Abstract
Recently, we reported a rare leukocyte adhesion deficiency (LAD) associated with severe defects in integrin activation by chemokine signals, despite normal ligand binding of leukocyte integrins.1 We now report that the small GTPase, Rap1, a key regulator of inside-out integrin activation is abnormally regulated in LAD Epstein-Barr virus (EBV) lymphocyte cells. Both constitutive and chemokine-triggered activation of Rap1 were abolished in LAD lymphocytes despite normal chemokine signaling. Nevertheless, Rap1 expression and activation by phorbol esters were intact, ruling out an LAD defect in Rap1 guanosine triphosphate (GTP) loading. The very late antigen 4 (VLA-4) integrin abnormally tethered LAD EBV lymphocytes to its ligand vascular cell adhesion molecule 1 (VCAM-1) under shear flow due to impaired generation of high-avidity contacts despite normal ligand binding and intact avidity to surface-bound anti-VLA-4 monoclonal antibody (mAb). Thus, a defect in constitutive Rap1 activation results in an inability of ligand-occupied integrins to generate high-avidity binding to ligand under shear flow. This is a first report of an inherited Rap1 activation defect associated with a pathologic disorder in leukocyte integrin function, we herein term it “LAD-III.” (Blood. 2004;103:1033-1036)
Introduction
Leukocyte arrest at target endothelial sites is nearly exclusively mediated by integrin receptors.2 Circulating leukocytes maintain their integrins in largely nonadhesive states, rapidly modulated by a variety of inside-out signals.3 We recently described a human genetic deficiency of leukocyte adhesion to endothelium (LAD), which results from impaired ability of leukocyte integrins to generate high avidity to their endothelial ligands at vascular endothelial contacts in response to endothelial chemoattractant signals.1 Patient leukocytes, however, express intact integrins and G-protein-coupled chemokine receptors (GPCRs). A growing body of evidence implicates the Ras-related GTPase, Rap1, as a key regulator of integrin activation by virtually all major inside-out stimuli transduced to leukocytes and platelets.4-9 Like other GTPases, it cycles from an inactive guanosine diphosphate (GDP)-bound form to an active guanosine triphosphate (GTP)-bound form, through guanine nucleotide exchange factors (GEFs) and GTPase activating proteins (GAPs).10 Suppression of endogenous lymphocyte Rap1 activity by GAP overexpression impairs both very late antigen 4 (VLA-4) and leukocyte function-associated antigen 1 (LFA-1) adhesiveness even in the absence of de novo Rap1 activation by chemoattractants.11
In this study, we characterized an Epstein-Barr virus (EBV)-transformed B-cell line derived from the LAD patient peripheral blood leukocytes (PBLs) and from age-matched control lymphocytes. We show here that Rap1 is normally expressed in LAD cells, but exhibits an aberrant activation pattern. Suppressed Rap1 activation correlated with a defect in the ability of LAD VLA-4 to generate high-avidity tethers to its endothelial ligand, vascular cell adhesion molecule 1 (VCAM-1). These results establish the first genetic abnormality in Rap1 activation state linked to a profound defect in leukocyte integrin adhesiveness in vitro and in vivo.
Study design
Cells and reagents
PBLs isolated from the patient with LAD and from an age-matched healthy donor were transformed with EBV, and lymphoblastoid lines were maintained in culture as previously described.12 Recombinant soluble 7-domain human VCAM-1 (sVCAM-1)13 and the anti-α4 integrin subunit monoclonal antibody (mAb), HP1/2,14 were both the generous gift of Dr R. Lobb (Biogen, Cambridge, MA). Stromal cell-derived factor 1α (SDF-1α) was purchased from R&D Systems (Minneapolis, MN). Anti-Rap1A was from Transduction Laboratories (Lexington, KY). Anti-extracellular signal-related kinase 1/2 (ERK1/2) and phospho-ERK1/2 antibodies were purchased from NEB/Cell Signaling (Beverly, MA).
Cell activation, pull-down assays, and Western blot analysis
EBV-transformed cells (1.5 × 107 cells) derived from control and patients with LAD were suspended at 3 × 107 cells/mL with RPMI-1640 and then stimulated with SDF-1α or phorbol 12-myristate 13-acetate (PMA) (each at 100 nM) at 37°C for 30 seconds or 5 minutes, respectively. Cells were resuspended with ice-cold lysis buffer (50 mM Tris [tris(hydroxymethyl)aminomethane]-HCl, pH 7.5, 1% Triton X-100, 150 mM NaCl, 10 mM MgCl2, 1 mg/mL Aprotinin, 1 mM phenylmethylsulfonyl fluoride). Active GTP-bound Rap1 was assessed by pull-down assays using a GST-RalGDS-RBD fusion protein as described.15
Analysis of leukocyte tethering, accumulation, and resistance to detachment
Preparation of VCAM-1- or HP1/2 mAb-coated substrates for the laminar flow adhesion assays was performed as previously described.16,17 All shear-flow experiments were performed at 37°C. Lymphocytes suspended in binding medium (cation-free Hank's balanced saline solution [HBSS], containing 10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.4] and 2 mg/mL bovine serum albumin [BSA] supplemented with Ca2+ and Mg2+ at 1 mM each) were perfused through the chamber at controlled flow rates as described.18 All cellular interactions with the adhesive substrates were determined by manually tracking the motions of individual cells along a 0.9-mm field. For analysis of VLA-4 activation by chemokines at short stationary contacts, leukocytes were allowed to settle onto the substrate for 1 minute. Flow was then initiated and increased step-wise by a programmed set of flow rates, and the number of cells that remained bound was determined as previously described.18
Results and discussion
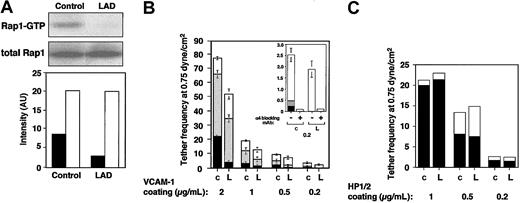
LAD EBV-transformed cells express normal Rap1 levels (Figure 1A). However, the basal GTP loading of Rap1 in these blastoid B lymphocytes was attenuated more than 3-fold in LAD-derived cells relative to control EBV-transformed lines (Figure 1A). LAD EBV cells also express normal VLA-4 levels with intact binding of soluble ligand.1 Nevertheless, VLA-4 in 2 independent LAD clones exhibited reduced ability to tether and arrest EBV cells on VCAM-1 under shear flow relative to several control EBV cells (Figure 1B and data not shown). In addition, the ability of VLA-4 to strengthen lymphocyte adhesion to high- and medium-density VCAM-1 in lymphocytes settled for 1 minute on the integrin ligand was reduced by 2- to 4-fold in LAD EBV cells (data not shown). Notably, VLA-4 tethers capable of immediately arresting tethered lymphocytes on high-density VCAM-1 were mediated by pre-existent high-affinity VLA-4 subsets susceptible to blockage by a low dose (1 ng/mL) of the VLA-4 binding peptide, Bio121117,19 (data not shown). Although LAD cells normally express such high-affinity VLA-4 subsets,1 these subsets failed to arrest LAD lymphocytes on VCAM-1 compared with the ability of these subsets to instantaneously arrest as many as 25% of tethered control cells (Figure 1B and data not shown). In contrast, LAD VLA-4 exhibited normal engagement and stabilization of tethers to the VLA-4 binding mAb, HP1/2, an anti-α4 mAb that binds VLA-4 independent of its affinity to native ligands and probes the ability of VLA-4 to cluster at subsecond adhesive contacts17,18 (Figure 1D). Thus, whereas occupancy and clustering of LAD VLA-4 with surface-bound mAbs were normal, high-affinity occupancy of LAD VLA-4 with surface-bound VCAM-1 was dramatically impaired.
Constitutive Rap1 activation and spontaneous VLA-4-mediated cell tethering and arrest on VCAM-1 are impaired in LAD EBV B cells. (A) GTP-bound Rap1 in control and LAD EBV-transformed cells was detected with pull-down assays using GST-RalGDS-RBD (top). Total Rap1 levels were detected with anti-Rap1A (middle). (Bottom) Intensity levels of Rap1 GTP (▪) and total Rap1 (□) were assessed by an image analyzer and are shownin arbitrary units (AU). Rap1 GTP of LAD cells was reduced to 34 ± 7% of that in control cells (mean ± SD of 3 independent determinations). (B) VLA-4-mediated cell capture (transient tethers), rolling, and arrest on VCAM-1 are defective in LAD EBV (L) lymphocytes compared with control EBV cells (c). The categories of different tethers are expressed as percent of the total cells in direct contact with the adhesive substrates. White, gray, and black boxes represent transient, rolling, and arrest categories, respectively. Results are given as mean ± range of determinations in 2 independent experiments. (Inset) Enlarged graph showing categories of cells interacting with the lowest VCAM-1 concentration (0.2 μg/mL). Most of the adhesive interactions were blocked with the α4-specific mAb HP1/2, both on this low-density (shown) as well as on the higher VCAM-1 (not shown) substrates. (C) VLA-4-mediated tethering (□) and arrest (▪) to different densities of surface-bound α4-specific mAb HP1/2 are normal in LAD cells. Shown is 1 representative experiment of 3. Bio1211 at 1 to 10 ng/mL did not interfere with either type of tether to the surface-bound mAb in either control or LAD cells (not shown).
Constitutive Rap1 activation and spontaneous VLA-4-mediated cell tethering and arrest on VCAM-1 are impaired in LAD EBV B cells. (A) GTP-bound Rap1 in control and LAD EBV-transformed cells was detected with pull-down assays using GST-RalGDS-RBD (top). Total Rap1 levels were detected with anti-Rap1A (middle). (Bottom) Intensity levels of Rap1 GTP (▪) and total Rap1 (□) were assessed by an image analyzer and are shownin arbitrary units (AU). Rap1 GTP of LAD cells was reduced to 34 ± 7% of that in control cells (mean ± SD of 3 independent determinations). (B) VLA-4-mediated cell capture (transient tethers), rolling, and arrest on VCAM-1 are defective in LAD EBV (L) lymphocytes compared with control EBV cells (c). The categories of different tethers are expressed as percent of the total cells in direct contact with the adhesive substrates. White, gray, and black boxes represent transient, rolling, and arrest categories, respectively. Results are given as mean ± range of determinations in 2 independent experiments. (Inset) Enlarged graph showing categories of cells interacting with the lowest VCAM-1 concentration (0.2 μg/mL). Most of the adhesive interactions were blocked with the α4-specific mAb HP1/2, both on this low-density (shown) as well as on the higher VCAM-1 (not shown) substrates. (C) VLA-4-mediated tethering (□) and arrest (▪) to different densities of surface-bound α4-specific mAb HP1/2 are normal in LAD cells. Shown is 1 representative experiment of 3. Bio1211 at 1 to 10 ng/mL did not interfere with either type of tether to the surface-bound mAb in either control or LAD cells (not shown).
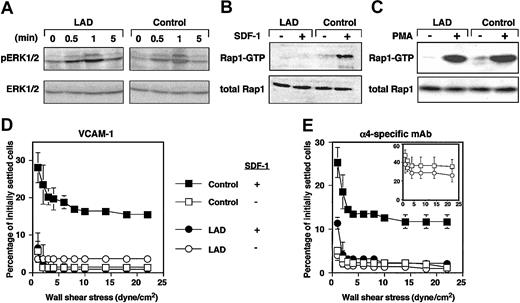
Chemokines and phorbol esters trigger, within seconds or minutes, Rap1 exchange of GDP for GTP.10,20 We therefore tested whether Rap1 derived from LAD cells exhibited defects in de novo activation by the prototypic chemokine, SDF-1α (CXCL12). Despite normal CXCR4 expression1 and SDF-1-triggered signaling monitored by phosphorylation of ERK1/2 (Figure 2A), SDF-1 activation of Rap1 was completely abolished in LAD EBV cells (Figure 2B). Strikingly, Rap1 activation by the phorbol ester, PMA, remained intact (Figure 2C). These findings rule out intrinsic activation defects of the GTPase in LAD cells resulting from improper GTP loading or abnormal GAP activities, which would have attenuated Rap1 activation by PMA in LAD cells. Indeed, we did not find abnormal expression of Spa1, a Rap1-specific lymphocyte GAP21 in LAD cells (data not shown). These results collectively suggest that a GPCR-regulated Rap1 GEF activity essential for Rap1 activation and VLA-4 avidity regulation is defective in LAD cells. Taken together with our previous findings on this LAD case,1 the present results indicate that basal and de novo chemokine-stimulated Rap1 activities control both spontaneous and chemokine-triggered VLA-4 adhesive activities in different hematopoietic cells. The possible cause of defective Rap1 activation could be dysfunctional GPCR-coupled Rap1-GEF. Calcium and diacyl-gycol (DAG)-regulated Rap1 GEFs (CalDAG-GEF) or phospholipase-C-generating DAG are candidates that could be involved in this LAD. However, we did not find significant differences in expression of phospholipase C isoforms including β1, β2, β3, γ1, or γ2 in LAD EBV cells (data not shown). The Rap1 GEF defective in LAD leukocytes is apparently distinct from the calcium and DAG-regulated Rap1 GEFs, CalDAG-GEFI or III, since the DAG analog PMA directly activates these GEFs and potently stimulated Rap1 activation in LAD EBV cells (Figure 2C). Alternatively, these GEFs may be normally expressed in LAD leukocytes but fail to undergo GPCR- or cytokine-dependent activation due to a loss of an essential scaffolding protein. Interestingly, GpIIbβ3 activation in LAD platelets by various GPCR agonists was largely abolished, despite normal collagen-induced activation of the β3 platelet integrin (M.A., in preparation, December 2003), suggesting defective Rap1 activation in platelets and their precursor cells, in addition to the defect observed here.
GPCR-mediated Rap1 activation and chemokine stimulation of VLA-4 avidity at prolonged contact are defective in LAD EBV cells. (A) A time course of SDF-1 stimulation (100 nM) of ERK1/2 phosphorylation in control or LAD EBV cells. Immunoblotting with anti-phosphospecific ERK1/2 (top row) and anti-ERK (bottom row) is depicted. SDF-1-induced ERK activation was blocked by pertussis toxin pretreatment of stimulated EBV cells (not shown). (B) Impaired SDF-1-induced Rap1 activation in LAD EBV cells. SDF-1-induced (100 nM, 30 seconds) stimulation of Rap1 activation in control and LAD cells. At this time point, maximal Rap1 activation was achieved in control cells. No Rap1 activation was observed in LAD cells at any SDF-1 dose between 0.5 to 5 minutes. (C) Similar PMA-induced Rap1 activation in control and LAD cells. Control and LAD cells were untreated (-) or treated (+) with 100 ng/mL PMA for 5 minutes, when maximal Rap1 activation was observed in both cell types. Rap1-GTP was detected with pull-down assays using GST-RalGDS-RBD (B-C, top panels). Total Rap1 is also shown (B-C, bottom panels). (D-E) Defective stimulation of VLA-4 avidity by surface-bound SDF-1 in LAD cells. Resistance to detachment by incremented shear forces by control or LAD cells settled for one minute at stasis on VCAM-1 (panel D, coated at 2 μg/mL) or on the α4-specific mAb HP1/2 (panel E, coated at 0.02 μg/mL), each coimmobilized with either inactivated SDF-1 (-) or intact SDF-1 (+), at 2 μg/mL. (E, inset) Shear resistance of control and LAD cells settled for 1 minute at stasis on high-density HP1/2 (coated at 0.2 μg/mL). Results are mean ± range of determinations in 2 fields and experiments are each representative of 3.
GPCR-mediated Rap1 activation and chemokine stimulation of VLA-4 avidity at prolonged contact are defective in LAD EBV cells. (A) A time course of SDF-1 stimulation (100 nM) of ERK1/2 phosphorylation in control or LAD EBV cells. Immunoblotting with anti-phosphospecific ERK1/2 (top row) and anti-ERK (bottom row) is depicted. SDF-1-induced ERK activation was blocked by pertussis toxin pretreatment of stimulated EBV cells (not shown). (B) Impaired SDF-1-induced Rap1 activation in LAD EBV cells. SDF-1-induced (100 nM, 30 seconds) stimulation of Rap1 activation in control and LAD cells. At this time point, maximal Rap1 activation was achieved in control cells. No Rap1 activation was observed in LAD cells at any SDF-1 dose between 0.5 to 5 minutes. (C) Similar PMA-induced Rap1 activation in control and LAD cells. Control and LAD cells were untreated (-) or treated (+) with 100 ng/mL PMA for 5 minutes, when maximal Rap1 activation was observed in both cell types. Rap1-GTP was detected with pull-down assays using GST-RalGDS-RBD (B-C, top panels). Total Rap1 is also shown (B-C, bottom panels). (D-E) Defective stimulation of VLA-4 avidity by surface-bound SDF-1 in LAD cells. Resistance to detachment by incremented shear forces by control or LAD cells settled for one minute at stasis on VCAM-1 (panel D, coated at 2 μg/mL) or on the α4-specific mAb HP1/2 (panel E, coated at 0.02 μg/mL), each coimmobilized with either inactivated SDF-1 (-) or intact SDF-1 (+), at 2 μg/mL. (E, inset) Shear resistance of control and LAD cells settled for 1 minute at stasis on high-density HP1/2 (coated at 0.2 μg/mL). Results are mean ± range of determinations in 2 fields and experiments are each representative of 3.
Rap1 is not constitutively associated with the plasma membrane of circulating lymphocytes and therefore probably cannot undergo de novo activation by chemokines at subsecond lymphocyte-endothelial contacts.10,20 Correspondingly, Rap1 does appear to contribute to subsecond SDF-1 triggering of VLA-4 tethers to low-density VCAM-1 or to the VLA-4-binding mAb, HP1/2, in EBV cells, since these tethers were normally triggered by SDF-1 in LAD EBV cells.1 In order to determine whether the de novo Rap1 activation defect perturbs VLA-4 activation by chemokine during more prolonged contacts, lymphocytes were settled for one minute on VCAM-1- or HP1/2-coated substrates containing SDF-1. Consistent with a role of Rap1 in SDF-1-stimulated VLA-4 adhesiveness at one-minute contacts, SDF-1-augmented VLA-4 adhesiveness to both ligand (Figure 2D) and immobilized VLA-4-binding mAb (Figure 2E) took place only in control but not in LAD EBV cells. Despite normal PMA stimulation of Rap1 in LAD EBV cells (Figure 2C), these cells failed to develop high-avidity VLA-4 binding to VCAM-1 in response to the pleotropic agonist.1 Collectively, these data suggest that de novo Rap1 activation by PMA is insufficient to overcome the constitutive defects in VLA-4 adhesiveness to VCAM-1 in the LAD lymphocytes. Although the constitutive defects in LAD VLA-4 adhesiveness were rescued on immobilized anti-VLA-4 mAb (inset in Figure 2E and data not shown), SDF-1 still failed to augment LAD VLA-4 adhesiveness to the mAb at one-minute static contacts (Figure 2E). This result is in agreement with the failure of SDF-1 to de novo activate Rap1. Taken together, our findings suggest that Rap1 effectors of VLA-4 and possibly of other integrins have a dual function in EBV lymphocytes. Rap1 apparently recycles between its perinuclear compartments and the plasma membrane where it modifies one or more of these cytoskeletal effectors, which in turn augment the ability of VLA-4 once occupied by VCAM-1 to stabilize and strengthen adhesion both under shear flow and at stationary contacts. These or other effectors may also translate exogenous chemokine and cytokine signals to Rap1 into further stimulation of integrin avidity at prolonged static contacts. Our results thus establish the first human genetic abnormality in Rap1 activation, which is linked to a profound defect in leukocyte integrin avidity regulation by ligand as well as by in situ GPCR-transduced activation. We propose that the designation LAD-III, which we have suggested for this syndrome,22 be used as a collective term for any similar or other congenital defect in leukocyte integrin activation of adhesion that is not the result of abnormal integrin expression or structure.
Prepublished online as Blood First Edition Paper, October 9, 2003; DOI 10.1182/blood-2003-07-2499.
Supported by the Israel Science Foundation and the Minerva Foundation, Germany (R.A.) and by a grant-in-aid from the Ministry of Education, Science, Sport, and Culture of Japan (T.K.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr S. Schwarzbaum for helpful discussions and editorial assistance. R. Alon is the Incumbent of the Tauro Career Development Chair in Biomedical Research.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal