Abstract
Plasmacytoid dendritic cells (pDCs) respond to unmethylated cytosine-phosphate-guanosine (CpG) motifs present in bacterial DNA or unmethylated synthetic oligodeoxynucleotides (CpG). In order to assess the function of pDCs in human newborns, interferon-α (IFN-α) production induced by CpG 2216 and phenotypic maturation of pDCs in response to CpG 2006 were compared in cord blood and adult blood. We first observed that neonatal pDCs displayed decreased up-regulation of CD80, CD83, CD86, and CD40, whereas HLA-DR and CD54 up-regulation did not differ significantly between adults and neonates. We then found that the production of IFN-α in response to CpG was dramatically impaired in cord blood. This neonatal defect was detected both at protein and mRNA levels and was still present in blood of 4-day-old babies. Further experiments on enriched pDCs confirmed that these cells are intrinsically deficient in CpG-induced IFN-α production at birth. These findings might be relevant to the increased susceptibility of human newborns to infections as well as to the use of CpG oligodeoxynucleotides as vaccine adjuvants in the neonatal period. (Blood. 2004;103:1030-1032)
Introduction
Human plasmacytoid dendritic cells (pDCs) represent the dominant source of type I interferon (IFN) among peripheral blood mononuclear cells (PBMCs) exposed to different microbial products or synthetic unmethylated cytosine-phosphate-guanosine (CpG) oligodeoxynucleotides (ODNs).1,2 Indeed, human pDCs express Toll-like receptor 9 (TLR9), which is responsible for recognition of CpG motifs present in bacterial DNA or ODNs.3,4 Recently, 2 classes of CpG ODNs with differential effects on pDCs were described. CpG-A ODNs were shown to trigger the production of high levels of type I IFN, whereas CpG-B ODNs elicited only low levels of type I IFN but induced efficient pDC maturation as evidenced by the up-regulation of major histocompatibility complex (MHC) class II and costimulatory molecules.5
In line with their action on pDCs, CpG ODNs were shown to be strong inducers of T helper 1 (Th1)-type and cytotoxic T-cell responses.5 CpG ODNs are therefore considered as potential adjuvants to enhance immune responses to vaccines aiming at the induction of cell-mediated immunity.6 A recent study in mice suggested that CpG might be of special interest for early life immunization, which is hampered by neonatal defects of antigen-presenting cells (APCs) and Th2 polarization of neonatal T-cell responses.7,8 Indeed, administration of CpG ODNs was shown to induce interleukin-12 (IL-12) release, to overrule Th2 polarization, and to allow Th1 responses to vaccine antigens in mouse newborns.9 However, the clinical significance of this observation requires further investigation as murine and human DC subsets differ in their expression of TLR and their responses to CpG ODNs.5,10,11 We therefore decided to explore the responses of human neonatal pDCs to CpG ODNs. The impaired maturation of pDCs induced by CpG-B ODNs in cord blood led us to further compare the production of IFN-α elicited by CpG-A in neonatal versus adult blood.
Study design
Adult fresh blood was obtained from healthy volunteers and umbilical cord blood samples were collected from healthy full-term neonates at the department of obstetrics of the Erasme Hospital (Brussels, Belgium). Venous blood samples (1 mL) were also obtained from a series of 4-day-old neonates after parental agreement. All procedures were approved by the ethics committee of the faculty of medicine at the Université Libre de Bruxelles.
Phenotype of plasmacytoid DCs and IFN-α production in whole blood
Whole blood (1 mL) was incubated at 37°C with 50 μg/mL CpG 2006 (CpG-A ODN) or CpG 2216 (CpG-B ODN). The following ODNs were provided by Tib Molbiol (Berlin, Germany): ODN 2216, 5′-ggGGGACGATCGTCgggggG-3′; ODN 2006, 5′-TCgTCgTTTTgTCgTTTTgTCgTT-3′. As a control, we used the following nonstimulatory GpC ODN: 5′-TgCTgCTTTT gTgCTTTTgTgCTT. The ODN concentration used in the whole-blood assays was selected on the basis of a previous dose-response study.12 After overnight incubation with CpG 2006, phenotype of plasmacytoid DCs was analyzed by 4-color flow cytometry as previously described.13 Briefly, whole-blood cells were incubated for 15 minutes at 4°C with 7 μL of a cocktail of fluorescein isothiocyanate (FITC)-conjugated monoclonal antibodies (mAbs) specific for CD3, CD14, CD16, CD19, CD20, CD34, and CD56 receptors; 5 μL APC-conjugated anti-CD11c mAb; 5 μL peridinin chlorophyll alpha protein (PerCP)-conjugated anti-HLADR mAb; and one of the following phycoerythrin (PE)-conjugated mAbs: anti-CD123, anti-CD80, anti-CD86, anti-CD54 (Becton Dickinson, Mountain View, CA), anti-CD40 mAb (Biosource Europe, Nivelles, Belgium), or anti-CD83 mAb (ImmunoTech, Marseille, France). Expression of HLA-DR, CD40, CD54, CD80, CD83, and CD86 was quantified on lineage-/HLADR+/CD11c- DCs after appropriate gating. The relative frequencies of pDCs in blood were expressed as the percentages of lineage-/HLADR+/CD11c- DCs among the mononuclear cells gated during acquisition according to forward scatter (FSC)/side scatter (SSC) criteria. In parallel, plasma was collected for determination of IFN-α levels by a commercial enzyme-linked immunosorbent assay (ELISA) kit with a detection threshold of 10 pg/mL (Biosource Europe). In some experiments, plasmacytoid dendritic cells (pDCs) were enriched from mononuclear cells obtained by Ficoll-Hypaque centrifugation, followed by magnetic cell sorting (MACS) using microbeads coated with anti-BDCA-4 mAb (blood dendritic cell antigen [BDCA-4] dendritic cell isolation kit; Miltenyi Biotec, Palo Alto, CA). Resulting cell preparations routinely contained more than 50% BDCA-2+ CD123+ cells as measured by staining with PE-conjugated anti-CD123 mAb and FITC-conjugated mAb specific for BDCA-2 (Miltenyi Biotec). Enriched pDCs were then stimulated for 48 hours with CpG 2216 or control ODNs (5 × 104 cells in 200 μL medium containing 10 μg/mL ODNs), followed by determination of IFN-α levels in culture supernatants by ELISA.
Quantification of IFN-α mRNA in whole blood by real-time PCR
β-Actin and IFN-α mRNA quantification in whole blood was performed using a method previously described.14,15 Briefly, mRNA was extracted from whole blood samples using the MagNa Pure LC mRNA Isolation Kit (Roche Diagnostics, Brussels, Belgium). Reverse transcription (RT) and real-time polymerase chain reaction (PCR) were then carried out using LightCycler-RNA Master Hybridization Probes (one-step procedure) on a Lightcycler device (Roche Diagnostics). The oligonucleotide sequences for IFN-α2A used were as follows: forward 5′-TGCCTGAAGGACAGACATGA-3′, reverse 5′-GGAGGACAGAGATGGCTTGA-3′; probe: 5′-(6-Fam)TGGTTGCCATCAAACTCCTCCTGG(Tamra [tetramethyl rhodamine])(phosphate)-3′. Primers used for β-actin were described previously.14
Statistical analysis
Data were compared using unpaired Mann-Whitney U test.
Results and discussion
Incomplete maturation of neonatal plasmacytoid DCs exposed to CpG
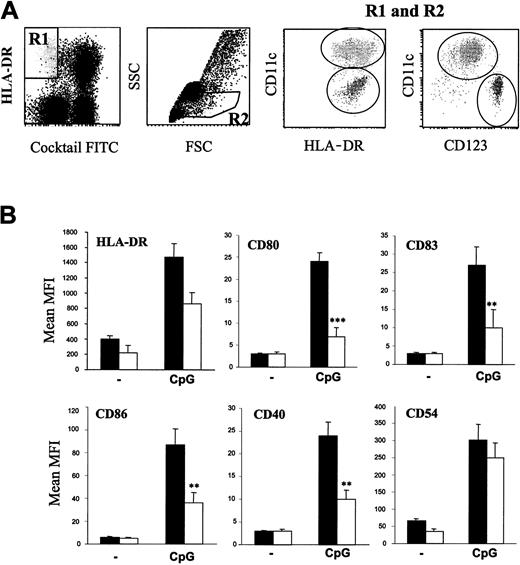
In order to mimic as much as possible the in vivo environment, the effects of CpG ODNs on pDCs were analyzed in whole blood. As shown in Figure 1A, we first verified that the vast majority of the lineage-/HLA-DR+/CD11c- cells were CD123high and therefore correspond to pDCs. We next observed using these flow cytometry parameters that the relative frequencies of pDCs in adult and neonatal blood were similar. Indeed, percentages (mean ± SEM) of pDCs among mononuclear cells were 0.90 ± 0.2% (n = 5) and 0.74 ± 0.2% (n = 5) in adult and cord blood, respectively. On the basis of previous studies,5 CpG-B ODN 2006 was selected to induce phenotypic maturation of pDCs. As shown in Figure 1B, CpG-B indeed induced a clear up-regulation of HLA-DR, CD80, CD83, CD86, CD40, and CD54 on pDCs in adult blood. In cord blood, neonatal pDCs also responded to CpG as all markers of maturation were significantly up-regulated compared with resting pDCs (P < .01 for each surface molecule). However, the intensity of the changes depended on the surface molecules considered. Whereas CpG-induced CD54 and HLA-DR up-regulation did not significantly differ between neonatal and adult DCs, the magnitude of CD80, CD83, CD86, and CD40 up-regulation was lower in cord blood pDCs (Figure 1B).
Flow cytometry analysis of pDCs in whole blood. (A) Blood DCs were gated from whole-blood cells by assembling R1 and R2 regions. Plasmacytoid DCs were identified as CD11c-/HLA-DR+ and CD123high. (B) Cord blood and adult blood samples (1 mL) were incubated overnight with or without 50 μg CpG 2006 and analyzed by flow cytometry for surface expression of the indicated molecules on pDCs identified as Lin-/CD11c-/HLA-DR+ cells. Histograms represent the mean ± SEM of median fluorescence intensities in 10 independent experiments on different donors. **P < .01; ***P < .001.
Flow cytometry analysis of pDCs in whole blood. (A) Blood DCs were gated from whole-blood cells by assembling R1 and R2 regions. Plasmacytoid DCs were identified as CD11c-/HLA-DR+ and CD123high. (B) Cord blood and adult blood samples (1 mL) were incubated overnight with or without 50 μg CpG 2006 and analyzed by flow cytometry for surface expression of the indicated molecules on pDCs identified as Lin-/CD11c-/HLA-DR+ cells. Histograms represent the mean ± SEM of median fluorescence intensities in 10 independent experiments on different donors. **P < .01; ***P < .001.
CpG-induced IFN-α secretion is deficient in neonatal blood
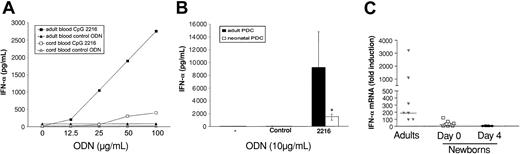
According to previous studies,2,5 the CpG-A 2216 was used to elicit IFN-α secretion. First, we established in adult whole blood that CpG 2216 induced the production of IFN-α in a dose-dependent manner. In contrast, IFN-α levels remained low in cord blood whatever the CpG dose considered (Figure 2A). Indeed, after addition of 50 μg/mL CpG to 18 adult blood and 35 cord blood samples, IFN-α plasma levels expressed as median (25th-75th quantiles) were 360 (267-582) pg/mL and 39 (0-167) pg/mL in adults and newborns, respectively.
IFN-α production is inhibited in neonates. (A) Induction of IFN-α release in whole blood by CpG 2216. Adult and cord blood samples were cultured with graded doses of CpG 2216 or control ODNs. After 20 hours, plasma samples were assayed by ELISA for detection of IFN-α levels. Shown is 1 representative experiment out of 3. (B) IFN-α production by enriched pDCs upon stimulation with CpG 2216. Adult and neonatal enriched pDCs were cultured with 10 μg/mL CpG 2216 or control ODNs. After 48 hours, supernatants were assayed by ELISA for detection of IFN-α levels. The figure shows medians (25th-75th quantiles) of 6 adult samples and 6 cord blood samples. *P < .05. (C) Determination of IFN-α mRNA levels by real-time PCR. Whole blood (1 mL) was incubated in the absence or presence of 50 μg CpG 2216. After 20 hours, mRNA was extracted, reverse transcribed, and amplified by real-time PCR using primers specific for IFN-α and β-actin. IFN-α mRNA levels were quantified and normalized against β-actin levels. The figure shows individual values and medians of 7 adult samples, 10 cord blood samples, and 8 blood samples from 4-day-old babies.
IFN-α production is inhibited in neonates. (A) Induction of IFN-α release in whole blood by CpG 2216. Adult and cord blood samples were cultured with graded doses of CpG 2216 or control ODNs. After 20 hours, plasma samples were assayed by ELISA for detection of IFN-α levels. Shown is 1 representative experiment out of 3. (B) IFN-α production by enriched pDCs upon stimulation with CpG 2216. Adult and neonatal enriched pDCs were cultured with 10 μg/mL CpG 2216 or control ODNs. After 48 hours, supernatants were assayed by ELISA for detection of IFN-α levels. The figure shows medians (25th-75th quantiles) of 6 adult samples and 6 cord blood samples. *P < .05. (C) Determination of IFN-α mRNA levels by real-time PCR. Whole blood (1 mL) was incubated in the absence or presence of 50 μg CpG 2216. After 20 hours, mRNA was extracted, reverse transcribed, and amplified by real-time PCR using primers specific for IFN-α and β-actin. IFN-α mRNA levels were quantified and normalized against β-actin levels. The figure shows individual values and medians of 7 adult samples, 10 cord blood samples, and 8 blood samples from 4-day-old babies.
In parallel, we verified that a nonstimulatory control ODN did not elicit IFN-α synthesis in adult or cord blood (Figure 2A).
In order to exclude the presence of an inhibitory factor in cord blood, we then compared the effects of CpG 2216 on pDCs enriched from adult and cord blood. Using the BDCA-4 isolation kit, we obtained cell populations containing 50% to 90% pDCs. First, we confirmed that pDCs represent the major source of IFN-α upon CpG stimulation as PBMC depletion of BDCA-4-positive cells decreased the production of IFN-α by more than 95% (data not shown). The neonatal defect in IFN-α production was still present when enriched pDCs were used as responders as shown in Figure 2B. These data are in line with previous studies that documented a decreased production of IFN-α by cord blood mononuclear cells exposed to viruses.16,17
In a final set of experiments, we used a quantitative RT-PCR method to determine whether the neonatal defect in IFN-α production was already apparent at the mRNA level. As this method requires only small volumes of blood, it could also be applied to samples from 4-day-old neonates. On the basis of preliminary experiments on adult blood, IFN-α mRNA levels were measured after 20 hours of incubation with CpG 2216. As shown in Figure 2C, a major defect in CpG-induced IFN-α mRNA accumulation was found in neonatal blood collected either at birth or 4 days after delivery (in both cases, P < .001 compared with adults).
We conclude that beside the reduced production of IFN-γ production by CD4+ T cells18,19 and the impaired synthesis of IL-12p70 by myeloid DCs,12,20 deficient responses of pDCs also contribute to the immaturity of cell-mediated immunity in human newborns. These findings might be relevant to the increased susceptibility to infections as well as to the use of CpG oligodeoxynucleotides as vaccine adjuvants in the neonatal period.
Prepublished online as Blood First Edition Paper, September 22, 2003; DOI 10.1182/blood-2003-04-1216.
Supported by the Centre Interuniversitaire de Vaccinologie sponsored by GSK Biologicals and the Région Wallonne, the Neovac project of the European Commission, and an Interuniversity Attraction Pole of the Belgian government. S.G. is a research fellow of the FNRS (Fonds National de la Recherche Scientifique).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal