Abstract
During apoptotic cell death, biochemical processes modify self-proteins and create potential autoantigens. To maintain self-tolerance in the face of natural cell turnover, the immune system must prevent or control responses to apoptosis-associated autoantigens or risk autoimmunity. The molecular mechanisms governing this process remain largely unknown. Here, we show that expression of the immunoregulatory protein CD200 increases as murine dendritic cells (DCs) undergo apoptosis. We define CD200 as a p53-target gene and identify both p53- and caspase-dependent pathways that control CD200 expression during apoptosis. CD200 expression on apoptotic DCs diminishes proinflammatory cytokine production in response to self-antigens in vitro and is required for UVB-mediated tolerance to haptenated self-proteins in vivo. Up-regulation of CD200 may represent a novel mechanism, whereby immune reactivity to apoptosis-associated self-antigens is suppressed under steady state conditions. (Blood. 2004;103: 2691-2698)
Introduction
As a result of natural cell turnover, cells in peripheral tissues continually undergo apoptosis. These apoptotic cells are associated with a distinct lack of inflammation, leading to the initial perception that the process of apoptosis is immunologically silent and passive.1 However, it is now clear that apoptotic cells do not just quietly disappear. Instead, they actively inhibit immune responses by providing immunoregulatory signals directly to cells of the immune system. For example, when added to human lymphocyte cultures stimulated with lipopolysaccharide, apoptotic cells inhibit production of proinflammatory cytokines such as tumor necrosis factor α (TNF-α), interleukin 1 (IL-1), and IL-12 and promote the production of the anti-inflammatory cytokine IL-10.2 T-cell activation is inhibited when apoptotic cells are added to splenocytes in the presence of Con A.3 Macrophages that have ingested apoptotic cells increase production of anti-inflammatory cytokines and inhibit production of proinflammatory cytokines.4 Furthermore, immature dendritic cells (DCs) that have taken up apoptotic cells have a compromised ability to mature into immunostimulatory antigen-presenting cells (APCs).5
Given their immunosuppressive capacity, it has been suggested that apoptotic cells generated during natural cell turnover play a central role in the establishment and maintenance of peripheral self-tolerance.6 Immature DCs and monocytes traffic through tissues, phagocytose apoptotic cells, and migrate to lymphoid tissue7 ; DCs that have ingested apoptotic cells process and present apoptotic cell-derived peptides on major histocompatibility complex (MHC) class I and class II molecules8 ; and DCs that have taken up apoptotic cells fail to initiate productive T-cell responses, possibly through the induction of regulatory T cells.9,10 Patients with autoimmune diseases have abnormal immune responses to self-peptides generated during apoptosis, suggesting that mechanisms exist in healthy individuals that suppress immunoreactivity to apoptosis-associated self-antigens.11
DCs are among the cells that continually turnover in peripheral tissues,12-14 and apoptotic DCs have been shown to play a role in apoptosis-linked peripheral tolerance through their interaction with lymph node-resident DCs.6,13 The molecular mechanisms that govern this process, however, have not been identified.
CD200, formerly known as OX-2, is a highly conserved type I transmembrane glycoprotein that is expressed on a variety of cell types, including DCs.15 Expression of the receptor for CD200 (CD200R) is restricted to myeloid-derived APCs and certain populations of T cells.16,17 Several studies have shown that CD200 imparts an immunoregulatory signal through CD200R, leading to the suppression of T-cell-mediated immune responses. Increased allograft survival following portal vein immunization with alloantigen correlates with an increase in CD200 expression on both hepatic and splenic DCs.18 Tolerance in this setting is reversed with a monoclonal antibody to CD200.19 CD200+ DCs or soluble CD200 decrease type-1 cytokine production (interferon γ [IFNγ] and IL-2) and promote production of type-2 cytokines (IL-4, IL-10, and TGFβ) in vitro and prolong allograft and xenograft survival in vivo.20,21 CD200-deficient mice have a compromised capacity to down-regulate APC activation in the steady state, resulting in chronic central nervous system inflammation, an exaggerated inflammatory response to trauma and an increased susceptibility to develop both experimental autoimmune encephalitis and collagen-induced arthritis.22
Taken together, these and other studies provide evidence that CD200-CD200R signaling results in the down-regulation of immune responses and that in the absence of CD200 tolerance to peripheral self-antigens is easily broken.
The molecular mechanism(s) controlling CD200 expression and the immunologic context (ie, steady state, inflammation) in which DCs express CD200 are not yet fully defined. Experiments in our laboratory revealed that CD200 expression in vivo and in vitro was increased on DCs undergoing apoptosis. Here, we examined the hypothesis that CD200 expression increases with apoptosis and that CD200 on apoptotic DCs mediates an immunoregulatory signal that leads to immune tolerance. We show that CD200 expression increases as murine DCs undergo apoptosis, that the gene for CD200 contains functional p53 response elements, that both p53- and caspase-dependent pathways control expression of CD200 during apoptosis, that CD200 expression on apoptotic DCs diminishes proinflammatory cytokine production in response to apoptosis-associated self-antigens in vitro, and that CD200 is required for UVB-mediated tolerance to haptenated self-proteins in vivo.
Materials and methods
Mice
C57BL/6 mice (B6) were purchased from Jackson Laboratories (Bar Harbor, ME). CD200-/- mice (derived in the B6 background22 ) were provided by Dr Jonathan Sedgwick (DNAX Research Institute, Palo Alto, CA). Age- and sex-matched mice were used in all experiments.
DC isolation, induction of apoptosis, and flow cytometry
Isolation of splenic DCs was carried out as previously described.23 Briefly, spleens were minced and collagenase digested. CD11c+ DCs were isolated by double column selection using CD11c microbeads and an AutoMACS immunomagnetic cell separator (Miltenyi Biotec, Auburn, CA). Positive fractions were consistently more than 97% CD11c+. DCs were cultured in Dulbecco modified Eagle medium (DMEM) with 5% heat-inactivated fetal bovine serum (FBS) supplemented with 5 × 10-5 M 2-mercaptoethanol (Sigma, St Louis, MO), HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer (10 mM), sodium pyruvate (1 mM), penicillin (100 U/mL), streptomycin (100 μg/mL), l-glutamine (2 mM), and minimum essential medium amino acids (referred to hereafter as complete DMEM) at 37°C in 5% CO2. All components were from Gibco BRL (Grand Island, NY) unless otherwise specified. DCs were cultured in complete DMEM at 1 × 106 cells/mL in round-bottom 96-microwell plates to induce apoptosis. Flow cytometry reagents include the following: anti-CD200 antibody (3B6; phycoerythrin [PE]; Tetralink, Buffalo, NY; and/or OX-90; biotin), rat immunoglobulin M (IgM) isotype control (R4-22; PE), I-Ab (KH74; biotin), CD80 (16-10A1; PE), CD86 (GL1; fluorescein isothiocyanate [FITC]), CD40 (HM40-3; FITC), or CaspACE FITC-VAD-FMK (Promega, Madison, WI). All antibodies were from BD PharMingen (San Diego, CA) unless otherwise stated. Annexin V versus 7-amino-actinomycin D (7-AAD) staining was performed according to the manufacturer's instructions (BD PharMingen) after antibody staining. On the basis of their Annexin V versus 7-AAD staining pattern, CD11c+ DCs were sorted into nonapoptotic and apoptotic cells on a fluorescence-activated cell sorting (FACS) DiVa cell sorter (BD Immunocytometry Systems). Sorted fractions were then analyzed for CD200 expression.
RT-PCR
CD11c+ cells were isolated (as described in “DC isolation, induction of apoptosis, and flow cytometry”) and cultured in complete DMEM. At various times after culture, cells were harvested, and total RNA was extracted with TRIzol according to manufacturer's instructions (Life Technologies, Rockville, MD). RNA was quantified and equal amounts (∼1 μg) were reverse transcribed into cDNA with oligo(dT) primers using Thermoscript reverse transcriptase-polymerase chain reaction (RT-PCR systems (Gibco BRL) according to the manufacturer's instructions. Semiquantitative RT-PCR was performed by using CD200 (5′-AGTGGTGACCCAGGATGAA-3′ 5′-TACTATGGGCTGTACATAG-3′) or β-actin (5′-GAGTCCTGTGGCATCCACG-3′ 5′-CTAGGAGCATTTGCGGTGGAC-3′) primer sets. In this procedure, a maximum of 40 PCR cycles was consecutively reduced by 5 or fewer cycles to determine relative quantities of CD200 or β-actin cDNA. Densitometry was performed directly on agarose gels using Alpha Imager 2200 v5.5 software on an Alpha Imager 2200 (Alpha Innotech, San Leandro, CA).
Quantitative real-time RT-PCR was performed on RNA isolated at various times after culture by using preformulated CD200 and 18S rRNA Gene Expression Assay systems according to manufacturer's protocol (Applied Biosystems, Foster City, CA). Reactions were carried out in an Opticon-2 Continuous Fluorescence Detector (MJ Research, Boston, MA). Data were analyzed by using the comparative Ct method (Applied Biosystems).
Identification of p53REs and p53 luciferase studies
Nucleic acid sequences were obtained from the National Center for Biotechnology Information “Entrez nucleotide” sequence database (http://www.ncbi.nlm.nih.gov/entrez). Accession numbers were as follows: murine CD200 gene, AH006102; human CD200 gene, Contig no. NT_00595.14 (region spanning 2791475-2821185). Sequences were analyzed for the consensus p53REs as described in “Results” with Lasergene sequence analysis software (DNASTAR, Madison, WI).
The following pairs of oligonucleotides encompassing the first putative p53 response element (p53RE no. 1) and the second putative p53 response element (p53RE no. 2) from the first intron of the human CD200 gene were commercially synthesized: p53RE no. 1a, 5′-AGCTAAACTTGCTGAGGAAGAGGCATGAAT-3′; p53RE no. 1b, 5′-GATCATTCATGCCTC-TTCCTCAGCAAGTTT-3′; p53RE no. 2a, 5′-AGCTGAGCTTGTCCATTCTGCATTCTTCATCATGGCC-3′; p53RE no. 2b, 5′-GATCGGCCATG-ATGAAGAATGCAGAATGGACAAGCTC-3′. As control, the following oligonucleotides encompassing randomly chosen sequence from the first intron of the human CD200 gene were also commercially synthesized: control-a, 5′-AGCTTAGTGCTTATTTCTACTGGCAGTATT-3′; control-b, 5′-GATCAATACTGCCAGTAGAAATAAGCACTA-3′. Oligonucleotides of each pair were annealed, resulting in double-stranded oligonucleotides with extremities compatible with a 5′HindIII and 3′BglII digestion product. Double-stranded oligos were cloned into the pLUC-MCS plasmid (Stratagene, La Jolla, CA) digested with HindIII and BglII. Either of these constructs (pLUC-MCS-p53RE no. 1, pLUC-MCS-p53RE no. 2, or pLUC-MCS-control) were cotransfected into p53-deficient NCI-H1299 cells24 (American Type Culture Collection, Rockville, MD) with or without pFC-p53 (Stratagene), a plasmid that constitutively expresses wild-type human p53. Cotransfections were carried out in 6-well culture plates using PolyFect transfection reagent according to manufacturer's instructions (Qiagen, Valencia, CA). Equivalent amounts of a vector that constitutively expresses green fluorescent protein (GFP) were used in replicates of each experiment to control for transfection efficiency. Luciferase activity was measured 24 to 48 hours after transfection by using a luciferase assay kit according to the manufacturer's instructions (Stratagene) with an AutoLumat LB953 bench top luminometer (Berthold Systems, Aliquippa, PA).
Autologous mixed lymphocyte reaction (MLR) cultures
CD11c+ cells were incubated for 24 hours in complete DMEM to induce apoptosis before the addition of responder cells. Approximately 70% of DCs were Annexin V+ at the time responder cells were added. For caspase inhibition experiments, CD11c+ cells were cultured in complete DMEM in the presence or absence of 200 μM Z-VAD-FMK (Promega) for 18 hours. Cells were washed 3 times and used as stimulator cells. CD4+ responder T cells were obtained from the spleens of B6 mice by double-column selection using anti-CD4 microbeads and the AutoMACS. Cells were routinely more than 98% CD4+ after separation. CD11c+ stimulator cells (5 × 105) were added to 2 × 105 CD4+ responder T cells. All cultures were carried out in complete DMEM in triplicate round-bottom microwells. Culture wells were pulsed with 1 μCi/mL (0.037 MBq/mL) [3H]-thymidine at 72 hours and thymidine incorporation was measured 12 hours later.
Cytokine analysis
Cytokine levels were measured from culture supernatants using cytometric bead arrays (CBA; BD Biosciences, San Diego, CA) according to the manufacturer's protocol. “Experimental” values were expressed as a percentage of CD200-/- controls for each cytokine. Normalized values from replicate experiments were pooled and compared with the CD200-/- control (ie, 100%) by Student t test.
UVB contact hypersensitivity studies
Contact hypersensitivity experiments were done as previously described.25 Briefly, the abdomens of mice were shaved, and groups of 5 mice were irradiated with 400 J/m2 UVB using a bank of 4 UVP-AB-111 midrange UV tubes (UVP, Upland, CA) emitting between 280 and 360 nm with a peak at 302 nm. Mouse ears were protected from UVB. Five control mice were shaved but not exposed to UVB. Mice were irradiated 4 consecutive times over 4 days. Twenty-four hours later, irradiated and control mice were sensitized by applying 25 μL 0.5% DNFB (2,4-dinitro-fluorobenzene; Sigma) diluted in acetone/olive oil (4:1) on the shaved abdominal skin. Five days later, mice were challenged with 5 μL 0.15% DNFB on each side of the right ear. Before challenge, baseline ear thickness was measured. After challenge, ear thickness was measured at 24 and 48 hours. Ear swelling was calculated by subtracting baseline values from the values recorded on subsequent days.
Results
CD200 expression increases during apoptosis
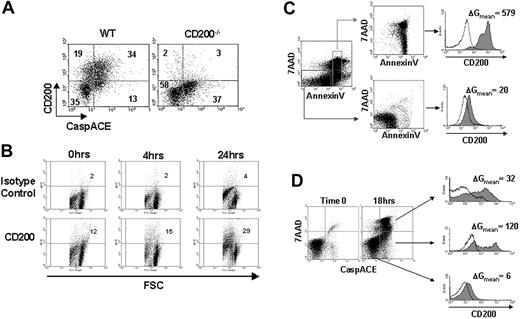
DCs continually undergo apoptosis in the steady state.13,23 By using CaspACE (FITC-Z-VAD-FMK), a cell-permeable reagent that labels apoptotic cells by binding specifically to activated caspases, we found that more than 70% of freshly isolated apoptotic CD11c+ DCs expressed CD200, as compared with 35% of nonapoptotic DCs (Figure 1A). We next examined both the kinetics and mechanism of CD200 expression on DCs as they undergo apoptosis. When cultured in vitro, splenic DCs spontaneously undergo apoptosis as a result of growth factor deprivation.26 By using this simple technique, we correlated CD200 expression with 3 established markers of apoptosis: reduction in cell size, inversion of phosphatidylserine, and activation of caspases. At various times, cultured DCs were collected and analyzed for forward light scatter (FSC) and CD200 expression. There were more than twice as many CD200+ cells after 24 hours of culture (29% versus 12%; Figure 1B) when approximately 70% of the cells were apoptotic based on Annexin V staining (Figure 2A). The high CD200-expressing population was restricted to the smaller cells identified by decreased FSC (Figure 1B). To confirm that apoptotic DCs expressed higher levels of CD200, we sorted Annexin V+ and Annexin V- cells and stained them with anti-CD200 monoclonal antibody (mAb; Figure 1C). Although a low level of CD200 expression was observed on some nonapoptotic DCs (Annexin V-/7-AAD-), there was a pronounced increase in CD200 expression on apoptotic DCs (Annexin V+/7-AAD-/+). Because activation of caspases is a hallmark of apoptotic cell death, we used CaspACE, along with 7-AAD and anti-CD200 mAb to assess CD200 levels on early and late apoptotic DCs. CD200 expression on nonapoptotic (CaspACE-/7-AAD-), early apoptotic (CaspACE+/7-AAD-), and late apoptotic (CaspACE+/7-AAD+) DCs after 18 hours of culture is shown in Figure 1D. Low levels of CD200 expression was observed on nonapoptotic DCs; however, DCs with activated caspases displayed a pronounced increase in CD200 expression.
Splenic DCs increase expression of CD200 on the cell surface as they undergo apoptosis. (A) Freshly isolated splenocytes from WT C57BL/6 or CD200-/- mice were quadruple stained with CaspACE, anti-CD200 mAb, CD11c, and 7-AAD. Cells shown are 7-AAD- and CD11c+. Numeric values denote the percentage of cells in each quadrant. (B) CD11c+ DCs were cultured in complete DMEM for 24 hours and assayed for cell size (FSC) versus CD200 expression. Quadrants are arbitrarily set on the basis of minimal (< 5%) staining with isotype control antibody. Numeric values denote the percentage of CD200+ cells. (C) CD11c+ cells were cultured in complete DMEM for 24 hours and sorted into either nonapoptotic (Annexin V-/7-AAD-) or apoptotic (Annexin V+/7AAD-/+) populations, and CD200 expression was examined. (D) CD11c+ cells were cultured in complete DMEM for 18 hours and triple stained with CaspACE, 7-AAD, and anti-CD200 mAb. Electronic gates were set on nonapoptotic (CaspACE-/7-AAD-), early apoptotic (CaspACE+/7-AAD-), or late apoptotic (CaspACE+/7AAD+) cells, and CD200 expression was examined. (C-D) Shaded and unshaded histograms represent staining with anti-CD200 and isotype control mAbs, respectively. ΔGmean values represent the change in MFI between anti-CD200 and isotype control staining. Results are representative of 3 replicate experiments.
Splenic DCs increase expression of CD200 on the cell surface as they undergo apoptosis. (A) Freshly isolated splenocytes from WT C57BL/6 or CD200-/- mice were quadruple stained with CaspACE, anti-CD200 mAb, CD11c, and 7-AAD. Cells shown are 7-AAD- and CD11c+. Numeric values denote the percentage of cells in each quadrant. (B) CD11c+ DCs were cultured in complete DMEM for 24 hours and assayed for cell size (FSC) versus CD200 expression. Quadrants are arbitrarily set on the basis of minimal (< 5%) staining with isotype control antibody. Numeric values denote the percentage of CD200+ cells. (C) CD11c+ cells were cultured in complete DMEM for 24 hours and sorted into either nonapoptotic (Annexin V-/7-AAD-) or apoptotic (Annexin V+/7AAD-/+) populations, and CD200 expression was examined. (D) CD11c+ cells were cultured in complete DMEM for 18 hours and triple stained with CaspACE, 7-AAD, and anti-CD200 mAb. Electronic gates were set on nonapoptotic (CaspACE-/7-AAD-), early apoptotic (CaspACE+/7-AAD-), or late apoptotic (CaspACE+/7AAD+) cells, and CD200 expression was examined. (C-D) Shaded and unshaded histograms represent staining with anti-CD200 and isotype control mAbs, respectively. ΔGmean values represent the change in MFI between anti-CD200 and isotype control staining. Results are representative of 3 replicate experiments.
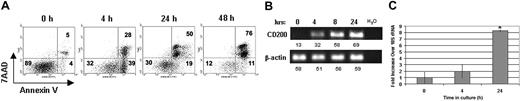
CD200 mRNA expression as DCs undergo apoptosis. (A) CD11c+ cells were isolated and cultured in complete DMEM to induce apoptosis. Numeric values denote (B) semiquantitative RT-PCR for CD200 and β-actin. Numbers adjacent to gel represent densitometry values for above spots. (C) Quantitative real-time RT-PCR for CD200 and18S rRNA were performed on mRNA isolated at various times after culture. Results are representative of 3 replicate experiments. *Statistically significant from 0 hour (P < .05) by Student t test. Error bars indicate ±1 SD.
CD200 mRNA expression as DCs undergo apoptosis. (A) CD11c+ cells were isolated and cultured in complete DMEM to induce apoptosis. Numeric values denote (B) semiquantitative RT-PCR for CD200 and β-actin. Numbers adjacent to gel represent densitometry values for above spots. (C) Quantitative real-time RT-PCR for CD200 and18S rRNA were performed on mRNA isolated at various times after culture. Results are representative of 3 replicate experiments. *Statistically significant from 0 hour (P < .05) by Student t test. Error bars indicate ±1 SD.
We examined CD200 mRNA levels in cultures of DCs undergoing apoptosis to determine whether increased cell surface CD200 expression correlated with increased mRNA. Splenic CD11c+ DCs were cultured in medium without growth factors to induce apoptosis and total RNA was isolated at various times for both semiquantitative and quantitative real-time RT-PCR analysis. Increasing levels of CD200 mRNA were observed during the 24-hour culture (Figure 2B), peaking at 24 hours when approximately 70% of DCs were apoptotic (Figure 2A). CD200 message was detected at time 0 only when 40 cycles of PCR amplification was used (data not shown), correlating with the low levels of CD200 expression on the cell surface at time 0 (Figure 1B). In quantitative real-time RT-PCR assays, we observed more than 8-fold increases in CD200 mRNA (relative to 18S rRNA) after 24 hours of culture (Figures 2C and 4C). Consistent with the up-regulation of CD200 at the transcriptional level during DC apoptosis, we did not detect an intracellular store of CD200 protein in freshly isolated (preapoptotic) DCs. (see Figure S1 on the Blood website; click the Supplemental Data Sets link at the top of the online article.)
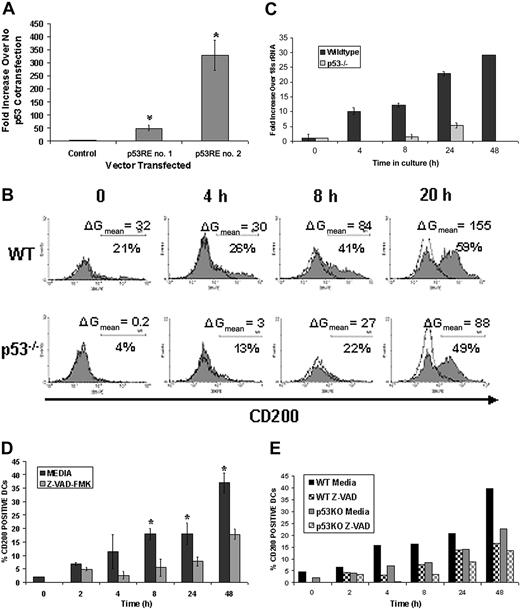
p53 and caspases drive CD200 expression during DC apoptosis. (A) The first and second putative p53REs (p53RE no. 1 and p53RE no. 2) or randomly chosen sequence (control) from the first intron of the human CD200 gene were cloned into a luciferase expression vector containing a minimal promoter element. NCI-H1299 cells were cotransfected with either of these constructs with or without a p53 expression vector (pFC-p53). Results shown are fold increases in luciferase activity over no pFC-p53 cotransfection for each vector. *P < .005 compared with control by Mann-Whitney U test. Results are representative of 5 replicate experiments. (B) CD11c+ DCs were isolated from wild-type (WT) and p53-/-C57BL/6 mice and cultured in complete DMEM for 48 hours to induce apoptosis. Cells were stained with CaspACE and anti-CD200 mAb in the presence of 7-AAD at various time points. Shaded and unshaded histograms represent staining with anti-CD200 and isotype control mAbs, respectively. Cells shown are gated on early apoptotic cells (CaspACE+/7AAD-). Results are representative of 2 replicate experiments. (C) Quantitative real-time RT-PCR was performed on cultured CD11c+ cells derived from either WT or p53-/- mice. Total RNA was normalized to 18S rRNA at each time point. Results are representative of 2 experimental replicates. (D) CD11c+ DCs were cultured in medium for 48 hours in the presence or absence of 100 μM Z-VAD-FMK and assayed for CD200 expression. *P < .05 as compared with Z-VAD-FMK treatment. Results are combined data from 3 replicate experiments. (E) DCs from WT and p53-/- mice were isolated as described earlier, cultured in medium with or without 100 μM Z-VAD-FMK, and examined for CD200 expression by flow cytometry. Error bars indicate ±1 SD (A, C-D).
p53 and caspases drive CD200 expression during DC apoptosis. (A) The first and second putative p53REs (p53RE no. 1 and p53RE no. 2) or randomly chosen sequence (control) from the first intron of the human CD200 gene were cloned into a luciferase expression vector containing a minimal promoter element. NCI-H1299 cells were cotransfected with either of these constructs with or without a p53 expression vector (pFC-p53). Results shown are fold increases in luciferase activity over no pFC-p53 cotransfection for each vector. *P < .005 compared with control by Mann-Whitney U test. Results are representative of 5 replicate experiments. (B) CD11c+ DCs were isolated from wild-type (WT) and p53-/-C57BL/6 mice and cultured in complete DMEM for 48 hours to induce apoptosis. Cells were stained with CaspACE and anti-CD200 mAb in the presence of 7-AAD at various time points. Shaded and unshaded histograms represent staining with anti-CD200 and isotype control mAbs, respectively. Cells shown are gated on early apoptotic cells (CaspACE+/7AAD-). Results are representative of 2 replicate experiments. (C) Quantitative real-time RT-PCR was performed on cultured CD11c+ cells derived from either WT or p53-/- mice. Total RNA was normalized to 18S rRNA at each time point. Results are representative of 2 experimental replicates. (D) CD11c+ DCs were cultured in medium for 48 hours in the presence or absence of 100 μM Z-VAD-FMK and assayed for CD200 expression. *P < .05 as compared with Z-VAD-FMK treatment. Results are combined data from 3 replicate experiments. (E) DCs from WT and p53-/- mice were isolated as described earlier, cultured in medium with or without 100 μM Z-VAD-FMK, and examined for CD200 expression by flow cytometry. Error bars indicate ±1 SD (A, C-D).
To determine whether inhibition of apoptosis altered CD200 expression, DCs were culture in medium supplemented with the pan-caspase inhibitor Z-VAD-FMK. Inhibition of DC apoptosis by blockage of activated caspases inhibited CD200 expression at all time points examined (Figure 4D).
Increased CD200 expression with apoptosis was not restricted to murine DCs. In C1498 leukemia cells, CD200 mRNA and protein were initially undetectable; however, there was a pronounced increase in both CD200 mRNA and cell surface protein expression as early as 8 hours after γ-irradiation, peaking at 48 hours, when more than 80% of the cells were apoptotic (Figure S2). CD200is a p53-target gene
The tumor suppressor protein p53 has been coined the “master regulator” of the apoptotic program because of its pivotal role in many of the signaling pathways initiated in apoptotic cells.27 The major function of p53 is that of a transcription factor. On being activated in the early stages of apoptosis, p53 binds to regulatory elements of various genes in a highly sequence-specific manner. p53-mediated gene regulation is thought to bring about many of the distinctive morphologic and physiologic characteristics associated with apoptosis. The target consensus sequence for p53-binding (p53 response element or p53RE) has been defined,28 and genes that contain this sequence either in the promoter region or downstream introns have been shown to be transcriptionally activated by p53.29 Translocation and/or accumulation in the nucleus are required for p53 to function as a transcription factor30 ; however, p53 activity has not been characterized in DC apoptosis. To determine whether p53 translocates to the nucleus in apoptotic DCs, we examined the intracellular localization of both p53 and caspases by immunofluorescent microscopy. CD11c+ DCs cultured for 24 hours in complete DMEM were triple-stained with 4′,6′diamedino-2-phenylindole (DAPI; to detect nuclear DNA), anti-p53 mAb, and CaspACE. Cells in which caspases had not been activated (CaspACE-negative) consistently showed cytoplasmic or perinuclear staining for p53; however; cells with activated caspases (CaspACE positive) consistently showed brighter p53 staining that colocalized with DNA (Figure S3).
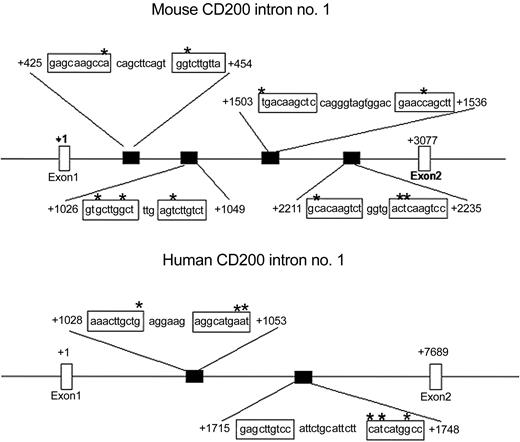
Given that CD200 mRNA expression increases and that p53 translocates to the nucleus with DC apoptosis, we performed a sequence analysis of both murine and human CD200 genes to search for consensus p53REs. Ap53RE consists of at least 2 copies of the sequence 5′-PuPuPu C(A/T)(T/A)GPyPyPy-3′ separated by 0 to 13 base pair (bp).28 Some mismatches in this sequence have been shown not to alter p53 binding.31 For our analysis, a maximum of 3 variations from this proposed consensus sequence was allowed with no more than one error in a single decamer if it was located in 1 of the 2 central nucleotides of a decamer. The C in position 4 and the G in position 7 were considered as invariant positions. These criteria are identical to those used by others to discover the p53REs in the Fas gene.32 We found 4 putative p53REs in the first intron of murine CD200 and 2 putative p53REs found in the first intron of human CD200 (Figure 3). Of note is the relatively conserved location of the second p53RE in mouse intron 1 with the first p53RE in human intron 1 from the initiation codon. We tested the ability of the 2 p53REs contained in the first intron of human CD200 to act as cis-enhancer elements in p53-mediated gene expression in vitro. The 25-bp sequence from 1028 to 1053 (p53RE no. 1) and the 33-bp sequence from 1715 to 1748 (p53RE no. 2) encompassing the first and second putative p53REs, respectively (Figure 3), were cloned into a luciferase reporter vector. A randomly chosen nucleotide sequence from the first intron of human CD200 also was cloned as a negative control. Each of these vectors was independently cotransfected into NCI-H1299 cells (which lack functional p53) with or without the pFC-p53 plasmid that constitutively expresses wild-type human p53. Increases in luciferase activity after cotransfection are indicative of functional p53REs capable of enhancing p53-mediated gene expression. In all experiments, no increase in luciferase activity was observed when pFC-p53 was cotransfected with control vector; however, cotransfection with vector containing either the first or second putative p53REs (p53RE no. 1 or p53RE no. 2) produced approximately 50- and 300-fold increases in luciferase activity, respectively (Figure 4A).
The genes for both human and murine CD200 contain p53REs. Intron no. 1 of murine CD200 has 4 p53REs, and intron no. 1 of human CD200 has 2 p53REs. *Variations from the consensus p53RE sequence. +1 represents the initiation codon.
The genes for both human and murine CD200 contain p53REs. Intron no. 1 of murine CD200 has 4 p53REs, and intron no. 1 of human CD200 has 2 p53REs. *Variations from the consensus p53RE sequence. +1 represents the initiation codon.
To determine if p53 was responsible for increasing expression of CD200 during DC apoptosis, we analyzed CD200 expression on cultured CD11c+ DCs isolated from the spleens of p53-/- and healthy (WT) C57BL/6 mice. DCs were cultured in complete DMEM and stained at various times with CaspACE or Annexin V-FITC and anti-CD200 mAb in the presence of 7-AAD. DCs from p53-/- mice showed slower kinetics and decreased overall expression of CD200 as compared with WT controls (Figure 4B). At 8 hours, the percentage of p53-/- DCs that stained positive for CD200 was approximately half that of WT DCs (22% versus 41%). Furthermore, the overall level of CD200 expression, as denoted by mean fluorescence intensity (MFI) of anti-CD200 staining, was decreased in p53-/- DCs (MFI = 55 versus 89 at 8 hours). After 20 hours of culture, DCs from p53-/- mice had comparable percentages of CD200+ cells (49% versus 59%); however, the expression levels of CD200 remained reduced (MFI = 86 versus 134 at 20 hours). Diminished CD200 expression in p53-/- DCs also was observed after 48 hours of culture (data not shown). To confirm that p53 increases transcription of CD200 during DC apoptosis, we performed quantitative RT-PCR on RNA isolated from cultured WT and p53-/- DCs. Decreased levels of CD200 mRNA were observed at all time points examined in p53-/- DCs (Figure 4C). In contrast, CD200 mRNA levels in WT DCs steadily increased with time and apoptosis.
Delayed expression of CD200 in p53-/- DCs suggests the existence of a p53-independent mechanism(s) for apoptosis-driven CD200 expression. Because caspases appeared to play a role in increasing CD200 expression (Figure 4D), we sought to determine whether activated caspases and p53 act in the same or complementary molecular pathways to increase CD200 expression in apoptotic DCs. Splenic CD11c+ DCs from p53-/- and WT mice were cultured with and without Z-VAD-FMK, and CD200 expression was measured over time. Cells were analyzed for Annexin V binding, 7-AAD uptake, and anti-CD200 staining (Figure 4E). As previously shown, DCs from p53-/- mice had diminished levels of CD200 on their surface compared with WT DCs. Treatment with Z-VAD-FMK further reduced CD200 expression in p53-/- DCs at all time points examined (Figure 4E), suggesting that p53 and caspases may act in different pathways in mediating CD200 expression during apoptosis.
CD200 expression with apoptosis alters T-cell activity in vitro
The autologous MLR is a T-cell-mediated immune response directed against autologous APCs. It consists of costimulation-dependent T-cell proliferation, primarily by CD4+ T cells, against self-peptides presented by self-MHC class II molecules expressed on the surface of DCs.33 Recently, published studies show that autoreactive T cells in autologous MLR cultures recognize and react to self-peptides that have been modified by the action of caspases in apoptotic cells and that DCs are responsible for processing and presenting these self-antigens to autologous CD4+ T cells.34,35 Abnormal autologous MLR responses are often observed in patients with autoimmunity, and, thus, this reaction is thought to represent a self-recognition mechanism that might be important in the establishment and maintenance of peripheral self-tolerance.11 Because CD200 expression increases with apoptosis (Figure 1) and CD200-/- mice have a compromised capacity to maintain peripheral tolerance to self-antigens,22 we tested whether CD200 expression on apoptotic DCs influences T-cell activation in autologous MLR cultures. We used apoptotic WT and CD200-/- DCs as stimulators and purified syngeneic (akin to autologous) CD4+ T cells as responders. We characterized the cell surface phenotype of both apoptotic CD200+/+ (WT) and apoptotic CD200-/- DCs prior to these assays to confirm that DCs from CD200-/- mice differ from WT primarily in their level of CD200 expression. CD200 expression was minimal on freshly isolated WT DCs but increased after 18 hours of culture. CD200 expression was not observed on fresh or cultured CD200-/- DCs. There was little to no difference in CD80, CD86, CD40, and MHC II expression between WT and CD200-/- DCs on either freshly isolated cells or cells cultured for 18 hours (Figure 5B). This finding is consistent with a previous study showing that BM-derived DCs from CD200-/- mice differ from WT only in their level of CD200 expression.22
CD200 expression on apoptotic DCs results in increased T-cell proliferation and diminished secretion of proinflammatory cytokines in caspase-dependent autologous MLR cultures. (A) WT CD11c+ DCs were cultured in complete DMEM with or without 200 μM Z-VAD-FMK for 24 hours, washed thoroughly, and then used as stimulators in autologous MLR cultures with purified CD4+ T cells from C57BL/6 mice as responders. *P < .05 versus medium control. (B) Cell surface protein expression on wild-type (WT) and CD200-deficient (CD200-/-) DCs. CD11c+ DCs were cultured in complete DMEM for 24 hours to induce apoptosis and stained with specific antibodies (shaded histograms) to detect cell surface protein expression by flow cytometry. Cells were more than 97% CD11c+, and approximately 70% were apoptotic by Annexin V staining. ΔGmean represents the change in MFI from isotype-matched controls (open histograms). (C) CD11c+ DCs were isolated from wild-type C57BL/6 (H-2b; CD200+/+) and CD200-deficient (H-2b; CD200-/-) mice and cultured in complete DMEM for 24 hours to induce apoptosis. Stimulators were more than 97% CD11c+, and approximately 70% were apoptotic by Annexin V staining at the time responders were added. Proliferation was measured at 72 hours by [3H]-thymidine incorporation. Results are representative of 4 replicate experiments. *P < .05 versus WT. (D) Cytokine levels were measured from supernatants at 72 hours by cytometric bead array. Results are the average of 4 replicate experiments. *Statistically significant from CD200-/- (P < .05) by Student t test. Error bars indicate ±1 SD (A, C-D).
CD200 expression on apoptotic DCs results in increased T-cell proliferation and diminished secretion of proinflammatory cytokines in caspase-dependent autologous MLR cultures. (A) WT CD11c+ DCs were cultured in complete DMEM with or without 200 μM Z-VAD-FMK for 24 hours, washed thoroughly, and then used as stimulators in autologous MLR cultures with purified CD4+ T cells from C57BL/6 mice as responders. *P < .05 versus medium control. (B) Cell surface protein expression on wild-type (WT) and CD200-deficient (CD200-/-) DCs. CD11c+ DCs were cultured in complete DMEM for 24 hours to induce apoptosis and stained with specific antibodies (shaded histograms) to detect cell surface protein expression by flow cytometry. Cells were more than 97% CD11c+, and approximately 70% were apoptotic by Annexin V staining. ΔGmean represents the change in MFI from isotype-matched controls (open histograms). (C) CD11c+ DCs were isolated from wild-type C57BL/6 (H-2b; CD200+/+) and CD200-deficient (H-2b; CD200-/-) mice and cultured in complete DMEM for 24 hours to induce apoptosis. Stimulators were more than 97% CD11c+, and approximately 70% were apoptotic by Annexin V staining at the time responders were added. Proliferation was measured at 72 hours by [3H]-thymidine incorporation. Results are representative of 4 replicate experiments. *P < .05 versus WT. (D) Cytokine levels were measured from supernatants at 72 hours by cytometric bead array. Results are the average of 4 replicate experiments. *Statistically significant from CD200-/- (P < .05) by Student t test. Error bars indicate ±1 SD (A, C-D).
Prior treatment of DC stimulators with Z-VAD-FMK reduced CD4+ T-cell proliferation in autologous MLR cultures by approximately 60% (Figure 5A), confirming that T-cell activation in these assays is partially dependent on caspase-mediated DC apoptosis. T-cell proliferation was significantly increased when CD200+/+ DCs were used as stimulators as compared with CD200-/- DCs (Figure 5C). This increase in proliferation was associated with more than double the amount of IL-2 production (average of 220% of CD200-/- controls; Figure 5D). In contrast, TNFα and IFNγ production was significantly reduced by -37% and -38%, respectively. The average production of IL-5 was increased by 277% when CD200+/+ DCs were used as stimulators as compared with CD200-/- controls (Figure 5D). We did not detect IL-4 in any of these assays.
CD200 is required for UVB-mediated tolerance in vivo
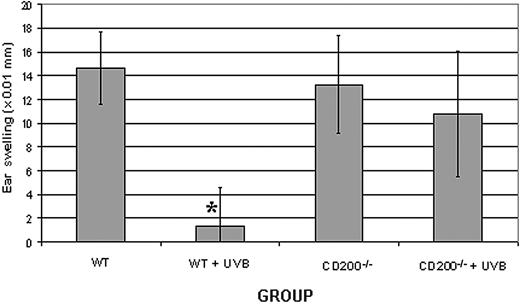
To assess the effects of apoptosis-associated CD200 expression on immune tolerance in vivo, we used a well-characterized system of UVB-induced immunosuppression of contact hypersensitivity. Low-dose UVB given prior to cutaneous sensitization with hapten systemically inhibits the contact hypersensitivity response of hapten-specific T cells.25 UVB-induced apoptosis of epidermal DCs (ie, Langerhans cells) is thought to play a role in tolerance induction in this model.36 WT and CD200-/- mice were given 4 consecutive doses of UVB and sensitized with DNFB on UVB-irradiated abdominal skin 24 hours later. Control mice for each group were not treated with UVB prior to sensitization. Five days later, nonirradiated ears were challenged with DNFB, and ear swelling was measured after both 24 and 48 hours to assess the magnitude of hapten-specific contact hypersensitivity. Exposure of WT mice to UVB prior to hapten sensitization significantly reduced contact hypersensitivity to DNFB when compared with WT mice that were not exposed to UVB (Figure 6). In contrast, CD200-/- mice were resistant to UVB-induced immunosuppression. When CD200-/- mice were exposed to UVB prior to hapten sensitization, contact hypersensitivity was not reduced (Figure 6). In a replicate experiment, ear swelling was significantly increased (P < .04) in CD200-/- mice that had received UVB when compared with CD200-/- nonirradiated controls (data not shown). Thus, CD200 is required for UVB-mediated tolerance in this contact hypersensitivity model.
CD200 is required for UVB-mediated suppression of contact hypersensitivity. Wild-type (WT) or CD200-deficient (CD200-/-) mice were either exposed (+ UVB) or not exposed to UVB prior to hapten sensitization and challenge. Ear thickness was measured at both 24 (not shown) and 48 hours. *Significantly different from all other groups (P < .01) by Student t test. Results shown are representative from 2 replicate experiments. Error bars indicate ±1 SD.
CD200 is required for UVB-mediated suppression of contact hypersensitivity. Wild-type (WT) or CD200-deficient (CD200-/-) mice were either exposed (+ UVB) or not exposed to UVB prior to hapten sensitization and challenge. Ear thickness was measured at both 24 (not shown) and 48 hours. *Significantly different from all other groups (P < .01) by Student t test. Results shown are representative from 2 replicate experiments. Error bars indicate ±1 SD.
Discussion
Here, we show that expression of CD200 increases with DC apoptosis and suggest that CD200 plays a role in the induction of apoptosis-associated immune tolerance. The genes for both murine and human CD200 contain p53REs that drive p53-mediated gene expression, and expression of CD200 during DC apoptosis was partially dependent on both p53 and activated caspases. Apoptotic DCs that expressed CD200 altered T-cell proliferation and cytokine production in autologous MLR cultures in vitro and CD200 was required for induction of UVB-mediated immune tolerance to hapten in vivo.
Steinman et al6 propose that phagocytosis of apoptotic cells in the absence of inflammation (ie, steady state) down-regulates APC activation and that this process plays a central role in the establishment and maintenance of peripheral self-tolerance. These researchers further speculate that apoptotic DCs may be the true mediators of tolerance. Although we provide no direct evidence that apoptotic CD200+ DCs mediate tolerance in vivo, we suggest an important role for CD200 in tolerance induction by apoptotic DCs on the basis of our data, showing that (1) DCs increase CD200 expression on undergoing apoptosis (Figure 1), (2) CD200 expression on apoptotic DCs skews the response of autologous T cells to decrease secretion of proinflammatory cytokines in vitro (Figure 5C), and (3) CD200 signaling is required for UVB-mediated tolerance in vivo (Figure 6). Others have shown that induction of apoptosis36-38 and alterations in Langerhans cell function39-41 are responsible for UVB-mediated tolerance.
A direct association between CD200 and apoptosis has not been reported in earlier studies that examined both CD200 tissue expression and CD200 function. However, CD200 expression is highest on thymocytes and migrating neurons.15 Both the thymus and the developing nervous system are intense areas of apoptosis. In the ovaries, another tissue with intense apoptosis, CD200 is expressed almost exclusively on structures that do not develop further, such as the granulosa of degenerating antral follicles and third-generation corpora lutea.42 Furthermore, CD200 expression on trophoblast tissue has been shown to play a role in tolerance induction at the maternal-fetal interface.43 Trophoblast tissue is highly apoptotic, and the mechanism of maternal-fetal tolerance is linked to apoptosis.44 Thus, CD200 may be constitutively expressed on some tissues and induced with apoptosis in others.
In some cases, studies on the functional activity of CD200 also can be linked to apoptosis. Increased allograft acceptance after immunization of mice with allogeneic cells injected into the portal vein has been attributed to increased CD200 expression on hepatic DCs.18 Tolerance induction by portal vein immunization has been reported to be due to retention and accumulation of tolerogenic apoptotic cells in liver sinusoids.45 A small, slow sedimenting population of BM-derived CD200+ DCs has been reported to inhibit type-1 and to stimulate type-2 cytokine production.21,46 Although the apoptotic status of the CD200+ DCs was not reported in these studies, their small size is characteristic of cells undergoing apoptosis (similar to CD200+ cells shown in Figure 1B).
Genes regulated by p53 have been shown to be involved in the processes of apoptosis, cell growth, DNA repair, and angiogenesis,29 but data are limited showing that p53 regulates genes involved in immune modulation. We found that p53 associates with DNA in apoptotic DCs (Figure S3) and that CD200 mRNA expression increases with DC apoptosis (Figure 2), suggesting to us that CD200 might be a p53-target gene. By using the same approach that was used to find p53REs within the Fas gene,32 we identified 4 putative p53REs in the first intron of murine CD200 and 2 putative p53REs in the first intron of human CD200 (Figure 3). As in that report, we did not find putative p53REs upstream of the transcription initiation site in the proposed promoter region. Interestingly, the second p53RE in the mouse CD200 gene is within 2 nucleotide base pairs from the first p53RE in the human CD200 gene relative to the initiation codon (Figure 3), suggesting the possibility that this is an evolutionarily conserved enhancer element. p53REs cloned from the human CD200 gene were functional in a p53-luciferase reporter system (Figure 4A), and CD200 expression was diminished in DCs from p53-/- mice (Figure 4B), confirming that CD200 is a bona fide p53-target gene.
Multiple lines of evidence suggest that CD200-CD200R is an immunomodulatory pathway leading to the suppression of T-cell-mediated immune responses as well as down-regulation of innate immunity.15,22,47 The data presented here suggest that CD200 facilitates the induction of tolerance to autoantigen in the context of apoptosis. Presumably, this occurs through ligation of CD200R. Whether CD200 interacts directly with T cells in a physiologically relevant manner or indirectly through CD200R+ APCs is not yet clear. In a recent report, Wright et al16 used CD200R-specific PCR primers and staining with new anti-CD200R mAbs to show that T cells express the receptor for CD200. We also found that murine T cells expressed CD200R mRNA and that they weakly bind soluble CD200, resulting in a novel cytokine expression profile in T-cell costimulatory assays in vitro (M.D.R., manuscript submitted, September 2003).
In the apoptosis-linked tolerance induction model proposed by Steinman et al,6 tissue-resident DCs in steady state are not stimulated and remain immature during migration to afferent lymph nodes. These short-lived migratory DCs subsequently undergo apoptosis in T-cell areas of the lymph node where longer-lived lymph node-resident DCs process them and their contents. Inaba et al13 propose that when self-antigens from noninflamed tissues (eg, during natural cell turnover) are brought to the T-cell areas by peripheral DCs, they are subsequently processed by lymph node-resident DCs in a regulatory and tolerogenic fashion.
Collectively, our findings, together with the finding that CD200-/- mice have increased numbers of constitutively activated APCs and are prone to induction of autoimmune disease,22 suggest that CD200 may play a role in apoptosis-linked peripheral tolerance to self-antigens. The mechanisms involved remain to be determined. In the context of natural cell turnover, induced as well as constitutive expression of CD200 may contribute to the maintenance of a noninflammatory milieu because CD200 plays a role in the homeostatic control of innate immune responses through regulation of macrophages and granulocytes.15,47 With identification of CD200-receptor expression on murine and human T cells, direct interaction between CD200+ DCs and T cells is an alternative mechanism. Failure to control peripheral immune response to self-proteins modified during natural cell death is a potential trigger for autoimmune disease.11 CD200-CD200R interaction may be an important pathway in such a setting.
Prepublished online as Blood First Edition Paper, November 26, 2003; DOI 10.1182/blood-2003-09-3184.
Supported by the Midwest Athletes Against Childhood Cancer Fund (Milwaukee, WI). M.D.R. was supported by the Medical Scientist Training Program of the Medical College of Wisconsin.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Jonathan Sedgwick, DNAX (Palo Alto, CA) for the generous gift of CD200-deficient mice.

![Figure 5. CD200 expression on apoptotic DCs results in increased T-cell proliferation and diminished secretion of proinflammatory cytokines in caspase-dependent autologous MLR cultures. (A) WT CD11c+ DCs were cultured in complete DMEM with or without 200 μM Z-VAD-FMK for 24 hours, washed thoroughly, and then used as stimulators in autologous MLR cultures with purified CD4+ T cells from C57BL/6 mice as responders. *P < .05 versus medium control. (B) Cell surface protein expression on wild-type (WT) and CD200-deficient (CD200-/-) DCs. CD11c+ DCs were cultured in complete DMEM for 24 hours to induce apoptosis and stained with specific antibodies (shaded histograms) to detect cell surface protein expression by flow cytometry. Cells were more than 97% CD11c+, and approximately 70% were apoptotic by Annexin V staining. ΔGmean represents the change in MFI from isotype-matched controls (open histograms). (C) CD11c+ DCs were isolated from wild-type C57BL/6 (H-2b; CD200+/+) and CD200-deficient (H-2b; CD200-/-) mice and cultured in complete DMEM for 24 hours to induce apoptosis. Stimulators were more than 97% CD11c+, and approximately 70% were apoptotic by Annexin V staining at the time responders were added. Proliferation was measured at 72 hours by [3H]-thymidine incorporation. Results are representative of 4 replicate experiments. *P < .05 versus WT. (D) Cytokine levels were measured from supernatants at 72 hours by cytometric bead array. Results are the average of 4 replicate experiments. *Statistically significant from CD200-/- (P < .05) by Student t test. Error bars indicate ±1 SD (A, C-D).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/7/10.1182_blood-2003-09-3184/6/m_zh80070459320005.jpeg?Expires=1767743178&Signature=4BVJngzg2PcTt8-0gvtz5v47L-NhSy~Tf2YPjLNqlo6EqNY~SewGBkirw6GVqXN91MYK4datDIKbpoUUiQv1LKKD5E3cMk2OPDeYBS0wIMhf-2g03Xw-KqH1q4jBpncjy-HdALoABvgO9a3ER9hUy2IFQSU8RmpGQZRd96hg-nH4kbZsk1dp0RXfLyhN73zn-wjll0ziEbaBDkgjUqI8WTVnponmZK~R2bPgCvPdAt28R9t9iCht0TVDc9nREANLS8l7s1s~iqxVVQPH2NjjUkFPBmCx1aRXzQxvxjWRbRnd1dxS8TRxsThb8e5h0OuXwvwf~QP-DuPQvRB3h2KkuA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal