCvKkb-4dNpTi1pH2uJJDCoVxqbyJPrFL5GwshAp1nzKGjRXutAR7XguCeeaNHrmpCTDgF4DEEtN9eIw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal