Abstract
Mutations in the proto-oncogene c-kit cause constitutive kinase activity of its product, KIT protein, and are associated with human mastocytosis and gastrointestinal stromal tumors (GISTs). Although currently available tyrosine kinase inhibitors are effective in the treatment of GISTs, there has been limited success in the treatment of mastocytosis. 17-Allylamino-17-demethoxygeldanamycin (17-AAG), a benzoquinoid ansamycin antibiotic, which binds to heat shock protein 90 (hsp90) causes destabilization of various hsp90-dependent kinases important in oncogenesis. Treatment with 17-AAG of the mast cell line HMC-1.2, harboring the Asp816Val and Val560Gly KIT mutations, and the cell line HMC-1.1, harboring a single Val560Gly mutation, causes both the level and activity of KIT and downstream signaling molecules AKT and STAT3 to be down-regulated following drug exposure. These data were validated using Cos-7 cells transfected with wild-type and mutated KIT. 17-AAG promotes cell death of both HMC mast cell lines. In addition, neoplastic mast cells isolated from patients with mastocytosis, incubated with 17-AAG ex vivo, are selectively sensitive to the drug compared to the mononuclear fraction. These data provide compelling evidence that 17-AAG may be effective in the treatment of c-kit-related diseases including mastocytosis, GISTs, mast cell leukemia, subtypes of acute myelogenous leukemia, and testicular cancer. (Blood. 2004;103:1078-1084)
Introduction
The proto-oncogene c-kit encodes the transmembrane type III tyrosine kinase, KIT protein,1 which is the receptor for stem cell factor (SCF).2-5 The subfamily of tyrosine kinases to which KIT belongs includes receptors for platelet-derived growth factor, macrophage colony-stimulating factor, and FLT3 ligand. These receptors are characterized structurally by 5 immunoglobulin-like extracellular domains and by an intracytoplasmic domain that contains an adenosine triphosphate (ATP)-binding domain and a phosphotransferase domain separated by an interkinase sequence.1,6 Under normal circumstances, SCF binds to KIT, inducing homodimerization of the receptor that leads to intrinsic kinase activity and results in autophosphorylation of tyrosine residues.7 KIT then becomes the docking site for various Src homology domain 2 (SH2) domain signaling molecules. KIT is expressed on melanocytes, mast cells, primitive hematopoietic cells, primordial germ cells, intraepithelial lymphocytes, and interstitial cells of Cajal.8,9
Activating mutations within c-kit, first identified by Besmer et al10 as the cellular homologue of viral oncogene v-kit, were later described in various neoplastic diseases including mastocytosis11 and gastrointestinal stromal tumors (GISTs).12 Activating mutations cause constitutive phosphorylation of KIT protein that is independent of ligand binding.13 Important downstream signaling pathways are inappropriately activated, and this is believed to contribute to the abnormal proliferation and survival of these neoplastic cells. Examples of signaling pathways activated by KIT include the Ras-Raf-MAP kinase cascade, the phosphatidylinositol-3-kinase-AKT cascade, the Janus kinase-signal transducer and activator of transcription (JAK/STAT) pathway, and Src family kinases.14,15
Mastocytosis, defined as a pathologic increase in the number of mast cells in tissue, is a heterogeneous disease varying in clinical significance from skin involvement alone to systemic involvement with infiltration of the gastrointestinal tract, spleen, and bone marrow.16 The childhood form is usually a self-limited cutaneous form not generally associated with mutated KIT protein, although exceptions have been published, whereas the sporadic adult systemic form of the disease is always associated with KIT-activating mutations.17 Two types of mutations have been well described.18 The first mutation is a single residue substitution of valine for aspartic acid at codon 816 (Asp816Val) and lies in the kinase domain of KIT.11 Mutations at this codon are present in the majority of cases of adult systemic mastocytosis.17 The second type of mutation includes single residue substitutions and in-frame insertions or deletions in the intracellular juxtamembrane region of KIT protein.19 It has recently been proposed that this region is an autonomously folded autoinhibitory domain that when compromised by mutation leads to increased activity of KIT.20 Juxtamembrane mutations are found consistently in diseases such as GISTs.21 HMC-1.2 cells, a human mast cell line, contain both types of mutations,22 whereas HMC-1.1 cells contain only the juxtamembrane mutation Val560Gly.23 As would be expected, HMC-1.1 cells are sensitive to specific tyrosine kinase inhibitors, whereas HMC-1.2 cells are relatively insensitive to these drugs.23,24 Therefore, it is likely that currently available specific tyrosine kinase inhibitors will be ineffective clinically in the treatment of mastocytosis.
Geldanamycin is a benzoquinoid ansamycin antibiotic, which binds to heat shock protein 90 (hsp90), and promotes the degradation of various hsp90 client proteins important in proliferation and survival of malignant cells.25-28 17-Allylamino-17-demethoxygeldanamycin (17-AAG) is a chemical derivative of geldanamycin, currently in clinical trials, which displays significant hsp90-dependent antitumor activity while possessing a favorable toxicity profile.29 hsp90 client proteins are frequently mutated or chimeric proteins that depend on hsp90 for proper folding and stability.29 Recently, mutated FLT-3, another type III tyrosine kinase receptor, was identified as an hsp90 client protein, and hsp90 inhibition led to apoptosis of mutated FLT3-transformed 32D cells.30
In the present study, we show that 17-AAG at concentrations obtainable in vivo in humans significantly decreases levels of mutated KIT protein, KIT activity, and the activity of important downstream signaling pathways in human mast cells. HMC-1 cell lines show growth inhibition and undergo cell death as demonstrated by propidium iodide staining, following treatment with 17-AAG. Patient-derived neoplastic mast cells harboring the codon 816 mutation treated with 17-AAG ex vivo demonstrate sensitivity, in contrast to what has been described for specific tyrosine kinase inhibitors.23
Patients, materials, and methods
Cells, antibodies, and drugs
HMC-1 cells,22 derived from a patient with mast cell leukemia, were provided by Joseph Butterfield (Mayo Clinic, Rochester, MN). HMC-1.2 cells contain the 2 mutations Asp816Val and Val560Gly and HMC-1.1 cells contain a single mutation, Val560Gly.23 They were maintained in Iscove medium with 25 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) and l-glutamine (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS; Gemini Bio-Products, Clabasas, CA) and 1.2 mM α-thioglycerol (Sigma, St Louis, MO) and grown at 37°C in 5% CO2. Cos-7 cells were obtained from American Type Culture Collection (Rockville, MD) and were grown in Dulbecco modified Eagle medium (DMEM; Biosource International, Camarillo, CA) supplemented with glutamine and 10% FBS (Gemini Bio-Products) and grown at 37°C in 5% CO2. For Western blot analysis, anti-KIT antibody C-19 (Santa Cruz Biotechnology, Santa Cruz, CA) was used as the primary antibody to detect total KIT and antiphospho-KIT (Tyr719; Cell Signaling Technology, Beverly, MA) was used to detect phosphorylated KIT. KIT protein immunoprecipitation efficiency was increased significantly by combining several primary antibodies. Thus, anti-KIT antibodies M-14, H-300, C-19, Ab81 (Santa Cruz Biotechnology) and PC34 (Oncogene Research, San Diego, CA) were used for immunoprecipitation. Rat anti-hsp90 antibody (SPA-840) was purchased from Stressgen Biotechnology (San Diego, CA); goat anti-hsp70 (K-20) antibody from Santa Cruz Biotechnology; rabbit antiphospho-AKT antibody, rabbit anti-AKT, rabbit anti-STAT3, and rabbit antiphopho-STAT3 (Tyr705) from Cell Signaling Technology; and mouse antitubulin antibody from Sigma.
17-AAG was provided by the Cancer Therapy Evaluation Program (CTEP; Bethesda, MD) and imatinib mesylate was provided by Novartis (Basel, Switzerland).
Western blot analysis and coimmunoprecipitation
Cells grown to a concentration of approximately 1 × 106 cells/mL, in the presence or absence of 17-AAG, were spun down at 800g for 5 minutes. The pellet was washed once with cold phosphate-buffered saline (PBS), pH 7.0, recentrifuged and resuspended in TNSEV buffer (50 mM Tris [tris(hydroxymethyl)aminomethane), pH 7.5,2 mM EDTA [ethylenediaminetetraacetic acid], 100 mM NaCl,2 mM sodium orthovanadate,1% NP-40) supplemented with Complete protease inhibitors (Roche Molecular Biochemicals, Indianapolis, IN). After being placed on ice for 10 minutes, cell lysates were clarified by centrifuging at 13 000g at 4°C for 20 minutes. Protein concentration was determined using the bicinchoninic acid (BCA) protein assay kit (Pierce Chemical, Rockford, IL). Equal aliquots of clarified cell lysate were heated at 100°C for 5 minutes in sodium dodecyl sulfate (SDS) buffer (80 mM Tris-HCL, pH 7.5, 2% SDS, 10% glycerol, 100 mM dithiothreitol, and 0.0005% bromphenol blue). Equal amounts of total protein were separated by 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE), transferred onto nitrocellulose paper by electroblotting, and analyzed using horseradish peroxidase (HRP)-conjugated secondary antibody (Amersham Biosciences, Piscataway, NJ) in conjunction with Western blot chemiluminescence (Pierce Chemical). Blots were probed with antitubulin antibody to confirm equal loading of total protein whenever necessary.
Minor changes were made to a previously described method for coimmunoprecipitation.31 Cells were grown and washed as described (see “Cells, antibodies, and drugs”). They were then resuspended in TMNSV lysis buffer (50 mM Tris-HCL, pH 7.5, 20 mM Na2MoO4, 0.09% Nonidet P-40, 150 mM NaCl, and 2 mM sodium orthovanadate) supplemented with Complete protease inhibitors (Roche Molecular Biochemicals). Lysate protein (3 mg) was incubated with 3 μg of each anti-KIT antibody used for immunoprecipitation for 2 hours at 4°C. Protein G-Sepharose beads (Invitrogen) were added and rotated at 4°C for 2 hours. As a negative control, Protein G-Sepharose beads were incubated with 3 mg lysate protein in the absence of anti-KIT antibody. The beads were washed 3 times with TMNSV buffer and then resuspended in SDS sample buffer. Immunoprecipitated protein was boiled for 5 minutes, and then separated by 10% SDS-PAGE. Protein transfer and probing were done as described.
Cell growth assay
Cell growth was determined on 3 consecutive days in the presence or absence of 17-AAG using methylthiazol-tetrazolium (MTT) as described.32 Cells were suspended in medium on day -1, at a concentration of 7.5 × 105 cells/mL, in a 96-well plate. Twenty-four hours later (day 0), 17-AAG was added at various concentrations to sample plates. MTT was added to the medium each day and incubated for 4 hours at 37°C. Cells were then spun down at 2000g, medium was removed, and 120 μL dimethyl sulfoxide (DMSO) was added to wells. Plates were shaken for 30 minutes at 1500g and absorbance was measured at 560 nm using the ELx808iu Ultra Microplate Reader (Bio-Tek Instruments, Winooski, VT). Samples were done in sextant at each concentration and the mean absorbance was used to determine the relative cell viability (relative growth). Relative growth is defined as sample absorbance divided by control absorbance (obtained from “day 0” plates).
Viability assays
Cell death was determined by propidium iodide uptake. Cells grown to a concentration of 1 × 106 cells/mL were incubated in the presence of no drug, 1 μM 17-AAG, or 1 μM imatinib for 24 hours. Then 5 × 105 cells were washed with buffer solution (10 mM HEPES, pH 7.4, 150 mM NaCl, 2.5 mM CaCl2, 1.0 mM MgCl2, 4% BSA) and then resuspended in 500 mL of the same buffer. Propidium iodide (300 ng; Oncogene Research) was added and samples were analyzed immediately by flow cytometry using an argon ion laser tuned to 488 nm (Becton Dickinson FACSCalibur, San Jose, CA) to quantify propidium iodide fluorescence. Cells (10 000) were counted in each experiment and representative data from 2 separate experiments are shown as dot plots (P values were determined by χ2 testing).
Transfection experiments
Cos-7 cells were grown to about 60% confluence in 6-well plates. Transfections were performed using the Fugene 6 reagent (Roche Molecular Biochemicals) and manufacturer's guidelines were followed. PcDNA3 plasmid vectors coding for either wild-type or Asp816Val mutated KIT were provided by Dr Gunnar Nilsson (University of Uppsala, Sweden).33 The Fugene 6 reagent/DNA ratio was 2:1. Plasmid DNA (1.5 μg) was used in each well and transfection proceeded overnight. Medium was then exchanged for fresh medium. SCF (Peprotech, Rocky Hill, NJ) was added (to appropriate wells) at a concentration of 100 ng/mL 1 hour prior to addition of 1 μM 17-AAG (to appropriate wells). Cells were lysed with TNSEV buffer and Western blot analysis was performed as described (see “Western blot analysis and coimmunoprecipitation”).
Patients
After their informed consent was given, bone marrow aspirates were obtained from 4 patients (2 men, 2 women; age range, 45-57 years) with indolent systemic mastocytosis. The Asp816Val c-kit mutation was verified in all patients. Patients are participants in a National Institutes of Health research protocol that has been approved by the institutional review board.
Bone marrow mononuclear cell culture, treatment with 17-AAG, and flow cytometric analysis of mast cells
Bone marrow mononuclear cells were isolated on a Ficoll-Hypaque 1.077 density gradient and cultured in Stem-Pro serum-free medium (Invitrogen) supplemented with 100 ng/mL recombinant human SCF (Peprotech) for 7 to 9 days as described.24 A control group grown in the absence of SCF was added to the experiment to demonstrate that the neoplastic cells in mastocytosis are not dependent on exogenous SCF for survival. 17-AAG was added to the cultures at concentrations of 0.1 to 1 μM. At the end of the culture period, total cell counts and mast cells percentages were determined. Mast cells were detected by their characteristic appearance in flow cytometric analysis of the bone marrow sample stained with phycoerythrin-conjugated antihuman CD117 (BD Biosciences, San Jose, CA) as described.24
Results
Mutated KIT protein and KIT activity decrease in HMC-1.1 and HMC-1.2 cells following treatment with 17-AAG
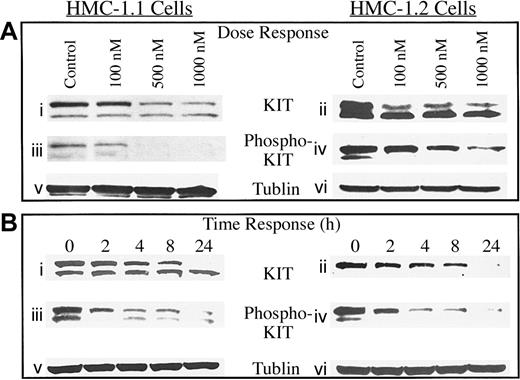
To determine the effects of 17-AAG on KIT protein in malignant human mast cells, HMC-1 cell lines were treated for 8 hours with increasing concentrations of 17-AAG and levels of total KIT protein and phosphorylated KIT protein were measured by Western blot analysis. As shown in Figure 1Ai-iv, there was a dose-dependent decrease in total KIT protein and KIT activity (as measured by KIT autophosphorylation) in both cell lines with a more profound effect at concentrations greater than 500 nM 17-AAG. This is relevant because concentrations of 1 μM can be achieved in vivo in humans as shown by preliminary data from phase 1 clinical trials.34
Dose and time response of KIT and phosphorylated KIT protein to 17-AAG in HMC-1.1 and HMC-1.2 cells. (A) Mast cells were incubated with increasing concentrations of 17-AAG for 8 hours, and levels of KIT protein (i-ii), phosphorylated KIT protein (iii-iv), and tubulin (v-vi) were measured as described in “Patients, materials, and methods.” (B) Mast cells were incubated for increasing time intervals with 1 μM 17-AAG and levels of KIT protein (i-ii), phosphorylated KIT protein (iii-iv), and tubulin (v-vi) were measured as described in “Patients, materials, and methods.” Tubulin protein was measured to confirm that equal amounts of total protein were added in each lane.
Dose and time response of KIT and phosphorylated KIT protein to 17-AAG in HMC-1.1 and HMC-1.2 cells. (A) Mast cells were incubated with increasing concentrations of 17-AAG for 8 hours, and levels of KIT protein (i-ii), phosphorylated KIT protein (iii-iv), and tubulin (v-vi) were measured as described in “Patients, materials, and methods.” (B) Mast cells were incubated for increasing time intervals with 1 μM 17-AAG and levels of KIT protein (i-ii), phosphorylated KIT protein (iii-iv), and tubulin (v-vi) were measured as described in “Patients, materials, and methods.” Tubulin protein was measured to confirm that equal amounts of total protein were added in each lane.
To examine the effect of increasing exposure time of human mast cells to 17-AAG, HMC-1 cells were incubated with 1 μM 17-AAG and cell lysate was collected for Western analysis over 24 hours. As seen in Figure 1Bi-vi, KIT protein in both malignant mast cell lines decreased as early as 2 hours following treatment and continued to decrease over 24 hours. Reduction in levels of phosphorylated KIT preceded the reduction of KIT protein, suggesting that hsp90 may have an important role in stabilizing the functional form of the protein. Importantly, the Asp816Val mutation did not appear to decrease sensitivity to the drug, as is seen with imatinib, which binds to the KIT kinase domain.
Proliferation and survival signaling pathways downstream of KIT protein are down-regulated following treatment with 17-AAG
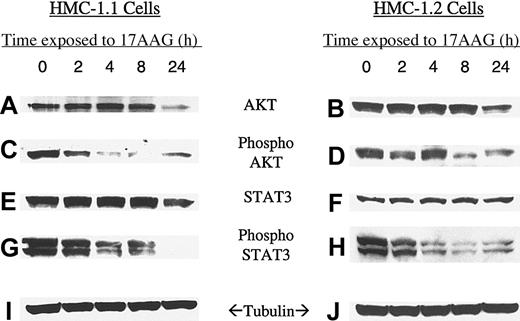
To determine whether 17-AAG affects downstream signaling pathways, we looked at levels of total AKT, activated AKT (phosphorylated AKT), STAT3, and activated STAT3 (phosphorylated STAT3). Activation of both pathways appears to be necessary for factor-independent growth and proliferation of Asp816Val KIT-mutated mast cells and MO7e cells35,36 and both signaling pathways play an important role in protecting cells from apoptosis.37,38 AKT has previously been shown to be degraded and deactivated by hsp90 inhibitors in breast cancer cell lines overexpressing HER-2.26,39-41 As can be seen in Figure 2, both phospho-AKT and phospho-STAT3 are rapidly down-regulated in human mast cell lines after treatment with 17-AAG. Inhibition of AKT and STAT3 is similar in both cell lines and correlates well with 17-AAG effects on KIT. In these HMC cell lines, loss of AKT and STAT3 activity precedes the more gradual decrease in their total levels. Unlike AKT, and supported by our data, STAT3 is not known to be a client protein of hsp90. Nevertheless, STAT3 is rapidly dephosphorylated following exposure of mast cells to 17-AAG.
Time response of KIT substrate molecules AKT and STAT3 to 17-AAG in HMC-1.1 and HMC-1.2 cells. Mast cells were incubated for increasing time intervals with 1 μM 17-AAG and levels of AKT (A,B), phosphorylated AKT (C,D), STAT3 (E,F), phospho-STAT3 (G,H), and tubulin (I,J) were measured as described in “Patients, materials, and methods.” Tubulin protein was measured to confirm that equal amounts of protein were added to each lane.
Time response of KIT substrate molecules AKT and STAT3 to 17-AAG in HMC-1.1 and HMC-1.2 cells. Mast cells were incubated for increasing time intervals with 1 μM 17-AAG and levels of AKT (A,B), phosphorylated AKT (C,D), STAT3 (E,F), phospho-STAT3 (G,H), and tubulin (I,J) were measured as described in “Patients, materials, and methods.” Tubulin protein was measured to confirm that equal amounts of protein were added to each lane.
hsp90 and hsp70 coimmunoprecipitate with KIT protein
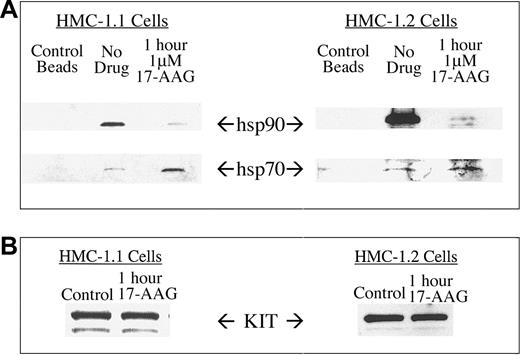
To study the interaction of KIT with the molecular chaperones hsp90 and hsp70, we immunoprecipitated mutated KIT protein from control and 17-AAG-treated HMC-1 cells and probed for associated chaperones. Although hsp90 was readily detectable in anti-KIT immunoprecipitates from untreated cells, treating cells with 1 μM 17-AAG for 1 hour caused hsp90 to dissociate with a concomitant increase in association of hsp70 (Figure 3). These findings are consistent with data published for other hsp90 client proteins, including v-src, p210Bcr-Abl, AKT, and HER-2.31,41,42 These data support the hypothesis that 17-AAG alters the chaperone complex associating with KIT client protein prior to stimulating the degradation of kinase.25
Coimmunoprecipitation of hsp90 and hsp70 with KIT protein. KIT protein was immunoprecipitated from HMC-1.1 and HMC-1.2 cells and associated hsp90 and hsp70 proteins were probed by Western blot analysis (A). Cells were treated with 1 μM 17-AAG for 1 hour. Protein G-Sepharose beads alone were used to exclude the possibility of nonspecific binding. Treatment with 17-AAG for 1 hour was chosen because of minimal change in total KIT levels during that time (B).
Coimmunoprecipitation of hsp90 and hsp70 with KIT protein. KIT protein was immunoprecipitated from HMC-1.1 and HMC-1.2 cells and associated hsp90 and hsp70 proteins were probed by Western blot analysis (A). Cells were treated with 1 μM 17-AAG for 1 hour. Protein G-Sepharose beads alone were used to exclude the possibility of nonspecific binding. Treatment with 17-AAG for 1 hour was chosen because of minimal change in total KIT levels during that time (B).
17-AAG inhibits growth of HMC-1.1 and HMC-1.2 cells
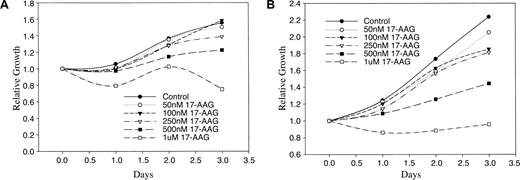
Viable cells will enzymatically convert soluble MTT into an insoluble formazan salt, which is then easily quantified by absorbance at 560 nm after solubilization in DMSO. Using this method we found that 17-AAG inhibited growth of HMC-1.1 and HMC-1.2 cells within 24 hours at concentrations similar to those resulting in loss of KIT and KIT activity (Figure 4). Similar to the dose response seen in Figure 1, this effect was most apparent at 17-AAG concentrations of 500 nM or greater. Again, both cell lines demonstrated a similar sensitivity to 17-AAG. Manual cell counting under similar conditions yielded similar results (data not shown).
Effect of 17-AAG on growth of HMC-1.1 and HMC-1.2 cells. HMC-1.1 cells (A) and HMC-1.2 cells (B) were grown in the presence of increasing concentrations of 17-AAG and data are shown as relative growth compared to control on day 0. Viability of the cells was determined by MTT assay as described in “Patients, materials, and methods.”
Effect of 17-AAG on growth of HMC-1.1 and HMC-1.2 cells. HMC-1.1 cells (A) and HMC-1.2 cells (B) were grown in the presence of increasing concentrations of 17-AAG and data are shown as relative growth compared to control on day 0. Viability of the cells was determined by MTT assay as described in “Patients, materials, and methods.”
17-AAG causes cell death in HMC-1.1 and HMC-1.2 cells
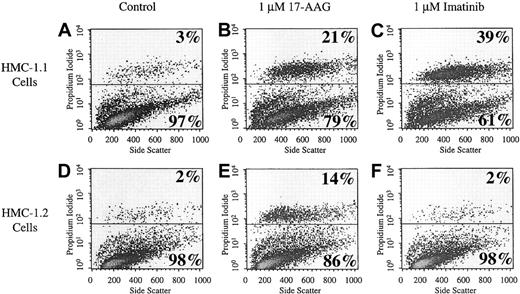
Analysis of cell death was performed by flow cytometry. Viable cells exclude propidium iodide and therefore appear in the lower half of the dot plots seen in Figure 5 (representative of 2 independent experiments). Nonviable cells cannot exclude propidium iodide and appear in the upper half of these plots. In the control group of HMC-1.1 and HMC-1.2 cells, 3% and 2% of the total population, respectively, are nonviable. Treatment of cells with 1 μM 17-AAG for 24 hours increased the percentage of dead cells to 21% and 14% for HMC-1.1 cells and HMC-1.2 cells, respectively (P < .001). In contrast, treatment with 1 μM imatinib mesylate for 24 hours causes cell death only in HMC-1.1 cells (P < .001) and not in HMC-1.2 cells. It has previously been shown that imatinib mesylate is ineffective in the treatment of HMC-1.2 cells because they harbor a kinase domain mutation in KIT.23 The data shown here further document the sensitivity of both HMC-1.1 and HMC-1.2 cells to 17-AAG, the latter of which is not sensitive to imatinib mesylate.
Cell death. Cell death of HMC-1.1 cells (A-C) and HMC-1.2 cells (D-F) after treatment with 17-AAG was determined by propidium iodide uptake. Cells were incubated with 1 μM 17-AAG for 24 hours. Whole cells were used for propidium iodide staining. The x-axis is in linear scale and represents side scatter. The y-axis is in log scale and represents fluorescence intensity corresponding to uptake of propidium iodide by the cells. Each plot is representative of 2 separately performed experiments.
Cell death. Cell death of HMC-1.1 cells (A-C) and HMC-1.2 cells (D-F) after treatment with 17-AAG was determined by propidium iodide uptake. Cells were incubated with 1 μM 17-AAG for 24 hours. Whole cells were used for propidium iodide staining. The x-axis is in linear scale and represents side scatter. The y-axis is in log scale and represents fluorescence intensity corresponding to uptake of propidium iodide by the cells. Each plot is representative of 2 separately performed experiments.
Patient neoplastic mast cells treated ex vivo are sensitive to 17-AAG
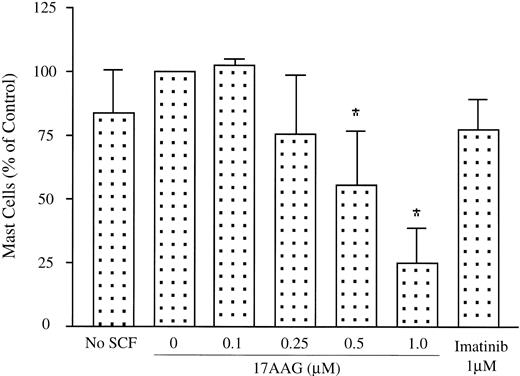
Because 17-AAG showed cytotoxicity against HMC-1.2 cells carrying the Asp816Val c-kit mutation, we next examined whether the drug exhibited a similar effect on human bone marrow mast cells isolated from patients with mastocytosis. Bone marrow mononuclear cells from 4 patients with indolent systemic mastocytosis were isolated by density gradient centrifugation and were each incubated with 0.1 to 1 μM 17-AAG for 7 to 9 days. As shown in Figure 6, a dose-dependent reduction in mast cell percentage, determined by flow cytometry, was observed. A significant reduction (P < .05) in mast cell percentage was observed at concentrations of more than 500 nM 17-AAG. The total numbers of mast cells also decreased in parallel to the percentage of the mast cells. At 1 μM 17-AAG, there was a 72% to 100% reduction in total numbers of mast cells (data not shown). Consistent with a previous report,24 imatinib did not cause a significant reduction in mast cell percentages using this assay.
Patient neoplastic mast cells are sensitive to 17-AAG. Bone marrow mononuclear cells isolated from 4 patients with systemic mastocytosis were separately incubated with increasing concentrations of 17-AAG as described in “Patients, materials, and methods.” Mast cell percentage of the total mononuclear fraction is shown. The control group, grown in the absence of SCF, demonstrates that the neoplastic cells in mastocytosis are not dependent on exogenous SCF for survival. Data are expressed as mean; n = 4; *P < .05.
Patient neoplastic mast cells are sensitive to 17-AAG. Bone marrow mononuclear cells isolated from 4 patients with systemic mastocytosis were separately incubated with increasing concentrations of 17-AAG as described in “Patients, materials, and methods.” Mast cell percentage of the total mononuclear fraction is shown. The control group, grown in the absence of SCF, demonstrates that the neoplastic cells in mastocytosis are not dependent on exogenous SCF for survival. Data are expressed as mean; n = 4; *P < .05.
KIT transiently transfected into Cos-7 cells is down-regulated following treatment with 17-AAG
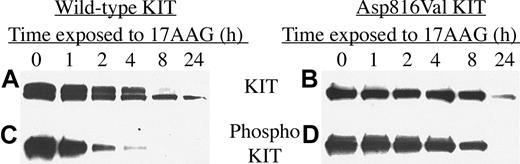
There are currently no widely available mast cell lines that contain either wild-type KIT or only the Asp816Val mutation, which is the most common KIT mutation in human mast cell disease. To test whether 17-AAG was effective on KIT harboring this mutation alone, as well as wild-type KIT, we transfected Cos-7 cells with plasmid coding for these proteins. Transfected cells were treated with 1 μM 17-AAG and incubated for up to 24 hours. As seen in Figure 7, and consistent with our earlier data, KIT activity is down-regulated before the total protein is degraded. Within 4 to 8 hours of drug exposure, Asp816Val KIT activity is significantly reduced, whereas total levels of KIT are only modestly affected. By 24 hours, phosphorylated KIT is undetectable, whereas total KIT protein is present by Western analysis, though at a reduced level. Both wild-type and kinase domain-mutated KIT are sensitive to 17-AAG, suggesting that the effect of 17-AAG on mutated KIT is a relative effect.
Wild-type and codon 816 mutated KIT protein are degraded and inactivated in transiently transfected Cos-7 cells. Cos-7 cells were transiently transfected with vectors coding for either wild-type or Asp816Val mutated KIT protein and then exposed to 1 μM 17-AAG for increasing lengths of time. Levels of KIT protein (A-B) and phosphorylated KIT protein (C-D) were measured as described in “Patients, materials, and methods.”
Wild-type and codon 816 mutated KIT protein are degraded and inactivated in transiently transfected Cos-7 cells. Cos-7 cells were transiently transfected with vectors coding for either wild-type or Asp816Val mutated KIT protein and then exposed to 1 μM 17-AAG for increasing lengths of time. Levels of KIT protein (A-B) and phosphorylated KIT protein (C-D) were measured as described in “Patients, materials, and methods.”
Discussion
Mastocytosis is one of several diseases in which mutated, constitutively activated KIT protein is implicated in disease pathogenesis. The data presented here suggest that 17-AAG is effective in targeting mutated KIT, causing its inactivation and degradation. Of particular importance is the fact that KIT harboring a kinase domain mutation remains sensitive to the effects of 17-AAG in the systems we tested. Several diseases related to mutations in the catalytic domain of kinases may not be sensitive to available specific kinase inhibitors. Indeed, kinase domain-mutated KIT is resistant to imatinib, unlike juxtamembrane-mutated KIT.23 In addition, many specific tyrosine kinase inhibitors bind to the kinase domain, and resistance to these drugs can occur if mutations occur in this region of the protein. This is exemplified by p210Bcr-Abl, an abnormal tyrosine kinase implicated in the pathogenesis of chronic myelogenous leukemia (CML). p210Bcr-Abl is an hsp90 client and is normally destabilized in the presence of hsp90 inhibitors.42,43 Evidence now suggests that one reason CML cells become resistant to the p210Bcr-Abl inhibitor imatinib mesylate is because of drug-induced or preexisting mutations within the catalytic domain leading to steric hindrance of drug binding.44,45 It has also been shown that CML cells harboring imatinib mesylate-induced kinase mutations retain their dependence on hsp90 and hence their sensitivity to 17-AAG.46,47
Our data demonstrate that mutated KIT protein and wild-type KIT protein are both sensitive to 17-AAG. Although, inhibition of wild-type KIT by 17-AAG might be expected to adversely effect in vivo hematopoietic progenitor cells, preliminary data from a phase 1 trial of 17-AAG report relatively few grade 3 and grade 4 bone marrow toxic events.34,48-50 This may be related to the actual level of basal KIT activation required for physiologic survival and regeneration of progenitor cells. In the transfection experiments described here, 100 ng/mL SCF was used, as is typical in such experiments. At this concentration there is a high level of KIT activation and thus a significant absolute reduction in activity following exposure to 17-AAG. Likewise, neoplastic mast cells, which may depend on high levels of intrinsic KIT activity, may demonstrate hypersensitivity to drug effects. 17-AAG may be less active in the context of in vivo physiologic levels of KIT activation where concentrations of SCF are reported to be about 3 ng/mL.51 Alternatively, hsp90 inhibitors may demonstrate some specificity toward neoplastic cells.52
The evidence presented here suggests that 17-AAG causes cell death in HMC-1 cells by down-regulating the active form of KIT, which leads to down-regulation of important substrate signaling molecules. This is supported by the sequence of molecular events that occurs on drug exposure. Before levels of KIT protein decrease, levels of phosphorylated KIT are dramatically reduced. Concurrent with this, activated AKT and activated STAT3 are down-regulated as evidenced by a sharp reduction in their phosphorylated forms (relative to their total levels) at 2 hours and later following drug exposure. Consistent with its chaperone function, hsp90 may play a role in maintaining the intrinsic activity of mutated KIT, and disruption of this interaction may thus lead to KIT inhibition and destabilization.
Alternatively, the growth inhibition and cell death seen in our experiments may be secondary to the direct effect of 17-AAG on other client proteins. Indeed, this is one of the most intriguing strengths of hsp90 inhibitors. AKT is a well-known client protein of hsp90 and may be directly affected by 17-AAG in HMC-1 cells. STAT3, on the other hand, is not a client protein of hsp90, which is supported by our observation that total levels of STAT3 are not affected by 17-AAG. Although not studied specifically in HMC-1 cells, STAT3 is activated via constitutively activated KIT in the murine mastocytoma cell line, FMA353 and the human myeloid cell line, MO7e cells.35 These reported observations imply that activation of STAT3 in HMC-1 cells is most likely secondary to mutated KIT and that the inactivation of KIT by 17-AAG is responsible for dephosphorylation of STAT3. Again, it remains conceivable that 17-AAG is causing STAT3 down-regulation indirectly through other upstream known hsp90 client proteins such as src or raf.
Inhibition of intrinsically activated KIT protein and its downstream signaling proteins might be expected to alter growth and survival. Indeed, growth and viability assays performed on both mast cell lines confirm this. In addition, using ex vivo cultures of patient cells isolated from bone marrow we show that 17-AAG is selectively cytotoxic to the neoplastic mast cell population. This effect occurs at 17-AAG concentrations of 500 nM and greater, which are drug levels that can be achieved in patients as reported in phase 1 trial preliminary results.34,48-50
Based on these data, we believe that a clinical trial using 17-AAG for the treatment of systemic mastocytosis is warranted. Although mastocytosis is a relatively indolent disorder, many patients experience considerable morbidity and would benefit from effective treatment, if available. The decision to treat an indolent disease with experimental therapy requires a careful assessment of potential benefits weighed against the known risks of the therapy. The currently known side effects of 17-AAG, at the dose and schedule planned for phase 2 trials, include anemia, anorexia, nausea, vomiting, and diarrhea.49 Data from completed phase 1 trials done at various institutions should be published soon.
Mastocytosis is often associated with other hematologic neoplasms such as myeloid leukemia. 17-AAG should also be considered for further investigation in these difficult situations. Although the primary treatment is always directed against the associated neoplasm, these cases often prove difficult to treat and the addition 17-AAG to primary therapy in the context of a clinical trial is attractive. Further, the importance of the type 3 tyrosine kinases, which include c-kit and FLT-3, in leukemogenesis has recently been emphasized in a comprehensive review by Reilly.54 Within this context the relevance of our findings is apparent and potentially important. Kit is expressed on approximately 80% of acute myeloid leukemias (AMLs)55 and codon 816 mutated kit is present in up to 40% of the core-binding factor acute leukemias.56 In addition, mutated FLT-3 consisting of internal tandem duplications (ITDs) is a known client protein of hsp90,30 and is present in a high proportion of AMLs.54 Taken together, 17-AAG is an appealing drug to investigate further in subtypes of AML. Most attractive, at least initially, would be those subtypes of AML expressing mutated KIT or mutated FLT-3.
Prepublished online as Blood First Edition Paper, October 9, 2003; DOI 10.1182/blood-2003-07-2477.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Joseph Butterfield at the Mayo Clinic (Rochester, MN) for providing the HMC-1 (HMC-1.2) cells and Dr Gunnar Nilsson (University of Uppsala, Sweden) for providing the PcDNA3 plasmid vectors. We would like to thank all members of our laboratories for stimulating discussion and continuous support.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal