Abstract
Induction of fetal hemoglobin (Hb F) is an important therapeutic tool in ameliorating complications of sickle cell disease. Nitric oxide has been implicated in the mechanism of Hb F synthesis induced by hydroxyurea (HU). This study examined whether zileuton (ZL), a structural analog of hydroxyurea, possessed Hb F–inducing properties and the potential role nitric oxide plays. ZL caused a dose-dependent increase in γ-globin expression in K562 cells. This effect was confirmed by a dose-dependent increase in Hb F synthesis in erythroid progenitors from individuals with sickle cell anemia and normal hemoglobin genotypes. l-arginine had no effect on Hb F production; however, it dose-dependently inhibited ZL's ability to induce Hb F. The nitric oxide synthase inhibitor NG-monomethyl–l-arginine (l-NMMA) inhibited l-arginine's effect and restored ZL-mediated increase in Hb F synthesis. In addition, 8-PCPT–cGMP (8-(4-chlorophenylthio)guanosine 3′,5′-cyclic monophosphate) inhibited ZL-mediated induction of Hb F synthesis. When comparing l-NMMA effects alone on ZL and HU, a partial reversal of increased Hb F synthesis was seen only with HU. Neither l-arginine alone nor l-arginine in combination with l-NMMA effected hydroxyurea-mediated induction of Hb F synthesis. This study demonstrates that ZL induces Hb F through a mechanism that involves l-arginine/nitric oxide/cGMP in a manner distinctly different from HU.
Introduction
Zileuton (ZL; (N-(1-benzo[b]thien-2-ylethyl)-N-hydroxyurea) is a specific inhibitor of 5-lipoxygenase (5-LO), which results in decreased formation of leukotrienes (LTB4, LTC4, LTD4, and LTE4).1 Structurally, ZL contains N-hydroxyurea (HU).2 This study investigated whether or not ZL can stimulate fetal hemoglobin (Hb F) synthesis in erythroid progenitors, as has been observed with HU. Currently HU is the only Federal Drug Administration–approved agent used to stimulate Hb F synthesis with proven efficacy in the treatment of sickle cell disease. HU has been documented to reduce the severity and frequency of painful vasoocclusive crisis, the incidence of acute chest syndrome,3,4 and mortality5 in sickle cell anemia (HbSS). Despite its use in HbSS, the mechanism of action by which HU induces Hb F remains uncertain. HU is cytotoxic and inhibits growth of burst forming unit-erythroid (BFU-E) colonies in a dose-dependent manner, while Hb F levels increase. The decrease in BFU-E colony growth observed with HU treatment is related to interruption of DNA synthesis in rapidly dividing late erythroid progenitors by inhibition of the enzyme, ribonucleotide reductase. This leads to a transient arrest of hematopoiesis. Suppression of BFU-E colony growth has been demonstrated to enhance premature commitment of earlier progenitor cells, accelerate erythropoiesis, and increase survival of sickle red blood cells containing Hb F.6-8
Nitric oxide has been implicated in the regulation of Hb F induction by HU, which was recently shown to react with heme proteins to generate nitric oxide.9-11 Ikuta et al12 demonstrated that an intracellular pathway, which includes soluble guanylate cyclase and cyclic guanosine monophosphate (cGMP)–dependent protein kinase, plays a role in the induction of γ-globin gene expression in 2 independent K562 erythroleukemic cell lines. Most recently, Cokic et al13 demonstrated that HU induces Hb F by the nitric oxide–dependent activation of soluble guanylate cyclase in K562 cells and human erythroid progenitors.
While, structurally, ZL contains HU, there are no published data that document whether ZL stimulates Hb F synthesis in primary erythroid progenitors.6-8 In the present study, we demonstrate ZL is a potent inducer of γ-globin mRNA in normal and sickle erythroid progenitors and in K562 cells. This study further demonstrates that ZL decreases BFU-E colonies in cell culture and stimulates Hb F synthesis similarly to that observed with HU. We postulated that ZL induced Hb F through a nitric oxide–dependent pathway, as previously suggested with HU. We found that ZL induction of Hb F synthesis occurs through a mechanism that does not involve the nitric oxide/cGMP pathway as demonstrated for HU. Furthermore, we observed that the nitric oxide/cGMP pathway does play a negative regulatory role in ZL induction of Hb F in contrast to HU.
Patients, materials, and methods
Materials
Bovine serum albumin (BSA, 66 000 molecular weight [mol wt]), and l-arginine were purchased from Sigma (St Louis, MO). ZL and HU were purchased from the University of South Alabama Pharmacy (Mobile, AL). NG-monomethyl–l-arginine (l-NMMA) was purchased from RBI (Natick, MA). Phorbol myristate acetate (PMA) and A23187 were dissolved in dimethyl-sulfoxide (DMSO). ZL was dissolved in 95% ethanol. HU, l-arginine, and l-NMMA were dissolved in sterile water. The 8-PCPT–cGMP (8-(4-chlorophenylthio)guanosine 3′,5′-cyclic monophosphate) was purchased from BIOLOG (Bremen, Germany) and dissolved in phosphate-buffered saline (PBS).
Blood collection
All participants gave informed consent and the University of South Alabama Institutional Review Board approved the protocol. During steady-state, blood samples (40 mL) were obtained from individuals with homozygous sickle cell anemia in the sickle cell clinics at the University of South Alabama. Whole blood samples were collected in syringes containing heparin and used within 48 hours of collection. Individuals who received a transfusion within 6 weeks of sample procurement were excluded from this study. Sickle cell anemia was documented by hemoglobin electrophoresis on each subject.
Cell culture
Human K562 erythroleukemic cells were grown in suspension cultures in RPMI-1640 containing 10% fetal bovine serum, penicillin (100 U/mL), and streptomycin (0.1 mg/mL) in a humidified incubator at 37° C, 5%CO2/95% air atmosphere. The following experimental conditions were analyzed in triplicate with 0.3 × 106 cells per well: an untreated control; ZL dissolved in 0.1% DMSO at final concentrations of 20, 40, 50, 75, or 100 μM; HU at 50, 75, or 100 μM; and 2 positive controls, hemin (50 μM; Sigma) and sodium butyrate (NB; Sigma, 2 mM). The K562 cells were induced for 48 hours then RNA isolated for further analysis. Cell viability was determined by 2% trypan blue exclusion at times 0 and 48 hours.
RNase protection analysis
RNA was isolated using RNA Stat-60 (TEL-TEST “B,” Inc, Friendswood, TX) according to the manufacturer's instructions. In brief, cell pellets were suspended in RNA Stat-60 (1 mL) and the RNA was precipitated with isopropanol. The mRNA levels were quantitated by RNase protection assay (RPA) with probes designed to yield protected fragments for human γ-globin (Huγ) and the internal control glyceraldehyde-3-phosphate dehydrogenase (GAPD) as previously described.14 Huγ mRNA levels were normalized to that of GAPD and compared with untreated K562 cells.
BFU-E colony growth
Mononuclear cells were isolated from the peripheral blood of each participant studied by density gradient centrifugation using Histopaque-1077 (Sigma). Erythroid progenitors were incubated in methylcellulose culture medium as previously described.14 ZL was added on day 0 at 25, 50, 75, or 100 μM concentrations for 14 days, at which time the number of BFU-E colonies were counted on an inverted microscope and harvested for hemoglobin determinations. We previously published similar experiments for HU to analyze Hb F inducibility in erythroid progenitors.6 Informed consent was obtained from all participants following the University of South Alabama Institutional Review Board guidelines.
Hemoglobin F determination
After 14 days of incubation, erythroid progenitors were collected, washed in PBS, and then lysed at room temperature in 200 μL of water. The supernatant was split into 2 tubes, 1 for total hemoglobin determination and 1 for Hb F determination. Fetal hemoglobin was measured as a percent of total hemoglobin, normalized to total protein by alkaline denaturation as published previously.6,14
Statistics
All results are presented as means ± SEM. Statistical analyses were performed using the unpaired Student t test and one-way analysis of variance (ANOVA). The Tukey test and Dunnett test15 were used for multiple comparisons when ANOVA indicated statistically significant differences between or within groups. Differences were considered to be significant when P is less than .05.
Results
ZL augments γ-gene mRNA levels in K562 cells
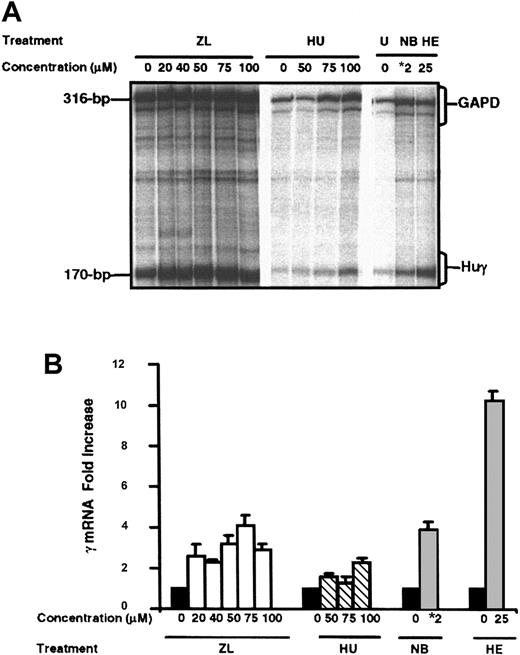
Several pharmacologic Hb F inducers such as HU, 5-azacytidine,16 erythropoietin,17 didox,18 and butyrate19 alter γ-globin gene expression in culture. Although the correlation between in vitro response and in vivo Hb F production is not necessarily direct, the induction of γ-globin gene activity in culture is a good indicator of potential clinical usefulness. Therefore, we analyzed the ability of ZL to induce γ-globin gene activity at the mRNA level. K562 cells were cultured in ZL (0 to 100 μM) or HU (50 μM to 100 μM) for 48 hours. Total cellular RNA was analyzed by RPA for γ-globin mRNA levels. We observed an increase in γ-globin mRNA for ZL at 20 μM with a maximal increase at 75 μM (Figure 1A). The level of γ-globin gene mRNA synthesis was quantitated relative to the internal control, GAPD. As shown in Figure 1B, we observed a 4.1-fold increase in γ-globin mRNA at a 75 μM ZL concentration compared with a 2.7-fold increase for HU at 100 μM (P < .05). Of note is the observation that ZL increased γ-globin mRNA 2.6-fold at the 20 μM concentration demonstrating comparable induction to 100 μM HU (Figure 1B).
γ-Globin gene induction by ZL in K562 cells. K562 cells were treated with ZL at 20 to 100 μM concentration or hydroxyurea (HU) 50 to 100 μM concentrations for comparison. γ-Globin mRNA levels were quantitated by RNase protection assay (RPA) with human γ (Huγ) and internal control gylcearaldehyde-3-phosphate dehydrogenase (GAPD) probes to yield protected fragments of 170 bp and 316 bp, respectively. (A) A representative RPA gel showing the mRNA bands for ZL-treated cells on the left and HU-treated cells in the middle. (B) Quantitative values for Huγ and GAPD mRNA production by phosphorimager analysis. The untreated K562 samples (0 μM) were normalized to one (▪). The total increase in γ mRNA was calculated as a ratio of GAPD to control for variations in sample loading for ZL- (□) or HU-treated (▧) K562 cells. Sodium butyrate (NB,  ) and hemin (HE,
) and hemin (HE,  ) were used as positive control for γ gene induction. *2 = NB at a 2 mM concentration. Error bars indicate SEM (n = 4 for each concentration/groups reported); each study done in triplicate.
) were used as positive control for γ gene induction. *2 = NB at a 2 mM concentration. Error bars indicate SEM (n = 4 for each concentration/groups reported); each study done in triplicate.
γ-Globin gene induction by ZL in K562 cells. K562 cells were treated with ZL at 20 to 100 μM concentration or hydroxyurea (HU) 50 to 100 μM concentrations for comparison. γ-Globin mRNA levels were quantitated by RNase protection assay (RPA) with human γ (Huγ) and internal control gylcearaldehyde-3-phosphate dehydrogenase (GAPD) probes to yield protected fragments of 170 bp and 316 bp, respectively. (A) A representative RPA gel showing the mRNA bands for ZL-treated cells on the left and HU-treated cells in the middle. (B) Quantitative values for Huγ and GAPD mRNA production by phosphorimager analysis. The untreated K562 samples (0 μM) were normalized to one (▪). The total increase in γ mRNA was calculated as a ratio of GAPD to control for variations in sample loading for ZL- (□) or HU-treated (▧) K562 cells. Sodium butyrate (NB,  ) and hemin (HE,
) and hemin (HE,  ) were used as positive control for γ gene induction. *2 = NB at a 2 mM concentration. Error bars indicate SEM (n = 4 for each concentration/groups reported); each study done in triplicate.
) were used as positive control for γ gene induction. *2 = NB at a 2 mM concentration. Error bars indicate SEM (n = 4 for each concentration/groups reported); each study done in triplicate.
K562 cell viability was decreased 10% when measured by trypan blue exclusion at 75 μM and 100 μM concentrations for both ZL and HU. The level of γ-globin gene induction was 3.9-fold and 10-fold for 2 μM sodium butyrate and 50 μM hemin, respectively.
ZL induces fetal hemoglobin in primary erythroid progenitors
The parent compound HU is known to induce Hb F production in vivo in sickle cell patients treated for an extended period and in primary erythroid cell culture. Therefore, we analyzed the capability of ZL to induce Hb F in HbSS erythroid progenitors, grown from peripheral blood mononuclear cells (MNCs). Each experiment was performed in triplicate. BFU-E colonies were grown in methylcellulose media supplemented with erythropoietin, interleukin-3, granulocyte-macrophage colony-stimulating factor, stem cell factor, and ZL at concentrations from 0 to 100 μM. The mean BFU-E colony number was 116.0 ± 15.2 without ZL compared with 13.3 ± 2.9 at 100 μM concentrations (P < .01; Table 1).
Effects of zileuton on BFU-E colony growth and fetal hemoglobin production in primary erythroid cultures
. | Zileuton concentrations, μM . | . | . | . | . | . | . | . | . | . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient no. . | 0 . | . | 25 . | . | 50 . | . | 75 . | . | 100 . | . | |||||||||
| . | BFU-E . | Hb F, % . | BFU-E . | Hb F, % . | BFU-E . | Hb F, % . | BFU-E . | Hb F, % . | BFU-E . | Hb F, % . | |||||||||
| 1 | 87 | 2 | 61 | 4 | 47 | 7 | 27 | 11 | 12 | 14 | |||||||||
| 2 | 135 | 7 | 91 | 11 | 72 | 14 | 43 | 18 | 7 | 21 | |||||||||
| 3 | 148 | 2 | 137 | 3 | 62 | 6 | 23 | 11 | 13 | 13 | |||||||||
| 4 | 93 | 3 | 86 | 5 | 66 | 7 | 34 | 9 | 21 | 16 | |||||||||
| Mean | 116 | 3.5 | 93.8 | 5.8 | 61.8 | 8.5 | 31.8 | 12.3 | 13.3 | 16.0 | |||||||||
| SEM | 15.2 | 1.2 | 15.8 | 1.8 | 5.3 | 1.8 | 4.4 | 2.0 | 2.9 | 1.8 | |||||||||
| P | — | — | > .05 | > .05 | < .01 | > .05 | < .01 | < .01 | < .01 | < .01 | |||||||||
. | Zileuton concentrations, μM . | . | . | . | . | . | . | . | . | . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient no. . | 0 . | . | 25 . | . | 50 . | . | 75 . | . | 100 . | . | |||||||||
| . | BFU-E . | Hb F, % . | BFU-E . | Hb F, % . | BFU-E . | Hb F, % . | BFU-E . | Hb F, % . | BFU-E . | Hb F, % . | |||||||||
| 1 | 87 | 2 | 61 | 4 | 47 | 7 | 27 | 11 | 12 | 14 | |||||||||
| 2 | 135 | 7 | 91 | 11 | 72 | 14 | 43 | 18 | 7 | 21 | |||||||||
| 3 | 148 | 2 | 137 | 3 | 62 | 6 | 23 | 11 | 13 | 13 | |||||||||
| 4 | 93 | 3 | 86 | 5 | 66 | 7 | 34 | 9 | 21 | 16 | |||||||||
| Mean | 116 | 3.5 | 93.8 | 5.8 | 61.8 | 8.5 | 31.8 | 12.3 | 13.3 | 16.0 | |||||||||
| SEM | 15.2 | 1.2 | 15.8 | 1.8 | 5.3 | 1.8 | 4.4 | 2.0 | 2.9 | 1.8 | |||||||||
| P | — | — | > .05 | > .05 | < .01 | > .05 | < .01 | < .01 | < .01 | < .01 | |||||||||
P value represent a comparison of BFU-E colonies and Hb F in the absence of zileuton (0 μM) versus 25 to 100 μM concentrations. BFU-E indicates burst-forming unit-erythroid; Hb F, fetal hemoglobin; and —, not applicable.
Despite the decline in BFU-E colonies, Hb F levels increased from 3.5% ± 1.2% to 16.0% ± 1.8% (P < .01), thus demonstrating the ability of ZL to induce Hb F production in primary erythroid progenitors. Pair-wise comparisons for BFU-E at 0 μM and 50, 75, and 100 μM were significant at P less than .01. Pair-wise comparisons for Hb F at 0 μM and 75 to 100 μM were significant at P less than .01. The correlation coefficient between the number of BFU-E colonies or percent Hb F versus the different ZL concentrations was –0.90 and +0.79, respectively (P < .05). These results are comparable to data we previously published for HU.6 A dose-dependent 8.7-fold decrease in BFU-E colony number (P < .05) was observed at 100 μM ZL compared with control. Similar studies performed with HU (0 and 100 μM) resulted in a 21.3-fold decrease for HU in BFU-E colonies grown from sickle cell anemia patients (P < .05). Despite less change in the number of BFU-E growth with ZL, we observed a 4.6-fold increase in Hb F compared with a 3.8-fold increase (5.1 ± 1.0 to 19.4 ± 1.5) for HU, suggesting comparable Hb F induction for both drugs.
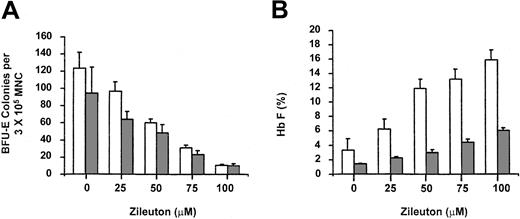
Next, the effect of ZL on γ-globin gene expression was assessed in erythroid progenitors obtained from healthy donors. Using the same protocol described in “Patients, materials, and methods,” BFU-E colonies from healthy donors were measured in the presence of ZL at 0 to 100 μM concentrations. In normal erythroid progenitors, we observed a decrease in BFU-E colony number from 94.0 ± 30.6 to 10.1 ± 2.4 (Figure 2A) and an increase in Hb F from 1.4% ± 0.05% to 6.0% ± 0.38% (Figure 2B). This pattern was similar to that obtained for sickle cell progenitors. Of note is the lower average BFU-E colony number for normal progenitors. This represents a 9.3-fold decrease and 4.3-fold increase in BFU-E and Hb F, respectively. Pair-wise comparisons of BFU-E colony growth for normal samples revealed significant decreases between ZL (0 μM) and the 50, 75, and 100 μM concentrations (P < .05). Similarly, Hb F levels were significantly increased at ZL concentrations of 50, 75, and 100 μM(P < .05).
BFU-E colony growth and fetal hemoglobin production in thepresence of zileuton for sickle cell and healthy individuals in methylcelluloseculture. Peripheral blood mononuclear cells were cultured as described in “Patients, materials, and methods.” The results are expressed as the number of BFU-E colonies per 3 × 105 mononuclear cells (MNCs). □ indicates sickle cell samples; and ▦, normal samples. Panel A demonstrates the BFU-E colonies on day 14 in the presence of zileuton at 0 to 100 μM concentrations. Panel B represents Hb F for sickle cell (□) and normal samples (▦). Note Hb F induction for both groups tested. Error bars represent SEM (n = 4 for each concentration/groups reported); each study done in triplicate.
BFU-E colony growth and fetal hemoglobin production in thepresence of zileuton for sickle cell and healthy individuals in methylcelluloseculture. Peripheral blood mononuclear cells were cultured as described in “Patients, materials, and methods.” The results are expressed as the number of BFU-E colonies per 3 × 105 mononuclear cells (MNCs). □ indicates sickle cell samples; and ▦, normal samples. Panel A demonstrates the BFU-E colonies on day 14 in the presence of zileuton at 0 to 100 μM concentrations. Panel B represents Hb F for sickle cell (□) and normal samples (▦). Note Hb F induction for both groups tested. Error bars represent SEM (n = 4 for each concentration/groups reported); each study done in triplicate.
l-arginine dose-dependently reverses the effect of ZL in sickle primary erythroid progenitors
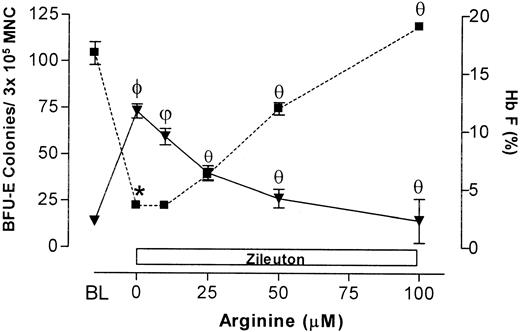
Based on data previously published with HU, the nitric oxide–dependent activation of soluble guanylate cyclase–protein kinase G pathway in primary erythroblast constitutes a mechanism that regulates expression of the γ-globin gene.12 In this study, the effect(s) of the nitric oxide donor, l-arginine,20,21 on ZL induction of Hb F in primary sickle erythroid progenitors in culture was assessed. l-arginine (0-100 μM) dose-dependently reversed ZL (50 μM) suppression of BFU-E colony growth and stimulation of Hb F synthesis (Figure 3). In contrast, l-arginine (100 μM) did not reverse the suppression of BFU-E colony growth and stimulation of Hb F synthesis seen with HU, 100 μM controls (Figure 4A-B). When sickle erythroid progenitors were treated with l-arginine (100 μM) alone, there was a 31% increase in BFU-E colony growth compared with the BFU-E control (P < .01; Figure 4). l-arginine had no effect on Hb F synthesis in the absence of ZL treatment of sickle erythroid progenitors in culture.
Effects of increasing concentrations of L-arginine on ZL-treated erythroid progenitors isolated from patients with homozygous sickle cell anemia. Cells were cultured on methylcellulose culture media and treated with ZL (100 μM) as described in “Patients, materials, and methods.” L-arginine (10-100 μM) was added at the beginning of the incubation period. The number of BFU-E colonies (▪) and levels of hemoglobin F (Hb F; ▾) were measured after 14 days of incubation. Data are expressed as mean ± SEM of 4 experiments done in triplicate. *P is less than .001 compared with baseline (BL); BL indicates the number of BFU-E colonies or percent Hb F measured in cultured cells not treated with ZL. The 0 μM point indicates the number of BFU-E colonies or percent Hb F measured in cultured cells treated with ZL alone. θ indicates that P is less than .01 c/w L-arginine (0 μM); ψ, P is less than .05 c/w L-arginine (0 μM); and ϕ, P is less than .001 c/w L-arginine (0 μM).
Effects of increasing concentrations of L-arginine on ZL-treated erythroid progenitors isolated from patients with homozygous sickle cell anemia. Cells were cultured on methylcellulose culture media and treated with ZL (100 μM) as described in “Patients, materials, and methods.” L-arginine (10-100 μM) was added at the beginning of the incubation period. The number of BFU-E colonies (▪) and levels of hemoglobin F (Hb F; ▾) were measured after 14 days of incubation. Data are expressed as mean ± SEM of 4 experiments done in triplicate. *P is less than .001 compared with baseline (BL); BL indicates the number of BFU-E colonies or percent Hb F measured in cultured cells not treated with ZL. The 0 μM point indicates the number of BFU-E colonies or percent Hb F measured in cultured cells treated with ZL alone. θ indicates that P is less than .01 c/w L-arginine (0 μM); ψ, P is less than .05 c/w L-arginine (0 μM); and ϕ, P is less than .001 c/w L-arginine (0 μM).
Effects of L-arginine and L-NMMA on HU-treated erythroid progenitors isolated from patients with homozygous sickle cell anemia. The experimental conditions were the same as in Figure 3. L-arginine (ARG; 100 μM) effects on hydroxyurea (HU)–treated (100 μM) erythroid progenitors in the absence and presence of the nitric oxide synthase inhibitor, NG-monomethyl–L-arginine (L-NMMA; 100 μM) were assessed. The number of BFU-E colonies (A) and percent Hb F (B) were measured as described in “Patients, materials, and methods.” Data are expressed as mean ± SEM. n = 4 in each group. Each experiment was done in triplicate. *P is less than .001 compared with control (CON).
Effects of L-arginine and L-NMMA on HU-treated erythroid progenitors isolated from patients with homozygous sickle cell anemia. The experimental conditions were the same as in Figure 3. L-arginine (ARG; 100 μM) effects on hydroxyurea (HU)–treated (100 μM) erythroid progenitors in the absence and presence of the nitric oxide synthase inhibitor, NG-monomethyl–L-arginine (L-NMMA; 100 μM) were assessed. The number of BFU-E colonies (A) and percent Hb F (B) were measured as described in “Patients, materials, and methods.” Data are expressed as mean ± SEM. n = 4 in each group. Each experiment was done in triplicate. *P is less than .001 compared with control (CON).
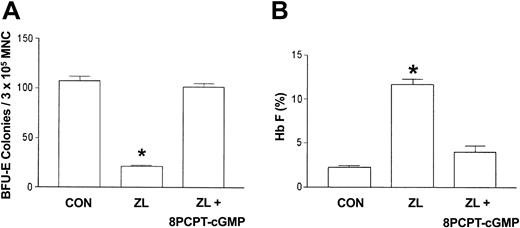
The 8-PCPT–cGMP reverses the effect of ZL in sickle primary erythroid progenitors
Since the l-arginine/nitric oxide synthase product, nitric oxide, activates soluble guanylate cyclase, which results in the accumulation of cGMP, we postulated that the cell-permeable cGMP analog, 8-PCPT–cGMP,22 would mimic l-arginine inhibition of ZL induction of Hb F and decrease of BFU-E colony growth. Using the same protocol described with l-arginine, sickle erythroid progenitors were treated with ZL (50 μM) in the absence or presence of 8-PCPT–cGMP (10 μM) and compared with ZL treatment and control (no treatment) groups. The 8-PCPT–cGMP reverses ZL (50 μM) suppression of BFU-E colony growth and stimulation of Hb F synthesis, similar to that observed with l-arginine (Figure 5A-B).
Effects of 8-PCPT–cGMP on ZL-treated erythroid progenitors isolated from patients with homozygous sickle cell anemia. The experimental conditions were the same as in Figure 3. The effect of 8-(4-chlorophenylthio)guanosine 3′,5′-cyclic monophosphate (8-PCPT–cGMP; 10 μM) on BFU-E colony growth (A) and Hb F synthesis (B) in zileuton-treated (ZL; 50 μM) sickle erythroid progenitors was assessed. Data are expressed as the mean ± SEM. n = 7 in control (CON) and ZL treatment groups; n = 4 in the ZL + 8-PCPT–cGMP treatment group. Each experiment was done in triplicate. *P is less than .001 compared with the CON and ZL + 8-PCPT–cGMP treatment groups.
Effects of 8-PCPT–cGMP on ZL-treated erythroid progenitors isolated from patients with homozygous sickle cell anemia. The experimental conditions were the same as in Figure 3. The effect of 8-(4-chlorophenylthio)guanosine 3′,5′-cyclic monophosphate (8-PCPT–cGMP; 10 μM) on BFU-E colony growth (A) and Hb F synthesis (B) in zileuton-treated (ZL; 50 μM) sickle erythroid progenitors was assessed. Data are expressed as the mean ± SEM. n = 7 in control (CON) and ZL treatment groups; n = 4 in the ZL + 8-PCPT–cGMP treatment group. Each experiment was done in triplicate. *P is less than .001 compared with the CON and ZL + 8-PCPT–cGMP treatment groups.
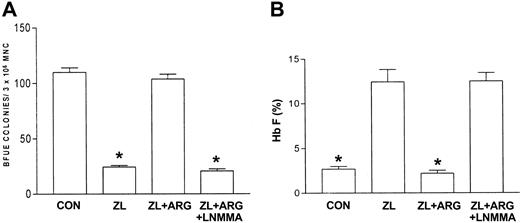
Nitric oxide synthase inhibition abrogates the l-arginine inhibitory effect on zileuton
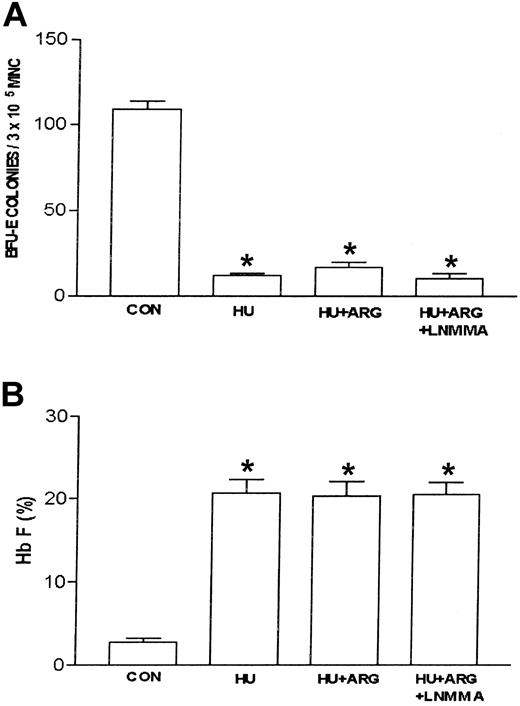
Nitric oxide synthase produces nitric oxide by converting l-arginine and molecular oxygen into citrulline and nitric oxide.20,21 The previous data suggested that l-arginine plays a negative regulatory role in ZL-mediated induction of Hb F. To test this, the nitric oxide synthase inhibitor, l-NMMA (100 μM), was used to determine if the inhibitory effect of l-arginine on ZL-induced Hb F synthesis was mediated by nitric oxide production. This dose of l-NMMA was chosen based on previous studies demonstrating nitric oxide synthase inhibition.23 Studies (n = 4, in triplicate) with l-NMMA alone were performed initially using sickle erythroid progenitors compared with controls (no l-NMMA). l-NMMA did not affect BFU-E colony growth or Hb F synthesis relative to control. Subsequent studies were performed comparing ZL or HU alone to ZL + l-NMMA and HU + l-NMMA. Interestingly, l-NMMA did not effect ZL-mediated inhibition of BFU-E colony growth or induction of Hb F synthesis. In contrast to ZL, l-NMMA did partially reverse the HU effects on BFU-E colony growth and Hb F synthesis (Table 2). Figure 6A-B demonstrates that l-NMMA reverses the l-arginine effect on ZL in sickle erythroid progenitors resulting in restoration of decreased numbers of BFU-E colonies and stimulation of Hb F synthesis.
Effects of NG-monomethyl-L-arginine on zileuton-and hydroxyurea-mediated BFU-E colony growth and fetal hemoglobin production in erythroid progenitors
. | ZL+LNMMA, 100 . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
. | n . | CON . | ZL, 50 μM . | μM . | HU, 100 μM . | HU+LNMMA, 100 μM . | |||||
| BFU-E | 4 | 124.4 ± 1.3 | 24.3 ± 2.3 | 27.5 ± 2.3 | 8.2 ± 0.9 | 20.8 ± 1.7* | |||||
| Hb F, % | 4 | 1.8 ± 0.2 | 11.5 ± 0.6 | 9.4 ± 0.7 | 19.7 ± 0.6 | 14.8 ± 1.1* | |||||
. | ZL+LNMMA, 100 . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
. | n . | CON . | ZL, 50 μM . | μM . | HU, 100 μM . | HU+LNMMA, 100 μM . | |||||
| BFU-E | 4 | 124.4 ± 1.3 | 24.3 ± 2.3 | 27.5 ± 2.3 | 8.2 ± 0.9 | 20.8 ± 1.7* | |||||
| Hb F, % | 4 | 1.8 ± 0.2 | 11.5 ± 0.6 | 9.4 ± 0.7 | 19.7 ± 0.6 | 14.8 ± 1.1* | |||||
BFU-E indicates burst-forming unit-erythroid; Hb F, fetal hemoglobin; CON, control; ZL, zileuton; LNMMA, NG-monomethyl-L-arginine; and HU, hydroxyurea.
P < .01 compared with HU alone.
Effects of L-arginine and L-NMMA on zileuton-treated erythroid progenitors isolated from patients with homozygous sickle cell anemia. The experimental conditions were the same as in Figure 3. l-arginine (ARG; 100 μM) effects on zileuton-treated erythroid progenitors in the absence and presence of the nitric oxide synthase inhibitor LNMMA (100 μM) were assessed. The number of BFU-E colonies (A) and percent Hb F (B) were measured as described in “Patients, materials, and methods.” Data are expressed as mean ± SEM. n = 7, control (CON); n = 7, zileuton (ZL)–treated; n = 5, ZL + L-arginine (ARG); and n = 4, ZL + ARG + LNMMA–treated erythroid progenitors. Each experiment was done in triplicate. *P is less than .001 compared with CON and ZL + ARG.
Effects of L-arginine and L-NMMA on zileuton-treated erythroid progenitors isolated from patients with homozygous sickle cell anemia. The experimental conditions were the same as in Figure 3. l-arginine (ARG; 100 μM) effects on zileuton-treated erythroid progenitors in the absence and presence of the nitric oxide synthase inhibitor LNMMA (100 μM) were assessed. The number of BFU-E colonies (A) and percent Hb F (B) were measured as described in “Patients, materials, and methods.” Data are expressed as mean ± SEM. n = 7, control (CON); n = 7, zileuton (ZL)–treated; n = 5, ZL + L-arginine (ARG); and n = 4, ZL + ARG + LNMMA–treated erythroid progenitors. Each experiment was done in triplicate. *P is less than .001 compared with CON and ZL + ARG.
These data, cumulatively, support the hypothesis that nitric oxide plays an inhibitory role in Hb F induction by ZL and supports previously reported studies that nitric oxide at least in part facilitates the synthesis of Hb F synthesis by HU.
Discussion
ZL is a selective inhibitor of 5-LO and structural analog of HU1,2 that is currently being used as an anti-inflammatory agent in the treatment of asthma.24-29 To date there are no published studies that address the potential significance of ZL as a therapeutic agent in the treatment of sickle hemoglobinopathies. HU is the only drug currently approved for the treatment of sickle cell disease (SCD) that is known to ameliorate its most common symptom, pain,3 and common complications associated with death, including the acute chest syndrome.30,31 The amelioration of symptoms by HU occurs at least in part through its ability to stimulate Hb F synthesis. Exactly how HU stimulates Hb F synthesis is not known.
In the present study, an erythroid cell culture system and K562 cells were used to study the effects of ZL on BFU-E colony growth, Hb F synthesis, and γ-globin mRNA synthesis. In addition, studies were performed that analyzed the role of the nitric oxide/cGMP pathway in regulating Hb F induction in primary sickle erythroid progenitors. In this study the major findings were as follows: (1) ZL induced γ-globin mRNA synthesis in K562 cells; (2) ZL induced Hb F synthesis in normal and sickle primary erythroid progenitors; (3) the nitric oxide donor, l-arginine, and cGMP analog, 8-PCPT–cGMP, blocked ZL-induced Hb F synthesis; and (4) the nitric oxide synthase inhibitor, l-NMMA, blocks the l-arginine inhibitory effect on ZL. These observations strongly support that the in vitro effects of ZL are at least in part negatively regulated through a nitric oxide–dependent pathway. Unlike ZL, l-arginine did not affect HU induction of Hb F. Furthermore, l-NMMA had no effect on ZL induction of Hb F. In contrast, l-NMMA partially abrogates the decrease in number of BFU-E colonies and increase in Hb F synthesis seen with HU. These observations strongly suggest that the role of nitric oxide signaling in regulating ZL and HU induction of Hb F synthesis occur through different mechanisms.
Previously, we and others demonstrated the ability of HU to induce γ-globin mRNA production at the transcriptional level.6,32 Thus the experimental data supports a multilevel mechanism for Hb F induction and the antisickling effects of HU therapy. In this study, a dose-response curve for ZL versus BFU-E colony number was established similar to that previously shown for HU.6 These data suggest cytotoxicity plays a role in the mechanism of action for Hb F synthesis by ZL as well. Our data clearly demonstrated a comparable ability of ZL to induce γ-globin gene expression at the transcriptional level and suggest ZL may have clinical utility similar to that reported with HU in the treatment of sickle hemoglobinopathies. Furthermore, the concentrations of ZL used in this study are pharmacologically achievable with standard dosing previously reported in patients with asthma.33
The nitric oxide–dependent activation of soluble guanylate cyclase–protein kinase G pathway in primary erythroblast constitutes a mechanism that regulates expression of the γ-globin gene12 by HU. In this study, the effect(s) of the nitric oxide donor, l-arginine,20,21 on ZL induction of Hb F in primary sickle erythroid progenitors in culture was assessed. l-arginine dose-dependently blocked ZL-mediated induction of Hb F and caused the BFU-E colony number and Hb F percentage to return to control values. This suggested that ZL mediated increased Hb F synthesis through a cytotoxic pathway that is nitric oxide dependent. l-arginine and molecular oxygen in the presence of nitric oxide synthase produce citrulline and nitric oxide, which activate soluble guanylate cyclase and increase intracellular cGMP accumulation.20 Administration of the cell-permeant cGMP analog, 8-PCPT–cGMP, mimicked larginine effects, suggesting that the upstream activation of nitric oxide is required to achieve γ-globin inhibition. The role of l-arginine as a nitric oxide donor is supported by results obtained with the nitric oxide synthase inhibitor, l-NMMA. l-NMMA in the presence of ZL and l-arginine resulted in the restoration of Hb F induction and a decrease in the number of BFU-E colonies by ZL. These findings cumulatively support that ZL increases Hb F synthesis and decreases the number of BFU-E colonies through a pathway that is at least in part negatively regulated by nitric oxide. In addition, the ZL dose-response data suggest cytotoxicity plays a role in the mechanism of action for Hb F synthesis by ZL and HU. In contrast to ZL, l-arginine did not have any effect on sickle erythroid progenitors treated with HU. However, l-NMMA partially antagonized HU induction of Hb F and the decrease in BFU-E colony number. These data demonstrate that nitric oxide plays a negative role in Hb F synthesis induced by ZL in contrast to a small but significant positive regulatory role in HU-mediated induction of Hb F synthesis.
Ikuta et al12 recently demonstrated that soluble guanylate cyclase activators or analogs increased γ-globin in K562 cells and human erythroid progenitors. In addition, hemin- and butyrate-mediated γ-globin induction was prevented by inhibiting soluble guanylate cyclase or cGMP-dependent protein kinase.12 Subsequently, Cokic et al13 demonstrated that S-nitrosocysteine, a nitric oxide donor, induced γ-globin mRNA and Hb F protein in K562 cells and human erythroid progenitors. Both S-nitrosocysteine and HU increased cGMP levels, and guanylate cyclase inhibitors abolished S-nitrosocysteine and HU-induced γ-globin expression. In this study, l-arginine (100 μM), a precursor to nitric oxide, did not stimulate Hb F synthesis over a 14-day period in cultured sickle erythroid progenitors. Whether or not l-arginine induces γ-globin mRNA in sickle erythroid progenitors was not assessed. Although l-arginine is a precursor to nitric oxide, studies performed using HU and l-arginine did not mirror the findings of a nitric oxide–dependent pathway reported by Cokic et al13 with the nitric oxide donors, S-nitrosocysteine and HU, on Hb F synthesis. However, the nitric oxide synthase inhibitor, l-NMMA, did partially antagonize the HU effects. The lack of an l-arginine effect in the presence of l-NMMA suggests that the basal level of nitric oxide may be more significant in defining the physiologic significance of nitric oxide in HU induction of Hb F synthesis. While Cokic et al13 suggest that nitric oxide releasing or potentiating agents may have potential therapeutic benefit, l-arginine is not supported by our study to be such an agent. Our data does not rule out the possibility that specific nitric oxide donors directly stimulate the γ-globin promoter, however, the data highlight the importance of evaluating potential γ-globin inducers in the context of l-arginine, the major dietary source of nitric oxide.
Prepublished online as Blood First Edition Paper, February 5, 2004; DOI 10.1182/blood-2003-08-2969.
Supported by Comprehensive Sickle Cell Program from the National Heart, Lung and Blood Institute and the Florence Foundation Research Career Development (contract grant no. P60 HL-38639).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Many thanks to Marilyn Chancellor and Kathy Billingsley for the preparation of this manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal