Abstract
Previous studies have shown that the multiple myeloma (MM) cell line and MM patient cells express high-affinity vascular endothelial growth factor (VEGF) receptor-1 or Fms-like tyrosine kinase-1 (Flt-1) but not VEGF receptor-2 or Flk-1/kinase insert domain-containing receptor (Flk-1/KDR) and that VEGF triggers MM cell proliferation through a mitogen-activated protein kinase (MAPK)-dependent pathway and migration through a protein kinase C (PKC)-dependent pathway. The present study evaluates the efficacy of the small molecule tyrosine-kinase inhibitor GW654652, which inhibits all 3 VEGF receptors with similar potency. We show that GW654652 acts directly on MM cells and in the bone marrow microenvironment. Specifically, GW654652 (1-10 μg/mL) inhibits, in a dose-dependent fashion, VEGF-triggered migrational activity and cell proliferation of MM cell lines that are sensitive and resistant to conventional therapy. As expected from our previous studies of VEGF-induced signaling and sequelae in MM cells, GW654652 blocked VEGF-induced Flt-1 phosphorylation and downstream activation of AKT-1 and MAPK-signaling cascades. Importantly, GW654652 also inhibits interleukin-6 and VEGF secretion and proliferation of MM cells induced by tumor cell binding to bone marrow (BM) stromal cells. The activity of a pan-VEGF receptor inhibitor against MM cells in the BM milieu, coupled with its lack of major toxicity in preclinical mouse models, provides the framework for clinical trials of this drug class to improve patient outcome in MM. (Blood. 2004;103:3474-3479)
Introduction
Elevated serum levels of angiogenic cytokines such as vascular endothelial growth factor (VEGF)1,2 and basic fibroblast growth factor (bFGF)1-4 have been reported in patients with multiple myeloma (MM) and are likely to contribute to increased microvessel density (MVD), which in turn is correlated with disease progression and poor prognosis.1,5,6 We and others have shown that VEGF is expressed and secreted by MM cell lines, patient cells, and bone marrow stromal cells (BMSCs) and that VEGF secreted by MM cells triggers interleukin-6 (IL-6) production from BMSCs, thereby augmenting paracrine MM cell growth. Conversely, IL-6 enhances the production and secretion of VEGF by MM cells.7,8 Importantly VEGF receptor-1 protein (Fms-like tyrosine kinase-1 [Flt-1]) but not VEGF receptor-2 (kinase insert domain-containing receptor KDR [Flk-1]) is expressed on MM cell lines and patient cells.2,9 In a previous study we have reported direct effects of VEGF on MM cell proliferation and migration. These effects are mediated by at least 2 signaling pathways, the extracelluar signal-regulated kinase kinase (MEK)-extracellular signal-regulated protein kinase (ERK) pathway and a protein kinase C-α (PKC-α-dependent cascade.10,11 Moreover, a recent report shows that VEGF-A expression is higher in normal plasma cells than in B cells; however, in contrast to MM cells, neither Flt-1 nor KDR is expressed in primary plasma cells. These data suggest that an autocrine VEGF loop is present in MM cells but not in primary plasma cells.12
Thalidomide (Thal), first used empirically to treat patients with refractory and relapsed MM based on its antiangiogenic activity, achieved responses in one third of patients.13 Our preclinical studies defined a role for MM-host interactions in regulating MM cell growth, survival, drug resistance, and migration in the BM. Importantly, Thal and its derivative, more potent, immunomodulatory drugs (IMiDs; Celgene, Warren, NJ) can overcome the growth and survival advantage conferred by the BM milieu, including down-regulating VEGF.14,15 Furthermore, in addition to stimulating BM angiogenesis, mediating autocrine and paracrine growth of MM cells, and triggering MM cell migration, VEGF inhibits the maturation of dendritic cells16,17 and increases osteoclastic bone-resorbing activity.18 These studies provided the basis for the use of IMiD CC-5013 (Revimid; Celgene, Warren, NJ) in a phase 1 dose-escalation trial in patients with relapsed and refractory MM, which demonstrated either response or stabilization of disease in 79% of patients.19 Taken together, these data indicate a complex pathophysiologic role for VEGF in the BM microenvironment in MM and provide the preclinical framework for novel therapeutics targeting VEGF and Flt-1. We have reported that the VEGF receptor tyrosine kinase inhibitor PTK787/ZK222584 (Novartis Pharmaceuticals, Basel, Switzerland) acts directly on MM cells and inhibits paracrine IL-6-mediated MM cell growth in the BM milieu, further supporting this view.9
The present study evaluates the efficacy of the indazolylpyrimidine GW654652 (GlaxoSmithKline, Research Triangle Park, NC), a small-molecule tyrosine kinase inhibitor that inhibits all 3 VEGF receptors with similar potency. Preclinical data demonstrated that GW654652 is a potent inhibitor of VEGF- and bFGF-mediated angiogenesis and VEGF-induced vascular permeability in vivo. In addition, daily oral dosing with GW654652 inhibits the growth of human tumor xenografts in vivo.20 Moreover, GW654652 has a good pharmacokinetic profile in murine and canine studies.21 The present study shows that these pan-VEGF receptor inhibitors act directly on MM cells and in the BM microenvironment to overcome drug resistance, providing the preclinical rationale for use of this drug class to improve patient outcome in MM.
Patients, materials, and methods
Materials
Recombinant human VEGF encoding the 165-amino acid residue variant of human VEGF was purchased from R&D Systems (Minneapolis, MN). Human plasma fibronectin was obtained from Gibco, Life Technologies (Grand Island, NY). The rabbit polyclonal antibody directed against amino acids within the extracellular domain of Flt-1, pERK, ERK-2, and pSTAT3 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit polyclonal antibodies raised against pAKT-1 (S473) and AKT-1 were purchased from Cell Signaling Technology (Beverly, MA). Antiphosphotyrosine 4G10 antibody was kindly provided by Dr Tom Roberts (Dana-Farber Cancer Institute, Boston, MA).
Cells and cell culture
All human MM (RPMI-Dox40, MM.1R, MM.1S, RPMI, U266) cell lines and primary MM cells were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS; Harlan, Indianapolis, IN), 100 U/mL penicillin, 10 μg/mL streptomycin, and 2 mM L-glutamine (Cellgro, Herndon, VA). Before cells were stimulated with VEGF, they were incubated overnight in RPMI 1640 with 2% FBS, followed by an additional 3 hours in RPMI 1640 without FBS. Human umbilical vein endothelial cells (HUVECs) from a pool of 5 healthy donors (Clonetics BioWhittaker, Walkersville, MD) were maintained in EGM-2MV media (Clonetics BioWhittaker).
Isolation of patient tumor cells
After appropriate informed consent was given, MM patient cells (96% CD38+CD45RA-) were obtained from BM samples by antibody-mediated negative selection using RossetteSep (StemCell Technologies, Vancouver, BC, Canada), as previously described.22
Cell lysis, immunoprecipitation, and Western blotting
Cells were washed 3 times with 1× phosphate-buffered saline (PBS) and were lysed with lysis buffer (10 mM Tris, 50 mM NaCl, Na-pyrophosphate) or RIPA lysis buffer supplemented with 1% triton, 1 mM sodium vanadate, 1 mM phenylmethylsulfonyl fluoride, and protein inhibitor cocktail (Boehringer Mannheim, Mannheim, Germany). Insoluble material was removed by centrifugation (15 000 rpm for 30 minutes at 4°C). Immunoprecipitation was performed as described previously.11 Briefly, Flt-1 (H-225) (Santa Cruz Biotechnology) antibody was used to immunoprecipitate Flt-1. Immunocomplexes were collected after overnight incubation at 4°C with protein A-Sepharose beads (Sigma, St Louis, MO). For Western blotting, cell lysates (30-100 μg per lane) or immunoprecipitates were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) before electrophoretic transfer onto Hybond C super (Amersham, Arlington Heights, IL).
Transwell migration assay
Cell migration was assayed using a Boyden-modified chamber assay, as described previously.11 Briefly, starved cells were added on an 8-μm pore size polycarbonate membrane precoated with fibronectin (10 μg/mL) separating the 2 chambers of a 6.5-mm transwell (Costar). VEGF (10 ng/mL or 100 ng/mL) was added to the lower chamber. After 2 to 5 hours, cells that migrated to the lower compartment were counted using a Coulter counter ZBII (Beckman Coulter, Brea, CA).
Time-lapse video microscopy
MM.1S cells were starved in RPMI medium containing 2% FBS for 16 hours, plated to fibronectin-coated tissue culture plates (35 × 10-mm plates; Becton Dickinson Labware, Bedford, MA), and stimulated with VEGF (100 ng/mL) in the presence or absence of GW654652. For image capturing, the Zeiss Axiovert 200 M inverted microscope (Zeiss, Goettingen, Germany), equipped with a 100-W halogen lamp, plan-neofluar, and plan-apochromat objectives from 10- to 100-fold magnification and an achromatic/aplanatic condenser, was connected to a Photometrics Cool-SNAP digital camera with incorporated cooled, charge-coupled device (CCD) technology. A 1392 × 1040 resolution sensor (ICX205AL 0.5-inch interline progressive-scan HAD CCD with microlenses; Sony) ensured that each image showed extraordinary detail.
The microscope, shutter (Uniblitz; Vincent Associates), and CCD camera were controlled by SlideBook 4.0 software. Animation, export to Quick Time movie and image analysis were performed using the SlideBook 4.0 software (Santa Monica, CA). During all experiments the temperature was maintained at 37°C using the BC-100 controller (Bionomic System).
MTT
The inhibitory effect of GW654652 on MM.1S cell growth was assessed using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT; Chemicon International, Temecula, CA), which is cleaved by viable cells to yield a dark blue formazan product, as described previously.23 Briefly, cells from 48-hour cultures were pulsed with 10 μL of 5 mg/mL MTT to each well for the last 4 hours of 48-hour cultures, followed by 100 μL isopropanol containing 0.04 N HCl. Absorbance was measured at 570 nm using a spectrophotometer (Molecular Devices, Sunnyvale, CA). Cell survival was estimated as a percentage of the value of untreated controls.
DNA synthesis and cell proliferation assay
Cell growth was assessed by measuring [3H]-thymidine uptake, as described in previous studies.11
Cell cycle analysis
ELISA
Cytokine levels were measured in supernatants from coculture systems, as described. VEGF and IL-6 concentrations were measured using commercially available enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems). The optical density of each well was detected by means of a microtiter plate reader at 450 nm. Each well was analyzed in triplicate.
Statistical analysis
Statistical significance of differences observed in VEGF-treated compared with control cultures was determined using an unpaired Student t test. The minimal level of significance was a P value less than .05.
Results
Effect of GW654652 on Flt-1 phosphorylation and VEGF-triggered activation of downstream signaling molecules
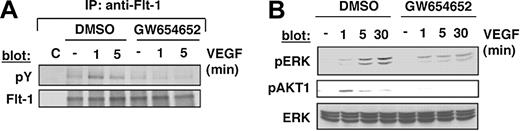
We and others have shown that MM cell lines and MM patient cells express high-affinity VEGF receptor-1 or Flt-1 but not VEGF receptor-2 or Flk-1/KDR2,9 ; moreover, VEGF triggers MM cell proliferation through an MAPK-dependent pathway and migration through a phosphoinositide 3-kinase (PI3-K)/PKC-dependent pathway.11 GW654652, an investigational small-molecule tyrosine kinase inhibitor, targets all 3 VEGF receptors with similar potency. Therefore, we first tested whether GW654652 can abrogate VEGF-induced tyrosine phosphorylation of Flt-1 in MM.1S cells. As shown in Figure 1A, VEGF-triggered up-regulation of Flt-1 tyrosine phosphorylation (1 and 5 minutes) in MM.1S cells was abrogated by pretreatment with GW654652 (10 μg/mL; 1 hour). Reprobing of the membrane with antisera directed against Flt-1 confirmed equal protein loading. Furthermore, GW654652 abrogated VEGF-induced phosphorylation of phospho-ERK and phospho-AKT-1 and downstream signaling molecules in the MAPK and the PI3-kinase pathways.
Effect of GW654652 on Flt-1 phosphorylation and VEGF-triggered activation of downstream signaling molecules. (A) MM.1S cells were starved overnight in RPMI 1640 with 1% FBS and for 3 hours in RPMI 1640 with no FBS. After pretreatment with GW654652 (1 hour; 10 μg/mL) or dimethyl sulfoxide (DMSO), MM.1S cells were stimulated with 100 ng/mL VEGF for 1 and 5 minutes. Flt-1 immunoprecipitates (IPs) from whole cell lysates were analyzed by Western blotting using antisera against phosphotyrosine residues. Equal loading was confirmed by immunoblotting with antisera directed against Flt-1. Nonspecific protein binding and detection were excluded by incubating protein A-Sepharose (PAS) beads with lysis buffer and Flt-1 antibody only (C, control). (B) MM.1S cells pretreated as in panel A were stimulated with 100 ng/mL VEGF in the presence and absence of GW654652 for 1, 5, and 30 minutes. Whole-cell lysates (60 μg) were analyzed by Western blotting using antisera against phospho-ERK (pERK) and phospho-AKT-1 (pAKT1). Immunoblotting for ERK-2 confirmed equal protein loading.
Effect of GW654652 on Flt-1 phosphorylation and VEGF-triggered activation of downstream signaling molecules. (A) MM.1S cells were starved overnight in RPMI 1640 with 1% FBS and for 3 hours in RPMI 1640 with no FBS. After pretreatment with GW654652 (1 hour; 10 μg/mL) or dimethyl sulfoxide (DMSO), MM.1S cells were stimulated with 100 ng/mL VEGF for 1 and 5 minutes. Flt-1 immunoprecipitates (IPs) from whole cell lysates were analyzed by Western blotting using antisera against phosphotyrosine residues. Equal loading was confirmed by immunoblotting with antisera directed against Flt-1. Nonspecific protein binding and detection were excluded by incubating protein A-Sepharose (PAS) beads with lysis buffer and Flt-1 antibody only (C, control). (B) MM.1S cells pretreated as in panel A were stimulated with 100 ng/mL VEGF in the presence and absence of GW654652 for 1, 5, and 30 minutes. Whole-cell lysates (60 μg) were analyzed by Western blotting using antisera against phospho-ERK (pERK) and phospho-AKT-1 (pAKT1). Immunoblotting for ERK-2 confirmed equal protein loading.
Effect of GW654652 on MM cell migration.
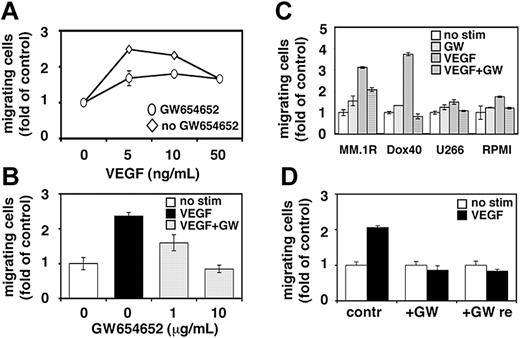
Migrational activity is required for homing of tumor cells to the BM, expansion within the BM microenvironment, and egress into the peripheral blood. Our and other studies have demonstrated the importance of MM cell migration for disease pathogenesis and progression.24-27 Having shown that VEGF-induced PI3-kinase signaling is blocked by GW654652, we next investigated whether VEGF-induced migration of MM cells can be abrogated by pretreatment with GW654652. Cell migration was assayed by measuring the transfilter migration activity of MM.1S cells seeded on membranes precoated with fibronectin. As reported previously, adding VEGF to conditioned medium in the lower chamber induced a dose-dependent increase in cell migration of growth factor-deprived MM.1S cells in the upper transwell chamber.11 Maximal (2.5-fold) migration activity induced by 5 ng/mL VEGF was significantly inhibited in MM.1S cells pretreated with GW654652 (10 μg/mL; 1 hour) (Figure 2A). Moreover, the inhibition of maximal VEGF-induced migration by GW654652 was concentration dependent (Figure 2B). Migrational activity was similarly blocked in MM.1R, RPMI-Dox40, U266, and RPMI cells (Figure 2C). To test the reversibility of GW654652-induced blockade of MM cell migration, VEGF-triggered MM.1S cell migration was determined after GW654652 removal and compared with migration in cells treated with GW654652 or left untreated. As shown in Figure 2D, MM cell migration was not recovered after GW654652 removal.
Effect of GW654652 on MM cell migration. (A-B) Growth factor-deprived MM.1S cells were pretreated with 10 μg/mL GW654652 (A) or 1 and 10 μg/mL GW654652 (B) or were left untreated. Cells were then plated on a fibronectin-coated polycarbonate membrane (8-μm pore size) in a modified Boyden chamber and were exposed for 4 hours to 5 to 50 ng/mL VEGF (A) or 5 ng/mL VEGF (B) in the lower chamber. (C) Growth factor-deprived MM.1R, RPMI-Dox40 (Dox40), U266, and RPMI cells were pretreated with 10 μg/mL GW654652 or were left untreated, plated on a fibronectin-coated polycarbonate membrane (8-μm pore size) in a modified Boyden chamber, and exposed to 5 ng/mL VEGF for 4 hours. (D) VEGF-triggered cell migration was determined in MM.1S cells after GW654652 removal (GW re) compared with migration in cells treated with GW654652 (GW) or left untreated (contr). At the end of treatment, cells on the lower part of the membrane were counted using a Coulter counter ZBII. Data represent mean ± SD for duplicate samples. Results shown are representative of 3 independent experiments.
Effect of GW654652 on MM cell migration. (A-B) Growth factor-deprived MM.1S cells were pretreated with 10 μg/mL GW654652 (A) or 1 and 10 μg/mL GW654652 (B) or were left untreated. Cells were then plated on a fibronectin-coated polycarbonate membrane (8-μm pore size) in a modified Boyden chamber and were exposed for 4 hours to 5 to 50 ng/mL VEGF (A) or 5 ng/mL VEGF (B) in the lower chamber. (C) Growth factor-deprived MM.1R, RPMI-Dox40 (Dox40), U266, and RPMI cells were pretreated with 10 μg/mL GW654652 or were left untreated, plated on a fibronectin-coated polycarbonate membrane (8-μm pore size) in a modified Boyden chamber, and exposed to 5 ng/mL VEGF for 4 hours. (D) VEGF-triggered cell migration was determined in MM.1S cells after GW654652 removal (GW re) compared with migration in cells treated with GW654652 (GW) or left untreated (contr). At the end of treatment, cells on the lower part of the membrane were counted using a Coulter counter ZBII. Data represent mean ± SD for duplicate samples. Results shown are representative of 3 independent experiments.
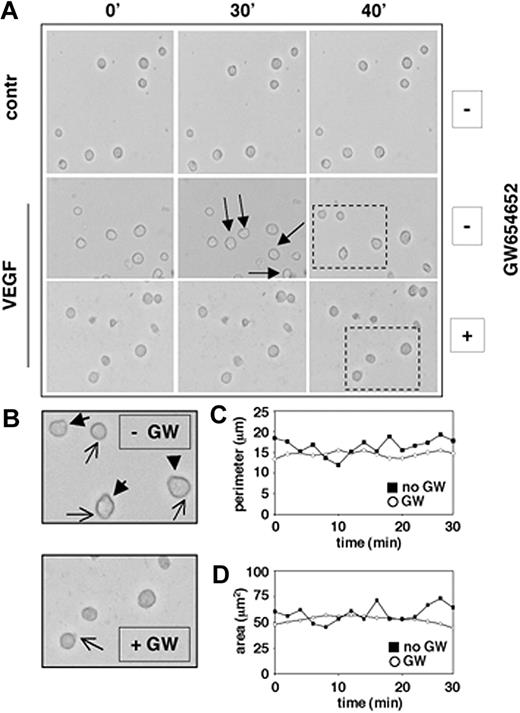
We have previously shown that VEGF induces significant increases in the migration of MM.1S cells adherent to fibronectin, suggesting a role for VEGF in the dynamic process of cell adhesion formation and release.10 Using phase-contrast time-lapse video microscopy (TLVM), we next investigated whether GW654652 can decrease the average VEGF-induced migrational rate of MM.1S cells seeded on fibronectin and can abrogate morphologic changes associated with VEGF-induced cell migration on fibronectin. Our results showed that the rate of MM cell movement was significantly decreased by GW654652 treatment (Figure 3A). MM cell shape also dramatically changed in the presence of GW654652: marked decreases in uropod formation at the trailing edges (closed arrows) and lamellipodia formation at the leading edges (open arrows) were observed in the presence of GW654652 (10 μg/mL), resulting in spherical cell morphology (Figure 3B). Subsequently, GW654652-induced changes in MM cell perimeter (Figure 3C) and cell surface area (Figure 3D) were quantitatively evaluated using the SlideBook 4.0 software. Taken together, these observations indicated that GW654652 markedly decreased the VEGF-induced migrational rate on fibronectin by reducing active, protruding structures in MM cells.
GW 654652 inhibits cell motility. MM.1S cells were pretreated with GW654652 (10 μg/mL) or with DMSO control (approximately 6 hours) and then plated on fibronectin-coated tissue-culture plates (35 × 10-mm plates; BD PharMingen), in the presence or absence of VEGF (100 ng/mL). Dynamic changes in MM cell morphology that mediate migration were assessed by phase-contrast TLVM. (A) Decreased cell motility after GW654652 treatment. Photographs were acquired at 2-minute intervals starting 3 hours after plating. Images shown are at 10-minute intervals, and arrows identify the movement of a single cell. Dashed rectangles identify those cells enlarged in panel B. Apostrophes indicate minutes. (B) Decreased ruffling/formation of lamellipodia and uropods after GW654652 treatment. Closed arrows indicate uropod formation, and open arrows indicate lamellipodia formation. (C-D) Cell perimeter (C) and surface area (D) illustrate morphologic changes on GW654652 treatment. Time-lapse micrographs were analyzed using SlideBook 4.0 software.
GW 654652 inhibits cell motility. MM.1S cells were pretreated with GW654652 (10 μg/mL) or with DMSO control (approximately 6 hours) and then plated on fibronectin-coated tissue-culture plates (35 × 10-mm plates; BD PharMingen), in the presence or absence of VEGF (100 ng/mL). Dynamic changes in MM cell morphology that mediate migration were assessed by phase-contrast TLVM. (A) Decreased cell motility after GW654652 treatment. Photographs were acquired at 2-minute intervals starting 3 hours after plating. Images shown are at 10-minute intervals, and arrows identify the movement of a single cell. Dashed rectangles identify those cells enlarged in panel B. Apostrophes indicate minutes. (B) Decreased ruffling/formation of lamellipodia and uropods after GW654652 treatment. Closed arrows indicate uropod formation, and open arrows indicate lamellipodia formation. (C-D) Cell perimeter (C) and surface area (D) illustrate morphologic changes on GW654652 treatment. Time-lapse micrographs were analyzed using SlideBook 4.0 software.
Effect of GW654652 on HUVECs and MM cell growth and survival
We next evaluated the direct effect of GW654652 on the survival and proliferation of HUVECs and MM cells using MTT assay and [3H]-thymidine uptake, respectively. VEGF receptors KDR and Flt-1 are expressed on the surfaces of endothelial cells.28 As shown in Figure 4A, the survival and proliferation of HUVEC cells were significantly decreased in 48-hour cultures with GW654652, and the IC50 was between 0.5 and 5 μg/mL. Importantly, the decrease in HUVEC survival and proliferation triggered by GW654652 was partially reversible after GW654652 removal. Moreover, MM cell survival (Figure 4B-C, left panels) and MM cell proliferation (Figure 4B-D, right panels) was significantly decreased by GW654652 treatment in dexamethasone (Dex)-sensitive MM.1S, Dex-resistant MM.1R, doxorubicin (Dox)-sensitive RPMI, and Dox-resistant RPMI-Dox40 (Dox 40) cells. In contrast to HUVECs, the effect of GW654652 on MM cell survival and proliferation was not reversed after GW654652 removal (Figure 4B-D). The IC50 of GW654652 on MM cells was 1 to 5 μg/mL.
Effect of GW654652 on HUVEC and MM cell growth. (A) Dose-related effects of GW654652 on survival (left) and proliferation (right) of HUVECs. HUVECs were cultured with control media or with GW654652 followed by no removal (▪) or removal (□) of GW654652. (B-C) Shown are dose-related effects of GW654652 on survival and proliferation of MM.1S (B) and MM.1R cells (C) and of RPMI-Dox40 (Dox40), U266, and RPMI cells (D). In each case, cell lines were cultured with control media or with the indicated concentrations of GW654652 (closed symbols). In MM.1S and MM.1R, cell assays were also performed after the removal of GW654652 (B-C; □). MTT cleavage (left) or [3H]-thymidine uptake (right) was measured during the last 8 and 4 hours, respectively, of 48-hour cultures. Values represent the mean ± SD absorbance or [3H]-thymidine uptake of triplicate cultures.
Effect of GW654652 on HUVEC and MM cell growth. (A) Dose-related effects of GW654652 on survival (left) and proliferation (right) of HUVECs. HUVECs were cultured with control media or with GW654652 followed by no removal (▪) or removal (□) of GW654652. (B-C) Shown are dose-related effects of GW654652 on survival and proliferation of MM.1S (B) and MM.1R cells (C) and of RPMI-Dox40 (Dox40), U266, and RPMI cells (D). In each case, cell lines were cultured with control media or with the indicated concentrations of GW654652 (closed symbols). In MM.1S and MM.1R, cell assays were also performed after the removal of GW654652 (B-C; □). MTT cleavage (left) or [3H]-thymidine uptake (right) was measured during the last 8 and 4 hours, respectively, of 48-hour cultures. Values represent the mean ± SD absorbance or [3H]-thymidine uptake of triplicate cultures.
Cell cycle profiles of MM cells exposed to GW654652
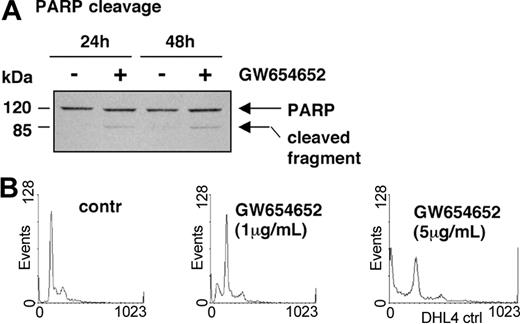
Having shown that GW654652 decreases MM cell survival, we next examined whether GW654652 induces poly (adenosine diphosphate [ADP]-ribose) polymerase (PARP) cleavage, a hallmark of apoptosis and activation of the protease cascade.29 As shown in Figure 5A, GW654652 (5 μg/mL) induced PARP cleavage, consistent with apoptosis and activation of proteases in MM.1S cells after 24 and 48 hours. We then examined cell cycle profile changes in MM cells induced by GW654652. The effect on cell cycle profile in U266 cells after 48-hour treatment with GW654652 (Figure 5B and Table 1) was representative of all MM cell lines investigated. A significant decrease in the S and G0/G1 phases, with a corresponding increase of cells in the sub-G1 phase, was triggered by GW654652.
PARP cleavage and cell cycle profile of MM cells exposed to GW654652. (A) GW654652 induces proteolytic cleavage of PARP. MM.1S cells were treated with GW654652 for 24 and 48 hours, followed by immunoblot analysis of the lysates with anti-PARP antibody. (B) Cell cycle profile of MM cells exposed to GW654652. U266 cells were cultured for 48 hours in the presence of GW654652 (1 and 5 μg/mL). Cultures in media alone served as negative control. Cells were stained with propidium iodide, and cell cycle profile was determined using flow cytometry.
PARP cleavage and cell cycle profile of MM cells exposed to GW654652. (A) GW654652 induces proteolytic cleavage of PARP. MM.1S cells were treated with GW654652 for 24 and 48 hours, followed by immunoblot analysis of the lysates with anti-PARP antibody. (B) Cell cycle profile of MM cells exposed to GW654652. U266 cells were cultured for 48 hours in the presence of GW654652 (1 and 5 μg/mL). Cultures in media alone served as negative control. Cells were stained with propidium iodide, and cell cycle profile was determined using flow cytometry.
Cell cycle profile of MM cells exposed to GW654652
. | Population, % . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| Treatment . | Sub-G1 . | G0/G1 . | S . | G2/M . | |||
| Control | 8.90 | 58.40 | 18.60 | 11.70 | |||
| GW (1 μg/mL) | 22.40 | 48.60 | 11.20 | 15.60 | |||
| GW (5 μg/mL) | 37.10 | 32.50 | 9.90 | 11 | |||
. | Population, % . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| Treatment . | Sub-G1 . | G0/G1 . | S . | G2/M . | |||
| Control | 8.90 | 58.40 | 18.60 | 11.70 | |||
| GW (1 μg/mL) | 22.40 | 48.60 | 11.20 | 15.60 | |||
| GW (5 μg/mL) | 37.10 | 32.50 | 9.90 | 11 | |||
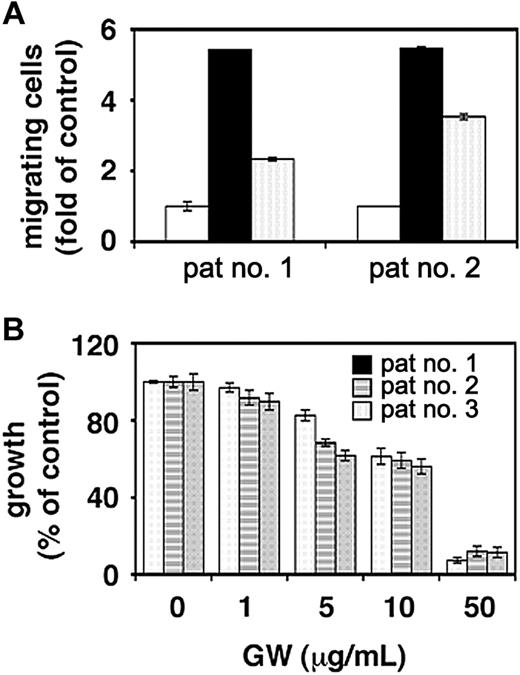
Effect of GW654652 on patient MM cell growth and migration
The effect of GW654652 on patient MM cell migration and survival was next investigated. VEGF-induced migrational activity in freshly isolated tumor cells from 2 MM patients was significantly down-regulated by GW654652 (Figure 6A). Furthermore, concentration-dependent decreases in survival were observed in MM cells from 3 patients cultured for 48 hours in the presence of GW654652, with an IC50 of approximately 10 μg/mL (Figure 6B).
Effect of GW654652 on patient MM cell survival and migration. (A) Effect of GW654652 on patient MM cell migration. Growth factor-deprived patient MM cells were pretreated with 1 μg/mL GW654652 or were left untreated, plated on a fibronectin-coated polycarbonate membrane (8-μm pore size) in a modified Boyden chamber, and exposed for 4 hours to 5 ng/mL VEGF added to the lower chamber. At the end of treatment, cells on the lower part of the membrane were counted using a Coulter counter ZBII. (open square) Control; (closed square) VEGF; (marked square) VEGF plus GW654652. (B) Effect of GW654652 on patient MM cell survival. Patient MM cells were cultured with control media or the indicated concentrations of GW654652. MTT cleavage was measured during the last 4 hours of 48-hour cultures. Values represent the mean ± SD absorbance of triplicate cultures.
Effect of GW654652 on patient MM cell survival and migration. (A) Effect of GW654652 on patient MM cell migration. Growth factor-deprived patient MM cells were pretreated with 1 μg/mL GW654652 or were left untreated, plated on a fibronectin-coated polycarbonate membrane (8-μm pore size) in a modified Boyden chamber, and exposed for 4 hours to 5 ng/mL VEGF added to the lower chamber. At the end of treatment, cells on the lower part of the membrane were counted using a Coulter counter ZBII. (open square) Control; (closed square) VEGF; (marked square) VEGF plus GW654652. (B) Effect of GW654652 on patient MM cell survival. Patient MM cells were cultured with control media or the indicated concentrations of GW654652. MTT cleavage was measured during the last 4 hours of 48-hour cultures. Values represent the mean ± SD absorbance of triplicate cultures.
Effects of GW654652 on the bone marrow microenvironment
The adherence of MM cells to BMSCs induced IL-6 secretion by BMSCs, resulting in increased proliferation of adherent MM cells. Furthermore, VEGF is expressed and secreted by MM cells, binds to VEGF receptors on BMSCs, and thereby stimulates production and secretion of IL-6 in BMSCs.7 Conversely, the production and secretion of VEGF by MM cells is enhanced by IL-6.7,8 Therefore, we examined the effect of GW654652 on paracrine MM cell growth and endothelial cell growth. First we tested the effect of GW654652 on VEGF and IL-6 production in a coculture system of MM cell lines (Figure 7A-B) or MM patient cells (Figure 7C-D) with BMSCs. A significant concentration-dependent reduction of VEGF and IL-6 production was observed in the presence of GW654652. Furthermore, GW654652 inhibited the proliferation of MM cells adherent to BMSCs (Figure 7E). Conditioned medium from an MM cell—BMSC culture with GW654652 was evaluated for its ability to block HUVEC growth. As expected, GW654652 induced concentration-dependent decreased growth of HUVECs (Figure 7F).
Effects of GW654652 on the bone marrow microenvironment. (A-B) GW654652 inhibits the secretion of VEGF and IL-6 induced by MM cell line binding to BMSCs. MM.1S cells were cultured in control media alone or with the indicated concentrations of GW654652. VEGF (A) or IL-6 (B) concentrations were measured using ELISA. (C-D) GW654652 inhibits the secretion of VEGF and IL-6 induced by patient MM cell binding to BMSCs. Patient MM cells were cultured in control media alone or with the indicated concentrations of GW654652. VEGF (C) or IL-6 (D) concentrations were measured using ELISA. (E) GW654652 inhibits proliferation of MM cells adherent to BMSCs. MM cells were cultured with or without BMSCs. GW654652 was added in the indicated concentrations, and proliferation was measured using [3H]-thymidine uptake. (F) Inhibition of HUVEC proliferation in the presence of conditioned medium from a MM-BMSC cell culture. Supernatants derived from MM-BMSC cultures pretreated with GW654652 were mixed with EGM-2MV media. HUVEC cell proliferation was measured using [3H]-thymidine uptake. Experiments were performed in triplicate. Data shown are the mean ± SD of experiments performed in triplicate.
Effects of GW654652 on the bone marrow microenvironment. (A-B) GW654652 inhibits the secretion of VEGF and IL-6 induced by MM cell line binding to BMSCs. MM.1S cells were cultured in control media alone or with the indicated concentrations of GW654652. VEGF (A) or IL-6 (B) concentrations were measured using ELISA. (C-D) GW654652 inhibits the secretion of VEGF and IL-6 induced by patient MM cell binding to BMSCs. Patient MM cells were cultured in control media alone or with the indicated concentrations of GW654652. VEGF (C) or IL-6 (D) concentrations were measured using ELISA. (E) GW654652 inhibits proliferation of MM cells adherent to BMSCs. MM cells were cultured with or without BMSCs. GW654652 was added in the indicated concentrations, and proliferation was measured using [3H]-thymidine uptake. (F) Inhibition of HUVEC proliferation in the presence of conditioned medium from a MM-BMSC cell culture. Supernatants derived from MM-BMSC cultures pretreated with GW654652 were mixed with EGM-2MV media. HUVEC cell proliferation was measured using [3H]-thymidine uptake. Experiments were performed in triplicate. Data shown are the mean ± SD of experiments performed in triplicate.
Discussion
Recent studies show that bone marrow angiogenesis increases, with progression from monoclonal gammopathy of undetermined significance (MGUS) to nonactive MM to active MM.1,5 Importantly the angiogenic factor VEGF is secreted by MM cells: our own and other studies demonstrate a paracrine tumor-stromal circuit of VEGF/IL-6 production and secretion7,8 and a direct effect of VEGF on MM cell proliferation and migration.10,11 Moreover, VEGF mediates endothelial cell proliferation, inhibits the maturation of dendritic cells,16,17 and increases bone-resorbing activity of osteoclasts by enhancing their survival.18 Using novel inhibitors of VEGF receptors that target MM cells and the BM stroma represents a new, biologically based treatment strategy.
The indazolylpyrimidine GW654652 is a small-molecule tyrosine kinase inhibitor that inhibits all 3 VEGF receptors with similar potency. Preclinical data demonstrate that GW654652 is a potent inhibitor of VEGF- and bFGF-mediated angiogenesis and of VEGF-induced vascular permeability in vivo. In addition, daily oral dosing with GW654652 inhibits the growth of human tumor xenografts (head and neck, colon, melanoma, and prostate) in vivo.20 Moreover, GW654652 has a favorable pharmacokinetic profile in murine and canine studies, suggesting potential chemical applicability in MM.21
The present study shows that the indazolylpyrimidine class of pan-VEGF receptor inhibitors acts directly on MM cells and in the BM microenvironment to overcome drug resistance. Specifically, we found that GW654652 inhibits VEGF-triggered migrational activity and the proliferation of MM cell lines, including those sensitive and resistant to conventional therapy, in a dose-dependent fashion. Furthermore GW654652 blocks VEGF-induced Flt-1 phosphorylation and downstream activation of AKT-1 and MAPK signaling pathways. GW654652 also acts in the BM microenvironment because it blocks HUVEC proliferation and inhibits IL-6 and VEGF secretion and proliferation of MM cells induced by MM cell binding to bone marrow stromal cells. Therefore, GW654652 offers the potential to overcome drug resistance and to increase patient outcome. The drugs that similarly overcome the MM growth, survival, and drug resistance advantages conferred by the BM microenvironment include CC-5013 and bortezomid (Velcade; Millenium Pharmaceuticals, Cambridge, MA), each of which has already shown promise in overcoming conventional drug resistance in MM.19,30
The inhibition of MM cell growth, survival, and migration was not reversed once the drug was removed after treatment with GW654652. Conversely, the effect on HUVEC survival and proliferation was partially reversed after drug removal. The higher sensitivity of MM cells may be attributed to lower VEGF receptor expression levels in MM cells compared with HUVECs. Alternatively, these data may indicate that long-term exposure of MM cells to GW654652 leads to the stable destruction of Flt-1 on MM cells.
Our study provides the preclinical rationale for evaluating indazolylpyrimidine VEGF receptor inhibitors as novel MM therapeutics. In addition, our data demonstrate the promise of agents targeting the MM cell, the MM cell-host interaction, and the BM milieu to improve patient outcome in MM. Furthermore, our results suggest the use of this drug class in cancers other than MM to target tumor cells and their microenvironments.
Prepublished online as Blood First Edition Paper, November 26, 2003; DOI 10.1182/blood-2003-10-3527.
Supported by a Multiple Myeloma Research Foundation Senior Research Grant Award (K.P.), National Institutes of Health grant PO-1 78378, and the Doris Duke Distinguished Clinical Research Scientist Award (K.C.A.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank G. Li for technical help and Drs A. Cardoso and M. Tavares for providing HUVECs.

![Figure 4. Effect of GW654652 on HUVEC and MM cell growth. (A) Dose-related effects of GW654652 on survival (left) and proliferation (right) of HUVECs. HUVECs were cultured with control media or with GW654652 followed by no removal (▪) or removal (□) of GW654652. (B-C) Shown are dose-related effects of GW654652 on survival and proliferation of MM.1S (B) and MM.1R cells (C) and of RPMI-Dox40 (Dox40), U266, and RPMI cells (D). In each case, cell lines were cultured with control media or with the indicated concentrations of GW654652 (closed symbols). In MM.1S and MM.1R, cell assays were also performed after the removal of GW654652 (B-C; □). MTT cleavage (left) or [3H]-thymidine uptake (right) was measured during the last 8 and 4 hours, respectively, of 48-hour cultures. Values represent the mean ± SD absorbance or [3H]-thymidine uptake of triplicate cultures.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/9/10.1182_blood-2003-10-3527/6/m_zh80090460840004.jpeg?Expires=1763602605&Signature=MeMR~V3HcZlImiyV4oc30xJq6Yo~vcsG9TUFfP5BoZ-LlYmaivdtk5Zeo0bly84dFc7gExSbrQd-L3w71TpPSFMG7PnmOuMuFT5-H1gVzCucs-Ph~rEWluqNm7vVNB9LXvodthVtbCoeXXq28LZV5dBSAzLOXv053-u350Nt52O2a6NLVPUnq3QQoZgm7Sn3nJqWP1uHrhzdD43yZ~DuUmOCHgY4MjLmdmYC~jQ1up8MIHbJopnVRSoAQe05GZgH~sKLCwBJUZDD5CShZ8XAjBYX~MvpkQhyavpk1UuM5kJGmth6cuPn1Yn3nwMLdgqUgZD6zp-n4NgPc63-~asF0g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. Effects of GW654652 on the bone marrow microenvironment. (A-B) GW654652 inhibits the secretion of VEGF and IL-6 induced by MM cell line binding to BMSCs. MM.1S cells were cultured in control media alone or with the indicated concentrations of GW654652. VEGF (A) or IL-6 (B) concentrations were measured using ELISA. (C-D) GW654652 inhibits the secretion of VEGF and IL-6 induced by patient MM cell binding to BMSCs. Patient MM cells were cultured in control media alone or with the indicated concentrations of GW654652. VEGF (C) or IL-6 (D) concentrations were measured using ELISA. (E) GW654652 inhibits proliferation of MM cells adherent to BMSCs. MM cells were cultured with or without BMSCs. GW654652 was added in the indicated concentrations, and proliferation was measured using [3H]-thymidine uptake. (F) Inhibition of HUVEC proliferation in the presence of conditioned medium from a MM-BMSC cell culture. Supernatants derived from MM-BMSC cultures pretreated with GW654652 were mixed with EGM-2MV media. HUVEC cell proliferation was measured using [3H]-thymidine uptake. Experiments were performed in triplicate. Data shown are the mean ± SD of experiments performed in triplicate.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/9/10.1182_blood-2003-10-3527/6/m_zh80090460840007.jpeg?Expires=1763602605&Signature=IQoVZ9p11S8Y1S959~xeggLlU8d9ZKgCkSX29i5DnfvLdOfq7yWFtXMIheALb6byYqEpebAdu3gw18XK4sjTLu~E0JQRHB~3Mo~naYiLyiOh7Q1xZmOzjr~6G0a0tHct8dxQAa36mt8qC8soaQtlc7lvD7CVnaSH4PwoKxE~j4-0FL0HWg7PGe~bdbcutPepB0SHMoQvpiCIq8Irti2HGTmCH2B3J4HnMaPjFw37Pyb0R3vXrO00pa7SXmrL7EwtLs9cy7Fxzs4Pa-hUEIwas7EKlY1n6gN3HJcpQp~-g91xoDtjwvDC2A3YOA3tuYQ1xYSfC6mAw253aC3sptz~0w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal