Abstract
Tissue type plasminogen activator (tPA) is a key enzyme in the fibrinolytic cascade. In this paper we report that tPA contains 2 independent epitopes that exert opposite effects on blood vessel tone. Low concentrations of tPA (1 nM) inhibit the phenylephrine (PE)–induced contraction of isolated aorta rings. In contrast, higher concentrations (20 nM) stimulate the contractile effect of PE. The 2 putative vasoactive epitopes of tPA are regulated by the plasminogen activator inhibitor-1 (PAI-1) and by a PAI-1–derived hexapeptide that binds tPA. TNK-tPA, a tPA variant in which the PAI-1 docking site has been mutated, stimulates PE-induced vasoconstriction at all concentrations used. The stimulatory, but not the inhibitory, effect of tPA on the contraction of isolated aorta rings was abolished by anti–low-density lipoprotein receptor–related protein/α2-macroglobulin receptor (LRP) antibodies. Administering tPA or TNK-tPA to rats regulates blood pressure and cerebral vascular resistance in a dose-dependent mode. In other in vivo experiments we found that the vasopressor effect of PE is more pronounced in tPA knockout than in wild-type mice. Our findings draw attention to a novel role of tPA and PAI-1 in the regulation of blood vessel tone that may affect the course of ischemic diseases.
Introduction
Tissue type plasminogen activator (tPA) is one of the 2 principal plasminogen activators in mammals. The activation of plasminogen by tPA or urokinase plasminogen activator (uPA) leads to the generation of the potent proteolytic enzyme plasmin, which cleaves blood clots; tPA–/– mice accumulate fibrin in several organs,1 and their capacity to cleave exogenous clots,1 including pulmonary microemboli, is decreased.2
Several groups have previously shown that in addition to its capacity to bind fibrin,3 tPA binds to cells within the blood vessel wall.4 According to these studies, tPA binds to at least 2 receptors on the cell surface, one of which was identified as plasminogen activator inhibitor-1 (PAI-1).4,5
Another receptor in the vasculature that plays an important role in the metabolism of tPA is the low-density lipoprotein receptor–related protein/α2-macroglobulin receptor (LRP), which mediates the internalization and degradation of tPA.5-7 The binding of tPA to LRP is enhanced by PAI-1, which, in addition to neutralizing the catalytic activity of tPA, increases its internalization and degradation by cells.8
In an experimental stroke model, the infarcted area in tPA–/– mice was smaller than in wild-type (WT) animals,9 indicating that endogenous tPA plays a deleterious role in the development of the damage induced by stroke. In contrast to the decreased sizes of ischemic cerebral infarcts seen in tPA–/– mice, the sizes of infarcts in plasminogen activator inhibitor type-1 knockout mice (PAI-1–/–) were increased.10 However, in urokinase plasminogen activator knockout mice (uPA–/–) no change in infarct size was found10 ; therefore, the combined data obtained from uPA–/– and PAI–/– mice suggest that the negative role of tPA in stroke development may not be related to the activation of plasminogen. Additional results also show that exogenous tPA increases the size of the infarcted area caused by stroke.9,10 More recent studies have shown that tPA induces vasodilatation of isolated cerebral blood vessels, an effect that was considered responsible for the intracranial bleeding that can follow thrombolytic treatment with tPA.11
In spite of the widespread use of tPA as a thrombolytic agent in acute myocardial infarction (MI) and its growing use in acute ischemic stroke, little is known about its ability to regulate vasoactivity. An effect on vasoactivity may play a critical role in the deleterious effect seen during the development of stroke or in the bleeding that may appear after the administration of tPA to patients with acute MI or stroke. Therefore, our aim was to study the effect of tPA on blood vessel contraction and dilatation and to attempt to elucidate the molecular basis of this effect. Our findings show that tPA has 2 independent epitopes with opposite effects on blood vessel tone; the activity of each epitope is abolished by PAI-1. We also report that phenylephrine causes a greater increase in blood pressure in tPA–/– mice than in WT animals and that administering tPA to rats regulates blood pressure and cerebral vascular resistance.
Materials and methods
Materials
Recombinant tPA (rtPA) and tPA variant TNK-tPA were obtained from Genentech (South San Francisco, CA). In addition, rtPA was obtained from American Diagnostica (Greenwich, CT). Phenylephrine (PE) and l-nitroarg-methyl ester (l-NAME) were purchased from Sigma (St Louis, MO). tPA–/–, uPA–/–, and uPAR–/– mice on a C57/black background and wild-type C57/black mice were purchased from Jackson Laboratories (Bar Harbor, ME). Four-month-old male mice were used in all studies. Recombinant 39-kDa receptor-associated protein (rRAP) was generously provided by Dr D. Strickland (American Red Cross, Rockville, MD). Anti-LRP antibodies were also provided by D. Strickland and by American Diagnostica. The peptides (EEIIMD and REIIMD) were synthesized as previously described.12 Human umbilical vein vascular smooth muscle cells (SMCs) were the kind gift of Dr D. Cines (Department of Pathology and Laboratory Medicine, University of Pennsylvania) and were prepared as previously described.6
Methods
Contractile response of isolated aortic rings. Experiments were performed as previously described.13-15 Mice were killed by exsanguination. Thoracic aortas were removed with care to avoid damage to the endothelia, dissected free of fat and connective tissue, and cut into transverse rings 5 mm in length.16 To study the role of endothelial cells in the performed experiments, the aorta rings were gently rotated on a stainless steel rod to remove the endothelium (denuded aorta). To record isometric tension, the rings were mounted in a 10-mL bath containing oxygenated (95% O2; 5% CO2) solution of Krebs-Henseleit (KH) buffer (144 mM NaCl, 5.9 mM KCl, 1.6 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, 25 mM NaHCO3, and 11.1 mM d-glucose). The rings were equilibrated for 1.5 hours at 37°C and maintained under a resting tension of 2 g throughout the experiment. Each aortic ring was then contracted by adding PE or endothelin in stepwise increments (from 10–10 M to 10–5 M). In other experiments, tPA or TNK-tPA was added at the indicated concentrations (see figure legends) 15 minutes before the addition of PE. To exclude any effect of the vehicle of tPA or TNK-tPA, control experiments were performed using dialyzed tPA and TNK-tPA; no difference between the dialyzed and nondialyzed tPA or TNK-tPA was found. When indicated, the nitric oxide (NO) synthase inhibitor l-NAME (1 μM final concentration) was added 10 minutes before tPA or TNK-tPA. In each experiment, rings exposed to KH buffer alone were analyzed in parallel. Isometric tension was measured with a force displacement transducer and was recorded online using a computerized system (ExperimentiaÆ, Budapest, Hungary).
The half-maximal effective concentration (EC50) was calculated by measuring the response of aorta rings (y-axis) to increasing concentrations of PE (x-axis). The lines that intersect with the y- and x-axes were drawn to determine the concentration of PE that induces 50% of its maximal effect. Each EC50 was calculated as the mean ± SD of 3 separate experiments.
Blood pressure recording in mice. Mean arterial blood pressure (MABP) was measured as previously described.13-15 Mice were anesthetized by intraperitoneal administration of 10 mg/kg–1 ketamine and 50 mg/kg xylazine. One cannula was placed in the left carotid artery to record the mean arterial blood pressure, and a second, through which PE (0.3 μg per animal) or saline was administered, was placed in the right jugular vein. Blood pressure was monitored continuously through a transducer placed in the left carotid cannula using the CARDIOSYS computerized system (ExperimentiaÆ).
Hemodynamic studies in rats
Sprague-Dawley rats (353 ± 4 g) were used for all experiments and were fed on regular chow with free access to drinking water. The study was conducted in accord with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The rats were anesthetized with thiobutibarbital (Inactin) (100 mg/kg intraperitoneally), and catheters were placed in the trachea (PE-250), external jugular vein, femoral artery, and vein (PE-50). Each animal was kept in a prone position, and normal saline was infused throughout the experiment at a rate of 5 mL/h. Mean arterial blood pressure was continuously monitored by a pressure transducer system connected to the arterial line. The skin above the Bregma suture was removed, and the periosteum and the external plate of the calvarium were slightly eroded. A laser Doppler flow probe (Perimed, Stockholm, Sweden) was placed perpendicularly to the bone 2 mm lateral and posterior to the Bregma, on the right side, for the determination of cortical cerebral blood flow. Online recordings of blood pressure and cerebral blood flow were stored, displayed, and analyzed with the use of a computerized system (MacLab/8; Analog Digital Instruments, Castle Hill, NSW, Australia). Mean values for each determinant were analyzed over 1-minute periods. Changes in regional cerebral vascular resistance were extrapolated from the relative changes in blood pressure divided by the relative changes in blood flow as described in Heyman et al.17 All values are expressed as the percentage of baseline measurements.
Experimental design
Following a baseline 30-minute period with stabilized hemodynamics, rats (n = 7-10 per group) were injected with tPA (2-10 mg/kg) alone or combined with the PAI-1–derived hexapeptide EEIIMD (0.5 mg). A third group was injected with TNK-tPA (2-10 mg/kg) in 0.5 mL, infused over 1 minute.
In a complementary second set of experiments, we studied the effect of tPA on carotid blood flow. Anesthetized rats were prepared as detailed above, but the measurement of cerebral cortical blood flow was substituted with the direct determination of blood flow at the common carotid artery using a perivascular transonic ultrasonic volume flow sensor (T-106; Transonic Systems, Ithaca, NY).
Binding, internalization, and degradation of tPA
Sodium iodide I 125 tPA or TNK-tPA was radiolabeled and characterized as described previously.18 SMC cells were grown to confluence in 48-well Falcon multiwell tissue culture plates (Becton Dickinson, Lincoln Park, NJ) to a final density of approximately 5 × 104 cells/well in Dulbecco modified Eagle medium (DMEM) media (Bet Haemek Israel), supplemented with 10% fetal calf serum (FCS). Cells were prechilled to 4°C for 30 minutes and washed twice with KH buffer. They were then incubated with KH buffer containing 20 nM 125I-tPA for 2 hours at 4°C. Washing the cells 4 times with binding buffer to remove unbound ligand terminated the incubation. Radiolabeled ligand bound to the cell surface was released by adding 50 μM glycine-HCl, pH 2.8 as described previously.12,18 Nonspecific binding was determined by measuring cell-associated radioactivity in the presence of 500 nM unlabeled tPA. Specific binding was defined as the difference between total and nonspecific binding.
To measure internalization, after the cells were incubated with 125I-tPA for 2 hours at 4°C and washed 4 times with binding buffer to remove unbound ligand, the cells were warmed to 37°C for another hour, and radiolabeled ligand bound to the cell surface was released with glycine-HCl, pH 2.8. After releasing cell surface–bound ligand, internalized tPA was determined by solubilizing the cells with 0.1 N NaOH and measuring the cell-associated radioactivity. To measure degradation, the media were removed after 18-hour incubation with radiolabeled ligands at 37°C, trichloroacetic acid was added to a final concentration of 10%, the precipitated protein was separated by centrifugation, and the radioactivity in the supernatant was measured as previously described.12,18
Statistical analysis
In vivo data are presented as mean ± SEM. One-way analysis of variance (ANOVA) with post-hoc Newman-Keuls analysis and paired t test were performed for the evaluation of repeated within-group hemodynamic measurements. Statistical significance was set at P less than .05.
Ex vivo experiments were performed in triplicate and were repeated a minimum of 3 times on different tissues. All data are presented as mean ± SD of the 3 experiments.
Results
tPA stimulates or inhibits contraction of aorta rings in a dose-dependent manner
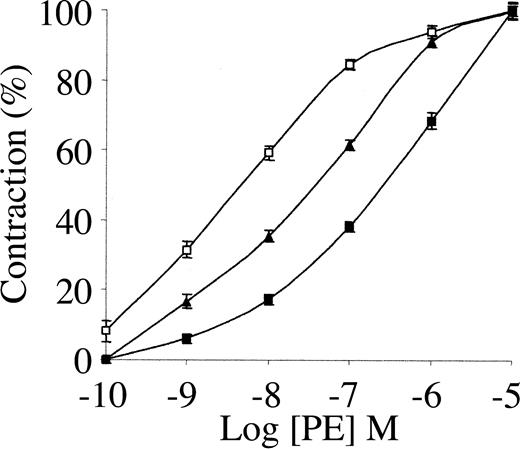
To study the involvement of tPA in the regulation of blood vessel tone under physiologic or therapeutic conditions, we examined its effect on the contraction of isolated rat aorta rings. As shown in Figure 1, 1 nM tPA inhibited PE-induced vasoconstriction, with EC50 increasing from 34 to 351 nM PE. The inhibitory effect of tPA on PE-induced vasoconstriction is in line with the data published by Cipolla et al.11 When endothelin was used instead of PE, tPA had a similar effect (not shown).
Effect of tPA on PE-induced contraction of isolated mouse aorta rings. Contraction of isolated aorta rings was induced by increasing concentrations of PE in the absence of tPA (▴) or in the presence of 1 nM (▪) or 20 nM tPA(□). Values are presented as mean ± SD.
Effect of tPA on PE-induced contraction of isolated mouse aorta rings. Contraction of isolated aorta rings was induced by increasing concentrations of PE in the absence of tPA (▴) or in the presence of 1 nM (▪) or 20 nM tPA(□). Values are presented as mean ± SD.
Because 1 nM tPA is within the physiologic range and is far below therapeutic concentrations, we examined the effect of a higher dose of tPA on PE-induced vasoconstriction. As shown in Figure 1, 20 nM tPA produced an effect opposite that seen at 1 nM; 20 nM tPA enhanced the vasoconstriction induced by PE, decreasing the EC50 of PE from 34 to 1.6 nM. At higher concentrations (50 nM) tPA induced a similar provasoconstrictive effect (data not shown).
tPA exerts its effects by acting on vascular endothelial cells
Because the endothelial cell layer plays a critical role in the regulation of blood vessel vasoactivity, we examined the effect of tPA on denuded aorta rings. We found no effect of tPA on the contraction of denuded aorta rings or on aorta rings pretreated with the NO synthase inhibitor l-NAME (data not shown).
TNK-tPA, a variant with stimulatory but not inhibitory effect on blood vessel contraction
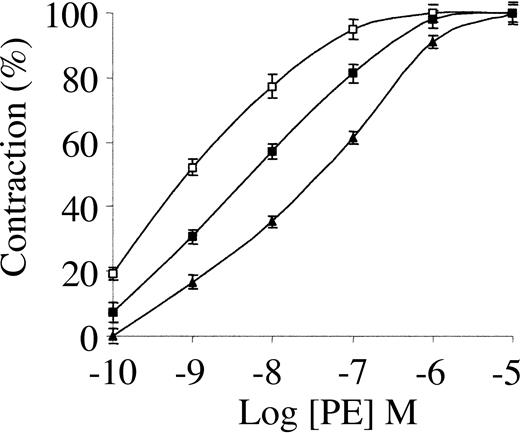
TNK-tPA is a bioengineered variant of tPA that has a longer half-life than tPA,19 an 80-fold greater resistance to PAI-1 than tPA, and a 14-fold greater relative fibrin specificity.19,20 In vitro, TNK-tPA is 8- and 13-fold more potent than tPA toward whole blood and platelet-enriched clots, respectively.19 In spite of these great advantages of TNK-tPA over tPA in vitro, TNK-tPA had no significant advantage over rtPA in clinical studies.21-23
To elucidate the basis for the discrepancy between TNK-tPA activity in vitro and in patients, we studied its vasoactivity. Figure 2 shows that low concentrations of TNK-tPA had an opposing effect to that of tPA on the contraction of aorta rings. The vasoconstriction induced by PE was stimulated to a similar extent by 1 nM TNK-tPA and by 20 nM tPA (compare with Figure 1). Figure 2 shows also that the stimulatory effect of TNK-tPA on PE-induced vasoconstriction was greater when its concentration was increased from 1 to 20 nM; 20 nM TNK-tPA decreased the EC50 from 34 to 0.63 nM. As in the case of tPA, TNK-tPA failed to induce any effect in the vasoconstriction of denuded aorta rings or aorta rings pretreated with l-NAME (not shown).
Effect of TNK-tPA on PE-induced contraction of isolated mouse aorta rings. Contraction of isolated aorta rings was induced by increasing concentrations of PE in the absence of TNK-tPA (▴) or in the presence of 1 nM (▪) or 20 nM TNK-tPA (□). TNK-tPA at both concentrations enhanced PE-induced vasoconstriction.
Effect of TNK-tPA on PE-induced contraction of isolated mouse aorta rings. Contraction of isolated aorta rings was induced by increasing concentrations of PE in the absence of TNK-tPA (▴) or in the presence of 1 nM (▪) or 20 nM TNK-tPA (□). TNK-tPA at both concentrations enhanced PE-induced vasoconstriction.
PAI-1 inhibits tPA-mediated vasoactivity
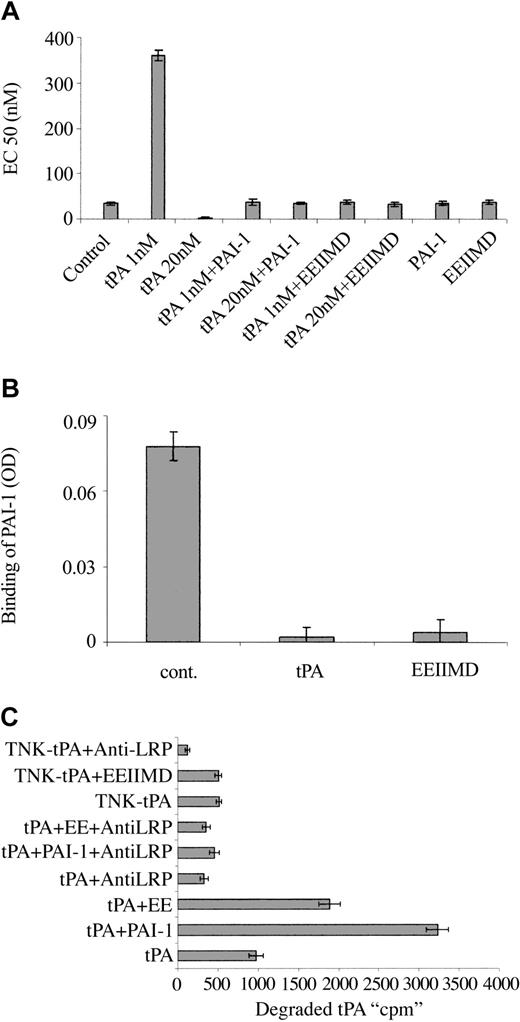
To understand the molecular basis for the differences between the vasoactivity of TNK-tPA and tPA, we asked whether the PAI-1 docking site, corresponding to amino acids 296 to 299,24,25 that had a tetra-alanine substitution (K296A/H297A/R298A/R299A) in TNK-tPA,19,26 was involved in tPA vasoactivity. To answer this question, we examined the effect of PAI-1 (the natural ligand of the docking site) on tPA vasoactivity. Figure 3A shows that both the tPA provasodilatation and provasoconstrictive effects are inhibited by equimolar concentrations of PAI-1.
Effect of PAI-1 and PAI-1–derived peptide on the vasoactivity of tPA. (A) EC50 of PE on the contraction of isolated aorta rings was determined in the absence (Control) or presence of tPA 1 nM (tPA 1 nM) or 20 nM (tPA 20 nM), tPA with an equimolar concentration of PAI-1 (tPA 1 nM + PAI-1), (tPA 20 nM + PAI-1), tPA with 2 μM EEIIMD (tPA 1 nM + EEIIMD), (tPA 20 nM + EEIIMD), 20 nM PAI-1 (PAI-1), or 2 μM EEIIMD (EEIIMD) alone. (B) Inhibition of the binding of PAI-1 to tPA by the PAI-1–derived peptide. The binding of PAI-1 (1 nM) to immobilized tPA was determined in the absence (cont.) or presence of 2 μM EEIIMD (EEIIMD). Equimolar concentration of tPA (tPA) served as a positive control. (C) PAI-1–derived peptide enhances LRP-mediated degradation of tPA. The degradation of WT tPA (tPA) or TNK-tPA (TNK-tPA) by SMCs was determined in the absence or presence of PAI-1 (PAI-1) or PAI-1–derived peptide (EE) and of anti-LRP antibodies (anti-LRP) (as specified in the labels).
Effect of PAI-1 and PAI-1–derived peptide on the vasoactivity of tPA. (A) EC50 of PE on the contraction of isolated aorta rings was determined in the absence (Control) or presence of tPA 1 nM (tPA 1 nM) or 20 nM (tPA 20 nM), tPA with an equimolar concentration of PAI-1 (tPA 1 nM + PAI-1), (tPA 20 nM + PAI-1), tPA with 2 μM EEIIMD (tPA 1 nM + EEIIMD), (tPA 20 nM + EEIIMD), 20 nM PAI-1 (PAI-1), or 2 μM EEIIMD (EEIIMD) alone. (B) Inhibition of the binding of PAI-1 to tPA by the PAI-1–derived peptide. The binding of PAI-1 (1 nM) to immobilized tPA was determined in the absence (cont.) or presence of 2 μM EEIIMD (EEIIMD). Equimolar concentration of tPA (tPA) served as a positive control. (C) PAI-1–derived peptide enhances LRP-mediated degradation of tPA. The degradation of WT tPA (tPA) or TNK-tPA (TNK-tPA) by SMCs was determined in the absence or presence of PAI-1 (PAI-1) or PAI-1–derived peptide (EE) and of anti-LRP antibodies (anti-LRP) (as specified in the labels).
Amino acid residues 350 to 355 in PAI-1 regulate tPA vasoactivity
In addition to the docking site, PAI-1 interacts with the catalytic site of tPA. To determine in a more specific way the role of the PAI-1 docking site in the vasoactivity of tPA, we examined the effect of the PAI-1–derived hexapeptide EEIIMD, which corresponds to the amino acid residues 350 to 355 of PAI-1 (the epitope in PAI-1 that interacts with the tPA docking site24,27 ). Figure 3A shows that 2 μM PAI-1–derived peptide, like full-length PAI-1, prevented the provasodilatory and the provasoconstrictive effects of rtPA. The control peptide REIIMD, which has almost no effect on PAI-1 binding to uPA or on uPA vasoactivity,12,25 did not affect tPA-mediated vasoactivity (not shown). The PAI-1–derived peptide EEIIMD had no effect on the catalytic activity of tPA (not shown), but it inhibited the binding of PAI-1 to tPA (Figure 3B).
PAI-1 is known to increase the LRP-mediated internalization and degradation of tPA; in view of the ability of the PAI-1–derived peptide to mimic the PAI-1 effect on tPA vasoactivity, we asked whether it also affects tPA metabolism. Figure 3C shows that the degradation of radiolabeled tPA is increased by PAI-1 and by PAI-1–derived peptide, although the effect of EEIIMD was markedly lower than that of PAI-1. As shown in Figure 3C, the degradation of tPA in the presence or absence of PAI-1 or EEIIMD was inhibited by anti-LRP antibodies but not by irrelevant IgG (not shown). PAI-1 and the PAI-1–derived peptide EEIIMD affected the internalization of radiolabeled tPA by cells in a manner comparable to that of tPA degradation (not shown). These results are in line with our previous report on the effect of the PAI-1–derived peptide on uPA internalization and degradation.12 The data presented in Figure 3C also show that TNK-tPA degradation is LRP-mediated, but at a much slower rate than WT tPA; furthermore, EEIIMD, like irrelevant IgG (not shown), had no effect on TNK-tPA degradation.
LRP mediates the provasoconstrictive effect of tPA
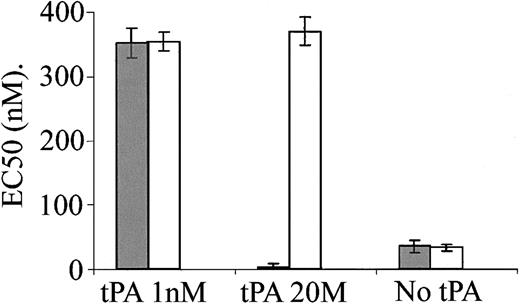
The data presented in Figure 3 indicate that PAI-1 inhibits the vasoactivity of tPA and increases its degradation. LRP is one of the major receptors of tPA in the liver and vasculature and mediates a significant part of tPA clearance from the circulation.28 PAI-1 is known to modify the interaction of tPA with LRP.7,8 LRP is also involved in signal transduction, including Ca++ mobilization,29 and it mediates uPA and defensin14,15 vasoactivity. We, therefore, examined the involvement of LRP in tPA-induced vasoactivity. Figure 4 shows that anti-LRP antibodies had no effect on the provasodilatory effect obtained at low concentration of tPA (1 nM). In contrast, anti-LRP antibodies abolished the provasoconstrictive effect obtained at 20 nM tPA. Furthermore, in the presence of anti-LRP antibodies, 20 nM tPA induced vasodilatation comparably to that induced by 1 nM tPA (Figure 4), supporting the existence of 2 independent tPA receptors and the conclusion that the vasodilatation is LRP-independent. Figure 4 also shows that anti-LRP antibodies have no effect on the contraction of aorta rings in the absence of tPA. rRAP induced an effect comparable to that of anti-LRP antibodies (data not shown).
Inhibition of tPA-induced vasoconstriction, but not vasodilatation, by anti-LRP antibodies. EC50 was calculated from the response of mouse aortic rings to increasing concentrations of PE and was determined in the absence or presence of 1 or 20 nM tPA and in the absence (▦) or presence of anti-LRP antibodies (□).
Inhibition of tPA-induced vasoconstriction, but not vasodilatation, by anti-LRP antibodies. EC50 was calculated from the response of mouse aortic rings to increasing concentrations of PE and was determined in the absence or presence of 1 or 20 nM tPA and in the absence (▦) or presence of anti-LRP antibodies (□).
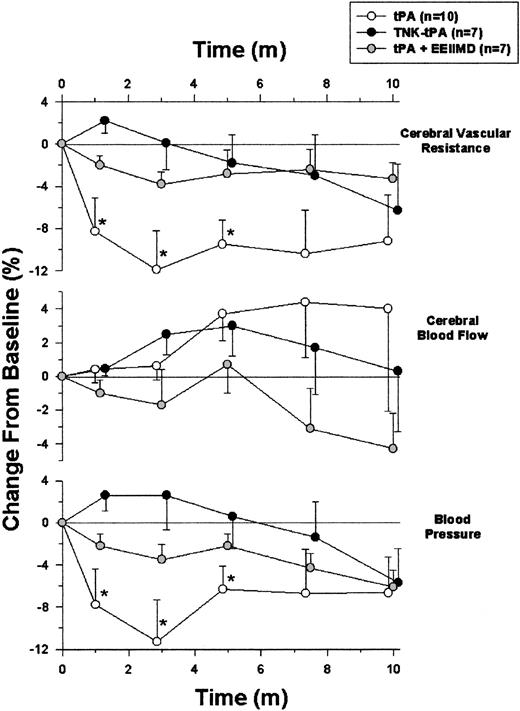
Exogenous tPA affects systemic blood pressure and cerebral vascular resistance in rats
After showing the effect of tPA and its inhibitor (PAI-1) on the contraction of isolated aorta rings, the next step was to examine the vasoactivity of the plasminogen activator and PAI-1 in-vivo. Figure 5 shows that the intravenous administration of 2 mg/kg tPA to anesthetized rats induced a rapid and partially reversible decline in blood pressure that was most pronounced by 3 minutes. When the animals were given the same amount of tPA together with 0.1 mg of the PAI-1–derived peptide EEIIMD, no significant hemodynamic change was detected. Injection of saline alone induced no change in rat blood pressure (not shown).
Effect of tPA on rat hemodynamics. Blood pressure and cortical cerebral blood flow were monitored in anesthetized rats given intravenous tPA alone (tPA) or in combination with the PAI-1–derived peptide EEIIMD (tPA + EEII) or TNK-tPA (TNK-tPA). Cerebral vascular resistance was extrapolated from the corresponding changes in blood pressure and regional flow. Values are presented as mean ± SEM. In tPA-treated rats but not in the other 2 groups, blood pressure and cerebral vascular resistance significantly declined. (*P < .05 vs baseline, ANOVA).
Effect of tPA on rat hemodynamics. Blood pressure and cortical cerebral blood flow were monitored in anesthetized rats given intravenous tPA alone (tPA) or in combination with the PAI-1–derived peptide EEIIMD (tPA + EEII) or TNK-tPA (TNK-tPA). Cerebral vascular resistance was extrapolated from the corresponding changes in blood pressure and regional flow. Values are presented as mean ± SEM. In tPA-treated rats but not in the other 2 groups, blood pressure and cerebral vascular resistance significantly declined. (*P < .05 vs baseline, ANOVA).
Taking into consideration the potential deleterious role of tPA in an experimental stroke model in which the infarcted area in tPA–/– mice was smaller than in WT animals,9 we asked whether tPA regulates cerebral circulation. Figure 5 shows that despite the decrease in blood pressure, cerebral blood flow was not significantly affected by tPA. Figure 5 also shows that tPA decreased cerebral vascular resistance significantly; this finding could explain the maintenance of cerebral blood flow in the presence of a decrease in blood pressure.
Using the alternative method in which carotid blood flow was monitored, tPA induced a modest, although consistent, vasoactive response; the calculated vascular resistance declined by 3.5% ± 0.3% below baseline, within 2 to 3 minutes of tPA administration (n = 19; P < .0002). When tPA was administered together with 0.1 mg EEIIMD, no significant change in vascular resistance was detected, as in the results shown in Figure 5.
The data presented in Figure 5 show that under the described experimental conditions, in vivo the vasodilatory effect of tPA is dominant between the 2 opposite effects seen ex vivo. It was, therefore, of interest to examine in vivo the vasoactivity of TNK-tPA, the tPA variant that ex vivo had only a vasoconstrictive effect (Figure 2). Figure 5 shows that TNK-tPA (2 mg/kg) had no vasodilatory effect in vivo and that its administration even induced a slight and statistically insignificant increase in blood pressure during the first 5 minutes. Moreover, cerebral vascular resistance did not decline significantly (Figure 5).
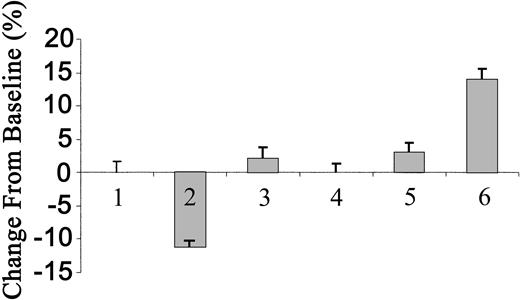
The inability of each subtype of tPA to induce an increase in blood pressure prompted us to ask whether the circulating concentrations were in the effective range. To answer this question, we determined the concentrations of radiolabeled tPA in the circulation, as previously described.2 We found that 3 minutes (when the maximal effect of tPA was achieved) after the injection of 2 mg/kg WT tPA, the concentration in the blood reached 166 nM. Taking into consideration the possibility that an endogenous ligand(s) may compete for the receptor mediating the vasoconstrictive effect of tPA, we increased the dose of tPA to 10 mg/kg. Figure 6 shows that at this concentration, WT tPA lost its vasodilatory effect and instead caused a statistically insignificant increase in blood pressure, whereas TNK-tPA induced a significant increase in the MABP of approximately 14% (n = 10; P < .0002).
Effect of tPA on blood pressure of rats. Blood pressure was determined in anesthetized rats 3 minutes after intravenous injection of saline (columns 1 and 4), 2 or 10 mg/kg tPA (columns 2 and 3, respectively), or 2 or 10 mg/kg TNK-tPA (columns 5 and 6, respectively). (Mean ± SD, *P < .0002 vs baseline.)
Effect of tPA on blood pressure of rats. Blood pressure was determined in anesthetized rats 3 minutes after intravenous injection of saline (columns 1 and 4), 2 or 10 mg/kg tPA (columns 2 and 3, respectively), or 2 or 10 mg/kg TNK-tPA (columns 5 and 6, respectively). (Mean ± SD, *P < .0002 vs baseline.)
Endogenous tPA participates in the regulation of blood pressure
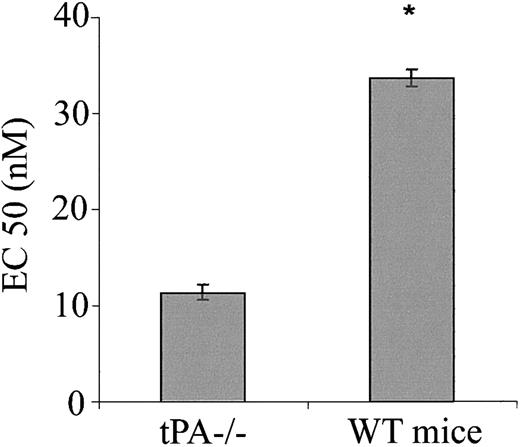
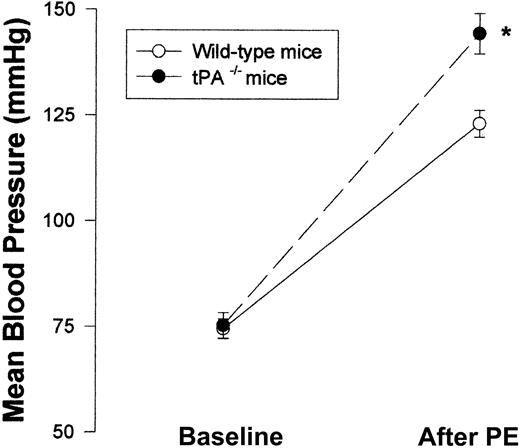
Having shown the effect of exogenous tPA on blood vessel tone in vitro and in vivo, we next asked whether endogenous tPAplays any role in the regulation of blood vessel vasoactivity. Figure 7 shows that aorta rings from tPA–/– mice had a greater response to PE than rings from WT mice with EC50 of 11.35 and 33.67 nM, respectively (n = 5; P < .0001). To examine the effect of endogenous tPA on blood pressure, we compared the blood pressure of tPA–/– mice to that of WT. No significant differences between the blood pressure of tPA–/– and WT mice were found; MABP was 75.33 and 74.5 mm Hg, respectively (Figure 8).
Effect of endogenous tPA on the contraction of isolated aorta rings. Contraction of aortic rings from WT or tPA–/– mice was determined in the presence of increasing concentrations of PE, and the EC50 of PE was calculated. (Mean ± SD, *P < .0001 vs tPA–/–.)
Effect of endogenous tPA on the contraction of isolated aorta rings. Contraction of aortic rings from WT or tPA–/– mice was determined in the presence of increasing concentrations of PE, and the EC50 of PE was calculated. (Mean ± SD, *P < .0001 vs tPA–/–.)
Effect of endogenous tPA on blood vessel vasoactivity. Mean blood pressure was monitored in WT and tPA–/– mice (n = 6 per group) at baseline and after intravenous injection of 0.1 μg PE. (Mean ± SD, *P < .0001 vs WT mice, 2-way ANOVA.)
Effect of endogenous tPA on blood vessel vasoactivity. Mean blood pressure was monitored in WT and tPA–/– mice (n = 6 per group) at baseline and after intravenous injection of 0.1 μg PE. (Mean ± SD, *P < .0001 vs WT mice, 2-way ANOVA.)
In contrast to the similar MABP seen in WT and tPA–/– mice, Figure 8 shows that after the intravenous injection of PE (0.1 μg), tPA–/– mice had a greater response than WT mice—MABP was 144.5 ± 4.76 compared with 123.17 ± 3.19.
Discussion
This study was designed to examine the effects of tPA and PAI-1 on blood vessel vasoactivity. Our data show that these 2 components of the fibrinolytic system do indeed regulate blood vessel tone. Ex vivo data in isolated aorta rings strongly suggest that tPA contains 2 distinct epitopes with opposite effects. One epitope inhibits the contraction induced by PE, whereas the other enhances it. The finding that TNK-tPA had no inhibitory effect but maintained the capacity to stimulate PE-induced vasoconstriction supports the contention that we are dealing with 2 independent epitopes.
Another interesting finding is the observation that PAI-1 abolished the effect of tPA on blood vessel tone. The involvement of PAI-1 in tPA-mediated vasoactivity clarified the molecular basis of plasminogen activator vasoactivity. Binding of PAI-1 to its docking site, situated outside the catalytic site (aa 296-299), seems to induce conformational changes in tPA that affect the exposure of epitopes that regulate blood vessel tone. This assertion is supported by the ability of the PAI-1–derived peptide (EEIIMD) to inhibit tPA vasoactivity without affecting its catalytic activity and, to some extent, by the inability of TNK-tPA (mutated at the docking site) to cause vasorelaxation.
It is clear that the effect of tPA is endothelial cell dependent and, more specifically, NO synthase dependent. The fact that only the provasoconstrictive effect of tPA was inhibited by LRP antagonists suggests that the binding of tPA to LRP leads to the inhibition of NO synthase. On the other hand, tPA seems to bind to another receptor present in endothelial cells that mediates the stimulation of NO synthase activity and thus inhibits the vasoconstrictive effect of PE.
The capacity of PAI-1 to inhibit the LRP-mediated vasoactivity of tPA is in line with its well-known effect on the tPA–LRP interaction and suggests that the binding of tPA to the (putative) receptor that mediates the provasodilatory effect is also regulated by PAI-1.
The normal blood pressure of tPA–/– mice, despite the increased vasoactivity of isolated aorta rings from the same animals, suggests that the physiologic effect of tPA on blood vessel tone is strictly regulated and may become evident only under special physiologic or pathologic conditions; This conclusion is supported by the increased response of blood pressure to PE in tPA–/– mice.
The finding that 2 mg/kg tPA induced vasodilatation is consistent with the ex vivo data that show the inhibitory effect of tPA on the contraction of isolated aorta rings. On the other hand, the inability of high-dose WT-tPA to induce clear vasoconstriction in vivo remains an open question. One reason for the apparent difference between the in vivo and ex vivo data could be that tPA exerts its opposite effects on blood vessel tone through 2 different receptors, each of which can function differently in vivo or in vitro. A change in the activity of either of the 2 receptors could be attributed to the availability of alternative ligands or regulators under in vivo conditions.
The finding that TNK-tPA induces vasoconstriction suggests that in vivo the signal transduction pathway that mediates the vasoconstrictive effect of tPA is functional and that its activity may become active under certain physiologic or pathologic conditions. Furthermore, the vasoconstrictive effect of TNK-tPA may explain the discrepancy between its performance as a thrombolytic agent in vitro and in patients. In other words, the inability of TNK-tPA to induce vasodilatation (and possibly to induce more vasoconstriction) could limit its delivery to the clot that has to be cleaved.
In conclusion, our findings describe a novel role for tPA and its inhibitor, PAI-1, in the regulation of blood vessel tone that could be of importance in the development of ischemic disorders. Evidently, additional studies are required to elucidate the detailed mechanisms by which tPA affects blood vessel vasoactivity.
Prepublished online as Blood First Edition Paper, September 25, 2003; DOI 10.1182/blood-2003-05-1685.
Supported by National Institutes of Health grants HL60169, HL66442, and HL67381.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal