Abstract
Although thalidomide (Thal) does not directly induce T-cell activation, it increases proliferation of T cells following CD3 activation. In this study, we examined the immunomodulatory effects of a more potent analog of Thal, immunomodulatory drug (IMiD), on T cells. Although IMiD3 does not directly stimulate proliferation of normal donor CD3+ T cells, it significantly costimulates proliferation of CD3+ T cells induced by CD3 ligation (stimulation index [SI], 2.4), immature dendritic cells (DCs; SI, 2.1), and mature DCs (SI, 2.6). T-cell proliferation triggered by DCs was abrogated by cytotoxic T lymphocyte antigen 4–immunoglobulin (CTLA-4–Ig), and IMiD3 partially overcomes this inhibitory effect. IMiD3 also overcomes the inhibitory effects of CTLA-4–Ig on Epstein-Barr virus (EBV) and influenza (Flu)–specific CD4 and CD8 T-cell responses, as measured by cytokine capture and enzyme-linked immunosorbent spot (ELISPOT) assay. IMiD3 did not induce up-regulation of CD28 expression on T cells, or of CD80-CD86 expression on dendritic cells. Importantly, IMiD3 triggers tyrosine phosphorylation of CD28 on T cells, followed by activation of nuclear factor κB (NF-κB), a known downstream target of CD28 signaling. These results therefore define the costimulatory mechanism whereby IMiD3 induces T-cell activation and provide the cellular and molecular basis for use of IMiD3 as an adjuvant in immunotherapeutic treatment strategies for multiple myeloma.
Introduction
Thalidomide (Thal) has been used to treat immune-mediated diseases such as erythema nodosum leprosum,1 chronic graft-versus-host disease,2 inflammatory bowel diseases,3 Behçet disease,4 and others.5 Although initial studies of the immunomodulatory effects of Thal suggested its principal effect to be tumor necrosis factor α (TNF-α) inhibition,6 more recent in vitro studies have shown that it increases natural killer (NK) cell cytotoxicity7 ; augments T-cell proliferation as well as increases interleukin-2 (IL-2) and interferon-γ (IFN-γ) production following CD3 ligation8 ; and modulates IL-12 production.9,10
The immunomodulatory drugs (IMiDs) are over 50 000 times more potent analogs of Thal,7 and IMiD3 has already achieved paraprotein responses in 71% of patients with relapsed and refractory relapsed multiple myeloma (MM).11 IMiDs directly induce apoptosis of MM cells via caspase-8 activation, block MM cell–bone marrow stromal cell interactions, inhibit secretion of cytokines in the bone marrow that are required for MM cell growth and survival,12,13 and inhibit angiogenesis.14 Importantly, IMiDs also increase NK cell cytotoxicity against MM cells.7 As for Thal, IMiDs stimulate T-cell proliferation as well as increase IL-2 and IFN-γ production following CD3 ligation and modulate IL-12 production.15 IMiD1 has also been shown to induce T-cell responses against colon cancer and melanoma in mouse models.16 To date, however, the mechanism whereby these agents trigger T-cell proliferation remains undefined.
In this study, we further characterized the immunomodulatory effects of IMiD3 on T cells. Our results confirm the costimulatory effect of IMiD3 on proliferation and cytokine secretion of T cells induced by CD3 ligation or dendritic cells (DCs). Importantly, we show for the first time that IMiD3 activates CD28 and overcomes cytotoxic T lymphocyte antigen 4–immunoglobulin (CTLA-4–Ig) blockade, thereby confirming that drug-induced costimulation is mediated via B7-CD28 pathway.
Study design
Cells
Peripheral blood was obtained from healthy volunteers by phlebotomy following written informed consent according to our institutional review board guidelines. Peripheral blood mononuclear cells (PBMCs) were isolated as previously described.7 CD3+ T cells were negatively selected as per the manufacturer's direction using a cocktail of antihuman monoclonal antibody (CD11b, CD16, CD19, CD36, and CD56)–labeled magnetic microbeads (Miltenyi Biotech, Auburn, CA). Immature dendritic cells (DCs) expressing CD86 were generated from adherent mononuclear cells using granulocyte-macrophage colony-stimulating factor (GM-CSF) at 1000 U/mL, and IL-4 at 10 ng/mL; mature DCs expressing CD80, CD83, and CD86 were produced by addition of TNF-α at 20 ng/mL on day 5, as described earlier.7
Drug preparation
IMiD3 (Celgene, Warren, NJ) was dissolved in dimethyl sulfoxide (DMSO) and stored at –20°C. IMiD3 (10 μM) was used in all experiments, with DMSO as a control.
Proliferation assay
CD3+ T cells from healthy donors incubated in the presence or absence of IMiD3 were seeded in triplicate in 96-well flat bottom plates at a concentration of 1 × 105 cells/well in 200 μL volume. Mature or immature DCs, 200 ng/mL antihuman CD3 (Research Diagnostics, Flanders, NJ), and 10 μg/mL CTLA-4–Ig (kindly provided by Dr John Gribben, Dana Farber Cancer Institute [DFCI]) were added as indicated. Cells were pulsed with 0.5 μCi/well (0.0185 MBq/well) [3H]thymidine ([3H]TdR; New England Nuclear, Boston, MA) for the last 12 hours of 72-hour cultures. Plates were harvested using an automatic cell harvester (Tomtec, Hamden, CT), and thymidine incorporation was measured using a liquid scintillation counter (Wallac, Turku, Finland). Colorimetric assays were also performed using 3-(4,5-dimethyl-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS; Promega, Madison, WI) to evaluate cell growth, as previously described.12
IFN-γ and IL-4 ELISPOT analysis
For IFN-γ and IL-4 enzyme-linked immunosorbent spot (ELISPOT) analysis, fresh unstimulated PBMCs from HLA-A2 donors were seeded at 1 × 105 cells/well into MultiScreen Filter Plates (Millipore, Bedford, MA) precoated with 10 μg/mL monoclonal antibody (mAb; Mabtech, Nacka, Sweden). Cells were cultured in the presence or absence of Epstein-Barr virus (EBV),17 influenza,18 and human T-cell leukemia virus type I (HTLV-1)19 –specific peptides (10 μg/mL) (New England Peptide, Fitchburg, MA) and then incubated in 5% CO2 at 37°C for 40 hours, together with IMiD3, CTLA-4–Ig, or anti-HLA class I mAb (1 μg/mL) (eBioscience, San Diego, CA). The purity of this commercially available peptide for EBV, influenza, and HTLV-1 was 80%, 83.5%, and 94.7%, respectively. DMSO and phytohemagglutinin (5 μg/mL) were used as negative and positive controls, respectively. After extensive washes, and incubation with biotinylated mAb (Mabtech) followed by incubation with streptavidin-alkaline phosphatase (Mabtech), 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium (Moss, Pasadena, MD) was added, until development of dark spots. Spots were analyzed using an automated ELISPOT reader (Carl Zeiss, Thornwood, NY).
IFN-γ secretion assay
For further characterization of IFN-γ secretion by T cells induced by IMiD3, PBMCs were incubated with IMiD3, in the presence or absence of antihuman CD3 (200 ng/mL). DMSO and staphylococcal enterotoxin B (SEB; Sigma, St Louis, MO) at 1 μg/mL were used as negative and positive controls, respectively. To detect IFN-γ–secreting cells, cells were labeled with IFN-γ Catch Reagent (Miltenyi Biotech) for 5 minutes on ice, and then incubated for 45 minutes at 37°C. IFN-γ was detected using secondary phycoerythrin (PE)–conjugated IFN-γ detection Ab. Cells were counterstained with mAb against CD8–fluorescein isothiocyanate (FITC) or CD4-FITC, and then analyzed using flow cytometry.
Immunoprecipitation/Western blotting
CD3+ T cells were cultured with IMiD3. Media alone and media containing 200 ng/mL antihuman CD28 Ab (Research Diagnostics) were used as negative and positive controls, respectively. Cells were then collected and lysed as described.12 Lysates were incubated with antihuman CD28 Ab (clone 9.3) for 4 hours at 4°C, and then immunoprecipitated for 2 hours with protein A/G Plus Agarose (Santa Cruz Biotechnology, Santa Cruz, CA). Immune complexes were resolved on 8% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and analyzed by immunoblotting with antiphosphotyrosine horseradish peroxidase (HRP)–conjugated Ab (Santa Cruz Biotechnology).
Electrophoretic mobility shift assay
CD3+ T cells were cultured with IMiD3. Media alone and media containing 5 ng/mL TNF-α (R&D Systems, Minneapolis, MN) were used as negative and positive controls, respectively. Cells were collected and nuclear extracts were prepared for electrophoretic mobility shift analysis (EMSA) using the Nuclear Extract Kit (Active Motif, Carlsbad, CA), as described earlier.20
Statistical methods
Student t test was used for statistical analysis of the results.
Results and discussion
IMiD3 costimulates T cells and overcomes CTLA-4–Ig blockade
We first evaluated the effect of IMiD3 on T-cell proliferation and cytokine production. As can be seen in Figure 1A, IMiD3 alone does not induce cell cycle changes in T cells. Following CD3 ligation on T cells, however, IMiD3 enhances G1 to S transition compared with CD3 ligation alone (Figure 1A). This effect was observed at 48 hours, and was more pronounced at 72 hours (data not shown). The mean increment in S-phase cells induced by IMiD3 and anti-CD3 is 47.8 ± 6.7% in 3 separate experiments. Similarly, IMiD3 augments IFN-γ secretion by CD4 and CD8 T cells induced by anti-CD3, as measured by cytokine capture assay, but has no effect by itself (Figure 1B).
IMiD3 costimulates T cells and overcomes CTLA-4–Ig blockade.(A) CD3+ T cells were negatively selected from healthy donors and incubated with IMiD3 alone (10 μM), anti-CD3 alone (200 ng/mL), IMiD3 and anti-CD3, or DMSO alone as a negative control. Cells were washed in phosphate-buffered saline (PBS), fixed and permeabilized in 70% ethanol, treated with 10 μg/mL DNase-free Rnase, and stained with propidium iodide. Cell cycle profile was determined by flow cytometry using Coulter EPICS XL-MCL (Coulter, Birmingham, United Kingdom). Panel A presents the proportion of cells in different stages of the cell cycle from 2 of 3 healthy donors. (B) CD3+ T cells were stimulated with IMiD3 alone (10 μM), anti-CD3 alone (200 ng/mL), IMiD3 and anti-CD3, or DMSO as a control. IFN-γ was detected using a cytokine secretion assay, and cells were counterstained for CD4 or CD8 FITC-conjugated antibody. The numbers indicate the proportion of cells staining positive for IFN-γ. (C) CD3+ T cells were incubated for 72 hours in the presence or absence of IMiD3 (10 μM), anti-CD3 (200 ng/mL), immature or mature dendritic cells, and CTLA-4–Ig (10 μg/mL), as indicated. Cells were pulsed with [3H]TdR for the last 12 hours, harvested, and [3H]TdR uptake was measured using a liquid scintillation counter. Results represent the mean ± standard deviation of the mean cpm from a triplicate assay. These results are representative of 3 independent experiments (*P < .05). (D) CD3+ T cells from HLA-A2 donors were incubated in ELISPOT plates with PBMCs for 40 hours in the presence or absence of IMiD3 (10 μM) or CTLA-4–Ig (10 μg/mL), as indicated. Cells were stimulated with EBV-, influenza-, or HTLV-1–specific peptides (10 μg/mL). Plates were washed and stained for IFN-γ. ▤ indicates EBV; ▧, influenza; and ▨, HTLV-1. Results represent mean ± standard deviation of the mean IFN-γ spot forming cells (SFC) from 3 independent experiments (*P < .05).
IMiD3 costimulates T cells and overcomes CTLA-4–Ig blockade.(A) CD3+ T cells were negatively selected from healthy donors and incubated with IMiD3 alone (10 μM), anti-CD3 alone (200 ng/mL), IMiD3 and anti-CD3, or DMSO alone as a negative control. Cells were washed in phosphate-buffered saline (PBS), fixed and permeabilized in 70% ethanol, treated with 10 μg/mL DNase-free Rnase, and stained with propidium iodide. Cell cycle profile was determined by flow cytometry using Coulter EPICS XL-MCL (Coulter, Birmingham, United Kingdom). Panel A presents the proportion of cells in different stages of the cell cycle from 2 of 3 healthy donors. (B) CD3+ T cells were stimulated with IMiD3 alone (10 μM), anti-CD3 alone (200 ng/mL), IMiD3 and anti-CD3, or DMSO as a control. IFN-γ was detected using a cytokine secretion assay, and cells were counterstained for CD4 or CD8 FITC-conjugated antibody. The numbers indicate the proportion of cells staining positive for IFN-γ. (C) CD3+ T cells were incubated for 72 hours in the presence or absence of IMiD3 (10 μM), anti-CD3 (200 ng/mL), immature or mature dendritic cells, and CTLA-4–Ig (10 μg/mL), as indicated. Cells were pulsed with [3H]TdR for the last 12 hours, harvested, and [3H]TdR uptake was measured using a liquid scintillation counter. Results represent the mean ± standard deviation of the mean cpm from a triplicate assay. These results are representative of 3 independent experiments (*P < .05). (D) CD3+ T cells from HLA-A2 donors were incubated in ELISPOT plates with PBMCs for 40 hours in the presence or absence of IMiD3 (10 μM) or CTLA-4–Ig (10 μg/mL), as indicated. Cells were stimulated with EBV-, influenza-, or HTLV-1–specific peptides (10 μg/mL). Plates were washed and stained for IFN-γ. ▤ indicates EBV; ▧, influenza; and ▨, HTLV-1. Results represent mean ± standard deviation of the mean IFN-γ spot forming cells (SFC) from 3 independent experiments (*P < .05).
As reported previously7,15 and seen in Figure 1C, IMiD3 does not induce T-cell proliferation. However, IMiD3 induces significant CD3+ T-cell proliferation in the presence of antihuman CD3 (stimulation index [SI], 2.4), immature DCs (SI, 2.1), or mature DCs (SI, 2.6) (Figure 1C). We next evaluated the mechanism whereby IMiD3 mediates this costimulatory effect. CTLA-4–Ig, which blocks costimulation through the B7-CD28 pathway, inhibits T-cell proliferation induced by immature and mature DCs (Figure 1C). Importantly, IMiD3 partially overcomes this inhibitory effect of CTLA-4–Ig. These results were also confirmed using MTS colorimetric assays (data not shown).
We next evaluated whether IMiD3 augments viral antigen-specific T-cell responses, and similarly determined whether CTLA-4–Ig or anti–HLA class I antibody could block these effects. As seen in Figure 1D, IFN-γ production by T cells is induced by EBV and influenza, but not by HTLV-1–related peptides. This IFN-γ secretion was inhibited by HLA class I blocking Ab W6/32 (data not shown) and partially inhibited by CTLA-4–Ig (Figure 1D). Interestingly, IMiD3 increases T-cell response to both EBV and influenza, but not to HTLV-1 peptides. As with T-cell proliferation, IMiD3 partially overcomes the inhibitory effect of CTLA-4–Ig on EBV, and influenza triggered IFN-γ secretion (Figure 1D). In contrast, IL-4 secretion was not induced by EBV and influenza peptides or by IMiD3 (data not shown).
Effect of IMiD3 on B7-CD28 costimulatory molecules
To delineate the mechanism whereby IMiD3 overcomes B7-CD28 blockade and thereby provides costimulation, we first evaluated the effect of drug on cell surface expression of costimulatory molecules on T cells and antigen-presenting cells (APCs). IMiD3 does not up-regulate CD28 expression on T cells or modulate cell surface expression of CD80 or CD86 on immature or mature DCs (data not shown).
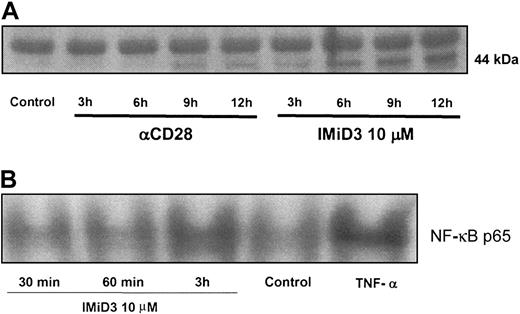
Since CD28 tyrosine phosphorylation represents one of the first steps of T-cell costimulation via this pathway, we next evaluated whether IMiD3 induces CD28 tyrosine phosphorylation on normal donor T cells. As seen in Figure 2A, IMiD3 induces tyrosine phosphorylation of CD28 as early as 3 hours, which is sustained up to 12 hours. As nuclear factor κB (NF-κB) is one of the downstream signals of CD28 activation,21 we therefore next determined whether IMiD3 induces NF-κB activation in T cells. CD3+ T cells were incubated with IMiD3 and analyzed for activation of NF-κB. As can be seen in Figure 2B, IMiD3 induces NF-κB activation at 3 hours; TNF-α serves as a positive control. CD3 ligation leads to activation of several signal transduction pathways, including activation of NFκB. In this study, we have not observed any difference in NFκB activation, with or without IMiD3, following CD3 ligation (data not shown). Additionally, it has been demonstrated that CD3 ligation provides a distinct message to the T cells separate from CD28 phosphorylation.
IMiD3 induces CD28 phosphorylation and NF-κB activation. CD3 T cells negatively selected from healthy donors were incubated for different time points in the presence or absence of IMiD3 (10 μM). (A) Cell lysates were immunoprecipitated with antihuman CD28 Ab. Immune complexes were resolved on SDS-PAGE, transferred on polyvinylidene fluoride (PVDF) membrane, and analyzed by blotting with antiphosphotyrosine Ab showing a 44-kDa band representing tyrosine phosphorylation. DMSO and anti-CD28 Ab were used as negative and positive controls, respectively. (B) Nuclear extracts were collected and prepared for EMSA as described previously.12 DMSO and TNF-α (5 ng/mL) were used as negative and positive controls, respectively. Densitometric measurement with data normalized to TNF-α as 100% showed 62.5%, 59%, and 90% activation at 30 minutes, 60 minutes, and 3 hours, respectively, following IMiD3 treatment with 64% activation for negative control.
IMiD3 induces CD28 phosphorylation and NF-κB activation. CD3 T cells negatively selected from healthy donors were incubated for different time points in the presence or absence of IMiD3 (10 μM). (A) Cell lysates were immunoprecipitated with antihuman CD28 Ab. Immune complexes were resolved on SDS-PAGE, transferred on polyvinylidene fluoride (PVDF) membrane, and analyzed by blotting with antiphosphotyrosine Ab showing a 44-kDa band representing tyrosine phosphorylation. DMSO and anti-CD28 Ab were used as negative and positive controls, respectively. (B) Nuclear extracts were collected and prepared for EMSA as described previously.12 DMSO and TNF-α (5 ng/mL) were used as negative and positive controls, respectively. Densitometric measurement with data normalized to TNF-α as 100% showed 62.5%, 59%, and 90% activation at 30 minutes, 60 minutes, and 3 hours, respectively, following IMiD3 treatment with 64% activation for negative control.
Thal, first developed in 1954 as a sedative agent during pregnancy and removed from the market due to related phocomelia, has been revived as an effective therapy for a number of inflammatory and immune disorders.1-5 Although the molecular mechanisms mediating its clinical benefits are unknown, it induces immunomodulation.6-10 Its more potent analog, IMiD3, has already achieved reasonable responses in the setting of relapsed refractory MM, highlighting the importance of defining the mechanisms whereby it can overcome drug resistance.11 In this study, we first confirmed that IMiD3 alone does not induce proliferation or cytokine secretion by T cells. However IMiD3 augments T-cell activation triggered by antihuman CD3 or DCs. Moreover, IMiD3 partially overcomes CTLA-4–Ig blockade of T-cell proliferation and cytokine secretion. These observations suggested that IMiD3 provides costimulatory help via the B7-CD28 pathway. Our data show that IMiD3 does not up-regulate expression of costimulatory molecules on either T cells (CD28) or on APCs (CD80-CD86), but rather directly induces tyrosine phosphorylation of CD28 on T cells.
CD28 is the major costimulatory molecule on T cells interacting with its ligands CD80 and CD86 on APCs, resulting in enhanced T-cell responses both by up-regulating cytokine secretion and inducing cell proliferation.22-24 When CD28 interacts with its ligands, protein tyrosine kinases such as Lck and Fyn phosphorylate CD28, leading to recruitment and activation of downstream targets including phosphatidylinositol 3-kinase, GRB-2-SOS, and NFκB.21 In our study, we show that IMiD3-triggered CD28 phosphorylation on T cells partially overcomes blockade of costimulation by CTLA-4–Ig and induces downstream NF-κB activation. CD28-responsive elements (CD28REs) are present in the promoters of genes encoding for IL-2 and IFN-γ,25 correlating with the effects of IMiD3 on secretion of the cytokines observed in our study.
To our knowledge, this is the first small molecule that provides direct costimulatory help to activate T cells. The cytoplasmic tail of CD28 lacks intrinsic catalytic activity, and the small molecule IMiD3 is also probably devoid of enzymatic activity; therefore, the role of protein tyrosine kinases such as Lck and Fyn in mediating the effects of IMiD3 is under active investigation. Thus further characterization of the mechanisms whereby CD28 tyrosine phosphorylation is triggered by IMiD3 will both delineate signaling and suggest molecular targets to generate effective immune responses. Moreover, these observations provide the preclinical rationale for the use of IMiD3 as an immunomodulatory agent to enhance efficacy of DC-based vaccinations.
Prepublished online as Blood First Edition Paper, September 25, 2003; DOI 10.1182/blood-2003-02-0361.
Supported by the Multiple Myeloma Research Foundation Awards (R.L., T.H., N.M., D.C., N.C.M., K.C.A.); the VA Merit Review Grant, the National Institutes of Health grant PO1-78378, and Leukemia and Lymphoma Society Scholar in Translational Research Award (N.C.M.); and the National Institutes of Health grants (RO1-50947 and PO1-78378) and Doris Duke Distinguished Clinical Research Scientist Award (K.C.A.)
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 1. IMiD3 costimulates T cells and overcomes CTLA-4–Ig blockade.(A) CD3+ T cells were negatively selected from healthy donors and incubated with IMiD3 alone (10 μM), anti-CD3 alone (200 ng/mL), IMiD3 and anti-CD3, or DMSO alone as a negative control. Cells were washed in phosphate-buffered saline (PBS), fixed and permeabilized in 70% ethanol, treated with 10 μg/mL DNase-free Rnase, and stained with propidium iodide. Cell cycle profile was determined by flow cytometry using Coulter EPICS XL-MCL (Coulter, Birmingham, United Kingdom). Panel A presents the proportion of cells in different stages of the cell cycle from 2 of 3 healthy donors. (B) CD3+ T cells were stimulated with IMiD3 alone (10 μM), anti-CD3 alone (200 ng/mL), IMiD3 and anti-CD3, or DMSO as a control. IFN-γ was detected using a cytokine secretion assay, and cells were counterstained for CD4 or CD8 FITC-conjugated antibody. The numbers indicate the proportion of cells staining positive for IFN-γ. (C) CD3+ T cells were incubated for 72 hours in the presence or absence of IMiD3 (10 μM), anti-CD3 (200 ng/mL), immature or mature dendritic cells, and CTLA-4–Ig (10 μg/mL), as indicated. Cells were pulsed with [3H]TdR for the last 12 hours, harvested, and [3H]TdR uptake was measured using a liquid scintillation counter. Results represent the mean ± standard deviation of the mean cpm from a triplicate assay. These results are representative of 3 independent experiments (*P < .05). (D) CD3+ T cells from HLA-A2 donors were incubated in ELISPOT plates with PBMCs for 40 hours in the presence or absence of IMiD3 (10 μM) or CTLA-4–Ig (10 μg/mL), as indicated. Cells were stimulated with EBV-, influenza-, or HTLV-1–specific peptides (10 μg/mL). Plates were washed and stained for IFN-γ. ▤ indicates EBV; ▧, influenza; and ▨, HTLV-1. Results represent mean ± standard deviation of the mean IFN-γ spot forming cells (SFC) from 3 independent experiments (*P < .05).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/5/10.1182_blood-2003-02-0361/6/m_zh80050457390001.jpeg?Expires=1763549386&Signature=3mf~-waC-NKpAcZ7zsrZ412j-1EXp3tChzycV1IXfJkhWe3cuK~xAraC0~Io0rGsPea4y-eS~TgQh3fmZtkAYAB02D2PBp~jl5u7Aw1rwupu4gM10FRt3hDfmGtNbywh~jzVp42yMDC3tZ29DVlV0yuxBEgVEnPYlc2KVzf-KpScL6KhyKliUkxEl5cyesdF7dkfX6C~iJZRlugZ~GhhMDsniLlid8NBNxIglUFjHyxsh4o~ucbTUjA3x7JZF-fvOZiY9dkjI26mRVtDetI6f66ExGu92Kp4KkS7CvnF~dP4A8y1IQZbQNwGxE1XzpP90uQm~5hTFrjlefR9LPhbwQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal