Abstract
Besides its well-recognized role in hemostasis and thrombosis, thromboxane A2 synthase (TXAS) is proposed to be involved in thrombopoiesis and lymphocyte differentiation. To evaluate its various physiologic roles, we generated TXAS-deleted mice by gene targeting. TXAS–/– mice had normal bone marrow megakaryocytes, normal blood platelet counts, and normal CD4 and CD8 lymphocyte counts in thymus and spleen. Platelets from TXAS–/– mice failed to aggregate or generate thromboxane B2 in response to arachidonic acid (AA) but produced increased prostaglandin-E2 (PGE2), PGD2, and PGF2α. AA infusion caused a progressive drop of mean arterial pressure (MAP), cardiac arrest, and death in wild-type (WT) mice but did not induce shock in TXAS–/– mice or in WT and TXAS–/– mice treated with antagonist to the thromboxane-prostanoid (TP) receptor. The TXAS–/– mice were able to maintain normal MAP upon AA insult when TP was present but were unable to do so when TP was blocked by an antagonist, suggesting a role of endoperoxide accumulation in influencing MAP. We conclude that TXAS is not essential for thrombopoiesis and lymphocyte differentiation. Its deficiency causes a mild hemostatic defect and protects mice against arachidonate-induced shock and death. The TXAS-deleted mice will be valuable for investigating the roles of arachidonate metabolic shunt in various pathophysiologic processes.
Introduction
Thromboxane A2 synthase (TXAS) is a 60-kDa transmembrane protein that is distributed in platelets, monocytes, and several other cell types.1-5 It belongs to the large cytochrome p450 (CYP) family.6 It is considered to be an atypical CYP as it does not possess mono-oxygenase activity. It is an isomerase catalyzing the conversion of prostaglandin H2 (PGH2) to thromboxane A2 (TXA2), HHT (l2-L-hydroxy-5, 8,10-heptadecatrienoic acid), and malondialdehyde (MDA). TXA2 is a potent stimulator of platelet activation and aggregation and vascular constriction.7 These actions of TXA2 have been demonstrated to be mediated by binding to a G-protein–coupled–specific receptor, the thromboxane-prostanoid (TP) receptor.8 The cellular and tissue distribution of TP receptors is closely correlated with that of TXAS.9 Since TXA2 has an extremely short half-life and acts as an autocoid, the concordant tissue distribution between TXAS and TP facilitates the rapid actions of TXA2. TXA2 plays a major role in hemostasis, thrombosis, vasoconstriction, and vascular cell proliferation. Patients whose platelets are defective in producing TXA2 have a bleeding disorder, whereas overproduction of TXA2 is associated with diverse vascular events including acute coronary syndrome, ischemic stroke, pulmonary hypertension, abnormal renal hemodynamics, and preeclampsia.10-18 The important role of TXA2 in hemostasis and vascular disorders is further supported by the defects observed in TP-deleted mice generated by gene targeting. TP–/– mice have normal embryogenesis, postnatal growth, and fertility but exhibit a mild bleeding disorder due to defective platelet aggregation and an alteration in hemodynamic response. TP–/– mice are less susceptible to atherogenesis, thrombosis, and the consequent vascular events.19,20
TXAS-deleted mice have not been reported. It might be anticipated that TXAS–/– mice would exhibit similar hemostatic defects and resistance to atherothrombosis as TP–/– mice. However, recent reports suggest that TXAS may have additional biologic activities, and therefore, its genetic deletion may have unexpected phenotypic changes that might not be shared by TP deletion. For example, it has been proposed that TXAS may be involved in platelet production.21 TXAS deletion may cause a shunt of PGH2 to other prostanoid synthetic pathways, which may elicit phenotypic changes independent of TXA2. It is, therefore, important to generate TXAS–/– mice and characterize their phenotypic changes. Genomic TXAS DNA was cloned by several laboratories including ours.22-24 It is surprisingly large in size (> 190 kb) despite a relatively small coding region. Several introns exceed 50 kb in size. In this study, we interrupted a 0.6-kb fragment harboring exon 9 and a portion of intron 9 from murine TXAS genomic DNA in embryonic stem (ES) cells and generated TXAS+/– and TXAS–/– mice. Our results show that TXAS–/– mice have a prolonged bleeding time and defective platelet aggregation. Metabolic analysis indicates a compensatory increase in PGE2, PGD2, and PGF2α synthesis. The TXAS–/– mice have normal megakaryocytes in bone marrow and blood platelet counts as well as normal CD4 and CD8 lymphocytes. TXAS–/– mice are resistant to arachidonate-induced shock and death and maintain a normal arterial blood pressure, as contrasted to a drop of blood pressure in TP-deleted mice.
Materials and methods
Targeting vector and disruption of the gene coding for TXAS
Isogenic genomic DNA coding for mouse TXAS was obtained by screening a 129sv mouse genomic DNA library (a gift from Dr Begue, Pasteur Institute, France) with a human TXAS cDNA fragment.2 An 8.1-kb BamHI fragment spanning a portion of intron 7 to intron 10 was isolated from the phage clone and subcloned into the unique BamHI site of pGEM7Zf (Promega, Madison, WI). A 0.6-kb fragment containing exon 9 and a portion of intron 9 was excised from the resultant plasmid by PstI digestion, and this gap region was filled with a 3.2-kb SalI fragment of phosphoglycerate kinase–hypoxanthine phosphoribosyltransferase (PGK-HPRT)25 modified at the SalI site by NsiI linkers. Consequently, the PGK-HPRT fragment was flanked by 2 TXAS DNA fragments, 6.1 kb and 1.4 kb, homologous to the 5′ and 3′ end, respectively, of the mouse genomic TXAS counterparts. The TXAS-floxed PGK-HPRT was subcloned into the unique BamHI site of pPNT-neo–, which was derived from pPNT26 by deleting the 2-kb NotI-XbaI fragment containing PGK-neo. The resultant targeting construct contains HPRT gene and herpes thymidine kinase (tk) gene from pPNT for selection of the targeted ES clones.
Gene targeting of ES cells and generation of TXAS knockout (KO) mice
The E14TG2a (HPRT–) ES cell line27 was cultured, propagated, and transfected with the targeting construct by electroporation as described previously.28 HAT (0.1 mM hypoxanthine, 4 μM aminopterin, and 0.16 mM thymidine) and ganciclovir (10 μM) were used to select for ES cell colonies grown from electroporation. Surviving cell colonies were isolated, established as clones, and genotyped by Southern blotting to ensure homologous recombination.29 Southern blotting and genomic DNA isolation were performed following standard procedures.30 Two probes, a 2-kb XbaI-XbaI fragment and a 0.6-kb XbaI-PstI genomic DNA fragment located at 5′ and 3′ to exon 9, respectively, were used for Southern blotting to identify the desired ES cell clones. The correct clones were subsequently introduced into blastocysts of C57BL/6J mice by microinjection using a technique previously described.31 Chimeric mice were bred with wild-type (WT) C57BL/6J and BALB/c mice to obtain heterozygous first generation (F1) mice, which were intercrossed to generate homozygous F2 mice. Genotyping of F1 and F2 mice was performed by Southern blotting and by polymerase chain reaction (PCR) with 35 cycles of amplifications at 94° C, 55° C, and 72° C for 1 minute each. The PCR reaction mixture contained 0.3- to 1-μg tail DNA, 200 μM deoxynucleoside triphosphate (dNTP), 200 nM each of the primers, and 2.5 U Taq DNA polymerase in 50-μL reaction buffers supplied by the manufacturer (Amersham Pharmacia Biotech, Cambridge, United Kingdom). The oligonucleotide primers used were 5′-TCCTCAGAGGCGGAGAGACTT-3′ (forward) in intron 8 paired with 5′-TTCCTATCAGTAACAGCATCTAAGAGG-3′ (reverse) in the HPRT gene for assessing the recombinant KO allele (the product size was 690 bp) and primer 5′-TGGATGCCCAGCACTCCATGAACT-3′ (forward) paired with 5′-AGGTCCACCTCTTTCAGAAGCC-3′ (reverse), both in exon 9, for assessing the WT allele (product size, 270 bp).
Northern blotting and reverse transcription–polymerase chain reaction (RT-PCR)
Total RNA was extracted from spleen and thymus using TriZol reagent (GibcoBRL, Gaithersburg, MD). For RT-PCR, 5 μg total RNA was first reverse transcribed into cDNA by reverse transcriptase (Superscript RT, GibcoBRL) using oligo dT as a primer. PCR was performed for 35 cycles of 94° C for 1 minute, 55° C for 2 minutes, and 72° C for 2 minutes using primers specific for TXAS (forward primer, 5′-AGGCTTCTGAAAGAGGTGGACCT-3′ in exon 9; and reverse primer, 5′-TGAAATCACCATGTCCAGATAC-3′ in exon 10) or glyceraldehyde-3-phosphate dehydrogenase (GAPDH; forward primer, 5′-GACCACAGTCCATGCCATCAC-3′; and reverse primer, 5′-TCCACCACCCTGTTGCTGTAG-3′). GAPDH was used as an internal control. The PCR products were analyzed by electrophoresis on a 2% agarose gel. Northern blotting was performed using the glyoxal method as described previously.29 A cDNA subfragment of 515 bp, corresponding to the 3′ end of the mouse TXAS cDNA, was obtained by RT-PCR using oligo dT for RT and primers 5′-AGGCTTCTGAAAGAGGTGGACCT-3′ in exon 9 and 5′-GACGGATCCTTCTATATCATCAGCGTGAC-3′ in exon 13 for PCR. The 515-bp fragment was labeled with α-32P–dCTP (deoxycytidine 5′-alpha 32P triphosphate) by random priming (rediprime II; Amersham Pharmacia Biotech) and used as a probe for Northern blot analysis. After washing away the unbound probes, the signal was visualized by autoradiography. The same membrane was subsequently stripped and reprobed with 32P-labeled mouse GAPDH cDNA as control for RNA integrity.
Complete blood count (CBC), clinical chemistry, and histology
Whole blood was collected by puncture of the retro-orbital plexus of mice using capillary tubes with or without an anticoagulant. CBCs and platelet counts were performed on EDTA (ethylenediaminetetraacetic acid) anticoagulated blood using an automated counting device (Sysmex KX-21; SYSMEX, Kobe, Japan). Serum blood chemistry was analyzed by an automated device (Hitachi 7170A Automatic Analyzer; HITACHI, Tokyo, Japan) at the Clinical Laboratory of the National Taiwan University Hospital as described previously.29 Parameters analyzed included levels of total protein, albumin, total bilirubin, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, amylase, creatine kinase, lactate dehydrogenase, and γ-glutamyltransferase. For histology analysis, kidney, thymus, lung, liver, spleen, and bone samples were fixed in 10% neutral-buffered formalin and processed according to standard methods. Bone marrow samples were decalcified in 11% hydrochloric acid, 5% to 10% polyvinylpyrrolidone, and less than 1% fluorinated surfactant (TBD-1; Shandon, Pittsburgh, PA) before section and staining with hematoxylin and eosin (H & E). Microscopic examination was performed on a light microscope (Nikon Model Microphot-XFA; Tokyo, Japan).
Electron microscopy
Electron microscopy of platelets was performed according to a modification of the procedure of Stenberg et al.32 Briefly, whole blood was collected from postcaval vein into citrated anticoagulant. Platelets were collected and fixed at 4° C overnight with 1.5% glutaraldehyde in 10 mM cacodylate buffer (pH 7.4). Platelets were then dehydrated through an ascending series of alcohols, infiltrated with propylene oxide, and embedded in epoxy resin. Ultrathin sections were stained with uranyl acetate and lead citrate and examined with a JEOL 100CX-II transmission electron microscope (JEOL, Peabody, MA) at an accelerating voltage of 60 kV.
Flow cytometry
Flow cytometry was performed as described previously31 with a fluorescence activation cell sorter (FACS; Becton Dickinson, San Jose, CA). For detection of the cells from thymus and spleen, each organ was ground into single-cell suspensions by a syringe in phosphate-buffered saline (PBS) followed by washing once in PBS. Approximately 2 × 105 cells from thymus, spleen, and peripheral blood of homozygous, heterozygous, and WT mice of 6 to 7 weeks old were subjected to specific surface protein staining by incubating with 1 μg of antibodies against lineage-specific markers at 4° C for 30 minutes. The lineage-specific antibodies included B220 for B lymphocytes; CD3, Thy1.2, CD4, or CD8 for T lymphocytes; and CD11b for dendritic cells/macrophages. Numbers of positively stained cells were expressed as percentages of total. All the antibodies were purchased from Pharmingen (San Diego, CA).
Bleeding time
Bleeding time was performed on mice at 12 weeks of age as described by Dejana et al.33 Mice were anesthetized with avertin and placed into a restraining device to free their tails in a vertical position throughout the test. An approximately 3- to 5-mm segment of the tail was transected using a disposable scalpel, and the remaining length of tail was immersed immediately into a 37° C solution of sterile isotonic saline. Bleeding time was measured from the time of tail transection until visible bleeding could no longer be detected. If bleeding continued longer than 10 minutes, the experiment was terminated and the bleeding site was cauterized. Animal experiments were conducted according to Declaration of Helsinki principles. The protocols were approved by the animal review board of National Taiwan University College of Medicine.
Platelet aggregation and secretion assays
Mice were anesthetized with avertin, and whole blood was collected from postcaval vein and mixed with one-tenth volume of 3.8% sodium citrate. The citrated blood was centrifuged at 200g for 15 minutes, the supernatant (platelet-rich plasma [PRP]) was collected, and the remaining fractions were centrifuged at 2000g to collect the platelet-poor plasma (PPP). For each group of mice, PRP from 2 to 4 mice were pooled and platelets were counted manually. Platelet aggregation was measured by adding arachidonic acid (AA; final concentration 1 mM), adenosine diphosphate (ADP; 10 μM), or collagen (0.2 mg/mL) to PRP (containing 3 × 108 platelets/mL) at 37° C in a platelet aggregation profiler (model PAP-4; Bio/Data Corp, Horsham, PA) with the maximal light transmission set by the absorbance of PPP. The aggregation was reported as change in light absorbance and monitored for more than 10 minutes after addition of each reagent.
Platelet release reaction was determined by the secretion of serotonin from platelet granules.34 Briefly, fresh platelets from PRP were incubated with 0.25 μM of 14C-serotonin (50 mCi/mM [1850 MBq/mM]; Amersham Pharmacia Biotech) for 30 minutes at 25° C. After washing twice with Tyrode solution (138 mM NaCl, 3 mM KCl, 12 mM NaHCO3, 1 mM MgCl2, 5 mM glucose, 0.4 mM Na2HPO4, and 10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid]) containing 1% bovine serum albumin (BSA), an aliquot (108 platelets in 1 mL) was incubated with 1 mM AA or 1 μM U46619 for 15 minutes at 37° C. One hundred microliters of the platelet suspension was added to 100 μL of a stop solution (10 mM EDTA and 200 mM formaldehyde). After incubation for 30 minutes on ice, the mixture was centrifuged at 10 000g for 2 minutes, and the radioactivity in the supernatant and the pellet was counted for β emissions in a scintillation counter. Percentage of 14C-serotonin release was calculated as supernatant cpm/total cpm × 100, where cpm indicates counts per minute.
Arachidonic acid (AA) metabolism
AA metabolic profile was analyzed by thin-layer chromatography (TLC) according to a previously described method35 with minor modifications. Briefly, citrated blood collected from 2 to 3 mice of each genotype was centrifuged at 200g for 10 minutes at room temperature to collect platelets. After washing twice in calcium-free Krebs-Henseleit solution (pH 7.4), platelets were resuspended in Krebs-Henseleit buffer and 1-mL platelet suspension was incubated for 10 minutes at 37° C with 5 μL of 0.1 mCi/mL (3.7 MBq/mL) [1-14C] AA (specific activity 48 mCi/mmol [1776 MBq/mmol]). The reaction was stopped by acidification to pH 3 with 1 normality (N) HCl and the reaction mixture was extracted 3 times with diethyl-ether, evaporated to dryness, and resuspended in 50 μL chloroform before applied to TLC on precoated silica gel plates (Merck, Darmstadt, Germany). The plates were developed in a chloroform-methanol–acetic acid–water ratio of 90:8:1:0.8 (vol/vol/vol/vol). The radioactivity of each metabolite was analyzed by a radio-chromatoscanner (System 200 Image scanner; BIOSCAN, Washington, DC). The 6-keto PGF1α and 2,3-dinor TXB2 were measured by enzyme immunoassay kits. The kit for 6-keto PGF1α assays was obtained from Amersham Pharmacia Biosciences and that for 2,3-dinor TXB2 was from Cayman Chemical (Ann Arbor, MI).
Blood pressure measurements
WT and TXAS–/– littermates were anesthetized with pentobarbital. Arterial pressure was monitored from the carotid artery by placing a string of polyethylene (PE-10) tubing in the artery and the pressure was recorded continuously at 50 recordings/second using Grass Model 79DEEG and polygraph Data Recording system (Grass Instrument, Quincy, MA). To evaluate the effect of AA and U46619 on blood pressure and cardiopulmonary conditions, AA (40 mg/kg body weight) or U46619 (2 mg/kg body weight) was infused intravenously via a jugular vein and mean arterial blood pressure was monitored continuously for 10 minutes. To assess the vascular response of TXAS–/– mice to AA in the presence of a TP antagonist, SQ29548 (2 mg/kg body weight) was infused via a tail vein followed by AA (40 mg/kg body weight) via a jugular vein and the mean arterial blood pressure was monitored.
Results
TXAS-deficient mice were viable and fertile
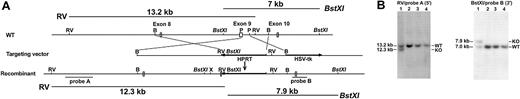
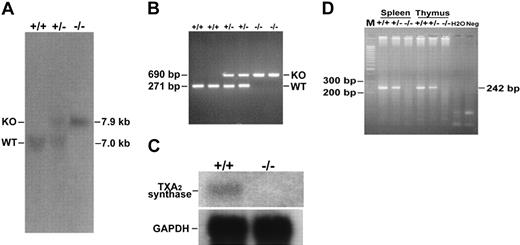
We deleted from the TXAS gene a 0.6-kb PstI fragment containing codons for amino acid residues 234-333 and the first 223 bp of intron 9 and replaced it with an HPRT cassette to terminate TXAS transcription (Figure 1A). The TXAS locus was successfully modified in the ES cell clones as demonstrated by Southern blotting shown in Figure 1B. The recombinant TXAS knockout (KO) allele exhibited a 12.3-kb EcoRV signal by a 5′ genomic DNA probe in contrast to a 13.2-kb band representing WT allele by the same analysis. When hybridized by a 3′ DNA probe, the KO and WT alleles gave an expected BstXI restriction pattern of 7.9 kb and 7.0 kb, respectively (Figure 1B), which is consistent with the results of hybridization with a 5′ probe. The TXAS-deleted ES cells were used to generate 2 chimeras, which transmitted the mutant TXAS allele to their offspring. Genotyping by Southern blotting of F1 using the 3′ probe revealed the presence of 7.9- and 7.0-kb bands, consistent with a heterozygous state (Figure 2A). Blotting of the F2 offspring showed only the 7.9-kb band, consistent with homozygosity. Similar results were obtained by using PCR analysis (Figure 2B). Confirmation of the TXAS-null status in the homozygous progeny was provided by Northern blotting and RT-PCR, showing absence of a full-length 1.9-kb TXAS mRNA (Figure 2C) and lack of a 242-bp PCR fragment (Figure 2D) in the homozygous TXAS–/– mice. These results led us to conclude that we have successfully generated a TXAS-deficient mouse. Of approximately 678 F2 progeny produced by intercross of the TXAS heterozygous (TXAS+/–) mutant mice, about an equal number of male and female offspring survived after weaning. The Mendelian ratio was 1:1.7: 0.8 for WT-heterozygous-homozygous mice. The heterozygous and homozygous mice were slightly lower than expected. The reason for this is not clear at the present time. The TXAS–/– mice exhibited no gross abnormalities in size, body weight, and anatomical features of major organs. Blood metabolic profile including levels of blood sugar, liver enzymes, blood urea nitrogen, creatinine, and electrolytes of TXAS–/– mice was not different from that of TXAS+/+ mice.
Targeted TXAS gene deletion strategy. (A) Exons 8 to 10 are depicted as open boxes. Filled arrows are the HPRT and tk genes. Transcription of HPRT gene is opposite to that of TXAS gene. Also shown are the hybridization probe A (5′), a 2-kb XbaI-XbaI fragment, and probe B (3′), a 0.6-kb XbaI-PstI fragment. The length of the DNA fragments of WT TXAS hybridized by the probes is indicated at the top and that of recombinant deletion mutant is indicated at the bottom. (B) Southern blot analysis of ES cell DNA digested by EcoRV and hybridized by probe A (left) or digested by BstXI and hybridized by probe B (right). The size of the bands for WT and mutant (KO) alleles is shown. Lane 1 shows an ES cell clone with WT and mutated bands, whereas lanes 2 to 4 show WT clones.
Targeted TXAS gene deletion strategy. (A) Exons 8 to 10 are depicted as open boxes. Filled arrows are the HPRT and tk genes. Transcription of HPRT gene is opposite to that of TXAS gene. Also shown are the hybridization probe A (5′), a 2-kb XbaI-XbaI fragment, and probe B (3′), a 0.6-kb XbaI-PstI fragment. The length of the DNA fragments of WT TXAS hybridized by the probes is indicated at the top and that of recombinant deletion mutant is indicated at the bottom. (B) Southern blot analysis of ES cell DNA digested by EcoRV and hybridized by probe A (left) or digested by BstXI and hybridized by probe B (right). The size of the bands for WT and mutant (KO) alleles is shown. Lane 1 shows an ES cell clone with WT and mutated bands, whereas lanes 2 to 4 show WT clones.
Genotype analysis, Northern blotting, and RT-PCR of TXAS–/– mice. Genotyping was determined by Southern blot (A) and PCR (B) analyses. Tail DNA was isolated from WT (+/+), heterozygous (+/–), or homozygous (–/–) TXAS deletion littermates; digested with BstXI; and hybridized to probe B as described in Figure 1B. Bands corresponding to WT (7.0 kb) and mutant (7.9 kb) alleles are indicated. For Northern blotting (C) and RT-PCR (D), total RNA from spleen and thymus tissues was analyzed as described in “Materials and methods.” Lanes +/+, +/–, and –/– are WT, heterozygous, and TXAS–/– littermates, respectively. The GAPDH fragment was used as an internal control and is shown at the bottom of panel C. Hybridization of the thymus RNA is shown with a 515-bp cDNA fragment of the exons 9 to 13 of TXAS. For RT-PCR, results of spleen and thymus samples are shown. M indicates 100-bp DNA ladders; and H2O and Neg, experiments in which reaction conditions were the same as the others except that RNA (H2O) or RT reaction (Neg) was omitted.
Genotype analysis, Northern blotting, and RT-PCR of TXAS–/– mice. Genotyping was determined by Southern blot (A) and PCR (B) analyses. Tail DNA was isolated from WT (+/+), heterozygous (+/–), or homozygous (–/–) TXAS deletion littermates; digested with BstXI; and hybridized to probe B as described in Figure 1B. Bands corresponding to WT (7.0 kb) and mutant (7.9 kb) alleles are indicated. For Northern blotting (C) and RT-PCR (D), total RNA from spleen and thymus tissues was analyzed as described in “Materials and methods.” Lanes +/+, +/–, and –/– are WT, heterozygous, and TXAS–/– littermates, respectively. The GAPDH fragment was used as an internal control and is shown at the bottom of panel C. Hybridization of the thymus RNA is shown with a 515-bp cDNA fragment of the exons 9 to 13 of TXAS. For RT-PCR, results of spleen and thymus samples are shown. M indicates 100-bp DNA ladders; and H2O and Neg, experiments in which reaction conditions were the same as the others except that RNA (H2O) or RT reaction (Neg) was omitted.
TXAS–/– mice had normal platelet production and T-cell development
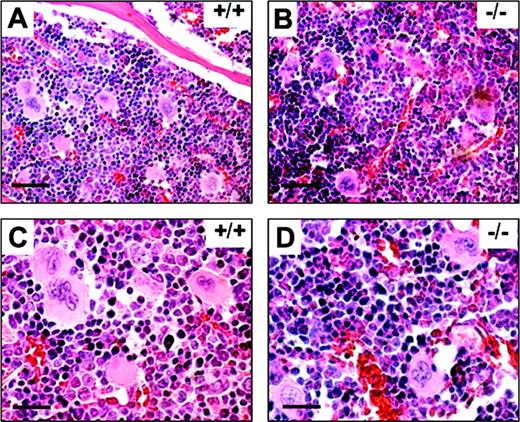
It has been suggested that TXAS is involved in platelet production. The evidence for this was indirect and based on an observation that nuclear factor–erythroid derived 2 (NF-E2)–null mice did not express TXAS with a correlated lack of platelet production.21 We were, therefore, interested in determining whether TXAS-null mice are capable of thrombopoiesis. We examined the bone marrow specimen from WT and TXAS–/– mice. As shown in Figure 3, bone marrow cellularity, megakaryocyte numbers, and morphology of TXAS–/– mice were not different from those of WT mice. The morphology of other cell lineages was also normal in TXAS–/– mice. Blood platelet count in TXAS–/– mice was slightly increased (840 ± 179 × 109/L and 786 ± 166 × 109/L for male and female mutants, respectively) but not significantly different from WT mice (755 ± 164 × 109/L and 686 ± 132 × 109/L). The morphology and granulation of peripheral platelets was also normal by electron microscopy (data not shown). Erythrocyte and leukocyte counts of TXAS–/– were also comparable to those of WT mice (data not shown). These findings indicate that TXAS is not essential for platelet production.
H & E staining of bone marrow sections. Bone marrow examinations of WT (+/+) and TXAS–/– (–/–) littermates are shown in low (A-B) and high (C-D) magnifications. Bars indicate 50 μm for panels A and B and 12.5 μm for panels C and D.
H & E staining of bone marrow sections. Bone marrow examinations of WT (+/+) and TXAS–/– (–/–) littermates are shown in low (A-B) and high (C-D) magnifications. Bars indicate 50 μm for panels A and B and 12.5 μm for panels C and D.
TXAS has been implicated in T-cell development in thymus.36,37 This notion had not been supported by direct evidence. We examined CD4- and CD8-bearing T cells in thymus and spleen by flow cytometry and the analysis revealed no significant difference in the subtypes of T cells between TXAS–/– and WT mice (Table 1). These results are consistent with the thromboxane receptor gene deletion data.19 Taken together, these results indicate that TXAS and TXA2 are not essential for thymus T-cell development.
Percentages of T-lymphocyte subsets in thymus and spleen by flow cytometry
. | Thymus, % . | . | . | Spleen, % . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subset . | +/+ . | +/- . | -/- . | +/+ . | +/- . | -/- . | ||||
| Thy 1.2+ | 92.9 ± 4.5 | 96.3 ± 3.6 | 96.4 ± 5.7 | 14.9 ± 9.4 | 9.8 ± 6.2 | 14.7 ± 8.4 | ||||
| CD4+CD8+ | 82.1 ± 11.4 | 72.8 ± 7.9 | 82.5 ± 3.9 | 9.3 ± 5.8 | 15.9 ± 10.3 | 9.7 ± 3.6 | ||||
| CD4-CD8- | 3.0 ± 2.1 | 4.6 ± 2.1 | 2.8 ± 1.3 | 7.9 ± 2.2 | 8.0 ± 3.0 | 6.7 ± 1.4 | ||||
| CD4+CD8- | 12.1 ± 7.2 | 17.9 ± 5.2 | 11.4 ± 1.4 | 57.3 ± 8.6 | 52.9 ± 9.5 | 59.0 ± 5.5 | ||||
| CD4-CD8+ | 3.8 ± 2.5 | 4.7 ± 1.4 | 3.3 ± 1.4 | 25.5 ± 4.1 | 23.2 ± 1.6 | 24.6 ± 2.7 | ||||
. | Thymus, % . | . | . | Spleen, % . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subset . | +/+ . | +/- . | -/- . | +/+ . | +/- . | -/- . | ||||
| Thy 1.2+ | 92.9 ± 4.5 | 96.3 ± 3.6 | 96.4 ± 5.7 | 14.9 ± 9.4 | 9.8 ± 6.2 | 14.7 ± 8.4 | ||||
| CD4+CD8+ | 82.1 ± 11.4 | 72.8 ± 7.9 | 82.5 ± 3.9 | 9.3 ± 5.8 | 15.9 ± 10.3 | 9.7 ± 3.6 | ||||
| CD4-CD8- | 3.0 ± 2.1 | 4.6 ± 2.1 | 2.8 ± 1.3 | 7.9 ± 2.2 | 8.0 ± 3.0 | 6.7 ± 1.4 | ||||
| CD4+CD8- | 12.1 ± 7.2 | 17.9 ± 5.2 | 11.4 ± 1.4 | 57.3 ± 8.6 | 52.9 ± 9.5 | 59.0 ± 5.5 | ||||
| CD4-CD8+ | 3.8 ± 2.5 | 4.7 ± 1.4 | 3.3 ± 1.4 | 25.5 ± 4.1 | 23.2 ± 1.6 | 24.6 ± 2.7 | ||||
Each value denotes mean ± SD from 5 mice.
+/+ indicates WT; +/-, heterozygous; and -/-, homozygous TXAS-deleted mice.
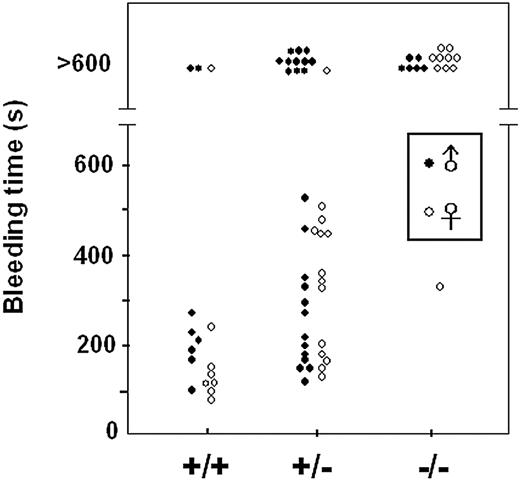
TXAS–/– mice exhibited prolonged bleeding time and defective platelet aggregation
All except one TXAS–/– mouse had a marked prolongation of bleeding time (Figure 4). The TXAS+/– mice had a significantly longer bleeding time than the WT mice. However, there was a wide distribution of bleeding time in the TXAS+/– mice (Figure 4). We next determined platelet aggregation induced by AA, ADP, and collagen and show the AA-induced aggregation as representative (Figure 5). TXAS–/– male and female mice showed an almost complete absence of AA-induced platelet aggregation with only a small primary wave of aggregation, whereas TXAS+/– had only a moderate reduction in platelet aggregation (Figure 5). By contrast, the maximal aggregation in response to ADP and collagen was not significantly different between TXAS-deleted and WT mice, but the lag period in response to collagen was slightly more prolonged in TXAS–/– mice (male/female, 218/240 s) than in WT (195/225 s) or heterozygous (200/225 s) mice. The TXAS–/– platelets had normal secretion function as judged by release of serotonin after activation (the percentage of 14C-serotonin release was 83.67 ± 6.1 and 84.47 ± 3.61 [n = 4] for WT and TXAS–/– platelets, respectively, after AA induction and 10.45 ± 0.13 and 14.00 ± 1.32 [n = 4], respectively, after U46619 activation). Analysis of eicosanoid levels produced by platelets after AA stimulation revealed an undetectable TXB2 level in male or female TXAS–/– mice (Figure 6). Interestingly, there was a compensatory increase in PGE2 levels and PGD2 and PGF2α became detectable in TXAS–/– mice compared with the WT and heterozygous mice (Figure 6). These results suggest that in the absence of TXAS, endoperoxides produced from AA by cyclo-oxygenase are shunted into PGE2 and, to a lesser extent, PGD2 and PGF2α synthetic pathways. The HHT level in TXAS–/– platelets was reduced but not completely absent. The residual HHT detected in TXAS–/– mice could represent nonenzymatic generation of HHT. We also measured TXB2 metabolite by enzyme immunoassay (EIA) and could not detect any TXB2 in TXAS–/– platelets stimulated with AA (data not shown).
Bleeding time by tail transection. WT (+/+), heterozygous (+/–), and homozygous TXAS–/– (–/–) littermates of male (•) and female (○) mice were analyzed for the duration of tail bleeding after transection. Bleeding time longer than 10 minutes is shown above as > 600 seconds.
Bleeding time by tail transection. WT (+/+), heterozygous (+/–), and homozygous TXAS–/– (–/–) littermates of male (•) and female (○) mice were analyzed for the duration of tail bleeding after transection. Bleeding time longer than 10 minutes is shown above as > 600 seconds.
Platelet aggregation in response to arachidonic acid. Platelets of male and female littermates were collected and counted as described in “Materials and methods.” The platelet number was adjusted to 3 × 108/mL and 400 μL was used. AA (final concentration 1 mM) was added to induce aggregation at 37° C. +/+ indicates WT; +/–, heterozygous; and –/–, homozygous littermates.
Platelet aggregation in response to arachidonic acid. Platelets of male and female littermates were collected and counted as described in “Materials and methods.” The platelet number was adjusted to 3 × 108/mL and 400 μL was used. AA (final concentration 1 mM) was added to induce aggregation at 37° C. +/+ indicates WT; +/–, heterozygous; and –/–, homozygous littermates.
Analysis of AA metabolites from AA-treated platelets by thin-layer chromatography (TLC). Washed platelets from 2 to 3 mice were used for each experiment. Five microliters of [1-14C]AA (0.1 mCi/mL [3.7 MBq/mL]) was added to washed platelets and incubated at 37° C for 10 minutes. The reaction was stopped by 27 μL 1 N HCl. Prostanoids were extracted with diethyl-ether before dissolving in 50 μL chloroform for TLC analysis. Developing condition was at room temperature for 80 minutes in 30 mL chloroform-methanol–acetic acid–water ratio of 90:8:1:0.8 (vol/vol/vol/vol). Commercially obtained metabolites were included as references (marked at the left hand side). +/+ indicates WT mice; +/–, heterozygous; and –/–, homozygous mutant.
Analysis of AA metabolites from AA-treated platelets by thin-layer chromatography (TLC). Washed platelets from 2 to 3 mice were used for each experiment. Five microliters of [1-14C]AA (0.1 mCi/mL [3.7 MBq/mL]) was added to washed platelets and incubated at 37° C for 10 minutes. The reaction was stopped by 27 μL 1 N HCl. Prostanoids were extracted with diethyl-ether before dissolving in 50 μL chloroform for TLC analysis. Developing condition was at room temperature for 80 minutes in 30 mL chloroform-methanol–acetic acid–water ratio of 90:8:1:0.8 (vol/vol/vol/vol). Commercially obtained metabolites were included as references (marked at the left hand side). +/+ indicates WT mice; +/–, heterozygous; and –/–, homozygous mutant.
TXAS gene deletion protected against AA-induced shock
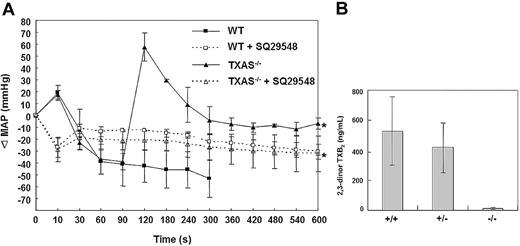
To determine whether TXAS plays a role in regulating blood pressure, we compared mean arterial pressure (MAP) between TXAS–/– and WT mice. The mean MAP values of WT male and female mice (n = 10) were 107.4 ± 5.8 and 106.2 ± 4.7 mm Hg, respectively. The respective male and female MAP values in TXAS–/– mice were 108.1 ± 3.8 and 106.1 ± 5.0 mm Hg, while those in TXAS+/– were 109.0 ± 3.3 and 105.6 ± 6.4 mm Hg. Thus, the basal blood pressure is not influenced by TXAS deletion. We next determined whether TXAS–/– mice were less susceptible to shock insults. We infused mice with AA intravenously and monitored blood pressure continuously. There was a transient rise in MAP at 10 seconds after AA infusion in WT mice but the MAP dropped rapidly thereafter and all 4 mice developed cardiac arrest at 2 minutes and died at 5 minutes after AA infusion (Figure 7A). On the other hand, all the TXAS–/– mice survived without cardiac arrest or death despite the same AA infusion. It is interesting to note that MAP in TXAS–/– mice dropped for the initial 90 seconds as in WT, but subsequently the blood pressure rose drastically at 120 seconds after AA infusion, declined gradually to the baseline, and maintained at baseline 300 seconds after infusion (Figure 7A). Neither the WT nor the TXAS–/– mice survived after infusion of a TXA2 analog and TP agonist, U46619. These results indicate that the protective effect of TXAS deletion is attributable to TXA2 deficiency without a concurrent defect in TP receptor. To determine whether blood pressure in TXAS-deleted mice is maintained by prostaglandin endoperoxides (PGG2 and PGH2) that are accumulated in the absence of TXAS, we pretreated TXAS-deleted or WT mice with SQ29548 and assessed the response of these 2 groups of mice to AA challenge. Both WT and TXAS-deleted mice were viable after AA treatment. However, compared with TXAS–/– mice without TP blockade, the SQ29548-treated TXAS-deleted mice, like the WT mice, exhibited a significantly lower MAP (Figure 7A). Thus, TXAS–/– mice with TP blockade resembled TP-deleted mice with respect to blood pressure changes.19
Hemodynamic response of mice challenged by intravenous injection of AA. (A) Changes in blood pressure after intravenous AA treatment with (- - -) and without (—) prior infusion of SQ29548. Mice were anesthetized and blood pressure was continuously monitored till MAP was in stable readouts. AA (40 mg/kg) was injected at time 0. When AA was injected without SQ29548, all the WT mice (n = 4) developed cardiac arrest at around 90 to 120 seconds and died at 300 seconds when MAP monitoring was stopped. All TXAS–/– mice (n = 4) survived and MAP was recorded for 10 minutes. All mice pretreated with SQ29548 (2 mg/kg) also survived after AA challenge and their MAP was also monitored for 10 minutes. Blood pressure was expressed as change in MAP (ΔMAP) before and after AA infusion. *The statistical P values were analyzed by analysis of variance (ANOVA) for TXAS–/– mice with and without SQ29548 and were 0.05, 0.02, 0.04, 0.02, 0.05, and 0.02 for 300, 360, 420, 480, 540, and 600 seconds, respectively. (B) Changes in plasma TXA2 after AA infusion. Plasma from mice at normal and steady-state condition (n = 11) and 5 minutes after infusion of AA (n = 4) was prepared from citrated blood containing indomethacin. Plasma 2,3-dinor TXB2 was measured by an EIA kit. Error bars represent ±1 SD.
Hemodynamic response of mice challenged by intravenous injection of AA. (A) Changes in blood pressure after intravenous AA treatment with (- - -) and without (—) prior infusion of SQ29548. Mice were anesthetized and blood pressure was continuously monitored till MAP was in stable readouts. AA (40 mg/kg) was injected at time 0. When AA was injected without SQ29548, all the WT mice (n = 4) developed cardiac arrest at around 90 to 120 seconds and died at 300 seconds when MAP monitoring was stopped. All TXAS–/– mice (n = 4) survived and MAP was recorded for 10 minutes. All mice pretreated with SQ29548 (2 mg/kg) also survived after AA challenge and their MAP was also monitored for 10 minutes. Blood pressure was expressed as change in MAP (ΔMAP) before and after AA infusion. *The statistical P values were analyzed by analysis of variance (ANOVA) for TXAS–/– mice with and without SQ29548 and were 0.05, 0.02, 0.04, 0.02, 0.05, and 0.02 for 300, 360, 420, 480, 540, and 600 seconds, respectively. (B) Changes in plasma TXA2 after AA infusion. Plasma from mice at normal and steady-state condition (n = 11) and 5 minutes after infusion of AA (n = 4) was prepared from citrated blood containing indomethacin. Plasma 2,3-dinor TXB2 was measured by an EIA kit. Error bars represent ±1 SD.
To confirm selective impairment of TXA2 synthesis in response to AA infusion in TXAS–/– mice, we measured the plasma levels of 2,3-dinor TXB2, a stable metabolite of TXA2, in TXAS–/– mice versus WT mice. Basal plasma 2,3-dinor TXB2 in untreated WT mice was barely detectable and was below the detection limit of the assay in untreated TXAS–/– mice (data not shown). The 2,3-dinor TXB2 levels were highly elevated in WT mice after AA infusion but remained very low in TXAS–/– mice (Figure 7B).
Discussion
Thromboxane A2 synthase occupies a key position in TXA2 biosynthesis. It is widely distributed in hematopoietic and lymphoid cells. Several important physiologic roles have been ascribed to TXAS but the evidence to support them was circumstantial. In this study, we report for the first time the generation of TXAS-deleted mice by gene targeting. Our findings indicate that TXAS is not essential for embryo development. The neonatal mice do not exhibit phenotypic abnormalities and have normal growth and fertility. These results are in accord with those of thromboxane receptor deletion. Taken together, these findings indicate that TXA2 is not essential for embryogenesis, reproduction, or growth. TXAS deletion provides additional information to TP deletion, as deletion of TXAS causes accumulation of prostaglandin endoperoxides and a shunting of the endoperoxides into other metabolic pathways, which may exert pathophysiologic actions that would not be detected with TP receptor deletion. To provide direct evidence for this, we used platelets as a model, as platelets are readily available from the genetically modified mice. We found that in contrast to predominant biosynthesis of TXB2 and HHT from exogenously added AA in WT mice, TXB2 was completely absent and HHT was markedly reduced with a compensatory increase primarily in PGE2 and to a lesser extent in PGD2 and PGF2α in homozygous TXAS-deleted mice. These results support the concept that the cellular levels of the terminal enzymes play an important role in regulating the extent and the composition of prostanoid syntheses in platelets and most likely also in other cell types.
It was reported that NF-E2, which was initially considered to be involved in erythropoiesis, is crucial for platelet production as NF-E2–/– mice had a complete absence of megakaryopoiesis.38 The role of NF-E2 in platelet production was reported to be independent of thrombopoietin. In search for genes that are responsible for NF-E2–induced megakaryopoiesis, Deveaux et al21 identified TXAS as a gene whose expression depends on NF-E2. TXAS expression was absent in NF-E2 knockout cells.21 These observations led the authors to postulate that TXAS may be involved in thrombopoiesis. We were intrigued by this proposal. We therefore analyzed bone marrow megakaryocytes and peripheral blood platelet counts. Our results indicate that thrombopoiesis is intact in TXAS–/– mice. Productions of other hematopoietic cells are also normal in the absence of TXAS. We conclude that NF-E2–mediated thrombopoiesis is independent of TXAS or TXA2.
The TXAS–/– mice mimic human TXAS deficiency in many aspects, such as prolonged bleeding time, defective AA-induced platelet aggregation, and altered AA metabolism,39,40 with absent TXA2 but more prominent PGE2, PGD2, and PGF2α productions.35 Our TXAS KO mice, like humans with TXAS deficiency, have a mild hemostatic defect without overt spontaneous bleeding. This is attributable to the TXA2-independent platelet aggregation induced by thrombin and collagen. Furthermore, accumulation of prostaglandin endoperoxides (PGG2 and PGH2), which stimulate platelets via the same TP receptors as TXA2, may compensate for diminished TXA2 production and provide control for hemostasis.35 The heterozygous TXAS+/– mice had variable hemostatic defects as evidenced by a wide range of bleeding time and mild aggregation defects. This is analogous to human TXAS deficiency. Hemostatic defects in human TXAS deficiency appear to be heterogeneous; some patients had near normal aggregation in response to AA39 while others were completely incapable of response to AA.34 Some had very severe bleeding episodes and others had mild bleeding problems.35,39,40 Whether this is due to different mutations in the TXAS gene resulting in variable degree of loss of TXAS activity is not conclusive because genetic defects of these patients were not fully characterized. Furthermore, evaluation of platelet abnormalities tended to rely on functional analysis without direct biochemical measurements.
Intravenous infusion of AA induces cardiac arrest and death in mice. This is due to cardiovascular collapse by aggregated platelets that occlude vital blood vessels. Platelet aggregates have been shown in these thrombotic models to be mediated at least in part by thromboxane coupling to its receptor(s).41-43 Blockade of TXA2 production and/or inhibition of thromboxane receptor activity by pharmacologic agents and deletion of TP receptor by gene targeting have been reported to prevent thrombotic death due to AA infusion.41-43 Our results indicate that TXAS plays a critical role in shock and death. We attribute the protective effect of TXAS deletion to absent TXA2 production. To address this, we treated TXAS-deleted mice with U46619, an agonist of TP receptor. U46619 abrogated the protective effect of TXAS deletion. These results support the notion that TXAS-derived TXA2 plays an essential role in shock and death. It should be noted that PGI2 levels as measured by its stable metabolite 6-keto–PGF1α are compensatorily increased in TXAS-deleted mice (data not shown). The elevated PGI2 may contribute to the protective action of TXAS deletion. However, it may function primarily to counter the action of TXA2.
We observed an initial decrease in blood pressure in TXAS–/– as well as in WT mice during the first 90 seconds after AA infusion but a paradoxical increase in MAP at 120 seconds in TXAS–/– mice, which declined slowly and returned to basal MAP 300 seconds after infusion (Figure 7A). The reason for the sharp rise in blood pressure at 120 seconds is unclear at the present time. Since the TP–/– mice have a significant drop in blood pressured without a paradoxical rise of MAP after AA infusion, we speculated whether the paradoxical rise could be secondary to accumulation of endoperoxides or shunting of endoperoxides into formation of a prostanoid that raises blood pressure. We have addressed this by pretreating TXAS-deleted mice with a selective TP receptor antagonist prior to AA challenge. The results support the notion that endoperoxides may contribute to maintenance of a normal blood pressure in TXAS-deleted mice. Interestingly, despite a marked rise of PGI2, it did not have a significant impact on blood pressure. These results provide a new insight into the importance of TXA2 and endoperoxides in controlling blood pressure.
Experimental evidence suggests that cyclo-oxygenase (COX) and PGE2 play important roles in neonatal development including the closure of ductus arteriosus (DA) after birth.44-47 According to the results of 2 reports,45,46 most of the prostaglandin E receptor 4 (EP4) receptor–null mice (95%) died within 48 hours after birth. Loftin et al44,47 reported that while the absence of only the COX-1 isoform did not affect the closure of DA, about 35% of COX-2 and 100% of COX-1/COX-2 double-deficient mice died with a patent DA within 48 hours and 12 hours of birth, respectively. Although both the EP4 receptor–deficient and COX-1/COX-2–double-deficient pups died in the postnatal period, the difference in postnatal survival suggests that the mechanisms are not identical. Therefore, it is proposed that in addition to COX-2/COX-1–derived PGE2 that is responsible for EP4 activation and remodeling of DA after birth, additional prostanoids, especially TXA2, may also play a role.44 In our TXAS-deficient mouse model we did not find developmental defects in the homozygous TXAS–/– mice. These results suggest that TXA2 is not involved in DA remodeling. However, as PGE2 production is increased in TXAS–/– mice, it is possible that PGE2 may compensate for the loss of TXA2. Whether TXA2 actively participates in regulating closure of DA requires further investigation. Our TXAS–/– mice will be useful in creating EP4–/– and TXAS–/– double-knockout mice, which will be valuable for assessing the involvement of prostanoids in regulating DA closure.
The activity of TXA2 may be controlled by antithrombotic agents that inhibit TXAS activity or antagonize TP receptor. The TXAS inhibitors offer an advantage over aspirin in that they may redirect arachidonate metabolism toward PGI2 and other protective eicosanoids. However, the therapeutic effect of these agents has been disappointing,48 partly because inhibition of TXAS also results in enhanced production of endoperoxides that can activate TP receptors (Figure 6). Dual inhibition of TXAS and TP may offer a better advantage.48,49 TXA2 synthase inhibitors used together with TP receptor antagonists have been shown to result in a profound antiplatelet effect that is considerably greater than low-dose aspirin therapy.50 However, despite promising results from animal models, the clinical efficacy of TXAS/TP inhibitors is not conclusive and there are even findings suggesting that these agents may inhibit platelet aggregation via other mechanisms besides inhibiting TXA2 activity.51 Thus, mice with double deletions of TXAS and TP receptors will be valuable not only for investigating the phenotype of complete absence of TXA2 activity but also for exploring the pharmacologic mechanisms related to these dual inhibitory agents.
Prepublished online as Blood First Edition Paper, March 16, 2004; DOI 10.1182/blood-2003-10-3661.
Supported by grants from the National Science Council, Taiwan (NSC89-2318-B002-006-M51; S.-W.L.); the National Health Research Institute, Taiwan (NHRI; DOH88-HR-811 and NHRI-GT-EX89; S.-W.L.); the Institute of Biomedical Sciences, Academia Sinica, Taiwan (IBMS-CRC86-T06, -CRC87-T05, -CRC88-T09, and -CRC91-T01; S.-W.L.); the National Taiwan University Hospital (90A01 and 91A06; S.-W.L.); the Taiwan Ministry of Education program for Promoting Academic Excellence of Universities (91-B-FA09-2-4; G.-Y.S. and H.-L.W.); the NHRI (NHRI-EX91-9108SC; P.-H.C.); and the US National Institutes of Health (P50 NS-23327 and R01 HL-50675; K.K.W.; and R01 HL60625; L.-H.W.).
I.-S.Y. and S.-R.L. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Dr Ying-Shuan E. Lee and Dr LanYang Ch'ang at the Academia Sinica and Dr Ming-Ching Shen at the Department of Internal Medicine, National Taiwan University Hospital (NTUH) for their support; Dr Chin-Tarng Lin at the department of pathology, NTUH for his help in interpreting the electron microscopy data; Dr C. F. Chen at the Department of Physiology for his help in measuring blood pressure; Ms Chou-Wen Ye, Huei-Jane Chen, Galan Hong, Ying-Hui Su, and Yin-Chi Wang for excellent technical assistance; the clinical chemistry laboratory and the Blood Bank of NTUH for analyzing blood chemistry and CBC; and the electron microscopy laboratory at the Chung-Gung Memorial Hospital for performing platelet electron microscopy.


![Figure 6. Analysis of AA metabolites from AA-treated platelets by thin-layer chromatography (TLC). Washed platelets from 2 to 3 mice were used for each experiment. Five microliters of [1-14C]AA (0.1 mCi/mL [3.7 MBq/mL]) was added to washed platelets and incubated at 37° C for 10 minutes. The reaction was stopped by 27 μL 1 N HCl. Prostanoids were extracted with diethyl-ether before dissolving in 50 μL chloroform for TLC analysis. Developing condition was at room temperature for 80 minutes in 30 mL chloroform-methanol–acetic acid–water ratio of 90:8:1:0.8 (vol/vol/vol/vol). Commercially obtained metabolites were included as references (marked at the left hand side). +/+ indicates WT mice; +/–, heterozygous; and –/–, homozygous mutant.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/1/10.1182_blood-2003-10-3661/6/m_zh80130463390006.jpeg?Expires=1765926304&Signature=3IH6hJXPqfw9MzyULkuPUR2bRzhmm58LcXzoucgIk65xDyMZkxH8SyDFPc3E3XN7HEIoiuqIbSWmbXRzdnzYNVyX9wqQG1M5zRiWH-vFT7Nn8VaLpqXdRscdazdvGhkCbIVdEkeUhbxQCPckcspLXVQu-ims4pFe6UgbVjJctxWpsWmavagqvTgCqV12sKw7h0uxHu1VlAM2ik9YYtvXx-wCTdBsl9Pk3Jc98ZpNqYYDeKJcMZ00RrOSXKl4IIliaDL9vKeei4V8rG6YURZk2zjU9hvJhunIOHrVJ5x2Y~RVJM0Vbf~AwPp9MHY~mYjMDgvOTV6ReOKBjSbM~g2LqA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal