Abstract
Despite the success of human leukocyte antigen (HLA) typing in allogeneic stem cell transplantation (SCT) it is rare to find an unrelated donor that is perfectly matched, making identification of “permissive” mismatches of paramount importance. Here, we describe novel associations between donor T-cell cytokine production during donor-antipatient mixed lymphocyte reactions (MLRs) and acute graft-versus-host disease (aGVHD). The data reveal positive correlations between both Th1-type and Th2-type cytokine production and GVHD and the assay established could potentially represent a useful tool for identification of permissible unrelated SCT donors. Associations between interleukin 13 (IL-13) levels and aGVHD were by far the strongest predictor of a GVHD (P = .0002). All patients suffering severe (grade III) aGVHD following SCT had donors who produced very high pretransplantation IL-13 responses, while those developing little or no aGVHD (grades 0-I) produced no IL-13 at all. IL-13 levels were independent of all other cytokines measured as well as cytotoxic T-lymphocyte precursor (CTLp) frequencies. The cytokines IL-5, interferon γ (IFN-γ), and tumor necrosis factor α (TNF-α) also predicted development of aGVHD (P < .05 for all 3), appearing to be coproduced in the assay and correlating with estimated CTLp frequencies. The data challenge the notion that aGVHD is purely a Th1-type cytokine-driven response, high-lighting a novel and highly significant link between the Th2-type cytokine IL-13 and aGVHD.
Introduction
Following stem cell transplantation (SCT), acute graft-versus-host disease (aGVHD) remains a serious cause of morbidity and mortality1,2 with its occurrence and severity being directly related to the degree of human leukocyte antigen (HLA) incompatibility between the patient and donor as well as to the presence of certain minor histocompatibility antigens. Therefore, an HLA-identical sibling is the marrow donor of choice. Unfortunately, such related matched donors are only available to approximately 30% of all patients who could benefit from SCT. The remaining 70% of patients require an alternative donor, such as an HLA “matched” volunteer unrelated donor (VUD).3-7
Most patients who require VUD SCT have a slim chance of finding a perfectly matched HLA donor. Although many patients find a donor matched for HLA-A, -B, and -DR alleles (60% of unrelated transplants), retrospective analysis using modern high-resolution typing techniques for HLA-A, -B, -C, -DR, -DP, and -DQ has shown the chance of finding a “perfectly HLA-matched” unrelated donor is in fact less than 10%.8 Thus, patients requiring unrelated SCT tend to have several potential donors available to them that are mismatched at one or more HLA loci and for many minor histocompatibility antigens.
It is well documented that some HLA mismatches can be well tolerated during SCT, leading to the concept that certain mismatches are “permissive.”9 Unfortunately, HLA typing does not provide the qualitative information needed to discriminate between individuals from these panels of prospective donors at the functional (GVH) level, so in order to avoid aGVHD, T-cell depletion procedures are often used that in turn give rise to transplantation problems including an increased chance of relapse. In order to identify permissible mismatches, a number of in vitro (donor-antipatient) cellular assays have previously been examined for the purpose of “fine-tuning” donor selection following initial HLA matching. These have included limiting dilution analysis (LDA) for measurement of cytotoxic T-lymphocyte precursor (CTLp) and helper T-lymphocyte precursor (HTLp) frequencies10-12 that has a greater sensitivity than the original assay measuring bulk mixed lymphocyte reaction (MLR) proliferation.13-19 The major factor that has prevented these LDA assays from being taken up for routine clinical prognostic use is the fact that they require a high degree of technical expertise and are time consuming to perform. Thus, there is a need for a simple reliable alternative.1
Recently we have re-examined the alloresponse with the aim of creating an assay capable of predicting aGVHD while remaining simple to perform. Through defining the optimal analysis of MLR cytokine responses we have revealed that the human alloresponse is far more heterogeneous than previously thought, with type-2 cytokines playing a major part in the overall composite cytokine profile produced.20 In particular, our studies identified interleukin 13 (IL-13) as a typical type 2 cytokine that is produced in abundance during unrelated, unmatched MLRs.
The potential benefits of measurement of cytokine profiles over traditional MLR proliferation or LDA for HTLp or CTLp frequencies are significant. Cytokine profiles provide both quantitative (cytokine levels) and qualitative (balance between type-1 and type-2 cytokines) data. The donor-antipatient MLR described here is both more sensitive and simpler to perform than the LDA currently used to complement HLA typing prior to SCT. The assay does not rely on indicator cell lines as required for the HTLp LDA or the use of radioactive isotopes used during CTLp LDA and uses commercially available antibodies for the enzyme-linked immunosorbent assays (ELISAs) that are easily standardized for consistency in different laboratories.
We have now tested pretransplantation donor-antipatient MLR responses through analysis of a broad composite Th1- and Th2-type profile for their potential value in the fine-tuning of donor selection prior to bone marrow transplantation (BMT). The data reveal that a “cluster” of cytokines, IL-5, tumor necrosis factor α (TNF-α), and interferon γ (IFN-γ) are coproduced in the assay and correlate with CTLp frequencies, while IL-13 levels provide an independent prognostic indicator that has by far the most significant association with aGVHD. Together, these studies reveal a complexity to the cytokine response during alloreactivity that was previously unrealized and should lead to a better understanding of the immune mechanisms regulating aGVHD and the development of an important new tool for the selection of unrelated donors for BMT.
Patients, materials, and methods
Culture conditions
For all experiments, complete culture medium consisted of RPMI 1640 supplemented with 100 IU/mL penicillin, 100 μg/mL streptomycin, 2 mM l-glutamine, and 1 mM pyruvate (all from Gibco Life Technologies, Paisley, United Kingdom). Cultured cells were maintained at 37°C in a humidified atmosphere of 5% CO2. During cell culture this medium was supplemented with 10% serum (Sigma, Poole, United Kingdom) heat inactivated at 56°C for 30 minutes prior to use.
Preparation of cells
Peripheral whole blood was collected in heparinized tubes (1 IU/mL blood) and mononuclear cells were isolated using Histopaque-1077 (Sigma) according to the manufacturer's instructions. For some experiments purified T cells were prepared using negative selection as previously described.20 In brief, whole peripheral blood mononuclear cells (PBMCs) were incubated in complete medium in tissue culture flasks for 30 minutes at 37°C and nonadherent cells were harvested. These cells were then resuspended in ice-cold complete medium containing an antibody cocktail (each at a final concentration of 10 μg/mL) of anti-CD11b, anti-CD19, anti-CD56, and anti-CD14 (DAKO, High Wycombe, United Kingdom). For preparation of CD4+ or CD8+ T cells either monoclonal antibody (mAb) OKT4 (anti-CD4) or UCHT1 (anti-CD8) was also included to remove either the helper (CD4+) or cytotoxic (CD8+) T cells. Excess antibody was then removed through 3 rounds of washing and T cells were purified using immunomagnetic beads coated with sheep antimouse immunoglobulin G (IgG; DYNAL, Oslo, Norway) according to the manufacturer's instructions. Purity of the T cells was confirmed to be more than 95% in each experiment using flow cytometry analysis.
Mixed lymphocyte reaction
For MLR cultures, responder cells were incubated with irradiated (30 Gy) allogeneic stimulator PBMCs (both at 1 × 105/well) in a total volume of 200 μL in 96-well round-bottomed plates (Nunc, Rochester, NY). Supernatants from quadruplicate wells were harvested on day 7 as previously optimized for the cytokines being investigated in this study20 and frozen at –30°C for later cytokine analysis.
Assessing the measurement of MLR cytokines for examining donor-antipatient responses
For testing the sensitivity of the assay, whole PBMCs were taken from SCT patients and each prospective donor for that patient that had been identified through preliminary HLA matching. Individual MLRs were performed using each of the prospective donors reacting against patient PBMCs in the MLR and cytokine profiles assessed in order to ascertain whether responses could distinguish between potential donors at the functional level both qualitatively (Th1-type versus Th2-type cytokine production) and quantitatively (cytokine levels). Further to this, the MLR cytokine response was examined for its capability of producing data that reflect both major histocompatibility complex (MHC) class I and class II differences between donor and patient. MLRs were performed using highly purified CD4+ or CD8+ donor T cells purified from whole PBMCs and responses compared to assess both helper (CD4+) and cytotoxic T-cell (CD8+) responses.
Patients and donors
For analysis of relationships between cytokine responses and aGVHD, sufficient stored PBMCs were available to study 24 patients with a diagnosis of chronic myelogenous leukemia (CML) who received VUD BMT from between February 22, 1995 and June 28, 1998 at the Hammersmith Hospital. At time of transplantation, 14 of the patients were in first chronic phase (CP), 6 were in accelerated phase (AP), and 4 were in blast crisis (BC). Informed consent was obtained from all participants, and approval for the study was obtained from the institutional review board of Hammersmith hospital. Pretransplantation conditioning regimens, GVHD prophylaxis, and management were as previously described.21 Donor identification involved HLA typing for class I antigens by serologic methods and DNA typing for DR and DQ alleles.19 In addition, the frequency in the donor's peripheral blood of alloreactive CTLp was used in donor selection.19 Further details of patients and donors are shown in Table 1 and of HLA typing in Table 2.
Details of patients and donors and transplantation outcome
Patient no. . | . | . | Disease state . | Survival . | . | Relapse . | . | Donor sex . | Patient CMV† . | Donor CMV† . | Acute GVHD‡ . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Sex . | Age, y . | . | Status* . | Days . | Status . | Days . | . | . | . | . | ||
| 1 | F | 34 | CP | 1 | 152 | — | — | M | + | + | 2 | ||
| 2 | M | 30 | CP | 0 | 2159 | — | — | M | - | - | 1 | ||
| 3 | M | 23 | CP | 0 | 1637 | Hem | 179 | M | - | - | 2 | ||
| 4 | M | 37 | CP | 1 | 134 | — | — | M | + | + | 1 | ||
| 5 | M | 21 | CP | 0 | 1697 | Hem | 297 | M | + | - | 2 | ||
| 6 | F | 43 | CP | 1 | 47 | Gf | 47 | M | - | - | 0 | ||
| 7 | M | 36 | CP | 1 | 69 | — | — | M | - | - | 2 | ||
| 8 | M | 23 | CP | 1 | 474 | Cyto | 291 | F | - | - | 3 | ||
| 9 | F | 16 | CP | 0 | 1270 | Cyto | 171 | M | - | - | 2 | ||
| 10 | F | 16 | CP | 0 | 886 | — | — | F | - | - | 2 | ||
| 11 | M | 35 | CP | 0 | 640 | Cyto | 140 | M | - | - | 1 | ||
| 12 | F | 39 | CP | 1 | 357 | — | — | F | - | + | 2 | ||
| 13 | M | 44 | CP | 0 | 465 | Hem | 154 | F | - | + | 1 | ||
| 14 | F | 35 | CP | 1 | 171 | — | — | M | + | - | 0 | ||
| 15 | F | 27 | AP | 0 | 1914 | Cyto | 182 | M | - | - | 1 | ||
| 16 | M | 23 | AP | 1 | 1263 | Cyto | 189 | F | + | + | 3 | ||
| 17 | F | 38 | AP | 1 | 234 | — | — | M | - | - | 2 | ||
| 18 | F | 15 | AP | 0 | 1610 | Hem | 258 | M | + | + | 2 | ||
| 19 | F | 39 | AP | 1 | 370 | Hem | 80 | F | - | - | 3 | ||
| 20 | F | 49 | AP | 1 | 57 | — | — | F | - | - | 3 | ||
| 21 | M | 36 | BC | 1 | 168 | — | — | M | - | + | 2 | ||
| 22 | F | 45 | BC | 1 | 189 | — | — | F | - | + | 3 | ||
| 23 | M | 26 | BC | 1 | 131 | Trans | 119 | M | - | + | 2 | ||
| 24 | M | 18 | BC | 1 | 198 | Hem | 147 | M | - | - | 2 | ||
Patient no. . | . | . | Disease state . | Survival . | . | Relapse . | . | Donor sex . | Patient CMV† . | Donor CMV† . | Acute GVHD‡ . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Sex . | Age, y . | . | Status* . | Days . | Status . | Days . | . | . | . | . | ||
| 1 | F | 34 | CP | 1 | 152 | — | — | M | + | + | 2 | ||
| 2 | M | 30 | CP | 0 | 2159 | — | — | M | - | - | 1 | ||
| 3 | M | 23 | CP | 0 | 1637 | Hem | 179 | M | - | - | 2 | ||
| 4 | M | 37 | CP | 1 | 134 | — | — | M | + | + | 1 | ||
| 5 | M | 21 | CP | 0 | 1697 | Hem | 297 | M | + | - | 2 | ||
| 6 | F | 43 | CP | 1 | 47 | Gf | 47 | M | - | - | 0 | ||
| 7 | M | 36 | CP | 1 | 69 | — | — | M | - | - | 2 | ||
| 8 | M | 23 | CP | 1 | 474 | Cyto | 291 | F | - | - | 3 | ||
| 9 | F | 16 | CP | 0 | 1270 | Cyto | 171 | M | - | - | 2 | ||
| 10 | F | 16 | CP | 0 | 886 | — | — | F | - | - | 2 | ||
| 11 | M | 35 | CP | 0 | 640 | Cyto | 140 | M | - | - | 1 | ||
| 12 | F | 39 | CP | 1 | 357 | — | — | F | - | + | 2 | ||
| 13 | M | 44 | CP | 0 | 465 | Hem | 154 | F | - | + | 1 | ||
| 14 | F | 35 | CP | 1 | 171 | — | — | M | + | - | 0 | ||
| 15 | F | 27 | AP | 0 | 1914 | Cyto | 182 | M | - | - | 1 | ||
| 16 | M | 23 | AP | 1 | 1263 | Cyto | 189 | F | + | + | 3 | ||
| 17 | F | 38 | AP | 1 | 234 | — | — | M | - | - | 2 | ||
| 18 | F | 15 | AP | 0 | 1610 | Hem | 258 | M | + | + | 2 | ||
| 19 | F | 39 | AP | 1 | 370 | Hem | 80 | F | - | - | 3 | ||
| 20 | F | 49 | AP | 1 | 57 | — | — | F | - | - | 3 | ||
| 21 | M | 36 | BC | 1 | 168 | — | — | M | - | + | 2 | ||
| 22 | F | 45 | BC | 1 | 189 | — | — | F | - | + | 3 | ||
| 23 | M | 26 | BC | 1 | 131 | Trans | 119 | M | - | + | 2 | ||
| 24 | M | 18 | BC | 1 | 198 | Hem | 147 | M | - | - | 2 | ||
M indicates male; F, female; CP, chronic phase; AP, accelerated phase; BC, blast crises; —, remission; Cyto, cytogenetic relapse; Hem, hematopoietic relapse; Trans, transient cytogenetic relapse; and Gf, graft failure.
1 indicates dead; and 0, alive.
- indicates negative; +, positive.
0 indicates none; 1, grade I; 2, grade II; and 3, grade III.
Patent and donor HLA types
Transplant . | HLA-A . | HLA-B . | HLA-Bw . | HLA-Cw . | HLA-DR . | HLA-DRB . | HLA-DQ . |
|---|---|---|---|---|---|---|---|
| No. 1 | |||||||
| Donor | 2 | 27,60 | 4,6 | NT | 4 | 53 | 3 |
| Patient | 2 | 27,60 | 4,6 | NT | 4 | NT | NT |
| No. 2 | |||||||
| Donor | 2,3 | 35 | 6 | NT | 0104,4 | NT | 1 |
| Patient | 2,3 | 35 | NT | 4 | 1 | NT | 5 |
| No. 3 | |||||||
| Donor | 1 | 8,37 | 4,6 | 6,7 | 1,15 | 51 | NT |
| Patient | 1 | 8,37 | 4,6 | NT | 1,15 | 51 | NT |
| No. 4 | |||||||
| Donor | 1,11 | 8,35 | 6 | 4,7 | 1,17 | 52 | 5,2 |
| Patient | 1,11 | 8,35 | NT | NT | 1,17 | 52 | NT |
| No. 5 | |||||||
| Donor | 2 | 7,60 | 6 | NT | 1501,0401 | 51 | NT |
| Patient | 2 | 7,60 | 6 | NT | 1501,0401 | 51,53 | NT |
| No. 6 | |||||||
| Donor | 2,29 | 44,14 | NT | NT | 13,b1 | NT | NT |
| Patient | 2,29 | 44,14 | NT | NT | 13,b1 | NT | NT |
| No. 7 | |||||||
| Donor | 2,24 | 7,44 | 6 | NT | 1501,9 | 51,53 | 6,9 |
| Patient | 2,24 | 7,44 | 4,6 | NT | 1501,9 | 51,53 | 6,9 |
| No. 8 | |||||||
| Donor | 2,28 | 51,53 | 4 | NT | 0403,1302 | 52,53 | 6,8 |
| Patient | 2,28 | 51,53 | 4 | NT | 0405,1302 | 52,53 | 6,8 |
| No. 9 | |||||||
| Donor | 3,25 | 7,44 | 4,6 | NT | 1501 | 51 | 6 |
| Patient | 3,25 | 7,44 | 4,6 | NT | 1501 | 51 | 6 |
| No. 10 | |||||||
| Donor | 2 | 18 | 6 | NT | 0110,11301 | 52 | 6,7 |
| Patient | 2 | 18 | 6 | NT | 0110,41301 | 52 | 6,7 |
| No. 11 | |||||||
| Donor | 1,2 | 8 | NT | 0701,0702 | 0301 | NT | 2 |
| Patient | 1,2 | 8 | NT | 701 | 301 | NT | 2 |
| No. 12 | |||||||
| Donor | 3,33 | 8,44 | NT | 0701,5 | 0401,1301 | NT | 6,7 |
| Patient | 3,34 | 8,44 | NT | 0701,0702 | 4,1301 | NT | 6,8 |
| No. 13 | |||||||
| Donor | 2 | 8,56 | NT | 1,0701 | 0301,0801 | NT | 2,4 |
| Patient | 2 | 8,56 | NT | 1,0701 | 0301,0801 | NT | NT |
| No. 14 | |||||||
| Donor | 2,26 | 44 | 5,9 | NT | 0401,1103 | NT | 7 |
| Patient | 2,26 | 44 | 5,9 | NT | 0401,1103 | 53,52 | 9,7 |
| No. 15 | |||||||
| Donor | 2,29 | 44,35 | 4,6 | NT | 0101,1104 | 52 | 1,7 |
| Patient | 2,29 | 44,35 | 4,6 | NT | 1,11 | 52 | 1,7 |
| No. 16 | |||||||
| Donor | 2,9(24) | 44,35 | 4,6 | NT | 0401,1401 | 52,53 | NT |
| Patient | 2,24 | 44,35 | 4,6 | NT | 4,14 | 52,53 | NT |
| No. 17 | |||||||
| Donor | NT | NT | NT | NT | NT | NT | NT |
| Patient | NT | NT | NT | NT | NT | NT | NT |
| No. 18 | |||||||
| Donor | 29,31 | 51,44 | 4 | NT | 0701,0801 | 53 | 2,4 |
| Patient | 29,31 | 51,44 | 4 | NT | 0701,0801 | 53 | 2,4 |
| No. 19 | |||||||
| Donor | 1,2 | 8,39 | 6 | NT | 0301,1201 | 52 | NT |
| Patient | 1,2 | 8,39 | 6 | NT | 0301,1201 | 52 | 2,7 |
| No. 20 | |||||||
| Donor | 2,3 | 51,62 | 4,6 | NT | 0101,0801 | NT | 4,5 |
| Patient | 2,3 | 51,62 | 4,6 | 3 | 0101,0803 | NT | 5,7 |
| No. 21 | |||||||
| Donor | 2,11 | 35,60 | 6 | NT | 1,4 | 53 | NT |
| Patient | 2,11 | 35,60 | 6 | NT | 1,4 | 53 | 5,8 |
| No. 22 | |||||||
| Donor | 2,31 | 44,35 | 4,6 | NT | 0401,0403 | 53 | NT |
| Patient | 2,31 | 44,35 | 4,6 | NT | 4 | 53 | NT |
| No. 23 | |||||||
| Donor | 1,28 | 35,14 | 6 | NT | 1,14,b1 | NT | NT |
| Patient | 1,28 | 35,14 | 6 | NT | 1,14,b1 | NT | NT |
| No. 24 | |||||||
| Donor | 3,26 | 7,35 | 6 | NT | 1501,1104 | 51,52 | 6,7 |
| Patient | 3,26 | 7,35 | 6 | NT | 1501,1104 | 51,52 | 6,7 |
Transplant . | HLA-A . | HLA-B . | HLA-Bw . | HLA-Cw . | HLA-DR . | HLA-DRB . | HLA-DQ . |
|---|---|---|---|---|---|---|---|
| No. 1 | |||||||
| Donor | 2 | 27,60 | 4,6 | NT | 4 | 53 | 3 |
| Patient | 2 | 27,60 | 4,6 | NT | 4 | NT | NT |
| No. 2 | |||||||
| Donor | 2,3 | 35 | 6 | NT | 0104,4 | NT | 1 |
| Patient | 2,3 | 35 | NT | 4 | 1 | NT | 5 |
| No. 3 | |||||||
| Donor | 1 | 8,37 | 4,6 | 6,7 | 1,15 | 51 | NT |
| Patient | 1 | 8,37 | 4,6 | NT | 1,15 | 51 | NT |
| No. 4 | |||||||
| Donor | 1,11 | 8,35 | 6 | 4,7 | 1,17 | 52 | 5,2 |
| Patient | 1,11 | 8,35 | NT | NT | 1,17 | 52 | NT |
| No. 5 | |||||||
| Donor | 2 | 7,60 | 6 | NT | 1501,0401 | 51 | NT |
| Patient | 2 | 7,60 | 6 | NT | 1501,0401 | 51,53 | NT |
| No. 6 | |||||||
| Donor | 2,29 | 44,14 | NT | NT | 13,b1 | NT | NT |
| Patient | 2,29 | 44,14 | NT | NT | 13,b1 | NT | NT |
| No. 7 | |||||||
| Donor | 2,24 | 7,44 | 6 | NT | 1501,9 | 51,53 | 6,9 |
| Patient | 2,24 | 7,44 | 4,6 | NT | 1501,9 | 51,53 | 6,9 |
| No. 8 | |||||||
| Donor | 2,28 | 51,53 | 4 | NT | 0403,1302 | 52,53 | 6,8 |
| Patient | 2,28 | 51,53 | 4 | NT | 0405,1302 | 52,53 | 6,8 |
| No. 9 | |||||||
| Donor | 3,25 | 7,44 | 4,6 | NT | 1501 | 51 | 6 |
| Patient | 3,25 | 7,44 | 4,6 | NT | 1501 | 51 | 6 |
| No. 10 | |||||||
| Donor | 2 | 18 | 6 | NT | 0110,11301 | 52 | 6,7 |
| Patient | 2 | 18 | 6 | NT | 0110,41301 | 52 | 6,7 |
| No. 11 | |||||||
| Donor | 1,2 | 8 | NT | 0701,0702 | 0301 | NT | 2 |
| Patient | 1,2 | 8 | NT | 701 | 301 | NT | 2 |
| No. 12 | |||||||
| Donor | 3,33 | 8,44 | NT | 0701,5 | 0401,1301 | NT | 6,7 |
| Patient | 3,34 | 8,44 | NT | 0701,0702 | 4,1301 | NT | 6,8 |
| No. 13 | |||||||
| Donor | 2 | 8,56 | NT | 1,0701 | 0301,0801 | NT | 2,4 |
| Patient | 2 | 8,56 | NT | 1,0701 | 0301,0801 | NT | NT |
| No. 14 | |||||||
| Donor | 2,26 | 44 | 5,9 | NT | 0401,1103 | NT | 7 |
| Patient | 2,26 | 44 | 5,9 | NT | 0401,1103 | 53,52 | 9,7 |
| No. 15 | |||||||
| Donor | 2,29 | 44,35 | 4,6 | NT | 0101,1104 | 52 | 1,7 |
| Patient | 2,29 | 44,35 | 4,6 | NT | 1,11 | 52 | 1,7 |
| No. 16 | |||||||
| Donor | 2,9(24) | 44,35 | 4,6 | NT | 0401,1401 | 52,53 | NT |
| Patient | 2,24 | 44,35 | 4,6 | NT | 4,14 | 52,53 | NT |
| No. 17 | |||||||
| Donor | NT | NT | NT | NT | NT | NT | NT |
| Patient | NT | NT | NT | NT | NT | NT | NT |
| No. 18 | |||||||
| Donor | 29,31 | 51,44 | 4 | NT | 0701,0801 | 53 | 2,4 |
| Patient | 29,31 | 51,44 | 4 | NT | 0701,0801 | 53 | 2,4 |
| No. 19 | |||||||
| Donor | 1,2 | 8,39 | 6 | NT | 0301,1201 | 52 | NT |
| Patient | 1,2 | 8,39 | 6 | NT | 0301,1201 | 52 | 2,7 |
| No. 20 | |||||||
| Donor | 2,3 | 51,62 | 4,6 | NT | 0101,0801 | NT | 4,5 |
| Patient | 2,3 | 51,62 | 4,6 | 3 | 0101,0803 | NT | 5,7 |
| No. 21 | |||||||
| Donor | 2,11 | 35,60 | 6 | NT | 1,4 | 53 | NT |
| Patient | 2,11 | 35,60 | 6 | NT | 1,4 | 53 | 5,8 |
| No. 22 | |||||||
| Donor | 2,31 | 44,35 | 4,6 | NT | 0401,0403 | 53 | NT |
| Patient | 2,31 | 44,35 | 4,6 | NT | 4 | 53 | NT |
| No. 23 | |||||||
| Donor | 1,28 | 35,14 | 6 | NT | 1,14,b1 | NT | NT |
| Patient | 1,28 | 35,14 | 6 | NT | 1,14,b1 | NT | NT |
| No. 24 | |||||||
| Donor | 3,26 | 7,35 | 6 | NT | 1501,1104 | 51,52 | 6,7 |
| Patient | 3,26 | 7,35 | 6 | NT | 1501,1104 | 51,52 | 6,7 |
NT indicates not tested.
Cytokine-specific ELISA
Paired unconjugated coating and biotinylated detection antibodies were used to measure IFN-γ (43-11 capture and 45-15 detection; Immunokontact, Abingdon, United Kingdom), IL-5 (JES1-5a10 capture and JES-5A10 detection; Immunokontact), IL-10 (JES3-9D7 capture and JES3-12G8 detection; Pharmingen, Hamburg, Germany), IL-13 (JES10-5A2 capture and B69-2 detection; Pharmingen), and TNF-α (Mab1 capture and MAb11 detection; Pharmingen). Recombinant cytokines were used as standards for ELISA in order to quantify levels in experimental supernatants. The standards used were human recombinant IL-5 (hrIL-5; AMS Biotechnology, Witney, United Kingdom), hrIL-10 (Genzyme, West Malling, United Kingdom), hrIL-13 (Becton Dickinson, Oxford, United Kingdom), hrIFN-γ (Appligene-Oncor, Watford, United Kingdom), and hrTNF-α (Serotec, Oxford, United Kingdom). ELISAs were performed as previously described.20
Statistical analysis
To examine the relationship between MLR cytokine levels and aGVHD, patients were grouped as being either nonsevere (grades 0-I) or moderate/severe (grades II-III only, as no patients developed grade IV aGVHD). Cytokine measurements were classed as either positive or negative, where a positive was defined as more than 3 standard deviations (from triplicate cultures) above the lower level of detection in the ELISA (typically 4 pg/mL). The resulting 2 × 2 contingency tables were analyzed using the Fisher exact test (Table 3). For assessment of correlations between MLR cytokines and CTLp frequencies, the Pearson rank correlation coefficient was used. P values obtained were 2 sided, and values less than .05 were considered statistically significant.
Statistical analysis of cytokine/GVHD relationships
. | Acute GVHD . | . | . | |
|---|---|---|---|---|
| Cytokine . | Grades 0—I . | Grades II—IV . | P* . | |
| Positive IL-5 | 0 | 10 | .005 | |
| Negative IL-5 | 7 | 7 | ||
| Positive IL-10 | 1 | 5 | .5 | |
| Negative IL-10 | 6 | 12 | ||
| Positive IL-13 | 0 | 14 | .0002 | |
| Negative IL-13 | 7 | 3 | ||
| Positive IFN-γ | 0 | 9 | .01 | |
| Negative IFN-γ | 7 | 8 | ||
| Positive TNF-α | 1 | 10 | .05 | |
| Negative TNF-α | 6 | 7 | ||
. | Acute GVHD . | . | . | |
|---|---|---|---|---|
| Cytokine . | Grades 0—I . | Grades II—IV . | P* . | |
| Positive IL-5 | 0 | 10 | .005 | |
| Negative IL-5 | 7 | 7 | ||
| Positive IL-10 | 1 | 5 | .5 | |
| Negative IL-10 | 6 | 12 | ||
| Positive IL-13 | 0 | 14 | .0002 | |
| Negative IL-13 | 7 | 3 | ||
| Positive IFN-γ | 0 | 9 | .01 | |
| Negative IFN-γ | 7 | 8 | ||
| Positive TNF-α | 1 | 10 | .05 | |
| Negative TNF-α | 6 | 7 | ||
Ascertained using the Fisher exact test (to 1 significant figure).
Results
Antipatient cytokine responses can discriminate between similarly HLA-matched potential donors
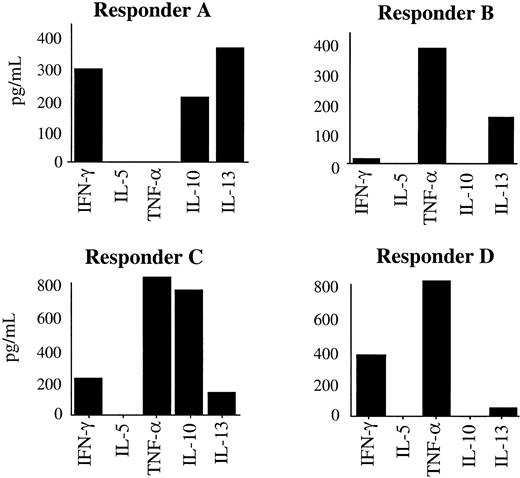
The sensitivity of the MLR cytokine profile was first tested to examine whether the antipatient responses of different potential donors for a transplant could be distinguished from each other through the cytokine profiles they produced. Potential donors were identified through donor registries (HLA-A, -B, and -DR allele match searching). The results showed that such individual donors who were difficult to discriminate between, purely at the level of HLA typing, displayed remarkable heterogeneity in cytokine profiles despite having a similar degree of HLA-mismatching to the patient they were reacting against. The cytokine profile therefore had sufficient sensitivity for the aims of this project. Four independent experiments were performed; Figure 1 and Table 4 show a typical experiment where responses of 4 potential donors, who had been identified during the initial donor search, were compared.
MLR cytokine levels can discriminate between potential SCT donors. Comparison of cytokine profiles produced by 4 individual potential SC transplant donors that had been selected through preliminary HLA matching. Potential donor cells were responding in MLRs against the patient stimulator cells. Supernatants were analyzed on day 3 for IL-4 (no IL-4 was detected by ELISA, data not shown) and day 7 for IL-5, IL-10, IL-13, TNF-α, and IFN-γ. Data are representative of 4 similar experiments performed using different patients and their preliminary potential donor panels. Data are expressed as mean of triplicate cultures with standard error of the mean less than 10% for each point.
MLR cytokine levels can discriminate between potential SCT donors. Comparison of cytokine profiles produced by 4 individual potential SC transplant donors that had been selected through preliminary HLA matching. Potential donor cells were responding in MLRs against the patient stimulator cells. Supernatants were analyzed on day 3 for IL-4 (no IL-4 was detected by ELISA, data not shown) and day 7 for IL-5, IL-10, IL-13, TNF-α, and IFN-γ. Data are representative of 4 similar experiments performed using different patients and their preliminary potential donor panels. Data are expressed as mean of triplicate cultures with standard error of the mean less than 10% for each point.
HLA types of responders (potential donors) and stimulator (patient) for experiments depicted inFigure 1
. | A . | B . | Bw . | Cw . | DR . | DQ . | DRB . |
|---|---|---|---|---|---|---|---|
| Stimulator | 2 | 5, 18 | 4, 6 | — | 1101, 1301 | 6, 7 | 52 |
| Responder A | 2 | 5, 18 | 6 | — | 1101, 1301 | 7 | 52 |
| Responder B | 2, 26 | 18, 49 | 4, 6 | — | 1101, 1301 | 6, 7 | 52 |
| Responder C | 2, 26 | 18 | 6 | — | 1101, 1303 | 7 | 52 |
| Responder D | 2 | 2,28 | 18, 6 | — | 1101, 1301 | 6, 7 | 52 |
. | A . | B . | Bw . | Cw . | DR . | DQ . | DRB . |
|---|---|---|---|---|---|---|---|
| Stimulator | 2 | 5, 18 | 4, 6 | — | 1101, 1301 | 6, 7 | 52 |
| Responder A | 2 | 5, 18 | 6 | — | 1101, 1301 | 7 | 52 |
| Responder B | 2, 26 | 18, 49 | 4, 6 | — | 1101, 1301 | 6, 7 | 52 |
| Responder C | 2, 26 | 18 | 6 | — | 1101, 1303 | 7 | 52 |
| Responder D | 2 | 2,28 | 18, 6 | — | 1101, 1301 | 6, 7 | 52 |
— indicates not done.
MLR cytokine profiles detect both HLA class I and class II mismatches
Next, the assay was tested to examine to what extent the cytokines measured reflected either CD4+ or CD8+ T-cell responses. Donor-antipatient MLRs using purified CD4+ or CD8+ T cells responding against whole PBMCs were examined for cytokine levels produced. Due to limited numbers of responder (donor) T cells following purification we focused on IL-13, IL-5, and IFN-γ, which we have previously shown to display the most consistent kinetics of production during the allogeneic MLR.20 In total, 12 experiments were performed of which 3 typical responses are depicted in Figure 2 and Table 5. The data gained showed that both CD4+ and CD8+ T cells were capable of cytokine responses and therefore the cytokine MLR reflects both MHC class I and MHC class II mismatches. Additionally, the overall composition of the cytokine profile varied between CD4+ and CD8+ responses for the same MLR pairing. For example, in Figure 2B the CD4+ response was heterogeneous, producing IL-5, IL-13, and IFNγ, whereas the CD8+ response was dominated by only IL-13.
Cytokine profiles reflect both CD4+ and CD8+ T-cell responses. Cytokine profiles obtained from CD4+ and CD8+ responder T cells during SCT donor-antipatient MLRs. MLRs were performed using highly purified (> 95%) CD4+ or CD8+ donor T-cell responders against whole PBMC patient stimulator cells. Supernatants were harvested on day 7 of the MLR and assessed for IFN-γ, IL-5, and IL-13 levels using ELISA. Data are expressed as mean of duplicate cultures with standard error of the mean less than 10% for each point. Three typical responses are shown from a total of 12 experiments performed.
Cytokine profiles reflect both CD4+ and CD8+ T-cell responses. Cytokine profiles obtained from CD4+ and CD8+ responder T cells during SCT donor-antipatient MLRs. MLRs were performed using highly purified (> 95%) CD4+ or CD8+ donor T-cell responders against whole PBMC patient stimulator cells. Supernatants were harvested on day 7 of the MLR and assessed for IFN-γ, IL-5, and IL-13 levels using ELISA. Data are expressed as mean of duplicate cultures with standard error of the mean less than 10% for each point. Three typical responses are shown from a total of 12 experiments performed.
HLA types of responders (potential donors) and stimulator (patient) for experiments depicted inFigure 2
. | A . | B . | Bw . | Cw . | DR . | DQ . | DRB . |
|---|---|---|---|---|---|---|---|
| Responder A | 2,3 | 17, 35 | 4,6 | — | 1,15 | 5, 6 | 51 |
| Stimulator A | 2,3 | 17, 35 | 4,6 | — | 1,15 | 5, 6 | 51 |
| Responder B | 29,30 | 13, 50 | 4,6 | — | 0301, 0701 | 2 | 52, 53 |
| Stimulator B | 30,31 | 13, 50 | 4,6 | — | 0301, 0701 | 2 | 52, 53 |
| Responder C | 1,2 | 8, 18 | 6 | 6,7 | 0401, 1104 | — | 52, 53 |
| Stimulator C | 1,2 | 8, 18 | 6 | 6,7 | 0401, 1104 | — | 52, 53 |
. | A . | B . | Bw . | Cw . | DR . | DQ . | DRB . |
|---|---|---|---|---|---|---|---|
| Responder A | 2,3 | 17, 35 | 4,6 | — | 1,15 | 5, 6 | 51 |
| Stimulator A | 2,3 | 17, 35 | 4,6 | — | 1,15 | 5, 6 | 51 |
| Responder B | 29,30 | 13, 50 | 4,6 | — | 0301, 0701 | 2 | 52, 53 |
| Stimulator B | 30,31 | 13, 50 | 4,6 | — | 0301, 0701 | 2 | 52, 53 |
| Responder C | 1,2 | 8, 18 | 6 | 6,7 | 0401, 1104 | — | 52, 53 |
| Stimulator C | 1,2 | 8, 18 | 6 | 6,7 | 0401, 1104 | — | 52, 53 |
— indicates not done.
Pre-BMT donor-versus-patient MLR cytokine profiles
Twenty-four pre-BMT donor antipatient PBMC MLRs were performed and levels of the cytokines IL-5, IL-10, IL-13, IFN-γ, and TNF-α were analyzed. The clinical details of these patients were followed up at least 1 year after transplantation in order to assess retrospectively whether the cytokine profiles were associated with aGVHD. The cytokine ELISAs were performed without knowledge of the transplantation outcome. The results showing the grade of aGVHD in relation to the pre-BMT cytokine response are depicted in Figure 3. Positive correlations between increased aGVHD severity and cytokine levels were demonstrated for the cytokines IL-5, IL-13, TNF-α, and IFN-γ, with statistical significance analyzed in relation to the subsequent development of aGVHD using the Fisher exact test (Table 3). IL-13 was shown to be the best prognostic indicator of subsequent aGVHD development (P = .0002), followed by IL-5 (P = .005), IFN-γ (P = .01), and TNF-α (P = .05). IL-10 showed no correlation with aGVHD severity (P = .5).
Correlations between pretransplantation patient-antidonor cytokine responses and aGVHD. Cytokine profiles from pre-BMT GVH direction PBMC MLRs. MLRs were performed using whole PBMCs from donors reacting against irradiated whole PBMCs taken from the corresponding patient before transplantation. Supernatants were frozen on day 7 of the MLR for later assessment of the cytokines IL-5, IL-10, IL-13, IFN-γ, and TNF-α. All cytokines were measured by ELISA. Also shown are the calculated CTLp frequencies and grade of GVHD as assessed routinely during the BM transplant matching and during posttransplantation care at Hammer-smith Hospital. Units shown are pg/mL for cytokine levels and estimated CTLp frequencies as 1/(1000×n); 1000 indicates an extremely low estimated frequency of one per million.
Correlations between pretransplantation patient-antidonor cytokine responses and aGVHD. Cytokine profiles from pre-BMT GVH direction PBMC MLRs. MLRs were performed using whole PBMCs from donors reacting against irradiated whole PBMCs taken from the corresponding patient before transplantation. Supernatants were frozen on day 7 of the MLR for later assessment of the cytokines IL-5, IL-10, IL-13, IFN-γ, and TNF-α. All cytokines were measured by ELISA. Also shown are the calculated CTLp frequencies and grade of GVHD as assessed routinely during the BM transplant matching and during posttransplantation care at Hammer-smith Hospital. Units shown are pg/mL for cytokine levels and estimated CTLp frequencies as 1/(1000×n); 1000 indicates an extremely low estimated frequency of one per million.
Links between the levels of different cytokines detected in the MLRs were analyzed using the Pearson rank correlation coefficient. A clear correlation was observed between the cytokines IL-5, IFN-γ, and TNF-α (r > 0.97, P < .001 for all permutations). Relationships between these 3 cytokines and corresponding CTLp frequencies were also strong (IL-5, r = 0.96, P < .001; IFN-γ, r = 0.98, P < .001; and TNF-α, r = 0.97, P < .001). In contrast, IL-13 levels were independent of IL-5, IFN-γ, and TNF-α as well as CTLp frequencies (P > .05). No correlation was detected between IL-10 and any other cytokines or CTLp frequencies.
Donor cytokine responses were also tested against fully allogeneic third-party stimulators in order to confirm that they were capable of cytokine responses during an MLR. Similarly, patient cells were tested in their ability to stimulate control third-party allogeneic responder cells and for donor cells to produce cytokines. In each case, positive third-party control responses were confirmed (data not shown). This demonstrated that the stimulatory capacity of antigen-presenting cells (APCs) and sensitivity to stimulation of the responder T cells was “normal” to a third-party population. Furthermore, stimulator and responder cells were cultured alone in parallel with the MLRs to detect any autologous cytokine production that might occur. In each case, cytokine levels during the “autologous MLR” were not above background (data not shown).
Discussion
Following allogeneic SCT, donor-derived T cells that recognize and respond to patient APCs are responsible for the development of aGVHD. This immune response results in the production of inflammatory cytokines such as IFN-γ, TNF-α, and IL-1 that initiate and maintain aGVHD in a process that has been termed the “cytokine storm.”22 For some time it has only been the type-1 T-cell cytokines that have been considered to be of importance during the initiation of aGVHD since little or no anti-inflammatory/type-2 cytokines could be detected during the alloresponse in vitro.23 The primary alloresponse had traditionally been characterized by high levels of IL-2, IFN-γ, and TNF-α and little or no IL-4 or IL-10. However, our recent studies examining the detailed kinetics of both type-1 and type-2 responses revealed that type-2 cytokines (IL-5 and IL-13 in particular) are actually produced in abundance during the allogeneic MLR, appearing during the assay at a later time point than has previously been examined.20 Furthermore, we showed that for any given fully allogeneic responder-stimulator (R/S) combination the type-1/type-2 cytokine profile was consistent when repeated numerous times over a period of one year.20 These cytokine profiles, however, varied tremendously between different R/S combinations. The overall profile appeared to be specific to the individual pairing being examined rather than being intrinsic to just responder or stimulator characteristics alone.
Together, these observations raised the previously unconsidered possibility that type 2 cytokines such as IL-5 and IL-13 have an influence during the cytokine storm and may play a role in dictating graft outcome during SCT. In order to address this possibility we have investigated whether cytokine production in bulk culture donor-antipatient MLRs could be applied as a potential tool in donor selection. Firstly, we have demonstrated that the assay has the required sensitivity to discriminate between the donor-antipatient alloresponses of potential donors identified during HLA matching for unrelated transplants. Moreover, cytokine profiles produced during the MLR measured both HLA class I and class II responses and so reflect both cytotoxic and helper T-cell responses during the alloresponse.
Having demonstrated the sensitivity of the assay, we next investigated whether levels of cytokines produced in the MLRs would correlate with transplantation outcome and therefore be of potential use as a prognostic tool. Our hypothesis at the outset was that type-1 cytokines would be hostile while the production of high-level type-2 cytokines would be anti-inflammatory and therefore beneficial for the patient after transplantation. However, the results were surprising. First, higher levels of cytokine per se correlated with greater risk of aGVHD development regardless of whether the cytokine was classically type 1 (IFN-γ and TNF-α) or type 2 (IL-5 and IL-13). In fact, the cytokines IL-5, IFN-γ, and TNF-α were coproduced, showing strong positive correlations with each other (as well as with CTLp frequencies). This observation contradicts the classical Th1/Th2 paradigm that states that such coproduction of type-1 and type-2 is a rare phenomenon.4 The most striking finding was the very strong positive correlation between IL-13 and aGVHD and the fact that this correlation was independent of that seen for the IL-5, IFN-γ, and TNF-α cytokine cluster as well as from CTLp frequencies, thus suggesting a second, quite independent functional component in the determination and perhaps generation of aGVHD.
IL-13 shares a great deal of functional redundancy with IL-4 due to the fact that on most cell types these cytokines act through the same receptor (a heterodimer composed of the IL-4Rα and the IL-13Rα chains) and signal through a common STAT-3 (signal transducers and activators of transcription 3) pathway. It was surprising then, given that our laboratory has previously shown IL-4 to be negatively correlated with aGVHD, that IL-13 was strongly positively correlated with aGVHD severity. We also previously showed IL-4 production to be negatively correlated with CTLp estimates and to be associated with increased risk of leukemia relapse,11 whereas no link between IL-13 and CTLp or disease relapse was observed here. These discrepancies raise a number of questions with respect to the relative roles of IL-4 and IL-13 during transplantation and, in particular, with aGVHD.
More recent studies have shown that IL-13 not IL-4 induces inflammation associated with the skin, gut, and airways during allergic states and infectious disease. IL-13 but not IL-4 has been shown to act powerfully in mediating the airway hyper-responsiveness, mucus overproduction by airway epithelium, and airway eosinophilia occurring in a murine model of asthma24-26 and to be instrumental in inducing the fibrosis associated with allergic responses.27 Similar findings have been made during infectious diseases where IL-13 but not IL-4 was shown to be critical for initiating and maintaining the fibrosis occurring in the granulatomous reaction leading to the tissue destruction that causes the morbidity and mortality in murine schistosomiasis models.28 Furthermore, IL-13 is chemotactic for monocytes and also for eosinophils, functions that are not evident for IL-4.29-31 The data presented here are the first to define links between IL-13 production and aGVHD and it is possible that IL-13 that is secreted by donor T cells during aGVHD is directly responsible for at least some of the pathology associated with this unwanted inflammatory response. As T cells in the skin can be a major source of IL-1332 it may be that following SCT, donor IL-13–secreting T cells migrate to the skin causing both direct inflammation and recruitment of inflammatory cells through this cytokine.
Until recently it has been difficult to explain the inflammatory role of IL-13 during disease at the molecular level, however, it is now known that IL-4 and IL-13 can activate quite different signaling pathways, even when binding to the same receptor complex.33 Thus, while both cytokines activate a common STAT-3 signaling pathway that is responsible for much of their functional redundancy, IL-4 predominantly activates through a SHP-1 (Src homology 2 domain containing tyrosine phosphatase) phosphatase pathway, influencing the phosphatidylinositol 3 (PI3)–kinase family, while IL-13, through SHP-2 activation, modifies Janus kinase 1 (JAK-1), tyrosine kinase 2 (Tyk2), and insulin receptor substrate-2 (IRS-2).33 Although the functional consequences of these differences are not understood, SHP-2, activated preferentially by IL-13, serves as a linker to the mitogen-activated protein (MAP) kinase pathway affecting acute phase protein (APP) production34 and could play an important role in regulating aGVHD. No correlations could be found between cytokine levels and chronic GVHD, although to confirm that there are no correlations, a further larger study would need to be performed.
During analysis of the data gained in this study, we were aware that a number of factors may have contributed to the cytokine associations observed. First, the disease state at the time of transplantation could have been a major factor in the results obtained. At time of transplantation 14 patients were in chronic phase, 6 were in acute phase, and 4 were in blast crisis. In order to address the possibility that those patients in acute phase or blast crisis simply stimulate more cytokine due to their disease state we analyzed the chronic phase patients as a single group (using the Fisher exact test). In this group IL-13 was still significantly correlated with aGVHD severity (P < .05, n = 14) re-enforcing the potential benefit of this assay in donor selection regardless of disease state at time of transplantation. We also examined whether the absolute numbers of T cells (CD3+) in the responder populations was responsible for the cytokine differences observed. However, no correlation between the composition of the major responder (or, in fact, stimulator populations) and aGVHD could be identified, in agreement with our previous observation during the alloresponse. These data indicate that the allo-mismatches themselves are the major factor that dictates the cytokine response during our MLR assay.
There have been a limited number of studies examining donor-antirecipient cytokine responses prior to SCT. These studies have examined gene expression using reverse transcriptase–polymerase chain reaction (RT-PCR) rather than extracellular cytokine levels, presumably for the ease of automation of this method for routine testing. However, none of these studies have succeeded in predicting the severity of aGVHD.35-38 Possible explanations for the failure of these studies in producing a reliable prognostic test for GVHD are 2-fold. First, the expression of cytokine mRNA does not necessarily relate to cytokine secretion, and secondly, the measurement of cytokine levels in MLR supernatants represents the sum of a more complex set of interactions within the culture including both cytokine production and consumption. Although RT-PCR has not been successful, other techniques such as enzyme-linked immunospot (ELISPOT) and intracellular flow cytometry remain untested. These techniques could be very useful in that they would allow an estimate of the frequencies of responding cytokine-producing donor T cells.
In summary, this study has examined donor T-cell cytokine production in response to allogeneic patient cells, revealing data that highlight significant correlations between Th2-type as well as Th1-type cytokines in aGVHD. Previous to this work, proportional hazards analysis has indicated that the best predictor for aGVHD is the CTLp assay, in that a low pre-BMT donor-antipatient CTLp frequency is a good sign for the patient's prognosis.19 This CTLp assay has a greater predictive accuracy than even high-resolution HLA typing using insulin-enhancer factor (IEF) and restriction fragment length polymorphism (RFLP).19 A test based on measurement of allospecific GVH IL-5, IFN-γ, or TNF-α could effectively replace the currently used CTLp LDA. Furthermore, in IL-5 we have identified a cytokine that is far more sensitive than CTLp measurements and able to better predict aGVHD severity than this assay. Measurement of IL-13 was the best predictor of aGVHD by far and was independent of all other cytokines measured as well as estimated CTLp frequencies.
The study described here has a relatively small number of patients examined (N = 24) and will therefore require confirmation in larger studies as well as in other transplantation centers. Nevertheless, the statistical significance of the links between cytokines and GVHD are strong and it appears that IL-13 measurements in combination with one of the IL-5/IFN-γ/TNF-α cluster could provide reliable information in pretransplantation aGVHD prognosis. We raise the possibility that IL-13 production may be directly responsible for at least some of the pathology during aGVHD as has been recently shown for this cytokine during other inflammatory responses such as asthma and parasitic worm infections. If so then a therapy based on specific blocking of IL-13 (as has been successfully used in murine models of asthma) may be a distinct possibility for post-SCT aGVHD treatment.
Prepublished online as Blood First Edition Paper, September 25, 2003; DOI 10.1182/blood-2003-01-0192.
Supported by Action Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Professor Hans Stauss for helpful discussion and suggestions during the preparation of this manuscript.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal