Abstract
The CD8+ cell noncytotoxic anti-HIV response (CNAR) is associated with a long-term healthy clinical state in HIV-infected individuals. Over time CNAR is reduced concomitant with progression to disease. In studies to evaluate whether the interaction between CD8+ cells and dendritic cells (DCs) could increase CNAR, CD8+ cells from individuals who showed a decrease in this antiviral activity were cocultured with monocyte-derived dendritic cells matured with CD40 ligand. After coculture with these mature DCs, the CD8+ cells showed an increase in CNAR greater than that observed with CD8+ cells costimulated with CD3/CD28 antibodies. This antiviral response appeared to be mediated primarily by production of interleukin-15 (IL-15) by the mature DCs. Purified IL-15 also enhanced CNAR, whereas IL-12 showed no substantial effect. These studies provide another potential approach by which the immune system in HIV infection could be restored by cytokine therapy, particularly IL-15 administration. (Blood. 2004;103:2699-2704)
Introduction
CD8+ cells from healthy HIV-infected individuals demonstrate a unique ability to suppress HIV replication without killing the infected cell.1 The extent of this CD8+ cell noncytotoxic antiviral response (CNAR) is reduced in infected individuals who progress to disease.2-5 The cause for this loss in CNAR is not yet understood. One reason could be the decrease in a T helper 1 (TH-1) cell cytokine profile observed over time in HIV-infected individuals with a change toward the TH-2 type of immune response, that is, production of interleukin-4 (IL-4), IL-5, and IL-10.6 TH-1 cell cytokines, particularly IL-2, help maintain CNAR; TH-2 cytokines inhibit its activity.7,8 Since IL-2 production by lymphocytes results from their interaction with dendritic cells (DCs),9 a compromise in DC function may be responsible for the loss of CNAR over time. In this regard, previous studies have shown that HIV-infected individuals exhibit a decrease in DC function and numbers circulating in the blood.10,11
Another reason for reduced CD8+ cell activity can be low CD4+ cell counts, which also correlate with the loss of CNAR.2,3,5 Therefore, CD40 ligand (CD40L), a surface protein expressed on CD4+ cells following activation, may not be present at sufficient levels to mature DCs via CD40 to stimulate CD8+ cell immune responses, specifically, CNAR. Upon activation by CD40L, dendritic cells mature and secrete IL-15 and IL-12.12-16 IL-15 can regulate expansion and differentiation of CD8+ cells, particularly the memory subsets of CD8+ cells.17-21 IL-12 induces the differentiation of T cells into TH-1 type cells that secrete large amounts of interferon-γ, which can also increase CD8+ cell immune responses and CNAR.7 Therefore, improving DC/T-cell interactions might restore or enhance CNAR in HIV-infected individuals. In evaluating this possibility, we studied whether dendritic cells, matured by CD40L, could enhance CNAR in CD8+ cells from HIV-infected individuals.
Patients, materials, and methods
Subjects
HIV-infected subjects were recruited from the San Francisco Bay area. Their clinical state ranged from asymptomatic to those who were progressing to disease as reflected by a reduced CD4+ cell count.22 This study received the approval of the Committee for Human Research, University of California, San Francisco.
Subset isolation and cell culture
Blood samples from the volunteer subjects were collected in heparinized tubes by venipuncture, and peripheral blood mononuclear cells (PBMCs) were isolated from the heparinized blood by Ficoll Hypaque gradient separation.22 To obtain monocyte-derived dendritic cells, monocytes were isolated from the PBMCs of uninfected subjects with CD14+ immunomagnetic (IM) beads, as described by the manufacturer (Miltenyi Biotech, Auburn, CA). Isolated monocytes were cultured for 6 days at 106 cells/mL in complete RPMI 1640 medium, containing 10% heat-inactivated (56°, 30 minutes) fetal bovine serum (Gibco BRL, Rockville, MD), 1% penicillin/streptomycin, 2 mM glutamine, sodium pyruvate, and HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), and supplemented with granulocyte-macrophage colony-stimulating factor (GM-CSF; 100 ng/mL) and IL-4 (200 U/mL; provided by DNAX, Palo Alto, CA). For differentiation of monocyte-derived DCs (moDCs) into mature DC (mDCs), cells were incubated for 24 hours with trimer CD40L (0.5 μg/mL; Immunex, Seattle, WA).
CD8+ cells from PBMCs of seropositive subjects with reduced CNAR (< 50% suppression of HIV replication) were isolated by positive selection with immunomagnetic beads (IM) beads (Dynal, Lake Success, NY)5,22 and cultured at 5 × 104 cells/well in a 96-well round bottom plate in complete RPMI 1640 medium containing 10 U/mL recombinant human IL-2 (generously provided by Glaxo-Wellcome, Research Triangle Park, NC).5,22 Mature dendritic cells were added to each well at the indicated DC/CD8+ cell ratio and cocultured for 6 days before evaluating CD8+ cell anti-HIV activity. In some experiments, the cocultures were incubated with neutralizing antibodies (10 μg/mL) to IL-15 (Immunex) or IL-12 (R&D Systems, Minneapolis, MN).23 An isotype control antibody (R&D Systems) was used in the wells indicated for the control. In addition, in some studies, IL-15 (Immunex) and IL-12 (R&D Systems) were used at concentrations suggested in the literature.24,25
For the studies, CD8+ cells were either left unstimulated or stimulated with both anti-CD3 and anti-CD28 antibodies (R&D Systems).23 The anti-CD3 antibodies (1 μg/mL) were bound to 96-well round bottom plates for 2 hours at 37°C, washed twice with phosphate-buffered saline (PBS), and CD28 antibodies (1 μg/mL) were then added. The optimal amount of antibody was determined prior to beginning the studies. The concentration used was based on the concentration at which anti-CD3 and anti-CD28 induced optimal cell proliferation at 6 days. The CD8+ cells were then cultured at 5 × 104 cells/well in RPMI 1640 complete medium containing 10 U/mL recombinant human IL-2. Control unstimulated CD8+ cells were also cultured in the complete medium containing 10 U/mL recombinant human IL-2.
Acute infection assay
From the PBMCs of seronegative individuals, CD4+ cells were obtained by IM bead separation (Miltenyi Biotech) and cultured at 2 × 106 cells/mL in the RPMI 1640 complete medium, supplemented with 10% purified human IL-2 (Roche, Indianapolis, IN). Immediately after plating the CD4+ cells, phytohemagglutinin (PHA-L, 3 μg/mL; Sigma Chemicals, St Louis, MO) was added to the culture for 3 days at 37°C. The cells were then washed and inoculated with HIV-1SF33, a β-chemokine-insensitive, CXCR4-dependent viral isolate,2,5,22 at a titer at which the peak reverse-transcriptase (RT) activity26 was reached at 7 days in infected CD4+ cells cultured alone. After 1 hour, cells were washed and 105 cells were mixed with CD8+ cells in duplicate cultures in a 96-well plate at 2-fold different input CD8+ cell/CD4+ cell ratios (ranging from 0.25 to 2:1), in complete RPMI 1640 medium, supplemented with 100 U/mL recombinant human IL-2. Culture supernatants were monitored for RT activity and the percent suppression was calculated in comparison with infected CD4+ cells cultured alone.2,5,22
Flow cytometry analysis
After CD8+ cells and dendritic cells were cocultured for 6 days, the CD8+ cells were removed from culture by IM beads and stained by standard procedures27 with conjugated antibodies to the cell surface markers, CD8+-fluorescein isothiocyanate (FITC), HLA-DR-FITC, CD45RO-phycoerythrin (PE), CD25-PE, and CD28-PE (Becton Dickinson Immunocytometry Systems, San Jose, CA). The cells were then analyzed by a fluorescence-activated cell sorter (FACS, Facsort; Becton Dickinson).
Cell proliferation assay
After CD8+ cells and dendritic cells were cocultured for 5 days, 10 μCi (0.37 MBq) 3 H-thymidine was added to each well of the CD8+ cell cocultures and incubated for an additional 16 hours. Cells were then harvested for analyses by a scintillation counter (Wallac, Gaithersburg, MD).
Results
Monocyte-derived dendritic cells enhance CNAR in HIV-infected subjects
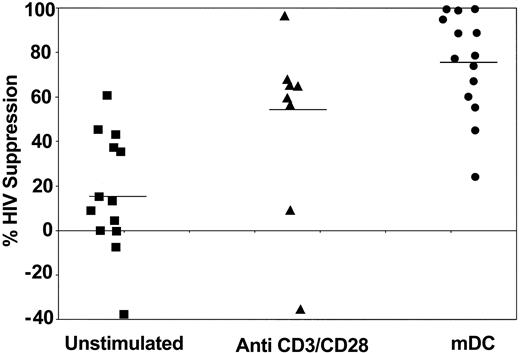
To determine whether CD8+ cell suppression of HIV replication can be improved after exposure to dendritic cells, CNAR was measured in HIV-seropositive donors whose CD8+ cells have shown reduced anti-HIV activity. These cells were either left unstimulated, stimulated with anti-CD3/anti-CD28 antibodies, or cocultured with allogeneic dendritic cells (Figure 1). Costimulation of the CD8+ cells with CD3 and CD28 antibodies had previously shown a substantial increase in CNAR.23 In the present studies, the CD8+ cells, previously cocultured with the allogeneic dendritic cells and then added to infected CD4+ cells at 1:1 and 0.5:1 CD8+cell/CD4+ cell ratios, suppressed HIV replication to a greater extent than the CD8+ cells treated by the other approaches. At a 1:1 ratio, the DC-stimulated CD8+ cells suppressed HIV replication by an average of 76%, compared with 48% by anti-CD3/anti-CD28 antibody-treated CD8+ cells (P = .0234) and 17% by unstimulated CD8+ cells (P = .0002; determined by exact Wilcoxon signed rank test; Figure 1). At a 0.5:1 ratio, the CD8+ cells after DC coculture suppressed HIV replication at an average of 55%, compared with 28% by CD8+ cells stimulated with anti-CD3/anti-CD28 antibodies and 1% observed with unstimulated CD8+ cells (data not shown). The average HIV suppression by CD8+ cells after PHA stimulation (at a 1:1 ratio) was 57%.
CD8+ cell noncytotoxic antiviral response after stimulation of CD8+ cells by different approaches. CD8+ cells from HIV-infected subjects were treated with 10 U/mL recombinant human IL-2 (control) (▪), polyclonally stimulated by anti-CD3 and anti-CD28 antibodies (▴), or cocultured with allogeneic CD40L-matured monocyte-derived DCs (•) (1:5 DC/CD8+ cell ratio) for 6 days. The CD8+ cells were then removed and cocultured with HIV-1-infected CD4+ cells, at a ratio of 1:1 CD8+/CD4+ cells.5 Culture supernatants were assayed for reverse-transcriptase (RT) activity on days 5 and 7.24 The extent (percent) of CNAR was determined by comparing the average percentage of RT activity in supernatants from duplicate cocultures with the average RT activity in fluids from HIV-infected CD4+ cells cultured alone. Bars indicate average suppression resulting from each treatment.
CD8+ cell noncytotoxic antiviral response after stimulation of CD8+ cells by different approaches. CD8+ cells from HIV-infected subjects were treated with 10 U/mL recombinant human IL-2 (control) (▪), polyclonally stimulated by anti-CD3 and anti-CD28 antibodies (▴), or cocultured with allogeneic CD40L-matured monocyte-derived DCs (•) (1:5 DC/CD8+ cell ratio) for 6 days. The CD8+ cells were then removed and cocultured with HIV-1-infected CD4+ cells, at a ratio of 1:1 CD8+/CD4+ cells.5 Culture supernatants were assayed for reverse-transcriptase (RT) activity on days 5 and 7.24 The extent (percent) of CNAR was determined by comparing the average percentage of RT activity in supernatants from duplicate cocultures with the average RT activity in fluids from HIV-infected CD4+ cells cultured alone. Bars indicate average suppression resulting from each treatment.
When the same subjects were studied, coculture of CD8+ T cells with matured dendritic cells (mDCs) gave a significantly greater increase in CNAR than did CD3/CD28 costimulation (P = .042, 95% CI = 2.1%-43.5%, signed ranked Wilcoxon test; Table 1). All these results indicate that stimulation of CD8+ cells by allogeneic DCs increases inhibition of HIV replication by CNAR better than PHA and anti-CD3/anti-CD28 antibody stimulation. The extent of this CNAR enhancement by DCs from different individuals did not correlate with the CD4+ cell counts of the infected individuals studied (data not shown).
Effect of CD3/CD28 costimulation and mDC coculture on the CD8+ cell noncytotoxic anti-HIV response
Subject . | αCD3/CD28 . | mDC . |
|---|---|---|
| 1 | 87.5 | 68.4 |
| 2 | 68.1 | 74.3 |
| 3 | 14.3 | 54.1 |
| 4 | 25.8 | 32 |
| 5 | 4.6 | 28 |
| 6 | 4.6 | 55.8 |
| 7 | 27.4 | 61.7 |
| 8 | 13.0 | 56.5 |
Subject . | αCD3/CD28 . | mDC . |
|---|---|---|
| 1 | 87.5 | 68.4 |
| 2 | 68.1 | 74.3 |
| 3 | 14.3 | 54.1 |
| 4 | 25.8 | 32 |
| 5 | 4.6 | 28 |
| 6 | 4.6 | 55.8 |
| 7 | 27.4 | 61.7 |
| 8 | 13.0 | 56.5 |
Data indicate percent of suppression of HIV-1 replication in infected CD4+ cells by CD8+ cells at a 1:1 CD8+ cell/CD4+ cell input ratio. The CD8+ cells were treated with antibodies to CD3 and CD28 or cocultured with mature dendritic cells (mDCs) (see “Patients, materials, and methods” for details on procedures). Control-infected CD4+ cells showed substantial HIV-1 replication (reverse transcriptase activity at > 2 × 106 cpm/mL of culture fluid).
Low input number of mature dendritic cells can enhance CNAR
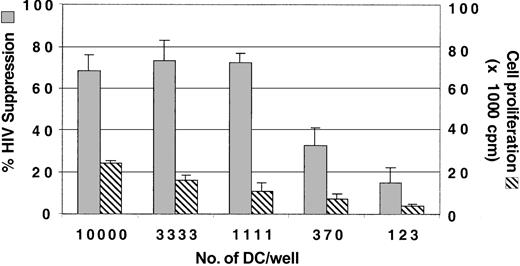
To determine the optimal number of allogeneic DCs needed for enhancing CNAR, the DCs were serially titrated in cocultures with CD8+ cells from the HIV-infected subjects (Figure 2). An input number of DCs ranging from 104 to 1.1 × 103 DCs (DC/CD8+ ratios of 1:5 to 1:45) in coculture with CD8+ cells enhanced CNAR. For our studies, we have used an input number of 104 DCs per well.
Optimization of the number of DCs for enhancement of CNAR. Monocytes were cultured with GM-CSF and IL-4 for 3 days and matured with CD40L trimer. In a 96-well plate, DCs at 3-fold dilutions were then added to 50 000 CD8+ cells/well. After 6 days of coculture, the CD8+ cells were removed and transferred into a coculture with HIV-1-infected CD4+ cells. Supernatants from duplicate cultures were assayed for RT activity.26 The extent of CNAR (▦) was determined by comparing these RT results with the average RT activity in supernatants of HIV-infected CD4+ cells cultured alone. Cell proliferation (▧) was measured as described in “Patients, materials, and methods.” Error bars indicate SD from the average suppression observed. Results are representative of 5 separate experiments.
Optimization of the number of DCs for enhancement of CNAR. Monocytes were cultured with GM-CSF and IL-4 for 3 days and matured with CD40L trimer. In a 96-well plate, DCs at 3-fold dilutions were then added to 50 000 CD8+ cells/well. After 6 days of coculture, the CD8+ cells were removed and transferred into a coculture with HIV-1-infected CD4+ cells. Supernatants from duplicate cultures were assayed for RT activity.26 The extent of CNAR (▦) was determined by comparing these RT results with the average RT activity in supernatants of HIV-infected CD4+ cells cultured alone. Cell proliferation (▧) was measured as described in “Patients, materials, and methods.” Error bars indicate SD from the average suppression observed. Results are representative of 5 separate experiments.
We also measured CD8+ cell proliferation 6 days after stimulation with each of the input numbers of allogeneic DCs. Cell proliferation was low with decreased concentrations of allogeneic DCs in the DC/CD8+ cell coculture; however, CNAR remained the same for the first 3 input quantities of allogeneic DCs (Figure 2). These results indicate that CNAR does not correlate with CD8+ cell proliferation.
Comparison of CNAR after coculture with allogeneic and autologous cells
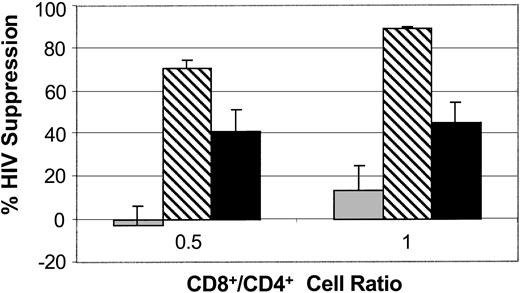
To assess the effect of mature autologous DCs on CNAR, we compared CNAR using CD8+ cells stimulated by DCs from allogeneic and the same (autologous) donor. A representative experiment is shown in Figure 3. At an input ratio of 1:1 CD8+/CD4+ cells, the CD8+ cells cocultured with allogeneic DCs suppressed HIV replication by 90%, whereas CD8+ cells cocultured with autologous DCs showed 42% suppression; CD8+ cells cultured alone showed minimal CNAR (15%). These results showed that in the inductive phase involving CD8+ cell interactions with DCs, allogeneic DCs increase CD8+ cell suppression of HIV replication better than autologous DCs.
Comparison of CNAR after stimulation with allogeneic compared with autologous DCs. CD8+ cells from HIV-infected subjects were either control (10 U/mL recombinant human IL-2; ▦), or cocultured with allogeneic (▧) or autologous (▪) CD40L-matured monocyte-derived DCs for 6 days. The CD8+ cells were removed and cocultured with HIV-1-infected CD4+ cells, at ratios of 0.5:1 and 1:1 (CD8+/CD4+ cells). Error bars indicate SD from the average suppression observed. Results are representative of 7 separate studies.
Comparison of CNAR after stimulation with allogeneic compared with autologous DCs. CD8+ cells from HIV-infected subjects were either control (10 U/mL recombinant human IL-2; ▦), or cocultured with allogeneic (▧) or autologous (▪) CD40L-matured monocyte-derived DCs for 6 days. The CD8+ cells were removed and cocultured with HIV-1-infected CD4+ cells, at ratios of 0.5:1 and 1:1 (CD8+/CD4+ cells). Error bars indicate SD from the average suppression observed. Results are representative of 7 separate studies.
Inhibition of IL-15 blocks the enhancement of CNAR by allogeneic dendritic cells
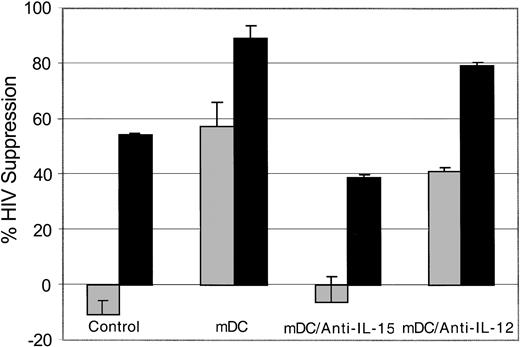
To identify a mechanism for the enhancement of CNAR following coculture with allogeneic DCs, we evaluated the CD8+ cell anti-HIV response when allogeneic DCs were mixed with CD8+ cells in the presence of neutralizing antibodies to IL-15 and IL-12. A representative experiment is shown in Figure 4. We noted that CNAR was reduced when antibodies to IL-15 were present in the CD8+ cell/allogeneic DC cocultures compared with the untreated and anti-IL-12-treated CD8+ cell cocultures. These results indicate that IL-15 is involved in the enhanced CNAR that results from allogeneic DC stimulation of CD8+ cells.
Role of IL-15 in mDC enhancement of CNAR. CD8+ cells from HIV-infected subjects were either untreated or treated (10 U/mL recombinant human IL-2), as indicated for 6 days. The mature allogeneic DCs (mDC) were cocultured with CD8+ cells at a ratio of 1:5. The CD8+ cells were then removed and cocultured with HIV-1-infected heterologous CD4+ cells, at a CD8+/CD4+ cell ratio of 0.5:1 (▦) or 2:1 (▪) in the presence or absence of antibodies to IL-15 or IL-12. Supernatants from duplicate cultures were assayed for RT activity. The extent of CNAR was determined by comparing these average RT results with the average RT activity in supernatants of duplicate cultures of HIV-infected CD4+ cells cultured alone. Error bars indicate SD from the average suppression resulting from each treatment. Results are representative of 7 separate experiments.
Role of IL-15 in mDC enhancement of CNAR. CD8+ cells from HIV-infected subjects were either untreated or treated (10 U/mL recombinant human IL-2), as indicated for 6 days. The mature allogeneic DCs (mDC) were cocultured with CD8+ cells at a ratio of 1:5. The CD8+ cells were then removed and cocultured with HIV-1-infected heterologous CD4+ cells, at a CD8+/CD4+ cell ratio of 0.5:1 (▦) or 2:1 (▪) in the presence or absence of antibodies to IL-15 or IL-12. Supernatants from duplicate cultures were assayed for RT activity. The extent of CNAR was determined by comparing these average RT results with the average RT activity in supernatants of duplicate cultures of HIV-infected CD4+ cells cultured alone. Error bars indicate SD from the average suppression resulting from each treatment. Results are representative of 7 separate experiments.
Increased activation of CD8+ cells and difference in cell subset proliferation after stimulation by allogeneic DCs
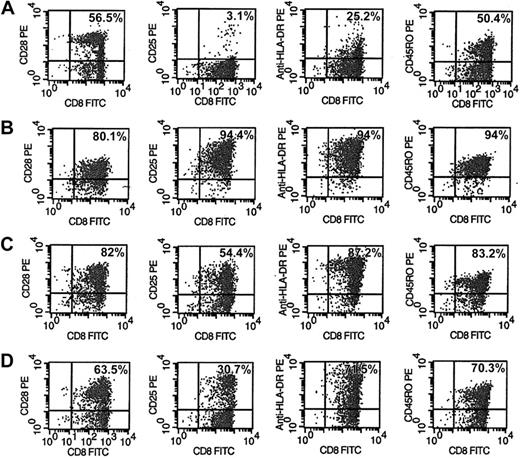
Phenotype and activation markers of CD8+ cells were evaluated after 6 days of various treatments of CD8+ cells (Figure 5). The CD8+ cells from HIV-seropositive donors were either unstimulated (Figure 5A), or stimulated with anti-CD3/anti-CD28 antibodies (Figure 5B), allogeneic DCs (Figure 5C), or allogeneic DCs in the presence of anti-IL-15 antibody (Figure 5D). A representative experiment is shown in Figure 5. The activation markers CD25 and HLA-DR expressed on CD8+ cells after stimulation with allogeneic DCs (Figure 5C) increased to 54% and 87%, respectively, compared with 3% and 25% observed with untreated CD8+ cells (Figure 5A). These results suggest that the extent of CNAR may correlate with higher levels of CD8+ cell activation. However, these activation markers on CD8+ cells after stimulation with anti-CD3/anti-CD28 antibodies also increased (94.4% for CD25; 94% for HLA-DR; Figure 5B) compared with unstimulated CD8+ cells. These data indicate that the significant difference in the extent of CNAR enhancement after stimulation with allogeneic DCs compared with anti-CD3/anti-CD28 antibodies does not necessarily correlate with the level of cell activation reflected by CD25 and HLA-DR expression.
Phenotype and activation changes in CD8+ cells after stimulation by allogeneic DCs with and without anti-IL-15 antibodies. CD8+ cells from HIV-infected subjects were either (A) control (10 U/m recombinant human IL-2), (B) polyclonally stimulated by anti-CD3 and anti-CD28 antibodies, (C) cocultured with allogeneic DCs (1:5 DC/CD8+ cell ratio), or (D) cocultured with allogeneic DCs in the presence of 10 μg/mL anti-IL-15 antibody for 6 days. CD8+ cells were removed and assessed by FACS for activation markers after staining with fluorophore-conjugated antibodies to CD28, CD25, HLA-DR, and CD45RO. Percentages reflect the number of CD8+ cells expressing that phenotype marker. Results are representative of 5 separate experiments.
Phenotype and activation changes in CD8+ cells after stimulation by allogeneic DCs with and without anti-IL-15 antibodies. CD8+ cells from HIV-infected subjects were either (A) control (10 U/m recombinant human IL-2), (B) polyclonally stimulated by anti-CD3 and anti-CD28 antibodies, (C) cocultured with allogeneic DCs (1:5 DC/CD8+ cell ratio), or (D) cocultured with allogeneic DCs in the presence of 10 μg/mL anti-IL-15 antibody for 6 days. CD8+ cells were removed and assessed by FACS for activation markers after staining with fluorophore-conjugated antibodies to CD28, CD25, HLA-DR, and CD45RO. Percentages reflect the number of CD8+ cells expressing that phenotype marker. Results are representative of 5 separate experiments.
In the presence of anti-IL-15 antibody, CD8+ cells stimulated with allogeneic DCs had decreased activation levels of CD25 and HLA-DR to 30.7% and 71.5%, respectively. These results indicate that activation of CD8+ cells may also depend on the presence of IL-15.
In allogeneic DC-stimulated CD8+ cells (Figure 5C), the CD8+ cell subsets, expressing CD28 or CD45RO, increased to 82% and 83%, respectively, compared with findings with unstimulated cells (56.5% and 50.4%, respectively). Similarly, these phenotype markers increased on CD8+ cells stimulated with anti-CD3/anti-CD28 antibodies to 80.1% and 94%, respectively. These results indicate that since both stimulations increased CD8+ cell subsets similarly, the substantial differences in their enhancement of CNAR may not depend on the expansion of either of these 2 subsets of CD8+ cells.
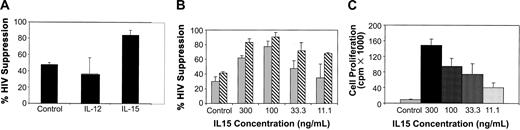
IL-15 alone can enhance CNAR compared with IL-12-treated CD8+ cells
To determine whether IL-15 alone can enhance CNAR, we measured CNAR using CD8+ cells receiving no treatment compared with CD8+ cells incubated with IL-12 (1 ng/mL) or IL-15 (150 ng/mL). Results from a representative experiment are presented in Figure 6. CNAR was substantially higher with CD8+ cells treated with IL-15 than with CD8+ cells cultured with IL-12 or left unstimulated (Figure 6A). The concentration of IL-15 required to enhance CNAR in CD8+ cells ranged from 100 to 300 ng/mL (Figure 6B). CD8+ cell proliferation also correlated with the concentration of IL-15 in the CD8+ cell culture (Figure 6C). However, CD8+ cells showed enhancement of CNAR at low concentrations of IL-15 (as low as 11.1 ng/mL) when cell proliferation was minimal (1:1 CD8+/CD4+ cell ratio tested; Figures 6B-C). These results again suggest that the enhancement of CNAR does not depend on the level of cell proliferation induced by IL-15.
Effect of IL-15 treatment on CNAR by CD8+ cells from HIV-infected subjects. (A) CD8+ cells from HIV-infected subjects were either treated (control) with 10 U/mL recombinant human IL-2, or treated with IL-12 (1 ng/mL) or with IL-15 (150 ng/mL) for 6 days, and then cocultured with infected CD4+ cells at a 0.5:1 ratio. (B) CD8+ cells were treated with the indicated concentrations of IL-15. Cells were then removed and cocultured with HIV-1 acutely infected CD4+ cells, at a ratio of 0.5:1 in panel A and in panel B (▦) and 1:1 in panel B (▧) (CD8+/CD4+ cells). Supernatants from duplicate cultures were assayed for RT activity. The extent (percent) of CNAR was determined by comparing these average RT results with the average RT activity from supernatants of duplicate cultures of HIV-infected CD4+ cells cultured alone. A bar indicates standard deviation from the average suppression of duplicate cultures. (C) Proliferation of cultured CD8+ cells either untreated (10 U/mL IL-2) or treated with the indicated doses of IL-15 was measured on day 5 after a 16-hour incubation with 3 H-thymidine. Results are representative of 5 separate experiments. Error bars indicate SD from the average suppression observed.
Effect of IL-15 treatment on CNAR by CD8+ cells from HIV-infected subjects. (A) CD8+ cells from HIV-infected subjects were either treated (control) with 10 U/mL recombinant human IL-2, or treated with IL-12 (1 ng/mL) or with IL-15 (150 ng/mL) for 6 days, and then cocultured with infected CD4+ cells at a 0.5:1 ratio. (B) CD8+ cells were treated with the indicated concentrations of IL-15. Cells were then removed and cocultured with HIV-1 acutely infected CD4+ cells, at a ratio of 0.5:1 in panel A and in panel B (▦) and 1:1 in panel B (▧) (CD8+/CD4+ cells). Supernatants from duplicate cultures were assayed for RT activity. The extent (percent) of CNAR was determined by comparing these average RT results with the average RT activity from supernatants of duplicate cultures of HIV-infected CD4+ cells cultured alone. A bar indicates standard deviation from the average suppression of duplicate cultures. (C) Proliferation of cultured CD8+ cells either untreated (10 U/mL IL-2) or treated with the indicated doses of IL-15 was measured on day 5 after a 16-hour incubation with 3 H-thymidine. Results are representative of 5 separate experiments. Error bars indicate SD from the average suppression observed.
We also compared the level of CD8+ cell activation after IL-15 treatment. After 6 days in culture, the activation markers CD25 and HLA-DR on CD8+ cells increased to 5.8% and 67.7%, respectively, compared with 1.5% and 35.5% for untreated CD8+ cells. In addition, the CD45RO subset of CD8+ cells increased slightly to 32.2% in the IL-15-treated CD8+ cells from the 20.3% observed in the untreated CD8+ cells (data not shown).
Discussion
Long-term survival of individuals with HIV infection has correlated with a CD8+ cell anti-HIV response that does not involve killing of the infected cell. This absence of CD8+ cell killing has been documented by the lack of CD4+ cell lysis and apoptosis and the lack of reduction in the number of virus-infected cells after 7 days of HIV suppression by CD8+ cells.1,28,29 Moreover, the antiviral activity is not major histocompatibility complex (MHC)-restricted and is observed with very low CD8+/CD4+ cell ratios (0.5-2:1).1,2,28
This CD8+ cell noncytotoxic antiviral response (CNAR) declines over time and appears to be lost prior to the reduction in CD4+ cell number and an increase in HIV replication in the infected host.1,30 Previous studies have indicated that IL-2 and CD3/CD28 costimulation of CD8+ cells from individuals who have decreased antiviral activity can restore this antiviral response.7,8,23 In the latter cases, the most likely mechanism is the induction of IL-2 production by CD8+ cells and the enhanced expression of the IL-2 receptor on the cell surface.
In approaches to mimic costimulation, we examined the ability of dendritic cells (DCs), the major activator of CD8+ cell responses,31,32 to restore CNAR in HIV-infected individuals who have reduced antiviral activity. The results from these present studies indicate that allogeneic DCs do show a greater ability to enhance CNAR than CD3/CD28-costimulated CD8+ cells and that this antiviral response appears mediated primarily by the production of IL-15 by the mature DCs (Figures 1, 2, 4, and 6). Other cytokines may also be involved. The observation with IL-15 is supported by the abrogation of this enhancement when the CD8+ cell/DC culture was performed in the presence of antibodies to IL-15 (Figure 4). Moreover, IL-15 treatment of CD8+ cells enhanced CNAR (Figure 6). This cytokine-mediated increase in CNAR was not observed with IL-12, but, as noted above, has been observed with IL-2 replacement.7,8 In this regard we have noted that treatment of individuals during acute infection with highly active antiviral therapy (HAART) reduces their CNAR.33 This antiviral activity, however, can be restored when HAART-treated subjects also receive IL-2 therapy (B. Martinez-Marino and J.A.L., unpublished observation, December 2003).
IL-15 has been important for the development and function of natural killer (NK) cells, NK-T cells, and intestinal intraepithelial lymphocytes.21 In association with IL-12, IL-15 was found to be responsible for optimal interferon-γ production by human NK cells.34 Its expression in antigen-presenting cells suggested a role in T-cell activation and stimulation.35,36 The present results indicate that IL-15 may indeed have a beneficial effect similar to that of IL-2 on the CD8+ cell noncytotoxic anti-HIV response. IL-15 receptor has its own unique alpha chain but shares the beta and gamma subunits of the IL-2 receptor. Conceivably these 2 cytokines act on CNAR in a similar manner. Importantly we found that cell proliferation was not a major determinant of this restoration of CNAR by mDCs and IL-15. Levels of CNAR were seen when limited CD8+ cell proliferation occurred (Figures 1, 6). Moreover, CNAR could be inhibited by antibodies to IL-15 back to levels present in unstimulated CD8+ cells (Figure 4), whereas cellular activation markers, although decreased by these antibodies, did not return to levels of unstimulated CD8+ cells (Figure 5). It is known that IL-15 enhances proliferation of memory cells and particularly those with an activated state.19,21,37 The phenotype analysis of IL-15-treated CD8+ cells supports this conclusion (Figure 5) first observed by others with CD8+ cytotoxic cells.21,38 Cell subset analyses also confirmed the preferential outgrowth of CD8+ cells with a memory phenotype (CD45RO; Figure 5).
An advantage of IL-15 therapy over IL-2 therapy is that IL-15 does not induce increased HIV replication within CD4+ cells.39,40 Thus, this cytokine may have a special value in therapy for HIV infection by enhancing cellular immune responses without affecting virus replication within the infected cells. The present studies indicate that allogeneic DCs were more effective at inducing CNAR than autologous DCs (Figure 3), most likely because the autologous cells were not pulsed with antigen to stimulate the CD8+ cell activity (Figure 3).
In summary the results of the present study place further emphasis on mechanisms by which the immune system could be beneficially manipulated either by cytokines such as IL-2 or IL-15 or by dendritic cell/CD8+ cell interaction. Importantly, they present new avenues for improving host control of HIV replication using the immune system instead of potentially toxic antiviral therapies.
Prepublished online as Blood First Edition Paper, December 18, 2003; DOI 10.1182/blood-2003-06-1913.
Supported by National Institutes of Health (NIH) grant R01 AI049926 and a grant from the Pendleton Foundation. JoAnn Castelli was recipient of support from an NIH training grant (T32 AI06395).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Steven Shiboski and M. Scott Killian for help in the statistical analyses.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal