Abstract
Induction of antigen-specific tolerance to transplantation antigens is desirable to control host-versus-graft and graft-versus-host reactions. Following molecular identification of a set of minor histocompatibility (H) antigens, we have used selected HY peptide epitopes for this purpose. Intranasal administration of individual major histocompatibility complex (MHC) class II-restricted HY peptides induces indefinite survival of syngeneic male skin grafts and allows engraftment of male bone marrow. Tolerance involves linked suppression to additional HY epitopes on test grafts. Long-term tolerance also requires suppression of emerging thymic emigrants. It does not involve deletion. HY peptide–specific CD4+ and CD8+ T cells expand on re-exposure to male antigen; these expansions are smaller in tolerant than control mice and fewer HY-specific cells from tolerant females secrete interferon γ and interleukin 10 (IL-10). Significantly, CD4+ cells from peptide-pretreated females fail to make IL-2 responses to cognate peptide, limiting expansion of the HY-specific CD8+ populations that can cause graft rejection. Consistent with this, tolerance induction by HY peptide is abrogated by coadministration of lipopolysaccharide. IL-10 does not appear to be critically involved because tolerance is inducible in IL-10–deficient mice. Adoptive transfer of tolerance into naive neonatal recipients by splenocytes from long-term tolerant donors provides evidence for involvement of regulatory cells.
Introduction
HY antigens are the male-specific, Y chromosome–encoded minor histocompatibility (H) antigens,1 others being encoded by autosomal genes. Antigenicity and allelism are created by sequence differences of peptides derived from intracellular proteins expressed at the cell surface following incorporation into major histocompatibility complex (MHC) class I and II molecules.2 A poorly understood mechanism of immunodominance operates to select only a small number of minor H epitopes as the focus of T-cell responses when grafts are exchanged between MHC-matched, multiple minor H-mismatched donor/recipient pairs.3-5 This raises the possibility of modulating the immune response to grafts by inducing linked suppression via tolerance to immunodominant epitopes.6 Clinical responses of patients undergoing female-to-male bone marrow transplantation suggest that HY responses can be immunodominant over autosomally encoded minor H antigens in humans7 ; thus induction of tolerance to HY antigens provides a model for inducing tolerance to autosomal minor H antigens via linked suppression.
Syngeneic male skin and bone marrow grafts are rejected by female mice of high responder H2b strains, such as C57BL/6 (B6) and F1 females with one H2b parent.1 Rejection requires both CD4+ and CD8+ T cells8 recognizing peptides derived from proteins encoded by the genes, Smcy, Uty, and Dby.9-12 Humans make male-specific T-cell responses to HY peptides from the same genes, SMCY, UTY, and DBY, strongly conserved across mammalian species, and from additional ubiquitously transcribed human Y chromosome genes, RPS4Y and DFFRY.13-19
We have previously described using immature, bone marrow–derived dendritic cells (BMDCs) pulsed with the immunodominant MHC class I (H2Db)–restricted HY peptide, WMHHNMDLI, encoded by the Uty gene, (HYDbUty)20 to induce tolerance to syngeneic male skin grafts in H2b mice. Following this treatment, about half the recipients tolerated first and second syngeneic male skin grafts indefinitely, and tolerance could be adoptively transferred into neonatal females. In contrast, immature DCs pulsed with the MHC class II (H2Ab)–restricted HY peptide, NAGFNSNRANSSRSS, derived from Dby, (HYAbDby) caused immunization, with accelerated graft rejection.21 The nondominant H2Db-restricted peptide, KCSRNRQYL (HYDbSmcy), pulsed onto immature BMDCs caused neither sensitization nor tolerance (E.J. et al, unpublished data, October 2001).
The intranasal route of administration of protein and peptide antigens induces unresponsiveness in mouse models of asthma22 and autoimmune diseases.23 This paper describes use of the intranasal route of administration of HY peptides to induce transplantation tolerance to HY in H2b mice. More than 90% of mice given the MHC class II–restricted HYAbDby peptide intranasally retained syngeneic male skin grafts indefinitely, accepting second syngeneic male but not third-party skin grafts. Syngeneic male bone marrow also engrafted in HY peptide–pretreated females under conditions in which controls did not engraft. Exposure to antigen in vivo or in vitro induced a much more limited clonal expansion of HY-specific T cells in tolerant compared with graft-rejecting mice. T cells from tolerant mice failed to secrete interleukin 2 (IL-2) and fewer cells were able to produce interferon γ (IFN-γ) and, unexpectedly, IL-10 in response to HY peptides compared with controls. Tolerance was transferable to naive mice with CD8-depleted splenocytes. The results suggest that induction of nonresponsiveness is due to limited clonal expansion and reduced cytokine production and that long-term maintenance of tolerance depends on a population of regulatory cells.
Materials and methods
Mice
C57BL/6J (B6) and CBA/Ca (CBA) mice (6-8 weeks old) were purchased from Harlan Olac (Bicester, United Kingdom). B6.Thy1.1, B6.IL10–/–, (B6×CBA/Ca)F1, and (B6×C3H.SW)F1 mice were bred in the Biological Services Unit at the Medical Research Council Clinical Sciences Centre (CSC).
Tissue-culture media and reagents
RPMI 1640 Medium (Gibco, Paisley, United Kingdom) was supplemented with 10% fetal calf serum (FCS; Autogen Bioclear, Calne, United Kingdom), HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; 10 mM), penicillin (100 IU/mL) and 100 μg/mL streptomycin (Gibco), and 5 × 10–5 M 2-mercaptoethanol and 2 mM l-glutamine (Gibco). Lipopolysaccharide (LPS) was purchased from Sigma (Poole, United Kingdom).
Peptides and tetramers
Peptides HYAbDby, NAGFNSNRANSSRSS; HYEkDby, REEALHQFRSGRKPI12 ; HYDbUty, WMHHNMDLI10 ; and HYDkSmcy, RRLRKTLL (P.J. Dyson et al, manuscript in preparation) were synthesized by the Central Research Resources unit of the CSC using 9-fluorenylmethyloxycarbonyl–protected amino acids and (2-(1-H-benzotriazol-2-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU) activation chemistry. Synthetic peptides were made as 1-mM stocks in phosphate-buffered saline (PBS) and filter sterilized.
Peripheral blood lymphocyte staining
Mice were tail-bled into Eppendorf tubes containing 200 μL 10 mM EDTA (ethylenediaminetetraacetic acid), 100 U heparin/mL in PBS, and 1 mL red blood cell (RBC) lysis buffer (Puregene; Flowgen, Ashbyde la Zouche, United Kingdom) added. Cell suspensions were mixed, incubated at room temperature for 15 minutes, and centrifuged (3500 rpm for 4 minutes). The cell pellet was resuspended in 50 μL PBS and transferred to tubes for staining. Tetramer, antibody staining, and analysis of 5,6-carboxyflourescein succinimidyl ester (CFSE) expression were performed as described herein.
Clonal expansion in vitro of tetramer-positive T cells from PBLs
Peripheral blood lymphocytes (PBLs), separated by Ficoll-Hypaque centrifugation, were stimulated (1 × 106/well) with irradiated syngeneic male spleen cells (5 × 106/well) and 10 IU/mL recombinant human IL-2 (hu-rIL-2) in 24-well plates. Cultures were sampled on day 7 for tetramer analysis, with the remaining cells left and restimulated on day 14; this protocol was repeated for 2 further rounds of culture. Before staining, dead cells were removed by Ficoll-Hypaque centrifugation. Cells were first incubated with 2.4G2 antibody against FcγII/III receptors, then stained simultaneously with anti-CD8–peridinin chlorophyll protein (PerCP) and HYDbUty tetramer–phycoerythrin (PE). After washing, the cells were analyzed by flow cytometry.
In vitro restimulation to establish T-cell lines
T-cell lines were generated by stimulating splenocytes (5 × 106/well) from tolerant mice with irradiated B6 male splenocytes (5 × 106/well) in 24-well plates for 4 weeks. They were maintained by repeated stimulation of recovered live cells (2 × 105/well) with irradiated B6 male splenocytes (5 × 106/well) and hu-rIL-2 (10 IU/mL) every 4 weeks. Seven days after the last restimulation, cells were harvested, and after removal of dead cells, analyzed for tetramers or for intracellular cytokine production.
Staining of intracellular cytokines for flow cytometric analysis
For stimulating cytokine production, splenocytes (4 × 106/mL) from graft-rejected or tolerant B6 females were cultured with irradiated B6 male splenocytes (4 × 106/mL), with or without hu-rIL-2 (10 IU/mL), in 24-well plates. Seven days later, the viable activated T cells (2 × 106/mL) were restimulated with irradiated unpulsed or peptide-pulsed B6 female T cell–depleted splenocytes (4 × 106/mL) in 24-well plates for 6 hours in the presence of monensin (GolgiStop; BD Biosciences Pharmingen). Cultured cells were incubated with 2.4G2 antibody, washed, and stained with anti-CD4–PE and anti-CD8–PerCP. After washing, cells were fixed, permeabilized with Cytofix/Cytoperm solution (BD Biosciences Pharmingen), washed with 1 × Perm/Wash solution (BD Biosciences Pharmingen), and resuspended in 1 × Perm/Wash solution containing anti-IFNγ–fluorescein isothiocyanate (FITC) or anti–IL-10–FITC or isotype-matched control antibodies. After a final wash, the cells were resuspended in staining buffer for flow cytometric analysis.
ELISPOT assays
These were performed as described previously.20
Intranasal peptide administration
PBS (20 μL) containing 3 to 100 μg peptide was administered intranasally on 3 consecutive days to B6, (B6xCBA/Ca)F1 or B6.IL10–/– females anesthetized with isoflurane. Control mice received intranasal PBS. An additional group was given HYAbDby peptide (3 μg in 20 μL PBS) plus 3 μg LPS on 3 consecutive days. The mice received syngeneic male grafts 10 days later.
Skin grafting
Skin grafting was conducted by the method of Billingham and Medawar25 using tail skin grafted onto the lateral thorax. After removal of the plaster casts, grafts were observed every 2 to 3 days and scored as rejected when less than 10% viable tissue remained.
Bone marrow grafting
Donor bone marrow was flushed with PBS from femurs and tibias of male B6.Thy1.1 mice and washed twice before injection. B6.Thy1.2 female recipients were pretreated by intranasal administration of 100 μg HY peptide or PBS on 3 sequential days, 10 days before low-dose irradiation (200 R), followed by 5 × 106 bone marrow cells injected intravenously in 0.2 mL PBS.
In vivo killing assay
Female and male B6 spleen cells (2 × 107/mL in PBS) were incubated with 5 μM or 0.5 μM CFSE (Molecular Probes, Cambridge, United Kingdom), respectively, at room temperature for 8 minutes in the dark. FCS (final concentration 20%) was added to stop the reaction. After washing, the cells were mixed, resuspended in PBS, and 2 × 107 cells in 0.2 mL were injected intravenously to each recipient. Peripheral blood was collected from individual mice at serial time points. After lysis of RBCs and blockade of FcR, PBLs were stained with HYDbUty tetramer–PE and anti-CD8–PerCP and analyzed for CFSE expression by fluorescence-activated cell sorting (FACS).
Transfer of tolerance to naive females
Pooled spleen cell suspensions were made from 3 to 5 long-term tolerant mice. One aliquot was reserved for the transfer of unseparated spleen cells and 3 other aliquots were treated to remove either CD8+, CD4+, or both subpopulations of cells using magnetic bead separation. Following treatment each aliquot was suspended at 5 × 107/mL, and 0.1 mL (5 × 106) cells injected intraperitoneally into neonatal females within 48 hours of birth. At 6 weeks of age the recipients were given syngeneic male skin grafts.
Statistical analysis
Statistical analysis was performed using the Mann-Whitney test and GraphPad Prism version 3.02 software (San Diego, CA).
Results
Induction of skin graft tolerance with intranasally administered HY peptides: minimal dosage and antigen specificity
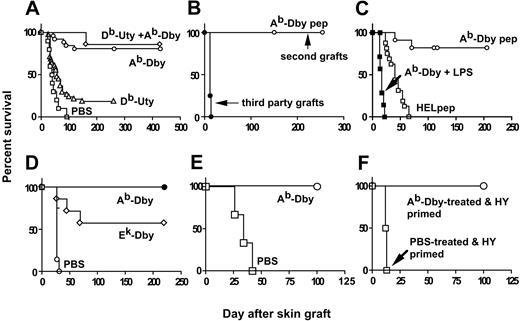
B6 female mice were pretreated intranasally with 100 μg HY peptide in PBS or PBS alone, 10 days before grafting with syngeneic male skin. Administration of the HYAbDby peptide alone, or together with the HYDbUty peptide, resulted in indefinite acceptance of the graft in most recipients (Figure 1A). Similar pretreatment with the HYDbUty peptide led to lower numbers of mice retaining their skin grafts, indicating that by this route the class I–restricted peptide was less effective. Placing a second B6 male and a third-party skin graft in the same graft bed (Figure 1B) on long-term (> 270 days) mice made tolerant by pretreatment with the HYAbDby peptide resulted in retention of the second syngeneic male grafts for the full observation period, but third-party, multiple minor-mismatched (B6×C3H.SW)F1 grafts from donors of either sex were rejected. The lowest dose of the HYAbDby peptide tested for tolerance induction was 3 μg. This induced long-lasting graft acceptance (Figure 1C). Specificity of tolerance induction was shown by intranasal administration of an H2Ab-binding peptide from the protein hen egg white lysozyme (HEL), 10 days before grafting with male skin. These recipients rejected their male grafts at the same tempo as naive females (Figure 1C).
Induction of transplantation tolerance to syngeneic male skin grafts by HY peptides. (A) Intranasal administration of HYAbDby alone or with HYDbUty peptide induces indefinite survival of syngeneic male skin grafts. Survival of syngeneic male skin grafts placed 10 days after intranasal pretreatment of B6 females with 3 × 100 μg HY peptide: HYAbDby (n = 25; ○), HYDbUty (n = 38; ▵), both peptides (n = 7; ⋄), PBS control (n = 10; □). Significant differences from PBS control: P < .0001 for the HYAbDby and HYAbDby plus HYDbUty groups, P = .0059 for the HYDbUty group. Data pooled from 3 experiments. (B) Tolerance induced by intranasal peptide treatment is transplantation antigen specific. Survival times of each of 2 secondary skin grafts placed on HYAbDby peptide–pretreated B6 mice retaining primary syngeneic male skin grafts more than 270 days (n = 14): syngeneic male graft (○), (B6/EiJ×C3H.SW)F1 graft (•; P > .0001). Data pooled from 2 experiments. (C) Administration of 3 μg HYAbDby peptide can tolerize, whereas HEL peptide has no effect and LPS abrogates tolerance. Survival times of syngeneic male skin grafts on B6 females given intranasally 3 × 3 μgHYAbDby (n = 21; ○), 3 × 100 μg HEL peptide (n = 16; □) or 3 × 100 μg HYAbDby peptide plus 3 μg LPS (n = 7; ▪). Significant difference of HEL peptide from HYAbDby control is P > .001. (D) Induction of tolerance in (B6×CBA)1 females. Survival times of syngeneic male skin grafts on (B6×CBA)F1 females following pretreatment with PBS (n = 7; ○), HYEkDby (n = 7; ⋄) or HYAbDby (n = 7; •) peptides (3 × 100 μg). (E) Role of IL-10. Survival times of syngeneic male skin grafts on B6.IL10–/– mice given intranasally 3 × 100 μg HYAbDby peptide (n = 6; ○) or PBS (n = 3; □). P = .0018. (F) Effect of male hematopoietic cell grafting on subsequent male skin grafts. Survival times of syngeneic male skin grafts on B6 mice pretreated intranasally with 3 × 100 μgHYAbDby peptide (n = 8; ○) or PBS (n = 8; □) followed by challenge at day 10 with male spleen cells, and grafting with male skin 60 days later; P = .0001.
Induction of transplantation tolerance to syngeneic male skin grafts by HY peptides. (A) Intranasal administration of HYAbDby alone or with HYDbUty peptide induces indefinite survival of syngeneic male skin grafts. Survival of syngeneic male skin grafts placed 10 days after intranasal pretreatment of B6 females with 3 × 100 μg HY peptide: HYAbDby (n = 25; ○), HYDbUty (n = 38; ▵), both peptides (n = 7; ⋄), PBS control (n = 10; □). Significant differences from PBS control: P < .0001 for the HYAbDby and HYAbDby plus HYDbUty groups, P = .0059 for the HYDbUty group. Data pooled from 3 experiments. (B) Tolerance induced by intranasal peptide treatment is transplantation antigen specific. Survival times of each of 2 secondary skin grafts placed on HYAbDby peptide–pretreated B6 mice retaining primary syngeneic male skin grafts more than 270 days (n = 14): syngeneic male graft (○), (B6/EiJ×C3H.SW)F1 graft (•; P > .0001). Data pooled from 2 experiments. (C) Administration of 3 μg HYAbDby peptide can tolerize, whereas HEL peptide has no effect and LPS abrogates tolerance. Survival times of syngeneic male skin grafts on B6 females given intranasally 3 × 3 μgHYAbDby (n = 21; ○), 3 × 100 μg HEL peptide (n = 16; □) or 3 × 100 μg HYAbDby peptide plus 3 μg LPS (n = 7; ▪). Significant difference of HEL peptide from HYAbDby control is P > .001. (D) Induction of tolerance in (B6×CBA)1 females. Survival times of syngeneic male skin grafts on (B6×CBA)F1 females following pretreatment with PBS (n = 7; ○), HYEkDby (n = 7; ⋄) or HYAbDby (n = 7; •) peptides (3 × 100 μg). (E) Role of IL-10. Survival times of syngeneic male skin grafts on B6.IL10–/– mice given intranasally 3 × 100 μg HYAbDby peptide (n = 6; ○) or PBS (n = 3; □). P = .0018. (F) Effect of male hematopoietic cell grafting on subsequent male skin grafts. Survival times of syngeneic male skin grafts on B6 mice pretreated intranasally with 3 × 100 μgHYAbDby peptide (n = 8; ○) or PBS (n = 8; □) followed by challenge at day 10 with male spleen cells, and grafting with male skin 60 days later; P = .0001.
Factors required for skin graft tolerance induction by intranasal HY peptides
Coadministration of LPS, an activator of the innate immune system, with the tolerogenic HYAbDby peptide resulted in priming rather than tolerance induction; these females rejected their male skin graft with an accelerated tempo (Figure 1C). Because H2k females are poor responders or nonresponders,1 presumably due to the low level of help provided by the H2Ek-restricted HY epitope, we have used high responder H2k×bF1 females to investigate the effects of H2k-resticted HY peptides on tolerance induction. Intranasal administration of the HYAbDby peptide was equally able to induce tolerance in (CBA×B6)F1 females as B6 females (Figures 1A,D). The HYEkDby peptide was also able to induce tolerance in about half the recipients (Figure 1D), despite the inability of this epitope to provide effective help. The requirement for IL-10 was tested using B6.IL10–/– mice pretreated intranasally with either PBS or HYAbDby peptide. The control, PBS-pretreated group rejected syngeneic male skin grafts with the same tempo as IL-10+/+ B6 mice, whereas the peptide-pretreated group showed robust tolerance (Figure 1E). The effects of priming peptide- or PBS-pretreated females with male hematopoietic cells on subsequently placed male skin grafts are shown in Figure 1F. PBS-pretreated mice that had rejected male spleen cells within 2 weeks (Figure 2) showed accelerated rejection of the male skin graft, whereas those pretreated with peptide and showing prolonged survival of male spleen cells were completely tolerant of the subsequently applied male skin graft.
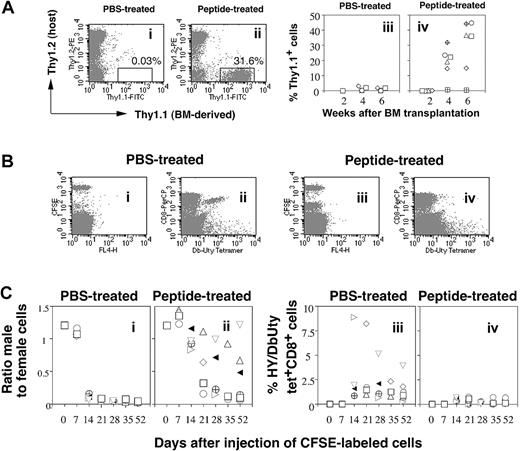
Induction of tolerance to male bone marrow and spleen cell engraftment by HY peptide. (A) Intranasal administration of HYAbDby peptide facilitates engraftment of syngeneic male bone marrow grafts. Appearance of B6.Thy1.1 male T cells in PBL of female B6 (Thy1.2) recipients pretreated intranasally with 3 × 100 μg HYAbDby peptide 10 days before irradiation (200 R) and with intravenous injection of 5 × 106 B6 Thy1.1 male bone marrow cells. FACS profiles of PBLs stained for each Thy1 allele, 2 weeks after bone marrow transplantation, of single representative PBS-treated mice (i), peptide-treated mice (ii), and percentage of donor-derived Thy1.1+ T cells in PBLs from recipient mice, PBS-treated, n = 6 (iii), and peptide-treated, n = 6 (iv) at 2, 4, and 6 weeks after bone marrow injection. Each symbol represents data from an individual mouse. Significant differences of peptide-treated from PBS control: P = .0021 at 4 weeks, P = .0028 at 6 weeks. (B) The in vivo cytotoxicity assay: FACS analysis of CFSE-label and tetramer. A mixture of male and female spleen cells labeled with different concentrations of CFSE (male, low; female, high) were injected intravenously into recipient mice: a PBS-treated naive female (i-ii) and an HYAbDby peptide–pretreated female (iii-iv). PBLs taken 14 days later were stained with CD8 and tetramer and examined by FACS. Panels i and iii show the remaining CFSE-labeled donor cells; panels ii and iv show tetramer-positive cells of recipient origin. (C) Intranasal HYAbDby peptide prolongs survival of syngeneic male spleen cells. B6 females were given intranasal PBS (n = 8) or 3 × 100 μgHYAbDby peptide (n = 8). Ten days later they received intravenously a mixture of differentially CFSE-labeled male and female splenocytes, and sequential PBL samples were analyzed as described. Panels i and ii show ratio of male to female cells, panels iii and iv show frequency of HYDbUty tetramer-positive cells within CD8+ T-cell population. Each symbol represents an individual mouse.
Induction of tolerance to male bone marrow and spleen cell engraftment by HY peptide. (A) Intranasal administration of HYAbDby peptide facilitates engraftment of syngeneic male bone marrow grafts. Appearance of B6.Thy1.1 male T cells in PBL of female B6 (Thy1.2) recipients pretreated intranasally with 3 × 100 μg HYAbDby peptide 10 days before irradiation (200 R) and with intravenous injection of 5 × 106 B6 Thy1.1 male bone marrow cells. FACS profiles of PBLs stained for each Thy1 allele, 2 weeks after bone marrow transplantation, of single representative PBS-treated mice (i), peptide-treated mice (ii), and percentage of donor-derived Thy1.1+ T cells in PBLs from recipient mice, PBS-treated, n = 6 (iii), and peptide-treated, n = 6 (iv) at 2, 4, and 6 weeks after bone marrow injection. Each symbol represents data from an individual mouse. Significant differences of peptide-treated from PBS control: P = .0021 at 4 weeks, P = .0028 at 6 weeks. (B) The in vivo cytotoxicity assay: FACS analysis of CFSE-label and tetramer. A mixture of male and female spleen cells labeled with different concentrations of CFSE (male, low; female, high) were injected intravenously into recipient mice: a PBS-treated naive female (i-ii) and an HYAbDby peptide–pretreated female (iii-iv). PBLs taken 14 days later were stained with CD8 and tetramer and examined by FACS. Panels i and iii show the remaining CFSE-labeled donor cells; panels ii and iv show tetramer-positive cells of recipient origin. (C) Intranasal HYAbDby peptide prolongs survival of syngeneic male spleen cells. B6 females were given intranasal PBS (n = 8) or 3 × 100 μgHYAbDby peptide (n = 8). Ten days later they received intravenously a mixture of differentially CFSE-labeled male and female splenocytes, and sequential PBL samples were analyzed as described. Panels i and ii show ratio of male to female cells, panels iii and iv show frequency of HYDbUty tetramer-positive cells within CD8+ T-cell population. Each symbol represents an individual mouse.
Induction of tolerance to male bone marrow and hematopoietic cell engraftment by intranasally administered HY peptide
B6 female recipient mice pretreated with either 100 μg HYAbDby peptide or PBS were given low-dose irradiation (200 R) 10 days later and intravenous injections of 5 × 106 bone marrow cells from B6.Thy1.1 male donors. PBL samples taken 14, 28, and 42 days later were stained with fluorescent antibodies specific for each Thy1 allele, and, on day 42, with HYDbUty tetramer. Engraftment of donor bone marrow was first observed in the peptide-pretreated group at day 28, with up to 32% of peripheral T cells of donor origin (Figure 2Aii) and these increased by day 42. In contrast, PBS-pretreated recipients had negligible levels of Thy 1.1 donor T cells (Figure 2Ai). Comparison of the percentage of Thy1.1 T cells in both groups (Figures 2Aiii,iv) showed that at days 28 and 42 the level of male bone marrow–derived T cells in the peptide-treated group was significantly higher than in the control.
PBS- and peptide-pretreated female B6 mice were given injections with syngeneic male and female spleen cells labeled with different concentrations of CFSE. CFSE is used in this assay as a marker of nondividing splenocytes, the 2 levels of staining allowing the relative numbers of 2 populations to be determined. PBL samples taken at the times indicated were assessed for the ratio of donor male-to-female CFSE-labeled cells, and for HY-specific tetramer responses, as illustrated in Figure 2B. PBLs from a PBS-treated naive female taken 14 days after injection of CFSE-labeled cells showed selective removal of male cells and a substantial level of HYAbDby-specific tetramers (Figure 2Bi,ii). A female receiving peptide pretreatment 10 days before injection of CFSE-labeled cells retained both male and female populations and gave only a small HYAbDby-specific tetramer response (Figure 2Biii,iv).
To investigate whether pretreatment with the HYAbDby peptide could affect acceptance of hematopoietic cells, we examined the effects of in vivo challenge with CFSE-labeled male and female splenocytes 10 days after intranasal administration of peptide or PBS. PBS-pretreated recipients rejected CFSE-labeled male cells within 14 days (Figure 2Ci) and this corresponded with the appearance of HYDbUty-specific tetramer responses (Figure 2Ciii). In contrast, removal of male CFSE-labeled cells was protracted in mice from the peptide-pretreated group and these recipients developed lower tetramer responses (Figure 2Cii,iv).
Investigation of cytokine production by HY-specific T cells during tolerance induction following intranasal peptide administration
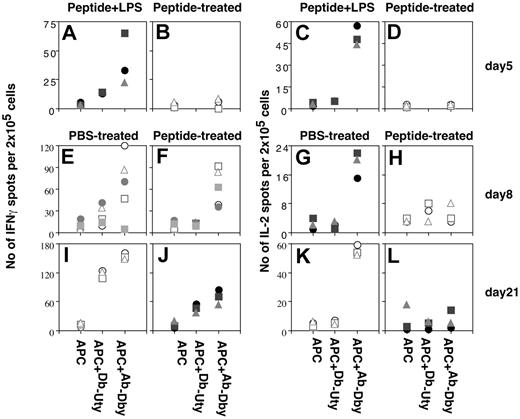
Early after intranasal peptide treatment and before skin grafting, cervical lymph node cells from females receiving pretreatment with HYAbDby peptide alone or with LPS (which causes immunization, Figure 1C) were examined by ELISPOT for IFN-γ and IL-2 secretion in response to HY peptides. Mice receiving peptide plus LPS had significant numbers of cells producing IFN-γ and IL-2 in response to the HYAbDby peptide, whereas cells from mice receiving peptide alone gave no response (Figure 3A-D; P = .035 and .0003 for IFN-γ and IL-2, respectively). Responses on days 8 and 21 after test grafting were examined by ELISPOT analysis of cells from graft draining lymph nodes from intranasal peptide or PBS-pretreated mice. Figure 3, panels E, F, I, and J, show clear evidence of HYAbDby-specific CD4+ cells making IFN-γ in both groups at each time point (P = .44 day 8; P = .05 day 21), but interestingly, in contrast to the PBS controls, those from peptide-pretreated mice did not make IL-2 following skin graft challenge (Figure 3G,H,K,L; P = .0056 day 8; P = .0003 day 21).
Ex vivo cytokine responses following intranasal peptide, before and after skin grafting. HYAbDby peptide and peptide plus LPS intranasally treated mice at day 5. Three mice from each group of mice, given 3 × 100 μg HYAbDby with (A,C) or without (B,D) 3 μg LPS were killed and cervical lymph nodes taken for ELISPOT analysis. Panels A and B show the IFN-γ response in the presence of irradiated female splenocytes alone or pulsed with HYDbUty or HYAbDby peptide; panels C and D show the IL-2 response. PBS- and HYAbDby peptide–pretreated mice 8 and 21 days after skin grafting. Two groups of B6 females (8 mice/group) pretreated with 3 × 100 μg HYAbDby peptide or PBS intranasally were given grafts with syngeneic male skin 10 days later. Recipients were killed 8 (5 mice per group) and 21 (3 mice per group) days later, and cell suspensions from graft-draining lymph nodes, from PBS-treated (E,G,I,K) and peptide-treated mice (F,H,J,L), were tested by ELISPOT assay for IFN-γ (E,F,I,J) and IL-2 (G,H,K,L) production in response to irradiated female splenocytes alone or pulsed with HYDbUty or HYAbDby peptide. Results are expressed as spots/2 × 105 cells. Each symbol represents an individual mouse.
Ex vivo cytokine responses following intranasal peptide, before and after skin grafting. HYAbDby peptide and peptide plus LPS intranasally treated mice at day 5. Three mice from each group of mice, given 3 × 100 μg HYAbDby with (A,C) or without (B,D) 3 μg LPS were killed and cervical lymph nodes taken for ELISPOT analysis. Panels A and B show the IFN-γ response in the presence of irradiated female splenocytes alone or pulsed with HYDbUty or HYAbDby peptide; panels C and D show the IL-2 response. PBS- and HYAbDby peptide–pretreated mice 8 and 21 days after skin grafting. Two groups of B6 females (8 mice/group) pretreated with 3 × 100 μg HYAbDby peptide or PBS intranasally were given grafts with syngeneic male skin 10 days later. Recipients were killed 8 (5 mice per group) and 21 (3 mice per group) days later, and cell suspensions from graft-draining lymph nodes, from PBS-treated (E,G,I,K) and peptide-treated mice (F,H,J,L), were tested by ELISPOT assay for IFN-γ (E,F,I,J) and IL-2 (G,H,K,L) production in response to irradiated female splenocytes alone or pulsed with HYDbUty or HYAbDby peptide. Results are expressed as spots/2 × 105 cells. Each symbol represents an individual mouse.
Cytokine production by HY peptide–specific cells from long-term tolerant mice
The numbers of HY tetramer–positive CD8+ T cells in PBLs from both HYAbDby peptide–pretreated tolerant recipients and PBS-pretreated controls were low (< 0.5% of CD8+ T cells). Following in vivo challenge with 5 × 106 male spleen cells intraperitoneally 7 days before testing, splenocytes from graft-rejected mice had greater expansions of tetramer-positive cells than tolerant mice (Figure 4Ai,ii; P = .0141). ELISPOT analysis showed graft-rejected mice had high numbers of IFN-γ–producing cells responding to male and to female antigen-presenting cells (APCs) pulsed with each of the 3 HY peptides (Figure 4Aiii), whereas tolerant mice gave lower responses (Figure 4Aiv; P = .0009, HYDbUty; P = .01, HYDbSmcy; P = .035, HYAbDby).
Clonal expansions and cytokine production following in vivo antigenic challenge of long-term tolerant B6 mice and their graft-rejecting controls. (A) Ex vivo data. Freshly isolated splenocytes from graft-rejected mice have a higher frequency of HYDbUty tetramer–positive CD8+ T cells and HY peptide–specific IFN-γ–producing cells than their tolerant counterparts. Seven PBS-treated graft-rejected mice (○), and 6 mice pretreated with 3 × 100 μg HYAbDby that retained syngeneic male skin grafts for more than 400 days (▵), were boosted intraperitoneally with 5 × 106 male splenocytes. Seven days later spleen cell suspensions from individual animals were analyzed by FACS for percentage of HYDbUty tetramer–positive CD8+ T cells (i-ii) and by ELISPOT for IFN-γ production in response to irradiated female or male B6 splenocytes alone, or irradiated female splenocytes pulsed with HYDbUty, HYDbSmcy, or HYAbDby peptide. The results are expressed as spots/2 × 105 cells (iii-iv). (B) After in vitro restimulation. Splenic cultures from graft-rejected, in vivo–boosted mice show large clonal expansions of HYDbUty tetramer–positive CD8+ T cells following in vitro restimulation, whereas responses of tolerant mice are lower; intracellular IFN-γ and IL-10 cytokine responses of T cells from the in vitro cultures from tolerant mice are also diminished. Splenocytes from the rejected (•) or tolerant (▴) mice, shown in panels Ai-ii, were incubated with irradiated B6 male spleen cells and IL-2 for 7 days. The cultured cells were analyzed by FACS and the results shown as a percentage of HYDbUty tetramer–positive CD8+ T cells (i-ii). The cultured cells were also analyzed for intracellular cytokine production after restimulation with unpulsed (○, ▵), or HYDbUty and HYAbDby peptide–pulsed APCs (•, ▴) for 6 hours in the presence of monensin. The cells were stained with anti-CD8 or anti-CD4, fixed, permeabilized, and then stained with anti–IFN-γ or anti–IL-10. Results are shown as the percentage of IFN-γ (iii-iv) or IL-10–producing cells (v-vi) in the CD8+ (iii,v) or CD4+ T-cell population (iv,vi). Note: the y-axes of panels iii to vi differ to facilitate identification of individual mice.
Clonal expansions and cytokine production following in vivo antigenic challenge of long-term tolerant B6 mice and their graft-rejecting controls. (A) Ex vivo data. Freshly isolated splenocytes from graft-rejected mice have a higher frequency of HYDbUty tetramer–positive CD8+ T cells and HY peptide–specific IFN-γ–producing cells than their tolerant counterparts. Seven PBS-treated graft-rejected mice (○), and 6 mice pretreated with 3 × 100 μg HYAbDby that retained syngeneic male skin grafts for more than 400 days (▵), were boosted intraperitoneally with 5 × 106 male splenocytes. Seven days later spleen cell suspensions from individual animals were analyzed by FACS for percentage of HYDbUty tetramer–positive CD8+ T cells (i-ii) and by ELISPOT for IFN-γ production in response to irradiated female or male B6 splenocytes alone, or irradiated female splenocytes pulsed with HYDbUty, HYDbSmcy, or HYAbDby peptide. The results are expressed as spots/2 × 105 cells (iii-iv). (B) After in vitro restimulation. Splenic cultures from graft-rejected, in vivo–boosted mice show large clonal expansions of HYDbUty tetramer–positive CD8+ T cells following in vitro restimulation, whereas responses of tolerant mice are lower; intracellular IFN-γ and IL-10 cytokine responses of T cells from the in vitro cultures from tolerant mice are also diminished. Splenocytes from the rejected (•) or tolerant (▴) mice, shown in panels Ai-ii, were incubated with irradiated B6 male spleen cells and IL-2 for 7 days. The cultured cells were analyzed by FACS and the results shown as a percentage of HYDbUty tetramer–positive CD8+ T cells (i-ii). The cultured cells were also analyzed for intracellular cytokine production after restimulation with unpulsed (○, ▵), or HYDbUty and HYAbDby peptide–pulsed APCs (•, ▴) for 6 hours in the presence of monensin. The cells were stained with anti-CD8 or anti-CD4, fixed, permeabilized, and then stained with anti–IFN-γ or anti–IL-10. Results are shown as the percentage of IFN-γ (iii-iv) or IL-10–producing cells (v-vi) in the CD8+ (iii,v) or CD4+ T-cell population (iv,vi). Note: the y-axes of panels iii to vi differ to facilitate identification of individual mice.
In vitro expansion of HY peptide–specific cells from long-term tolerant and graft-rejected mice: investigation of intracellular cytokine production
Tetramer-positive T cells from tolerant mice following in vivo boosting could be further expanded by in vitro culture for 7 days with male APCs in the presence (Figure 4Bii) or absence (not shown) of IL-2. However, these expansions were lower than in PBS-pretreated controls (Figure 4Bi; P = .01). Intracellular cytokine secretion by CD4+ and CD8+ T cells from graft-rejected and tolerant mice showed substantial differences (Figure 4Biii-vi). Although IFN-γ production by CD8+ T cells was independent of the presence of HY peptide in the 6-hour culture period prior to assay, the number of IFN-γ–producing CD8+ T cells was lower in cultures from tolerant mice than graft-rejected controls (Figure 4Biii; P = .0075). Not surprisingly, there was a direct correlation between the numbers of HYDbUty tetramer–specific cells and IFN-γ–producing CD8+ T cells in the in vitro–expanded cultures (Figure 4Bi-iii). Similarly, a higher proportion of CD4+ T cells from graft-rejected mice produced IFN-γ after peptide challenge, compared with tolerant mice (Figure 4Biv; P = .0001). Although only a small percentage of CD8+ T cells from graft-rejected or tolerant mice produced IL-10 (Figure 4Bv; P = .064, not significant), an unexpected finding was that the number of IL-10–producing cells in cultures of CD4+ T cells from graft-rejected mice was significantly higher than that from tolerant mice (Figure 4Bvi; P = .0007).
Capacity of HY-specific T cells from naive, immune, and tolerant females to expand following prolonged in vitro contact with antigen
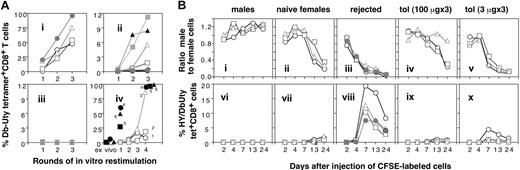
Expansions of HY-specific T cells from PBLs of 3 groups of mice and from spleen in a further group after in vitro culture with male APCs are shown (Figure 5). PBS-pretreated immune mice (Figure 5Ai) that had rejected their test male skin grafts showed substantial levels of HY-tetramer positive T cells after one round of restimulation, and all had high levels after the second and third restimulations. PBLs from tolerant mice (Figure 5Aii) took longer to show HY-specific tetramer positive cells, and these expansions were smaller. In contrast, PBLs from naive mice (Figure 5Aiii), not previously exposed in vivo to male tissues, did not generate HY tetramer expansions, even after 3 rounds of restimulation, in line with previous findings.26 In vitro restimulation of spleen cell cultures from 6 long-term tolerant mice showed that 3 required up to 4 rounds before the appearance of tetramer-positive T cells (Figure 5Aiv), cytokine responses to HY peptides (data not shown), and HY-specific cytotoxic function (data not shown).
Expansions of HY-specific T cells from PBLs and spleen. (A) Tetramer-positive T cells clonally expand with repeated in vitro restimulation. Panels i to iii show clonal expansion in vitro of tetramer-positive T cells from tolerant, memory, and naive mice. PBLs from 3 groups of mice, rejected (i; n = 4), tolerant (ii; n = 11), and naive (iii; n = 6), were stimulated with irradiated syngeneic male spleen cells plus IL-2. Cultures were sampled on day 7 for tetramer analysis, and the remaining cells restimulated on day 14, with sampling and restimulation repeated for 2 further rounds. Each symbol represents an individual mouse. The y-axes of panels ii and iii differ to facilitate identification of individual mice. Panel iv shows clonal expansions in vitro from spleen cells of long-term tolerant mice. T-cell lines were generated by repeated restimulation every 4 weeks of spleen cells from 6 tolerant mice (shown in Figure 4Bii) with irradiated male B6 splenocytes plus IL-2. Seven days after each restimulation, T cells were analyzed for the frequency of HYDbUty tetramer–positive CD8+ T cells (each symbol representing an individual mouse). (B) In vivo cytotoxic capacity of tolerant, naive, and sensitized mice. Five groups of B6 recipient mice (n = 3 or 4), naive males (i,vi), naive females (ii,vii), graft-rejected (iii,viii), and long-term (> 100 days) tolerant females following high-dose (3 × 100 μg; iv,ix), or low-dose (3 × 3 μg; v,x) intranasal HYAbDby peptide, were given intravenous injections on day 0 with a mixture of 1 × 107 female B6 splenocytes (labeled with 5 μM CFSE) and 1 × 107 male B6 splenocytes (labeled with 0.5 μM CFSE). PBLs taken between days 2 and 24 were analyzed for CFSE-labeled cells and HYDbUty tetramer–positive CD8+ T cells. The ratio of male to female cells is shown in panels i to v, the frequency of HYDbUty tetramer–positive cells within CD8 T-cell population in panels vi to x. Each symbol represents an individual mouse.
Expansions of HY-specific T cells from PBLs and spleen. (A) Tetramer-positive T cells clonally expand with repeated in vitro restimulation. Panels i to iii show clonal expansion in vitro of tetramer-positive T cells from tolerant, memory, and naive mice. PBLs from 3 groups of mice, rejected (i; n = 4), tolerant (ii; n = 11), and naive (iii; n = 6), were stimulated with irradiated syngeneic male spleen cells plus IL-2. Cultures were sampled on day 7 for tetramer analysis, and the remaining cells restimulated on day 14, with sampling and restimulation repeated for 2 further rounds. Each symbol represents an individual mouse. The y-axes of panels ii and iii differ to facilitate identification of individual mice. Panel iv shows clonal expansions in vitro from spleen cells of long-term tolerant mice. T-cell lines were generated by repeated restimulation every 4 weeks of spleen cells from 6 tolerant mice (shown in Figure 4Bii) with irradiated male B6 splenocytes plus IL-2. Seven days after each restimulation, T cells were analyzed for the frequency of HYDbUty tetramer–positive CD8+ T cells (each symbol representing an individual mouse). (B) In vivo cytotoxic capacity of tolerant, naive, and sensitized mice. Five groups of B6 recipient mice (n = 3 or 4), naive males (i,vi), naive females (ii,vii), graft-rejected (iii,viii), and long-term (> 100 days) tolerant females following high-dose (3 × 100 μg; iv,ix), or low-dose (3 × 3 μg; v,x) intranasal HYAbDby peptide, were given intravenous injections on day 0 with a mixture of 1 × 107 female B6 splenocytes (labeled with 5 μM CFSE) and 1 × 107 male B6 splenocytes (labeled with 0.5 μM CFSE). PBLs taken between days 2 and 24 were analyzed for CFSE-labeled cells and HYDbUty tetramer–positive CD8+ T cells. The ratio of male to female cells is shown in panels i to v, the frequency of HYDbUty tetramer–positive cells within CD8 T-cell population in panels vi to x. Each symbol represents an individual mouse.
In vivo anti-HY cytotoxic capacity of tolerant mice
To detect the in vivo cytotoxic potential of cells from tolerant mice, male and female cells labeled with differing concentrations of CFSE (Figure 2B) were injected intravenously into 3 to 4 individual mice in each of 5 recipient groups. This included males as control nonresponders, that maintained a ratio of male-to-female donor cells close to 1 throughout the experiment (Figure 5Bi). Naive female recipients gave a primary rejection response, with selective removal of male cells between day 7 and 14 (Figure 5Bii). PBS-pretreated mice that had rejected their test male skin grafts rejected CFSE-labeled male cells by day 7 (Figure 5Biii). In contrast, peptide-pretreated tolerant mice, retaining their syngeneic male test skin grafts over 150 days, rejected CFSE-labeled male cells more slowly, or, in some cases, retained them for the duration of the experiment (Figure 5Biv). In parallel, tetramer analysis showed rapid and substantial expansions of HYDbUty tetramer-positive cells in immune mice (Figure 5Bviii), slower, smaller expansions in naive mice (Figure 5Bvii), and low but detectable levels of these cells in tolerant mice (Figure 5Bix,x). Mice given the lower dose of peptide rejected their CFSE-labeled spleen cells more rapidly than naive females (compare Figure 5Bii and 5Bv) and generated more tetramer-positive cells than naive recipients or those receiving high-dose peptide (Figure 5Bvii,ix-x). Nevertheless, these mice remained tolerant of their skin grafts, as did those receiving high-dose peptide.
Transfer of tolerance to naive females
The finding that tolerance induced by intranasal administration of HYAbDby peptide was not associated with deletion of HY-specific T cells suggested a mechanism involving regulatory cells. Adoptive transfer of populations of splenic cells from long-term tolerant mice into neonatal females showed that transfer of either unseparated spleen cells or those depleted of CD8+ cells resulted in tolerance of the recipients grafted 6 weeks later with syngeneic male skin (Figure 6). In contrast, recipients of spleen cells depleted of CD4+ cells or of both CD4+ and CD8+ cells showed no prolongation of graft survival. Figure 6 shows one representative experiment: a second replicated the result. These data provide evidence for the involvement of regulatory cells in the maintenance of long-term tolerance induced with intranasal HYAbDby peptide.
Adoptive transfer of tolerance with splenocyte subpopulations. Survival times of syngeneic male skin grafts on B6 females injected intraperitoneally as neonates with 5 × 106 splenic cells from tolerant donors: either unseparated (n = 7; □), CD8 depleted (n = 4; ▵), CD4 depleted (n = 4; ⋄), or depleted of both cell subsets (n = 6; •).
Adoptive transfer of tolerance with splenocyte subpopulations. Survival times of syngeneic male skin grafts on B6 females injected intraperitoneally as neonates with 5 × 106 splenic cells from tolerant donors: either unseparated (n = 7; □), CD8 depleted (n = 4; ▵), CD4 depleted (n = 4; ⋄), or depleted of both cell subsets (n = 6; •).
Discussion
Intranasal delivery of the MHC class II–restricted HY peptides, HYAbDby and HYEkDby, induces robust antigen-specific tolerance in H2b and (H2b × H2k)F1 female mice to male skin grafts expressing an array of up to 5 additional MHC class I and class II peptides (Figure 1A-F), showing clear evidence for linked suppression.6 As little as 3 μg of the HYAbDby peptide given on 3 consecutive days is sufficient to cause skin graft tolerance (Figure 1C). This represents a considerably lower concentration than previously reported,22,27 and may be due to a low density of endogenous antigens on the test male grafts.28 Although much less effective, pretreatment by intranasal administration of HYDbUty peptide also causes tolerance with a similar phenotype in some recipients.
Our observation that intranasal peptide pretreatment alone causes not only skin graft tolerance, but also abrogation or delay in rejection of CFSE-labeled male spleen cells, and allows engraftment of male bone marrow stem cells (Figures 1, 2 and 5B) indicates that this may prove a good model for investigation for tolerance induction in stem cell transplantation (SCT). Furthermore, the finding that CFSE-labeled male hematopoietic cells are more sensitive to in vivo cytolysis than male skin grafts to rejection suggests that this minor H model may also be useful for the dissection of graft-versus-host (GvH) and graft-versus-leukemia (GvL) responses in SCT as investigated by Fontaine and colleagues.29
Induction of tolerance by the intranasal administration of the MHC class II–restricted HYAbDby peptide is likely to be a result of peptide presentation in the absence of a second immunostimulatory signal; clear evidence that presentation of peptide by resting DCs causes tolerance comes from a recent report in which expression of a lymphocytic choriomeningitis virus (LCMV)–derived CD8+ peptide epitope was induced on resting DCs in vivo, thus avoiding any in vitro manipulation,30 leading to tolerance not only to the peptide but also to subsequent viral infection. Following intranasal peptide administration, CD4+ cells from cervical lymph nodes failed to give a cytokine response on antigenic restimulation. In contrast, similar cells from mice given peptide plus LPS, which causes immunization (Figure 1C), gave significant peptide-specific IFN-γ and IL-2 responses (Figure 3A-D). To investigate the properties of the APCs in the draining lymph node, we gave fluorescently labeled peptide intranasally. However, the small proportion of labeled DCs in the cervical lymph node and spleen precluded the possibility of isolating sufficient numbers of cells for this. Nevertheless, CD11c+ cells from mice given intranasal peptide failed to cause proliferation of an HY peptide–specific T-cell clone, whereas the same population of cells from peptide plus LPS-treated mice was able to stimulate proliferation (not shown), giving an indication of the functional activation state of these cells.
Others have shown by adoptive transfer of T-cell receptor (TCR)–transgenic T cells that tolerance induction mediated by intranasal peptide is preceded by a wave of proliferation and cytokine production.22,31 Using a similar adoptive transfer approach with CFSE-labeled HY-specific TCR transgenic T cells, we found limited proliferation following intranasal peptide (compared with peptide plus LPS; not shown). However, administration of peptide to naive nontransgenic mice failed to trigger a response, suggesting intranasal peptide does not induce the low numbers of HYAbDby-specific precursor cells in unmanipulated mice to expand or produce cytokines. These results suggest that the initiation of tolerance induction in this model is due to peptide presentation by a population of “immature” DCs that fail to fully prime the CD4+ T cells, resulting in impaired proliferation and cytokine production.
Shortly after antigenic challenge in the form of a male skin graft (8 days), CD4+ cells from both peptide- and PBS-treated control mice made similar IFN-γ responses, providing evidence that the HYAbDby-specific CD4+ cells had not been deleted by peptide pretreatment (Figure 3E-F) The major difference between the mice that become tolerant and those that reject their grafts is the failure of HYAbDby-specific CD4+ cells from peptide-pretreated mice to produce IL-2 (Figure 3). Our finding that intranasal administration of either the HYAbDby or the HYEkDby peptide to (CBAxB6)F1 mice, where both HYAb-and HYEk-specific CD4+ cells are potentially able to provide help for HY-specific CD8+ cells, induces tolerance (Figure 1D) further shows that tolerance induction is a result of a dominant tolerance mechanism rather than functional disablement of a CD4+ helper population. We postulate that tolerance is induced as a result of incomplete activation of the HY-specific CD4+ cells by peptide presented on immature DCs. This leads to the generation of cells with an anergic phenotype that additionally fulfill a regulatory role,32,33 acting on APCs expressing additional HY epitopes at the site of the male graft. The presence of such regulatory cells early in the response has clearly been demonstrated using an in vivo adoptive transfer model34 ; however, neither the target nor mechanism of regulation has so far been identified.
We have also examined how tolerance, once established, is maintained. In long-term tolerant mice we found no evidence for significant levels of clonal deletion of HY-specific T cells. The low levels of HYDbUty tetramer–positive cells in tolerant mice are similar to those found in long-term memory mice.20 They, as well as HYAbDby-specific CD4+ T cells, can be expanded from tolerant mice following in vitro and in vivo restimulation; however, the expansions are considerably smaller than in mice that have rejected grafts (Figures 4Ai-ii,Bi-ii and 5Aiv). These more limited expansions might be due to apoptosis of the HY-specific cells as a result of activated cell death as has been shown in other models of transplantation tolerance and autoimmune disease.35-37 However, the results presented here suggest the mechanism is more likely to be ineffective priming. The lower level of expansion is also associated with a lower frequency of cytokine-producing cells, particularly striking for the CD4+ T-cell population (Figure 4Biv,vi). We were initially surprised that CD4+ T cells from tolerant mice failed to produce IL-10, particularly in the light of previous reports, using autoimmune models, demonstrating a role for IL-10 in tolerance induction.38-41 However, the finding that IL-10–/– mice can be made tolerant by intranasal administration of peptide (Figure 1E) as well as wild-type mice (Figure 1A) clearly shows that in this transplantation model, IL-10 does not play a crucial role. Our observations of a limited capacity for cytokine production by antigen-specific cells from tolerant mice are more consistent with the findings of a recent report,42 showing that although the ability of transgenic CD8+ cells to proliferate in response to antigen is indistinguishable whether the cells are transferred into naive or tolerant recipients, cytokine production and the cytotoxic capacity of transgenic cells transferred into tolerant hosts are impaired.
It is clear that tolerance in mice bearing long-term male skin grafts is maintained, despite the presence of circulating HY peptide–specific CD4+ and CD8+ T cells able to expand on in vivo challenge with male antigen. In addition, peptide-pretreated mice, challenged with male (CFSE-labeled) cells before skin grafting, were able to subsequently accept male skin grafts (Figure 1F) despite the inflammatory environment of the newly placed graft itself and again, the presence of circulating HY peptide–specific cells. Tolerance could be the result of (1) insufficient numbers of HY-specific CD4+ and CD8+ T cells to mediate skin graft rejection, or (2) qualitative, cell autonomous changes in class I– or II–restricted HY-specific cells, or (3) the presence of regulatory cells. We have evidence for more restricted expansions of HY-specific tetramer-positive cells in tolerant mice and their impaired ability to make cytokines in response to peptide. However, long-term tolerant mice will have naive HY-specific cells newly emigrating from the thymus, indicating that tolerance is not just a result of impaired quantitative or qualitative initial response to antigen but due to regulatory factors as evidenced by the ability of CD4+ cells adoptively transferred into neonatal recipients to render them, in turn, tolerant of syngeneic male skin grafts (Figure 6). Similar findings were made for mice made tolerant to male grafts following pretreatment with class I–restricted HY peptide–pulsed DCs.21 Waldmann and colleagues have clearly demonstrated the presence of regulatory T cells in their model of transplantation tolerance43-45 and a requirement for the sustained presence of antigen to maintain the tolerant state,46 a situation also manifest in our long-term tolerant mice.
Identification of the MHC class I and class II peptide epitopes responsible for male graft rejection in mice expressing the H2b and H2k haplotypes has given us the unique ability to dissect the immune response to this complex antigen and to induce antigen-specific tolerance that can be extended by linked suppression to include a substantial number of additional epitopes. Using a combination of tetramer staining, ELISPOT, and intracellular cytokine analysis, we have shown that peptide-induced tolerance does not cause deletion of HY-specific T cells. Rather, tolerance induction and the associated appearance of linked suppression is due to a defect in the ability of these cells to expand and produce proinflammatory cytokines. In the presence of potentially cytotoxic HY-specific CD8+ T cells that can be expanded both in vivo and in vitro by male antigen, maintenance of tolerance is associated with a population of CD4+ regulatory cells. An understanding of how this balance between responsiveness and nonresponsiveness is maintained will also be relevant for tumor immunotherapy, and further extending linked suppression to additional epitopes will be relevant for modulation of responses to both allografts and replacement therapies that introduce foreign protein antigens.
Prepublished online as Blood First Edition Paper, January 15, 2004; DOI 10.1182/blood-2003-11-3763.
Supported by funding from the Medical Research Council, United Kingdom.
E.S. and D.S. are joint senior authors.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We should like to thank Julian Dyson for his comments on the paper, Maggie Millrain for making the tetramers used in this study, and Vivien Tikerpae for her help with the manuscript.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal