Abstract
Upon plaque rupture or vascular injury, tissue factor (TF) protein in the vessel wall becomes exposed to flowing blood, initiating a cascade of reactions resulting in the deposition of fibrin and platelets on the injured site. Paradoxically, the growing thrombus may act as a barrier, restricting the convective and diffusive exchange of substrates and coagulation products between the blood and reactive vessel wall, thus limiting the role TF plays in thrombus growth. In this study, various in vitro, platelet-fibrin clots were prepared on TF:VIIa-coated surfaces and the rate at which factor (F) X in the well-mixed clot supernatant permeates the clot and is converted to Xa was monitored over several hours. The apparent diffusion coefficients of FX(a) in fibrin and platelet-fibrin clots at 37°C was 2.3 × 10–7 and 5.3 × 10–10 cm2/second, respectively, indicating that the mean time required for FX(a), and likely FIX(a), to diffuse 1 mm in a fibrin clot is 4 hours, and in the presence of platelets, 3.6 months. As complete human thrombotic occlusion has been observed within 10 minutes, an alternative source of procoagulant activity that can localize to the outer surface of growing thrombi, such as platelet factor XI or blood-borne TF, appears essential for rapid thrombus growth.
Introduction
In the generally accepted view of thrombosis, rupture or erosion of an atherosclerotic plaque exposes procoagulant regions on the vascular wall that bind platelets and initiate thrombus formation. The exposed material thought responsible for initiating coagulation is tissue factor (TF), which complexes with VII(a) to activate factor (F) IX and FX. Immediately following injury, FIX and FX are rapidly delivered to the vessel wall by flowing blood resulting in localized thrombin generation. However, as fibrin and platelets accrue to the injured surface, flow to the underlying TF region becomes restricted and substrates must percolate through an overlying platelet-fibrin mesh in order to become activated (Figure 1). Furthermore, to achieve effective prothrombinase activity (Xa:Va) near the outer surface of a growing thrombus, the products (IXa or Xa) must diffuse a substantial distance back through the overlying mass. Through its interaction with VIIIa, factor IX(a) plays a crucial role in amplifying the amount of Xa generated; However, since the only accepted source of Ixa in most coagulation models is its generation by TF:VIIa, IXa would have to percolate a substantial distance through the overlying platelets and fibrin in order to generate tenase activity near the surface of the growing thrombus. (Factor XI–mediated activation of IX through the intrinsic pathway is generally not regarded as an essential step in thrombosis and is addressed later.) Hence, to obtain occlusive thrombi, the substrates (IX and X) and their respective enzymes, either individually or working in concert, must travel several millimeters through a growing thrombus without the aid of convection. As diffusion of proteins the size of factors IX and X is slow, typically requiring several hours to reach a distance of one mm in water (20°C), 1 the time required to diffuse through a viscous, serum-filled medium along a tortuous path partially obstructed by platelets would likely be considerably longer.
In the accepted view of thrombosis, factors IX and X activated by TF at the vessel wall must diffuse away from the vessel wall in order to generate prothrombinase activity near the surface of a growing thrombus. This path becomes increasingly hampered with the accumulation of platelets and fibrin.
In the accepted view of thrombosis, factors IX and X activated by TF at the vessel wall must diffuse away from the vessel wall in order to generate prothrombinase activity near the surface of a growing thrombus. This path becomes increasingly hampered with the accumulation of platelets and fibrin.
Due to the enormous spatial and temporal complexity of thrombosis, most coagulation models have focused on a myriad of reaction rates and association constants that typically apply only to well-mixed systems.2 As the current view of thrombosis suggests that circulating zymogens are delivered to the periphery of a thrombus, activated at or near the underlying base of the thrombus, and then perform their function near the growing edge of the thrombus, the concept of a well-mixed system is doubtful. Recently, Kuharsky and Fogelson3 computationally modeled both coagulation reactions and platelet deposition, on a 10 × 10 μm2 patch of subendothelium with separate transport coefficients for the movement of proteins within the thrombus layer. The results suggested that physical obstruction of the TF surface may play a role in blocking TF:VIIa activity. Although factors affecting fibrin structure and to some extent platelet-fibrin structure have been studied,4 no one, to our knowledge, has attempted to physically model the transport of coagulation factors (or inhibitors) within a clot or thrombus. The transport of fibrinolytic agents has been investigated extensively with the conclusion that axial pressure gradients facilitate clot degradation by driving the convection of fibrinolytic agents through microchannels within a thrombus.5,6 The key difference between flow in thrombus growth and fibrinolysis is that clot lysis is achieved axially in the direction of the pressure gradient whereas thrombus growth occurs radially, perpendicular to microchannel flow. Thus, although it is conceivable that microchannels could serve as a conduit for the delivery of substrate from upstream, they would likewise result in removal of product by carrying it downstream as opposed to radially.
Work by several investigators has supported the notion of procoagulant activity resulting from FXa generation on the leading edge of a growing thrombus, a concept that we believe has not been included in comprehensive coagulation models and has not been established as a critical and essential piece of the coagulation-thrombosis scheme. Gailani and Broze7,8 demonstrated that low levels of thrombin can through FXI activate the intrinsic coagulation pathway on the surface of activated platelets. Others have found that most thrombi stain ubiquitously for TF antigen and that ex vivo thrombi formed on collagen surfaces exhibit TF activity.9 Hence the notion of blood-borne TF may also play a role in thrombosis. Clearly, the presence of meaningful levels of procoagulant activity or initiator complexes on the outer edge of growing thrombi would alter the way we perceive thrombosis. The objective of the present study is to construct model clots on top of TF-coated surfaces in order to measure the transport rates of FX(a) in the presence of platelets and fibrin. As FIX is similar in size and geometry to FX, one may infer its diffusional transport characteristics as well. By exploring the transport characteristics associated with the current paradigm of thrombosis, it may be possible to understand further the contribution of blood-borne or platelet procoagulant activity relative to thrombus growth and whether they are essential components of thrombosis.
Materials and methods
Proteins and reagents
Recombinant human FVIIa from Novo-Nordisk (Bagsvaerd, Denmark) was reconstituted to 6 μM in a solution of 50% glycerol and 50% HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid)–buffered saline (HBS; 10 mM HEPES, 140 mM NaCl, pH 7.4) and stored at –20° C. Human FX was isolated from plasma essentially as described by Miletich et al10 and stored at –20° C in 50% glycerol/50% HBS. Recombinant FXIII, a gift from Dr Paul Bishop of Zymogenetics (Seattle, WA), was reconstituted to 10 mg/mL in TANEP buffer (100 mM Tris-acetate, 150 mM NaCl, 1 mM EDTA [ethylenediaminetetraacetic acid], 0.1% PEG 8000, pH 7.5) and stored at –80° C.
Recombinant human TF (Genentech, San Francisco, CA) was incorporated into phospholipid vesicles (30% phosphatidylserine, 69% phosphatidylcholine, and 1% rhodamine-labeled phosphatidylethanolamine; all from Avanti, Alabaster, AL) according to the octylglucoside method.11 Final concentrations in the stock suspension of vesicles were 375 nM TF and 16.5 mM total phospholipid. Prior to each experiment, vesicles were extruded 33 times through a polycarbonate filter (0.1 μm pore size; Osmonics, Minnetonka, MN) yielding an average of 2 TF molecules per vesicle.
Preparation of TF-coated disks
Glass coverslips (1.5-cm diameter; Fisher Scientific, Hampton, NH) were cleaned by sonication in chromic-sulfuric acid for one hour, then 30 minutes in boiling Sparkleen. Coverslips were rinsed thoroughly with ultrafiltered, deionized water and dried under nitrogen. Prior to each experiment, the stock suspension of vesicles was diluted in HBS to 11.7 nM TF, 514 μM total phospholipid. A 100 μL drop of the suspension was placed on parafilm and overlaid with a clean glass coverslip. The coverslips were incubated for 2 hours at 37° C in a humidified environment, during which time the TF and phospholipids coadsorbed to the surface. The coated coverslips were washed vigorously with HBS, inserted into a 24-well plate containing HBS, and held in place with a silicone O-ring. Occasionally coverslips were mounted on slides and the rhodamine-labeled lipids visualized at ×100 in order to verify that a uniform layer was present.
Preparation of platelet-fibrin clots
Platelet-rich plasma was prepared from citrated blood of healthy donors by centrifuging once at 260g for 10 minutes, discarding the pellet, and then centrifuging at 180g for 10 minutes. Prostaglandin E1 (PGE1; 25 μM; Sigma, St Louis, MO) was added to the plasma supernatant and platelets were pelleted by centrifugation at 1200g for 15 minutes. Isolated platelets were washed twice in CGSa buffer (12.9 mM sodium citrate, 100 mM dextrose, 100 mM NaCl, and 1 U/mL apyrase) supplemented with 25 μM PGE1.
FXIII (2.78 mg/mL) was activated prior to experiments by incubating rFXIII with CaCl2 (14.7 mM) and thrombin (28 U/mL) for 30 minutes at 37° C, and subsequently quenching the thrombin activity with recombinant hirudin, 4600 U/mL. A typical clotting solution was prepared by mixing plasminogen-depleted fibrinogen (6 mg/mL; Enzyme Research Labs, South Bend, IN), activated recombinant FXIII (20 μg/mL), PGE1 (25 μM), cytochalasin-D (10 μM; Sigma), and CaCl2 (6.8 mM) in Hanks balanced salt solution (no. 10-527F; BioWhittaker, Walkersville, MD) supplemented with 5% bovine serum albumin (HBSS/BSA). In some experiments a fixed number of platelets or platelet-like microspheres (1.44 μm diameter; Polysciences, Warrington, PA) was added to the mixture prior to clot formation. A volume of clotting mixture corresponding to the desired clot thickness, typically 125 μL, was added to a 24-well plate containing a TF-phospholipid–coated glass coverslip. The platelets or microspheres were layered on top of coverslips by centrifuging a 5-well strip in a microplate carrier of a Sorvall swinging-bucket centrifuge, at 595g for 7 minutes. The microplate carrier was modified to position 5 wells cut out of a 24-well plate such that the surface of the coverslips would be normal to the relative centrifugal force. After the initial spin, 25 μL Reptilase-R (0.8 U/mL; Pentapharm, Basel, Switzerland), was gently added to each well and samples were centrifuged an additional 7 minutes at 595g. The second centrifugation served to flatten the meniscus that otherwise formed during clotting. All wells were then covered in HBSS/BSA and incubated for 30 minutes, allowing the clots to stabilize. In some experiments the platelets were fluorescently labeled with mepacrine (10 μM), and the clot structure visualized by confocal microscopy.
Measurement of FX(a) transport
To measure the transport of FX(a) through a given clot, clots were placed on an orbital shaker and the clot supernatant was replaced with an FX reaction mixture containing 150 nM FX, 10 nM rFVIIa, and 5 mM CaCl2 dissolved in HBSS/BSA (pH 7.4). Samples of the reaction mixture (25 μL) were periodically removed and stored in a 96-well plate containing 75μL of 50 mM EDTA. Each time a sample was extracted, the well was rapidly mixed with 4 × 25 μL flushes of the pipette tip, and the removed sample volume was replaced with an equal volume of fresh reaction mixture, thus preventing loss of available substrate (FX). Preliminary investigations indicated that in many cases the rate of FXa appearance over the first hour was not constant and slowly increased with time (transient period). After 2 hours of reaction time, the reaction mixture was replaced with new reaction mixture and samples continued to be taken over the next 4 hours. The rates of appearance of FXa over this interval were constant and were used to determine the steady-state flux of FXa from each clot. The concentration of FXa in each sample was measured by adding a chromogenic substrate, Spectrozyme-Xa (0.5 mM; American Diagnostica, Greenwich, CT), and measuring the rate of change in adsorbance at 405 nm using a Versamax microplate reader (Molecular Devices, Menlo Park, CA). Absorbance values were compared to a standard curve obtained using known concentrations of FX activated with Russell viper venom.
Calculation of FXa flux
To calculate the steady-state flux of FXa from clots, the total mass of FXa that appeared in the supernatant up to each time point was calculated, correcting for the FXa that was removed during earlier sample times, and plotted as a function of time. A least squares linear regression was fit to the steady-state data, and the flux (J [fmol/min/cm2]) was computed as the quotient of the slope of the fit (rate of FXa generation) and the average cross-sectional area of the clot. For wells that did not contain clots and consequently had high levels of FXa generation, only data from the initial 10 minutes were fit. Error bars in all figures represent standard error of the mean. Statistical differences in FXa fluxes were determined by analysis of variance, with significance set at .05.
Results
When glass coverslips coated with a TF and phospholipid bilayer were incubated with a reaction mixture containing FX, VIIa, and calcium, the concentration of FXa in the supernatant rapidly increased over the initial 10 minutes at a rate of 693 pM/min/cm2 and then continued to increase, although less rapidly as the available FX became depleted. In a duplicate well in which a 0.6 mm–thick fibrin clot was layered on top of the TF-phospholipid–coated surface, the rate at which FXa appeared in the supernatant was significantly slower, 21.2 pM/min/cm2. Following this 2-hour transient reaction period, the supernatants in the wells were replaced with fresh reaction mixture and the steady-state rates at which FXa appeared in the supernatants were measured. Figure 2 shows the FXa flux in wells containing no clot and in wells containing clots ranging from 0.3 mm to 2.6 mm in thickness. In all wells the presence of a thin, fibrin clot severely retarded the rate at which FXa reached the supernatant above the clot, with thicker clots resulting in decreased appearance of FXa.
Fibrin clots were constructed on top of TF-coated surfaces and the rate at which FXa emerged from the clot was measured as a function of clot thickness. Error bars represent SEM.
Fibrin clots were constructed on top of TF-coated surfaces and the rate at which FXa emerged from the clot was measured as a function of clot thickness. Error bars represent SEM.
To determine the effect of experimental mixing conditions on the appearance of FXa, wells containing a 0.6 mm–thick clot were mixed at 120 rpm or 360 rpm and the flux of FXa was measured. Wells mixed at 120 rpm exhibited an FXa flux of 41.3 ± 8.1 pM/min/cm2 whereas those mixed at 360 rpm had an FXa flux of 43.2 ± 6.0 pM/min/cm2. Hence, the rate of stirring was not a limiting factor in the transport of FXa to the surface of a fibrin clot. When FXIIIa was omitted from the clotting mixture, clots were fragile and eroded over the course of the experiment, resulting in much higher, but less reproducible, levels of FXa formation. Thus FXIIIa was essential to the model.
The effect of deposited platelets on FX(a) transport was investigated by layering platelets on the TF-phospholipid–covered surface. Figure 3 shows a confocal image of an experiment with mepacrine-labeled platelets illustrating the degree of packing as well as the height of the platelet layer. Although some platelets remained in the upper fibrin milieu, the average packing density with the platelet layer was 0.11 platelets/μm3 with clear gaps evident between adjacent platelets. The height of the platelet layer was calculated by assembling a sufficient number of 0.2 μm–spaced planar sections to visualize the distance from the rhodamine-labeled phospholipid surface to the uppermost height of the platelet layer. Typical experiments were performed with either 244 × 106 or 488 × 106 platelets resulting in layer heights of 14 μm or 27 μm, respectively.
Confocal images of model clots with a 26 μm–thick platelet layer. Left, vertical cross-section; right, 25 × 25 μm en face view.
Confocal images of model clots with a 26 μm–thick platelet layer. Left, vertical cross-section; right, 25 × 25 μm en face view.
Platelet layers decreased the transport of FXa. When a 14 μm–thick layer of platelets was added to the base of the fibrin clots, the steady-state appearance of FXa in the clot supernatant dropped to 1% of that observed in the absence of a clot, and to 13% of that observed in a fibrin clot alone. Doubling the thickness of the platelet layer to 27 μm further reduced the flux of FXa to 0.4% of that observed in the absence of a clot (Figure 4). When adenosine diphosphate (ADP) was added to a clot containing layered platelets (14 μm), the appearance of FXa in the supernatant fell to 0.3% of that detected in the absence of a clot. Thus, deposited platelets inhibit the net rate at which FX gets activated and reaches the clot surface, and the addition of a platelet agonist, such as ADP, appears to reduce further the rate at which FXa reaches the clot surface.
The addition of 13 μm– or 26 μm–thick platelet layers to fibrin clots, resulted in marked reductions in the appearance of FXa. In the absence of exogenous TF, no FXa generation was detected. P indicates platelets; F, fibrin.
The addition of 13 μm– or 26 μm–thick platelet layers to fibrin clots, resulted in marked reductions in the appearance of FXa. In the absence of exogenous TF, no FXa generation was detected. P indicates platelets; F, fibrin.
In similar experiments the physical impedance on FXa transport imposed by platelets was investigated by layering an equal number of polystyrene microspheres (488 × 106) on top of the TF-coated disk. No significant reduction in FXa transport was observed. When the number of microspheres was increased 5-fold, the appearance of FXa was reduced to 80% ± 20% of that observed in the presence of fibrin alone.
Discussion
In this investigation we measured the rate at which FXa diffuses through well-structured platelet-fibrin clots. Although we evaluated only Xa, the diffusion coefficient we determined would apply equally to any protein of similar dimensions. Thus, it is now possible to evaluate quantitatively the hypothesis that factors IXa and Xa, activated by surface-bound TF, diffuse through the nascent thrombus thereby promoting thrombus propagation. Our data are inconsistent with this formulation.
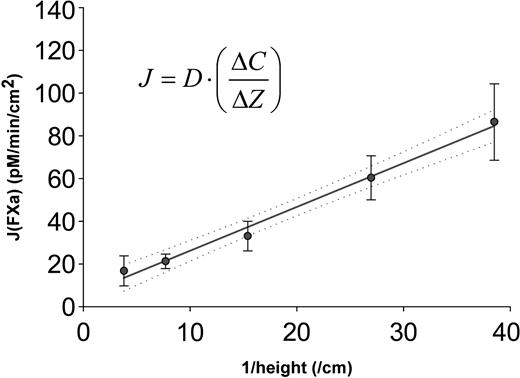
In order to apply diffusion laws to this system, we assumed that the activation of FX by TF:VIIa is rapid (kcat = 5-37/sec)12-14 compared with the flux of FX to the coverslip surface such that the FX near the coverslip surface is completely activated, and that at the time of our steady-state measurements, a linear concentration gradient (δC/δz) had developed across the fibrin clot. The apparent diffusion coefficient (Dapp) of Xa in a fibrin clot was estimated using the Fick First Law of Diffusion (J =–Dapp ×δC/δz), where J is the experimentally measured flux of Xa into the supernatant, and δC/δZ is evaluated as the difference in Xa concentration between the top (experimentally measured) and bottom (completely activated) of the clot (δC), divided by the clot thickness (δZ). The measured FXa fluxes were plotted against the inverse clot thickness (1/δz) and the slope of a least squares regression (Dapp ×δC) was used to evaluate Dapp (Figure 5), which we estimated to be 3.8 ± 0.16 × 10–7 cm2/second through a fibrin clot at 37° C containing 5% BSA (R2 = 0.975). The value typically used for computational modeling, 5.0 × 10–7 cm2/second,15 and our theoretical estimates based on direct observations and Stokes-Einstein approximations range from 5.9 × 10–7 cm2/second to 7.8 × 10–7 cm2/second at 37° C in water, depending on how the FX geometry is approximated.1 Hence the diffusion coefficient we infer experimentally in a fibrin gel medium is somewhat slower than theoretically predicted values in water. Correcting for differences in the viscosity of water (0.69 centipoise [cP]) and serum (1.15 cP) at 37° C, the calculated value for Dapp of FX in human plasma or serum at 37° C would be 2.3 × 10–7 cm2/second. Thus the mean time required for a molecule of FX(a) to diffuse a radial distance of 1 mm through a fibrin clot, or through plasma moving parallel to the vessel wall, would be 6.4 hours. The observation from Figure 5 that the Xa flux from thin clots, where the transport through the clot is most rapid, nicely fits the regression, suggests that local buildups of Xa or boundary layer effects are not appreciable.
The apparent diffusion coefficient of FX(a) in fibrin clots is estimated from a plot of FXa flux (J) versus inverse clot thickness.
The apparent diffusion coefficient of FX(a) in fibrin clots is estimated from a plot of FXa flux (J) versus inverse clot thickness.
The addition of relatively thin platelet layers (13 μmto27 μm) to the clot structure resulted in a marked decrease in the levels of FXa emanating from the clot. Two distinct mechanisms can be associated with this observation. First, platelets act as physical obstacles thereby increasing the mean free path by which an FX(a) molecule must travel in order to reach the clot surface. Second, an activated platelet contains approximately 200 to 400 FXa binding sites,16 thereby delaying its appearance in the supernatant. Both phenomena likely occur in the in vivo correlate, and both can be collectively characterized using an apparent diffusion coefficient of FX(a) in a platelet fibrin clot.17 Experimentally, the number of FXa molecules in the supernatant following the transient reaction period greatly exceeded the number of platelet FXa binding sites, and furthermore, the appearance of FXa in the supernatant over the subsequent 4-hour period was linear (typically r2 ≥ 0.99), and did not accelerate with time. Hence, the steady-state measurements of FXa appearance in the present study are not affected by FXa that binds to platelet receptors. This is because binding simply delays the steady state, but not its value, a phenomenon our group demonstrated in a tubular flow reactor.18
Substitution of the 27-μm platelet layer with an equal layer of 1.5-μm in diameter, polystyrene microspheres did not significantly reduce the appearance of FXa, and adding 5 times the thickness of microspheres only marginally decreased its appearance. Thus the physical impedance imposed by platelets likely results from their asymmetric shape and their ability to spread over surfaces when activated. Impeded diffusion by packed spheres and asymmetric “flakes” has been studied theoretically, with results indicating that tightly packed prolate spheroids with a 10:1 aspect ratio would reduce diffusion to 0.7% of that observed in the absence of obstructions. A similar layer of packed spheres would reduce the apparent diffusion to 42% of that observed in the absence of spheres. Thus, diffusion around spheres is 60-fold greater than around asymmetric “flakes.” Thus, the discoid shape, or the flattening out and spreading of activated platelets, likely accounts for the marked reduction in the rate at which FXa reaches the clot surface.
By knowing the thickness of the fibrin and platelet-fibrin layers, the supernatant FXa concentrations as a function of time, and the previously determined Dapp of FX(a) in fibrin, it was possible to estimate the Dapp of FX(a) in the presence of platelets and fibrin. By treating the diffusion through the fibrin and the platelet-fibrin layers as resistances in series, the Dapp of FX(a) through a platelet-fibrin clot was calculated to be 8.97 ± 1.1 × 10–10 cm2/second. Correcting for differences between the viscosities of water and serum at 37° C, the Dapp for FX(a) in a platelet-fibrin clot saturated with serum would be 5.3 × 10–10 cm2/second. Thus the mean time required for a molecule of FX to diffuse a distance of 1 mm to reach the vessel wall would be 3.6 months; an additional 3.6 months would be required for the vessel wall–generated FXa to reach the surface of the clot (Figure 6). These considerations are fully in accord with discoid platelets layered over the source of TF.17 Hence diffusion of Xa in the presence of platelets is approximately 1/500th that in the fibrin medium, which is slow already.
FX(a) transport time. Correcting for differences in experimental and serum viscosities, the mean time required for FX(a) to diffuse a round-trip distance of 1 mm is 7.8 hours in a fibrin clot (—•—) and approximately 6 months in a platelet-fibrin clot (—○—).
FX(a) transport time. Correcting for differences in experimental and serum viscosities, the mean time required for FX(a) to diffuse a round-trip distance of 1 mm is 7.8 hours in a fibrin clot (—•—) and approximately 6 months in a platelet-fibrin clot (—○—).
It is well accepted that FIX plays an essential role in amplifying the TF signal. However, as it is roughly the same size as FX, it would be subjected to similar diffusional limitations. After FXa and FIXa have been activated at the vessel wall, and an initial layer of platelets and fibrin have been deposited, it becomes increasing difficult for unactivated FIX or FX to reach TF:VIIa on the vessel surface. In a similar fashion, incoming FX will have difficulty reaching the FIXa:VIIIa, which is initially bound to a platelet a few microns away from the vessel wall. With further deposition of platelets and fibrin, the average time required for a molecule of FIX or FX to work its way through a platelet-fibrin mass becomes unreasonably long, especially in the presence of inhibitors such as tissue factor pathway inhibitor (TFPI), antithrombin III, and activated protein C, none of which were addressed in this study. In this study, the total amount of TFPI present in 488 × 106 platelets would have reduced the amount of active TF on the coverslip surface by only 15%, assuming every molecule of TFPI binds to TF.
Under superphysiologic concentrations of rVIIa, VIIa binds to platelets (50-100 nM Kd), and generates low levels of Xa in a mechanism that is believed largely to be TF independent (see Hoffman19 for a review); a phenomenon that explains the success achieved with high-dose rVIIa treatment of hemophiliacs. In our experimental model, using 2 nM rVIIa, we observed no measurable Xa generation by platelet-fibrin clots in wells containing phospholipid-coated coverslips (no TF), although it is conceivable that very low levels of Xa were generated that were insufficient to overcome binding to endogenous platelet TFPI and platelets (ie, no steady state). However, the fact that very low levels of extrinsic activity on the platelet surface help hemophiliacs achieve acceptable hemostasis is evidence that very low levels of procoagulant activity on the platelet surface can have dramatic effects.
The present findings show that while vessel wall TF may serve as a nidus for thrombosis, it is unlikely that it, or the products that it generated, serve as the driving force that allows thrombi to grow several millimeters, especially considering that spontaneous thrombus formation of 2 mm to 3 mm in diameter have been observed in vivo on a timescale of minutes.20 Therefore, low levels of procoagulant activity on the surface of a growing thrombus would likely have a much stronger impact on the rate of thrombus growth than saturating quantities of vessel wall–bound TF, cached beneath a blanket of platelets. Blood-borne sources of procoagulant activity that could explain the rapid growth of large-diameter thrombi may include circulating TF (either soluble, vesicle associated, or cell associated), thrombin-activated platelet factor XI, or something else. Currently, however, these proteins are not regarded as playing an essential key step in the pathophysiology of thrombosis. The procoagulant activity exerted by such a cofactor or enzyme directly on an activated platelet surface would allow for the rapid formation of prothrombinase complexes at the site where fibrin formation and platelet recruitment would be most effective. If diffusional transport of FIX and FX are determinants of thrombus growth, one would expect that the rate of growth would decrease with increasing platelet accumulation, especially since the time required for diffusion increases with the square of the distance. The presence of an alternative source of procoagulant activity would explain the lack of such an observation. Further studies that clarify the mechanism(s) by which different coagulation proteins come in to spatial contact with each during the dynamic events associated with thrombus formation are warranted.
Prepublished online as Blood First Edition Paper, March 11, 2004; DOI 10.1182/blood-2003-12-4352.
Supported in part by National Institutes of Health grants NIH-HL29019 and NIH-HL54469, and funding from a National Institutes of Health/National Cancer Institute (NIH-NCI) shared resources grant (1 R24 CA095823-01).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Microscopy was performed at the MSSM-Microscopy Shared Research Facility, supported, in part, with funding from a National Institutes of Health/National Cancer Institute (NIH-NCI) shared resources grant (1 R24 CA095823-01).






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal