Abstract
Long-term multilineage chimerism is achieved in CD45 congenic mice receiving high bone marrow doses with or without mediastinal irradiation (MI). Increased donor chimerism results in MI-treated compared with nonirradiated animals, suggesting that MI makes “space” for engraftment of donor pluripotent hematopoietic stem cells (PHSCs). We have now examined whether space is systemic or whether increased engraftment of donor marrow in locally irradiated mice is confined to the irradiated bones. While increased donor chimerism was observed in irradiated bones compared with nonirradiated bones of MI-treated animals 4 weeks following bone marrow transplantation (BMT), these differences were minimal by 40 weeks. MI-treated chimeras contained more adoptively transferable donor PHSCs in the marrow of both irradiated and distant bones compared with non-MI–treated chimeras. Similar proportions of donor PHSCs were present in irradiated and nonirradiated bones of locally irradiated mice at both 4 and 40 weeks. Irradiated bones contained more donor short-term repopulating cells than distant bones at 4 weeks, but not 40 weeks, after BMT. Our study suggests that local proliferation of donor PHSCs in mice receiving local irradiation rapidly leads to a systemic increase in donor PHSC engraftment.
Introduction
The development of minimally myelosuppressive regimens is a major goal for the purpose of using allogeneic bone marrow transplantation (BMT) to treat malignant and nonmalignant diseases and induce organ allograft tolerance. Regimens that employ nonmyeloablative doses of total body irradiation (TBI)1-5 or busulfan6 in conjunction with T-cell–depleting or costimulatory blocking reagents have been developed in rodents for this purpose. In addition, previous studies have shown that myelosuppressive conditioning can be removed from these regimens if a markedly increased dose of BM cells is used.7-9 However, the level of chimerism achieved without myelosuppression is lower than that achieved in the presence of a myelosuppressive component to the conditioning. Myelosuppressive conditioning promotes engraftment by creating “space,” which is a poorly understood concept that may include both physical factors and systemic secretion of cytokines and other marrow growth–promoting factors. Following irradiation, endothelial cells may enhance hematopoietic stem cell proliferation by producing cytokines such as granulocyte-macrophage colony stimulating factor (GM-CSF), stem cell factor, interleukin 1α (IL-1α), IL-6, and tumor necrosis factor-α (TNF-α).10-12 On the other hand, stem cell proliferation has been shown to be enhanced by myeloablation,13 whereas the initial homing to the marrow microenvironment or survival of hematopoietic stem cells has been shown to be impaired by irradiation.14
Ly5 is a CD45 isoform expressed on all leukocyte lineages, which exists in 2 allelic forms. Significant alloresistance and skin graft rejection is not detectable in the Ly5.2 to Ly5.1 congenic strain combination.15 In this combination, we previously observed that engraftment of a high dose of congenic bone marrow can be achieved without any host conditioning, but that the level of donor pluripotent hematopoietic stem cell (PHSC) engraftment was markedly increased by local irradiation to the thymic area.16 We made use of Ly5 congenic B6 mice to assess the mechanisms responsible for this creation of space by local irradiation.
Materials and methods
Animals
Female C57BL/6 (B6:H-2b, CD45.2) and female Ly-5 congenic B6.Ly5.2 (expressing the Ly-5.1 [CD45.1] allele according to the nomenclature of Morse et al17 ) mice were obtained from the Frederick Cancer Research Facility (Frederick, MD). For simplicity, C57BL/6 mice are described here as B6 mice and mice of the congenic strain are referred to as CD45.1 mice. All mice were maintained in a specific pathogen-free environment in sterilized microisolator cages in which they received autoclaved feed and autoclaved acidified drinking water. Recipients in each experiment were age matched and were 7 to 14 weeks old.
Conditioning and BMT
On day 0, some recipient CD45.1 mice received 7 Gy of selective irradiation to the thymic area (referred to as mediastinal irradiation, MI) or lower leg irradiation from a 60Co irradiator at a rate of 0.55 Gy/minute, as previously described.1 After completion of conditioning on day 0, 2 × 108 untreated bone marrow cells (BMCs) from B6 mice were administered intravenously.
Adoptive transplantation
BMCs were harvested from several bones, including those from irradiated and nonirradiated areas, at the time that the mice were killed and 9-10 × 106 BMCs were injected to lethally irradiated (10 Gy) CD45.1 mice. Equal numbers of BMCs from different sites of individual BM transplant recipients were transferred to 2 adoptive recipients, permitting paired statistical analysis of results in these animals.
Flow cytometric analysis of multilineage chimerism
The level of donor reconstitution of various lineages was evaluated by 2-color flow cytometric analysis using a FACScan (Becton Dickinson, Mountain View, CA) as previously described.6 Peripheral white blood cells (WBCs) were prepared by hypotonic shock. To block nonspecific Fcγ receptor (FcγR) binding of labeled antibodies, 10 μL undiluted culture supernatant of 2.4G2 (rat antimouse FcγR monoclonal antibody [mAb])18 was added to the first incubation, and these were stained with 20 μL undiluted culture supernatant of A20-1.7 (anti-CD45.1 mAb; mouse immunoglobulin G2a [IgG2a]) or 104-2.1 (anti-CD45.2 mAb; mouse IgG2a)17 (hybridomas kindly provided by Dr S. Kimura, Sloan-Kettering Cancer Institute, New York, NY) for 30 minutes at 4°C. Cell-bound mAbs were detected with fluorescein isothiocyanate (FITC)– or biotin-conjugated rat antimouse IgG2a mAb (Zymed Laboratories, Mundelein, IL), which was incubated for 30 minutes at 4°C. For biotin labeling, a 10-minute incubation with phycoerythrin streptavidin was performed. Cells were also incubated with FITC-conjugated or biotin-conjugated mAbs, including anti-CD4, anti-CD8, anti-B220 (all purchased from PharMingen, San Diego, CA), and anti–macrophage antigen-1 (anti-MAC1; Caltag Labs, San Francisco, CA) for 30 minutes at 4°C. Negative control mouse IgG2a mAb HOPC1-FITC, with no reactivity to mouse cells, was prepared in our laboratory. Two-color flow cytometric analysis (FCM) was used to distinguish donor and host cells of particular lineages, and the percentage of donor cells was calculated as previously described,16 by subtracting control staining from quadrants containing donor and host cells expressing a particular lineage marker and by dividing the net percentage of donor cells by the total net percentage of donor plus host cells of that lineage. Dead cells were excluded using propidium iodide staining.
Statistical analysis
Statistical significance was determined using unpaired Student t test in the initial mediastinal irradiation experiment and paired Student t test in the adoptive transplantation experiments. A P value of less than .05 was considered to be statistically significant.
Results
Local irradiation facilitates systemic repopulation by donor marrow cells, including PHSCs
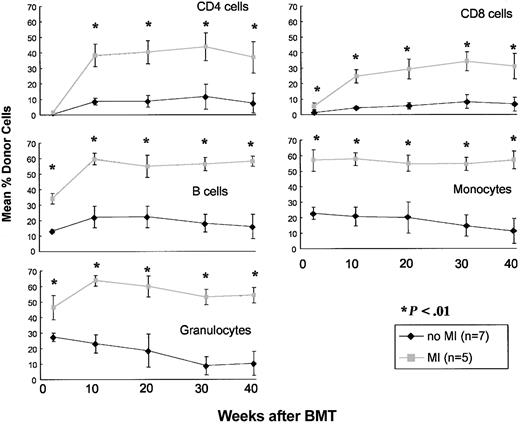
To examine local versus systemic effects of local MI, CD45.1 mice either did or did not receive 7 Gy MI (day 0) before receiving a high dose (2 × 108) of B6 BMCs. Mice treated with MI (n = 5) showed significantly higher chimerism (P < .05) than the mice not receiving MI (n = 7) in peripheral blood in all the lineages examined (up to 40 weeks after BMT; Figure 1), consistent with our previous results.16 To determine whether or not this enhanced chimerism induced by MI was confined to bones in the irradiated area or was systemic, mice were killed 40 weeks after BMT and marrow was harvested from the irradiated area (the sternum and upper thoracic vertebrae) or a nonirradiated area (tibiae and femora). Marrow chimerism levels in both sites were markedly greater in MI-treated mice than in nonirradiated controls (P < .05; Table 1). Marrow chimerism levels in irradiated bones (sternum and vertebrae) were not significantly higher than those in nonirradiated bones (legs) in MI-treated mice (Table 1). Chimerism in the spleen (including CD4, CD8, and B cells) and thymus of MI-treated mice was also significantly higher (P < .05) than that in nonirradiated mice (Table 1). Thus, MI enhances long-term donor chimerism in areas not exposed to irradiation as well as the area originally exposed to irradiation. The multilineage nature of this enhanced chimerism suggests that greater numbers of donor-derived PHSCs were present in MI-treated compared with control mice by 40 weeks after BMT.
Effect of mediastinal irradiation on donor chimerism in peripheral blood in an Ly5-congenic strain combination. CD45.1 congenic B6 mice received 200 × 106 B6 BMCs and either no MI ( ; n = 7) or 7-Gy MI (▦; n = 5). Mean percentages of donor CD4 cells, CD8 cells, B cells, monocytes, and granulocytes in WBCs are shown at the indicated time points. Statistically significant differences in chimerism in all lineages was seen between the no MI group and the 7-Gy MI group at all time points (except the CD4 cell lineage at 2 weeks). Error bars indicate SD.
; n = 7) or 7-Gy MI (▦; n = 5). Mean percentages of donor CD4 cells, CD8 cells, B cells, monocytes, and granulocytes in WBCs are shown at the indicated time points. Statistically significant differences in chimerism in all lineages was seen between the no MI group and the 7-Gy MI group at all time points (except the CD4 cell lineage at 2 weeks). Error bars indicate SD.
Effect of mediastinal irradiation on donor chimerism in peripheral blood in an Ly5-congenic strain combination. CD45.1 congenic B6 mice received 200 × 106 B6 BMCs and either no MI ( ; n = 7) or 7-Gy MI (▦; n = 5). Mean percentages of donor CD4 cells, CD8 cells, B cells, monocytes, and granulocytes in WBCs are shown at the indicated time points. Statistically significant differences in chimerism in all lineages was seen between the no MI group and the 7-Gy MI group at all time points (except the CD4 cell lineage at 2 weeks). Error bars indicate SD.
; n = 7) or 7-Gy MI (▦; n = 5). Mean percentages of donor CD4 cells, CD8 cells, B cells, monocytes, and granulocytes in WBCs are shown at the indicated time points. Statistically significant differences in chimerism in all lineages was seen between the no MI group and the 7-Gy MI group at all time points (except the CD4 cell lineage at 2 weeks). Error bars indicate SD.
Chimerism in lymphohematopoietic tissues of BM transplant recipients 40 weeks after BMT with or without MI
. | Bone marrow . | . | Spleen . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|
. | Sternum, vertebrae . | Legs . | CD4 cells . | CD8 cells . | B cells . | Thymus . | |||
| MI-treated mice* | 60.5 ± 5.72 | 56.32 ± 5.54 | 30.65 ± 5.92 | 31.85 ± 6.17 | 49.96 ± 2.96 | 37.91 ± 10.60 | |||
| Non-MI-treated mice | 12.37 ± 9.70 | 12.19 ± 10.33 | 5.17 ± 4.64 | 5.76 ± 4.43 | 13.66 ± 7.18 | 4.78 ± 3.32 | |||
. | Bone marrow . | . | Spleen . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|
. | Sternum, vertebrae . | Legs . | CD4 cells . | CD8 cells . | B cells . | Thymus . | |||
| MI-treated mice* | 60.5 ± 5.72 | 56.32 ± 5.54 | 30.65 ± 5.92 | 31.85 ± 6.17 | 49.96 ± 2.96 | 37.91 ± 10.60 | |||
| Non-MI-treated mice | 12.37 ± 9.70 | 12.19 ± 10.33 | 5.17 ± 4.64 | 5.76 ± 4.43 | 13.66 ± 7.18 | 4.78 ± 3.32 | |||
Values presented as mean % donor cells ± standard deviation. Populations are as follows: n = 5 for MI-treated mice, and n = 7 for non-MI-treated mice.
Significantly higher than non-MI-treated animals (P < .05).
To confirm the increased number of donor-derived PHSCs in nonirradiated sites of long-term chimeras that received MI compared with animals that did not receive irradiation, BMCs were harvested from the femora, tibiae, sternum, and upper thoracic vertebrae of 40-week chimeras in both groups and adoptively transferred to lethally irradiated (10 Gy) CD45.1 mice. Mice that received BMCs from MI-treated chimeras showed significantly higher (P < .05) levels of B6 (CD45.2) chimerism in all WBC lineages examined, including T cells, B cells, granulocytes, and monocytes, compared with mice that received BMCs from nonirradiated mice (Figure 2). This difference remained significant for the duration of follow-up (20 weeks). However, there were no significant differences in the groups that received BMCs from irradiated bones (sternum and vertebrae) or nonirradiated bones (legs) of MI-treated mice at any time point. Mice were killed 27 weeks after adoptive transfer and chimerism was examined in the hematopoietic tissues. The levels of B6 cells in BM, spleen, and thymus were significantly higher (P < .05) in mice that received BMCs from MI-treated mice than in the tissues of mice that had received marrow from nonirradiated mice (Table 2). However, there was no significant difference in the chimerism level between mice that received BMCs from an irradiated area or a nonirradiated area in peripheral blood, BM, spleen, and thymus (Figure 2; Table 2). These data demonstrate clearly that MI results in a systemic increase in the number of donor-derived PHSCs in BM transplant recipients.
Repopulation by adoptively transferred BMCs in lethally irradiated recipients. BMCs were harvested from legs of long-term chimeras (prepared with [ ;n = 5] or without [♦;n = 7] MI) or from sternum and vertebrae of chimeras prepared with MI (▴; n = 5) 40 weeks after BMT. BMCs (1 × 107) were injected to lethally irradiated (10 Gy) CD45.1 mice. Mean percentages of donor CD4 cells, CD8 cells, B cells, monocytes, and granulocytes in WBCs are shown at the indicated time points. Statistically significant differences were seen in chimerism levels in every lineage between recipients of marrow from the no irradiation group versus the 7-Gy MI group (except CD4 cell lineage at 2 weeks). Error bars indicate SD.
;n = 5] or without [♦;n = 7] MI) or from sternum and vertebrae of chimeras prepared with MI (▴; n = 5) 40 weeks after BMT. BMCs (1 × 107) were injected to lethally irradiated (10 Gy) CD45.1 mice. Mean percentages of donor CD4 cells, CD8 cells, B cells, monocytes, and granulocytes in WBCs are shown at the indicated time points. Statistically significant differences were seen in chimerism levels in every lineage between recipients of marrow from the no irradiation group versus the 7-Gy MI group (except CD4 cell lineage at 2 weeks). Error bars indicate SD.
Repopulation by adoptively transferred BMCs in lethally irradiated recipients. BMCs were harvested from legs of long-term chimeras (prepared with [ ;n = 5] or without [♦;n = 7] MI) or from sternum and vertebrae of chimeras prepared with MI (▴; n = 5) 40 weeks after BMT. BMCs (1 × 107) were injected to lethally irradiated (10 Gy) CD45.1 mice. Mean percentages of donor CD4 cells, CD8 cells, B cells, monocytes, and granulocytes in WBCs are shown at the indicated time points. Statistically significant differences were seen in chimerism levels in every lineage between recipients of marrow from the no irradiation group versus the 7-Gy MI group (except CD4 cell lineage at 2 weeks). Error bars indicate SD.
;n = 5] or without [♦;n = 7] MI) or from sternum and vertebrae of chimeras prepared with MI (▴; n = 5) 40 weeks after BMT. BMCs (1 × 107) were injected to lethally irradiated (10 Gy) CD45.1 mice. Mean percentages of donor CD4 cells, CD8 cells, B cells, monocytes, and granulocytes in WBCs are shown at the indicated time points. Statistically significant differences were seen in chimerism levels in every lineage between recipients of marrow from the no irradiation group versus the 7-Gy MI group (except CD4 cell lineage at 2 weeks). Error bars indicate SD.
Engraftment of PHSCs from MI-treated or nonirradiated mice
. | . | Spleen . | . | . | . | ||
|---|---|---|---|---|---|---|---|
| Source . | Bone marrow . | CD4 cells . | CD8 cells . | B cells . | Thymus . | ||
| From legs of non-MI-treated mice, n = 7 | 17.27 ± 12.27 | 9.33 ± 5.97 | 5.00 ± 4.86 | 15.41 ± 8.65 | 7.80 ± 5.56 | ||
| From legs of MI-treated mice, n = 5* | 45.01 ± 11.31 | 34.02 ± 8.30 | 29.71 ± 13.43 | 44.51 ± 16.16 | 35.56 ± 15.77 | ||
| From sternum and vertebrae of MI-treated mice, n = 5* | 49.38 ± 10.64 | 42.24 ± 7.60 | 42.32 ± 8.53 | 54.20 ± 9.90 | 46.42 ± 24.2 | ||
. | . | Spleen . | . | . | . | ||
|---|---|---|---|---|---|---|---|
| Source . | Bone marrow . | CD4 cells . | CD8 cells . | B cells . | Thymus . | ||
| From legs of non-MI-treated mice, n = 7 | 17.27 ± 12.27 | 9.33 ± 5.97 | 5.00 ± 4.86 | 15.41 ± 8.65 | 7.80 ± 5.56 | ||
| From legs of MI-treated mice, n = 5* | 45.01 ± 11.31 | 34.02 ± 8.30 | 29.71 ± 13.43 | 44.51 ± 16.16 | 35.56 ± 15.77 | ||
| From sternum and vertebrae of MI-treated mice, n = 5* | 49.38 ± 10.64 | 42.24 ± 7.60 | 42.32 ± 8.53 | 54.20 ± 9.90 | 46.42 ± 24.2 | ||
BMCs were harvested from long-term chimera 40 weeks after BMT. BMCs (1 × 107) were injected to lethally irradiated (10 Gy) CD45.1 mice. Mice were killed 27 weeks after adoptive transfer. Values are presented as mean % donor cells ± standard deviation.
Significantly higher than non-MI-treated animals (P < .05).
Local irradiation increases donor chimerism most markedly in the irradiated bones early (4 weeks) following BMT, but chimerism is also increased in distant sites
We next examined whether a local effect of irradiation on donor chimerism might be more readily apparent early following BMT, before engrafted PHSCs had equilibrated throughout the recipient, and repeated the above experiment so that animals could be studied at an earlier time point, 4 weeks following BMT. CD45.1 mice treated with or without MI received a high dose of B6 BMC (2 × 108) and were killed 4 weeks after BMT for examination of marrow chimerism. The level of chimerism in the irradiated area (sternum) was significantly higher than that in the nonirradiated area (legs) at this time point (Table 3). However, the difference in chimerism between the irradiated and nonirradiated areas was relatively small (mean 55.91% vs 44.42% donor), and chimerism was markedly higher at both sites in irradiated mice than in nonirradiated mice (Table 3). Thus, even as early as 4 weeks after BMT, local irradiation greatly increased donor chimerism at sites distant from the irradiated area. The difference between chimerism levels at irradiated and nonirradiated sites was very small by 40 weeks after BMT and remained markedly higher in irradiated than in nonirradiated animals (Table 1).
Chimerism in irradiated or nonirradiated bones 4 weeks after BMT
. | Mean ± SD % donor cells . | P . |
|---|---|---|
| No irradiation, n = 4 | NS | |
| Sternum and upper limbs | 18.47 ± 7.39 | |
| Legs | 22.40 ± 8.05 | |
| Leg irradiation, n = 4 | < 0.05 | |
| Sternum and upper limbs | 37.07 ± 4.30 | |
| Legs | 58.97 ± 9.01 | |
| MI, n = 4 | < 0.05 | |
| Sternum, irradiated bones | 55.91 ± 4.21 | |
| Legs, nonirradiated bones | 44.42 ± 5.64 |
. | Mean ± SD % donor cells . | P . |
|---|---|---|
| No irradiation, n = 4 | NS | |
| Sternum and upper limbs | 18.47 ± 7.39 | |
| Legs | 22.40 ± 8.05 | |
| Leg irradiation, n = 4 | < 0.05 | |
| Sternum and upper limbs | 37.07 ± 4.30 | |
| Legs | 58.97 ± 9.01 | |
| MI, n = 4 | < 0.05 | |
| Sternum, irradiated bones | 55.91 ± 4.21 | |
| Legs, nonirradiated bones | 44.42 ± 5.64 |
P values were as follows: sternum and upper limbs, no irradiation versus leg irradiation, P < .01; sternum and upper limbs, no irradiation versus MI, P < .0005; legs, no irradiation versus leg irradiation, P < .01; legs, no irradiation versus MI, P < .01. P values presented in the table were calculated for the subgroups in their respective categories.
The early increase in donor chimerism in irradiated compared with nonirradiated bones does not reflect increased PHSC chimerism
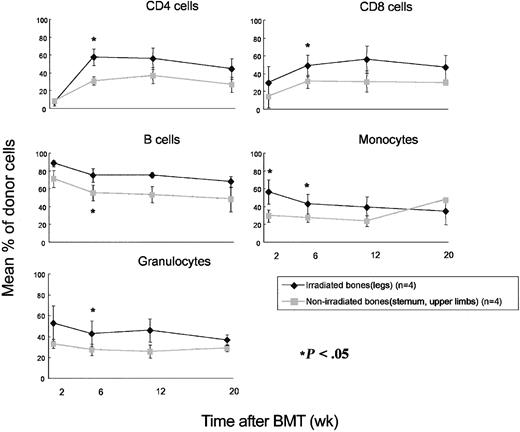
To specifically examine PHSC chimerism in irradiated and nonirradiated areas at an early time point after BMT, BMCs were collected from irradiated or nonirradiated bones at 4 weeks after BMT, analyzed for chimerism, and adoptively transferred to secondary lethally irradiated CD45.1 mice. For this experiment, local irradiation was delivered to the legs instead of the mediastinum. Chimerism results confirmed that the levels of donor bone marrow chimerism were significantly higher in the locally irradiated (legs) bones than in the nonirradiated bones (sternum, upper limbs) of the same animals (Table 3). This difference (mean 58.97% vs 37.07% chimerism in irradiated and nonirradiated areas, respectively) was even greater than that observed in the MI experiment described above (see Table 1). Again, bone marrow chimerism in both sites was significantly higher in irradiated mice than in nonirradiated mice at this time point (Table 3). Adoptive transfer of BMCs (0.9-1 × 107 cells) from irradiated legs to lethally irradiated (10 Gy) CD45.1 mice led initially to significantly higher levels of B6 cells in all lineages compared with those in mice that received the same number of BMCs from nonirradiated bones of the same donors (Figure 3). However, these differences diminished over time in all lineages and were no longer significant by 20 weeks following transfer. These results suggest that the difference in chimerism at irradiated versus nonirradiated sites at 4 weeks largely reflected a difference in the chimerism of short-term progenitor cells, rather than a difference in PHSC chimerism.
Repopulation in lethally irradiated secondary recipients following transfer of BMCs from irradiated and nonirradiated bones of the same chimeras 4 weeks after BMT. BMCs (9-10 × 106) were injected to lethally irradiated (10 Gy) CD45.1 mice. Mean percentages of donor CD4 cells, CD8 cells, B cells, monocytes, and granulocytes are shown at the indicated time points. *P < .05 between the 2 groups. Error bars indicate SD.
Repopulation in lethally irradiated secondary recipients following transfer of BMCs from irradiated and nonirradiated bones of the same chimeras 4 weeks after BMT. BMCs (9-10 × 106) were injected to lethally irradiated (10 Gy) CD45.1 mice. Mean percentages of donor CD4 cells, CD8 cells, B cells, monocytes, and granulocytes are shown at the indicated time points. *P < .05 between the 2 groups. Error bars indicate SD.
The thymus does not play a role in increasing donor PHSC chimerism in recipients of MI
Our previous studies demonstrated that irradiation to the mediastinal area enhanced the long-term level of donor thymopoiesis, which is derived from a different progenitor pool than hematopoiesis of other lineages.16 To rule out a specific role for the thymus in the enhancement of PHSC chimerism seen in recipients of MI, we compared chimerism in euthymic (EuT; n = 4) and thymectomized (ATX; n = 5) CD45.1 mice that received 7 Gy MI prior to B6 BMT. Four weeks following BMT, mice were killed and tissue chimerism was investigated. The level of donor CD4 and CD8 cells in the spleen was significantly higher in EuT mice compared with ATX mice (data not shown), suggesting that donor thymopoiesis had occurred by this time in the EuT group. However, there was no difference in the level of BM chimerism in legs of ATX and EuT mice (ATX: 40.49 ± 11.46, n = 5; EuT: 44.42 ± 5.64, n = 4). Irradiated sites (sternum) showed similar levels of donor chimerism in both groups (ATX: 51.24 ± 12.42, n = 5; EuT: 55.9 ± 4.21, n = 4). Thus, the increased chimerism at both local and distant sites in recipients of MI is not due to an effect of the irradiated thymus. Additional groups of EuT and ATX mice received BMT without irradiation, and these groups showed similar levels of chimerism, which again were significantly lower than those in MI recipients.
Discussion
In murine recipients of CD45 congenic bone marrow cell numbers that are clinically relevant, a dose of 1.5 to 3 Gy of TBI was shown to be required to reliably permit PHSC engraftment.2 However, long-term chimerism has been achieved by administering a high dose of male bone marrow cells (2 × 108) to syngeneic female BALB/c recipients.19 Other long-term studies in a CD45 congenic strain combination showed that this long-term chimerism included all myeloid and lymphoid lineages, indicating that PHSC engraftment had occurred.16 It was observed in these studies that T-cell and thymocyte chimerism was always lower than that of other lineages and other hematopoietic tissues, suggesting that nonidentical PHSC pools reconstituted the T-cell versus other lineages at equilibrium.16 This disparity between thymocyte chimerism and other lineages was overcome by 7 Gy of thymic irradiation, suggesting that elimination of an intrathymic progenitor pool might have enhanced donor-derived thymopoiesis.16 In the course of these studies, we also observed that the level of donor repopulation of all hematopoietic lineages increased in all hematopoietic tissues when 7 Gy of thymic irradiation was given, suggesting that space for PHSC engraftment was produced by local irradiation to the mediastinal area. Those results are confirmed here (Figure 1).
We have now attempted to determine whether this space-creating effect of local irradiation is due to the opening up of physical niches for donor stem cell engraftment in the irradiated site or instead reflects a systemic effect of irradiation, perhaps via the secretion of cytokines that promote proliferation of hematopoietic progenitors and PHSCs. Both early (4 weeks) and late (40 weeks) after BMT, the level of donor chimerism and number of PHSCs, as demonstrated in adoptive transfer experiments, was higher in nonirradiated bones of mice that received local irradiation at the time of original transplantation than in the bones of animals that had received no irradiation.
Results in short-term (4 weeks) chimeras revealed more marked differences in the level of donor chimerism in bones of the irradiated field (legs or mediastinum) than in nonirradiated bones (sternum and upper limbs, or legs, respectively) compared with the long-term studies. Nevertheless, adoptive transfer of marrow from irradiated sites 4 weeks after BMT led to increased chimerism in secondary recipients compared with that in recipients of marrow from nonirradiated sites only in the first few months following BMT. This increase was seen in both myeloid and lymphoid lineages. At later time points, no difference could be seen in the level of donor chimerism in any lineage in the adoptive recipients of marrow from irradiated versus nonirradiated areas. Thus, by 4 weeks after BMT, the only local effect of local irradiation that could be seen was an increase in the representation of donorderived short-term repopulating cells and not PHSCs. Even by this early time point, the major effect of local irradiation on donor PHSCs was a systemic increase in their number, to similar degrees at irradiated and nonirradiated sites.
Our data are consistent with a model in which local irradiation leads to the release of high levels of cytokines that promote the proliferation of PHSCs. Local destruction of resident PHSCs in irradiated sites may give donor PHSCs an initial competitive advantage in those sites. While irradiation has been shown to impair the initial (ie, within 24 hours) homing or survival of purified PHSCs in the irradiated marrow,14 this does not preclude the possibility that short-term progenitors engraft and/or proliferate preferentially in such sites and that the PHSCs that succeed in engrafting there may undergo extensive proliferation (presumably after the initial 24 hours when lower numbers of PHSCs were detected in marrow of irradiated compared with nonirradiated mice in the study of Plett et al14 ). Local irradiation might destroy the endothelial barrier to marrow migration at irradiated sites, as has been reported,20,21 and/or express proliferative cytokines and activating adhesion molecules at increased concentrations. While systemic cytokine release following local irradiation could theoretically lead to enhanced proliferation of donor stem cells that home initially to nonirradiated sites, it is difficult to envision why donor cells would preferentially (over host cells) respond to such cytokines in nonirradiated sites. If PHSC proliferation is increased distant to the site of irradiation, only preferential expansion of the donor could explain the markedly increased levels of donor chimerism in sites distant from the irradiation in mice that received local irradiation compared with nonirradiated mice. Thus, we favor a model whereby the small number of PHSCs (and the other progenitors) that succeed in engrafting in irradiated sites undergo extensive local proliferation and then later enter the circulation to increase donor engraftment in nonirradiated sites. Thus, the relative proportion in the entire animal of donor compared with host PHSCs is increased due to their local proliferation in irradiated sites, and their systemic redistribution and ultimate equilibration, apparently by 4 weeks, results in higher relative levels of donor PHSCs in other sites. This scenario is consistent with the hypothesis that stem cell engraftment reflects “competition” between stem cells for a limited number of niches.22
Recently, Zhong et al13 reported that bone marrow implanted directly into the femur of untreated mice initially redistributed in a similar manner to that in mice receiving lethal TBI but that PHSC proliferation was increased in TBI-treated mice. This proliferation was most marked between 3 and 7 days after transplantation, then ceased by 4 weeks. The increased proliferation was associated with an increase in the number of donor-derived cells in marrow remote from the site of its initial injection.13 However, because irradiation was given to the entire body, that study did not distinguish whether or not irradiation increases donor stem cell proliferation only in irradiated sites or systemically. As is discussed in the preceding paragraph, the preferential increase in donor-derived PHSCs induced by local irradiation in our study suggests that proliferation occurs largely in the irradiated site and that these expanded donor PHSCs redistribute and equilibrate throughout the animal within 4 weeks.
PHSCs circulate even in unmanipulated animals,23 and it is possible that this circulation may be augmented by stimuli induced by local irradiation. In this case, an alternative explanation for our result is that recipient PHSCs might leave their niches in nonirradiated sites in response to local irradiation and thereby open up niches in nonirradiated sites for injected donor PHSCs. We cannot distinguish between these possibilities at the present time.
Although CD45.2 mice have been shown to be capable of mounting an immune response to congenic CD45.1 donors (transplantation in the opposite direction to the one used here),24 the immune resistance in this combination was readily overcome by increasing the donor marrow dose. Since our studies involved much higher doses than those reported,24 and studies in the Ly5 congenic combination in the same direction used here showed no evidence of immune recognition,15 we do not believe that immune responses are likely to contribute to the phenomena reported here.
The ability to increase donor-derived PHSC engraftment with a dose of local irradiation that does not cause significant cytopenias7 suggests an approach to achieving lasting mixed chimerism and donor-specific tolerance with minimal myelosuppression. The use of mediastinal (thymic) irradiation has the advantage of overcoming intrathymic alloresistance,3,25 creating space in the thymus that enhances early donor thymopoiesis16 and increasing the proportion of donor PHSCs. It has been shown to be an important factor allowing the induction of mixed chimerism and tolerance in a nonhuman primate model26 and may ultimately prove to have similar advantages in the application of the nonmyeloablative mixed chimerism approach for the induction of allograft tolerance in humans.
Prepublished online as Blood First Edition Paper, October 30, 2003; DOI 10.1182/blood-2003-09-3249.
Supported by National Institutes of Health RO1 grant no. HL49915.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs Christene Huang and Yong-Guang Yang for helpful review of the manuscript, Mr Orlando Moreno for expert animal care and technical assistance, and Ms Robin Laber for expert assistance with the manuscript.

![Figure 2. Repopulation by adoptively transferred BMCs in lethally irradiated recipients. BMCs were harvested from legs of long-term chimeras (prepared with [;n = 5] or without [♦;n = 7] MI) or from sternum and vertebrae of chimeras prepared with MI (▴; n = 5) 40 weeks after BMT. BMCs (1 × 107) were injected to lethally irradiated (10 Gy) CD45.1 mice. Mean percentages of donor CD4 cells, CD8 cells, B cells, monocytes, and granulocytes in WBCs are shown at the indicated time points. Statistically significant differences were seen in chimerism levels in every lineage between recipients of marrow from the no irradiation group versus the 7-Gy MI group (except CD4 cell lineage at 2 weeks). Error bars indicate SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/5/10.1182_blood-2003-09-3249/6/m_zh80050457750002.jpeg?Expires=1769137551&Signature=geLhwKin1Qil02ZpwhUHvspZuQKyGjcNaKUT~jLPtSHAWB-nLeSD5TWTllqBwmTSts5eiBkeRqkk~1t4R5fT30RsCGYma2qdNiD5xqxkZXb8Byps2oSGRpIU3JZ8e1HKW8yT5JLgvLQf6aezoZF9WsCcSAqAtFme5FOlx5E9rriz-OPIyAKKuvCxvLi5K~pcgxDePCvf5-6szVys~NvC~NEa3wGI1XqsPhrVkRthoaJkFZvaXlc1x7Chhbgq7jhkVRUPFDkUneChfwr7O9SAr7kMRq0wQ~Y2AYbg0-9quPQvMemZ~Q50JqKQ6-15yuzFn8qR2r2JhwbTTsg1nFclAg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal