Abstract
Sepsis represents a growing concern in high-risk patients and there has been a lack of effective preventives and therapies. Bacterial/permeability increasing protein (BPI) is a human neutrophil granule-associated defense molecule specific for Gram-negative bacteria and their products. To develop a BPI-transgene–based prophylactic or therapeutic modality, we have developed a recombinant, replication-deficient adenoviral vector expressing full-length human BPI protein (AdhBPI). The expression of BPI is under control of a murine cytomegalovirus (CMV) promoter. Using in vitro and in vivo systems, AdhBPI-mediated gene transfer led to extracellular secretion of BPI protein, which effectively neutralized endotoxin (lipopolysaccharide [LPS]) and markedly reduced the production of proinflammatory cytokines tumor necrosis factor α (TNF-α) and macrophage inflammatory protein 2 (MIP-2) by freshly isolated murine alveolar macrophages. By using a mouse model of nonlethal sepsis elicited with LPS, we demonstrated that in vivo gene transfer of BPI was able to markedly inhibit the effect of a large dose of LPS on cytokine responses when injected intraperitoneally. Furthermore, such in vivo BPI gene transfer also improved the survival of mice suffering from lethal septic shock elicited by intraperitoneal injection of d-galactosamine and LPS. Thus, our results suggest that human BPI gene transfer vector has the potential to be used as a therapeutic agent for septic conditions.
Introduction
Sepsis is a heterogeneous condition with approximately 400 000 to 500 000 cases presented in the United States annually.1,2 The mortality rate for all septic episodes is estimated between 30% to 50%, and approximately 70% of all septic episodes involve Gram-negative bacteria.2,3 The Centers for Disease Control and Prevention has reported an increase in the incidence of sepsis over the last decade, which can be attributed to an increase in the number of patients at high risk for developing sepsis.1 High-risk patients include those who are immunocompromised from HIV infection, undergoing cancer therapy, or on immunosuppressive drugs, and those undergoing invasive surgical procedures. These patients are at greater risk of exposure to bacteria, especially in the clinical setting.4
The goal of sepsis management is to reduce or control the detrimental systemic inflammation responses to the infection using support therapies, while treatment therapies, such as antibiotics, attempt to eliminate the infection.5 With the increase in incidence of both drug-resistant bacteria and immunocompromised septic patients, current aggressive therapies are ineffective or even detrimental, and new therapies must be developed to increase the survival of septic patients.5-9 Moreover, it may be possible to prophylactically reduce the number of sepsis-induced mortalities by introducing a set of criteria to identify and treat high-risk individuals prior to surgery or cancer therapy.
Various novel sepsis therapies currently under development or under evaluation in clinical trials include anticoagulant therapy, therapies directed at the neutralization of lipopolysaccharide (LPS), and cytokine therapies. Anticoagulant therapy aims to improve multiple organ failure by attenuating intravascular coagulation.10 In this regard, the use of recombinant activated protein C has been shown to increase survival of patients.10,11 However, activated protein C therapy addresses only one portion of the complex sepsis condition and does not address the important initial proinflammatory cytokine cascade elicited by LPS from Gram-negative bacteria. On the other hand, while therapies directed at the neutralization of proinflammatory cytokines, such as tumor necrosis factor α (TNF-α), are promising in experimental models, they are largely ineffective in clinical trials.12-17 The interleukin-10 (IL-10) cytokine therapy aiming to counter the expression and effect of TNF-α has also proved ineffective.18 Such cytokine therapies are thus unlikely to be the best strategy. Sepsis therapies directed at neutralizing LPS by using anti-LPS antibodies have been in clinical trials for a number of years but have demonstrated disappointing outcomes.19-22
Bactericidal/permeability-increasing protein (BPI) is a 55- to 60-kDa protein found in the primary granule of human neutrophils. The gene coding for full-length BPI contains 1464 base pairs and the translated protein is 456 amino acids in length.23 Different from many other defense molecules, BPI has a specific effect on Gram-negative bacteria and LPS. The amino terminus of BPI is biologically active and binds with high affinity to shed both LPS and LPS on the bacterial outer membrane.24-29 The carboxy terminus of the protein is inserted into the granule membrane, but has no transmembrane signaling capability.25,26,30 BPI is capable of LPS neutralization, direct Gram-negative bacterial killing, and enhancing bacterial phagocytosis by phagocytes (opsonization). BPI binds LPS with a much higher affinity than natural endogenous LPS-binding protein (LBP).25,26 These properties of BPI make this molecule an attractive agent to be used for immunointervention strategies for preventing and/or treating sepsis. Indeed, several studies have evaluated the effect of recombinant BPI protein in experimental models of sepsis and endotoxemia in rabbits, rats, pigs, and mice.31-36 Furthermore, phase 1/2/3 clinical trials using recombinant BPI have been performed or are currently under way.32,37-40 In most cases, the regimen entails a continuous infusion of 2 to 8 mg/kg recombinant BPI for a period of 2 days.32,37-40 These clinical trials are designed to evaluate the efficacy of the amino terminal portion of recombinant BPI in either preventing sepsis in high-risk patients or treating septic patients. The completed trials have thus far demonstrated its safety and encouraging beneficial effects. However, like many other immunotherapies involving the use of recombinant proteins, administration of recombinant BPI in large doses is very costly, cannot be given for desired periods of time, and is unable to maintain a constant optimal therapeutic level due to its high cost and the short half-life of recombinant protein in vivo.
Gene transfer represents an effective means to derive immunotherapeutic molecules in vivo, and a single dose of transgene product, depending on the form of vector, is able to achieve a high, sustainable level of such molecules. However, BPI transgene-based modality has not been developed and evaluated for its therapeutic potential in models of endotoxemia or sepsis. In the current study, we have sought to develop and characterize a recombinant replication-deficient adenovirus encoding the whole-length human BPI protein, and evaluate its efficacy in neutralizing LPS and attenuating LPS-induced proinflammatory responses both in vitro and in vivo.
Materials and methods
Construction of recombinant adenoviral vector expressing human BPI
Since normal mature human neutrophils contain preformed BPI protein and express little BPI mRNA, we set out to extract the total cellular RNA from a patient's blood sample, which contained enriched neutrophil progenitor cells. This patient (65 years old) suffered multiple myeloma and was treated with cyclophosphamide chemotherapy on day 0, followed by stem cell factor (SCF) and granulocyte colony-stimulating factor (G-CSF) treatment from day 1 to day 11.41 G-CSF therapy following chemotherapy causes the rapid mobilization of progenitor myeloid cells from the bone marrow to aid in the repopulation of the immune cells destroyed by chemotherapy.42 Consequently, this patient had high levels of CD34+ progenitor cells mobilized into the peripheral blood by SCF and G-CSF therapy on day 11 after cyclophosphamide treatment, and these CD34+ cells represented a rich source of BPI mRNA for polymerase chain reaction (PCR) amplification.
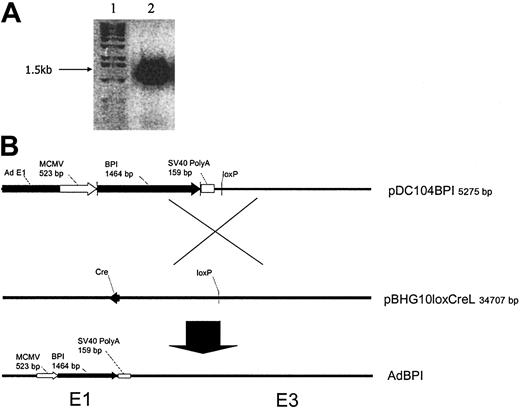
Human BPI cDNA was generated and amplified by using a pair of primers (5′ CCC GGG CCA CCA TGA GAG AGA ACA TGG CCA GG and 3′ AAG CTT TCA TTT ATA GAC AAC GTC TGC AC) through reverse transcription (RT)–PCR of total cellular RNA extracted from the blood sample. The shuttle plasmid pDC104 containing a murine cytomegalovirus (CMV) promoter (523 bp), multicloning sites, and SV40 poly A signal (159 bp) inserted into the left end E1 region of human type 5 adenovirus genome was double-digested with SmaI and HindIII. Using T4 ligase (Invitrogen, Carlsbad, CA), human BPI cDNA (1464 bp) was subcloned into the SmaI-HindIII site of pDC104 to obtain the recombinant plasmid pDC104BPI. The pDC104BPI plasmid was cotransfected into human embryonic kidney 293 cells with pBHG10loxCre plasmid containing the right end regions of human type 5 adenovirus genome, which has a partial deletion in the E3 region. The recombinant replication-deficient adenovirus AdhBPI was rescued through Cre-lox–mediated site-specific recombination (Figure 1B).43,44 The replication-deficient adenovirus AdDl70.3 was used as a control throughout this study.45 High titers of recombinant adenoviruses were amplified, purified, titrated, and stored as previously described.46
Construction of recombinant replication-deficient adenoviral vector expressing human BPI. (A) Human BPI cDNA (1.5 kb) was amplified from RNA of human blood CD34+ leukocytes by RT-PCR and visualized in 1% agarose gel by electrophoresis (lane 1, DNA size marker; lane 2, BPI cDNA product by RT-PCR). (B) Recombinant shuttle plasmid with BPI cDNA inserted into the E1 region of adenoviral genome (pDC104BPI) was cotransfected with viral rescue plasmid pBHG10loxCreL into 293 cells. Recombinant AdhBPI virus was rescued by homologous recombination between 2 loxP sites present in pDC104BPI and pBHG10loxCreL, which was catalyzed by Cre recombinase encoded by Cre cDNA in pBHG10loxCreL.
Construction of recombinant replication-deficient adenoviral vector expressing human BPI. (A) Human BPI cDNA (1.5 kb) was amplified from RNA of human blood CD34+ leukocytes by RT-PCR and visualized in 1% agarose gel by electrophoresis (lane 1, DNA size marker; lane 2, BPI cDNA product by RT-PCR). (B) Recombinant shuttle plasmid with BPI cDNA inserted into the E1 region of adenoviral genome (pDC104BPI) was cotransfected with viral rescue plasmid pBHG10loxCreL into 293 cells. Recombinant AdhBPI virus was rescued by homologous recombination between 2 loxP sites present in pDC104BPI and pBHG10loxCreL, which was catalyzed by Cre recombinase encoded by Cre cDNA in pBHG10loxCreL.
Characterization of adenovirus vector expressing human BPI
To verify the presence of hBPI cDNA in the genomic DNA of recombinant AdhBPI, 293 cells were transduced with AdhBPI and allowed to lyse (4-8 days). Viral DNA was then extracted using a pronase digestion protocol as previously described.46 Total DNA (5 μg) was size-separated by electrophoresis. The separated DNA was transferred to a nylon membrane and analyzed for BPI DNA expression by Southern hybridization.
To examine the adenovirus BPI transgene mRNA expression in vitro, 293 cells were transduced with AdhBPI (2 × 108 plaque-forming units [pfu]) for 12 hours in a tissue-culture flask. RNA was extracted from 293 cells using Trizol (Invitrogen). To examine the transgene mRNA expression in vivo, intramuscular gene transfer was performed with AdhBPI as previously described.47 Briefly, 5 × 107 pfu AdhBPI diluted in 50 μL phosphate-buffered saline (PBS) was injected into the calf muscle of anesthetized mice using a 26-gauge needle. RNA was extracted from murine muscle tissue as previously described.48 Total RNA (5 μg) from both in vitro and in vivo AdhBPI-infected samples were separated by electrophoresis and analyzed for transgene mRNA expression by Northern hybridization.48
To examine the AdhBPI transgene protein expression, human alveolar epithelial A549 cells were transduced with AdhBPI (2 × 108 pfu) for 18 hours prior to S35 metabolic labeling of proteins. Virus was washed off the cell layer and cells were starved in Dulbecco modified Eagle medium (Invitrogen) containing 4500 mg/L d-glucose, but no l-glutamine, sodium pyruvate, l-methionine, or l-cystine for 30 minutes, followed by incubation with S35 Translabel (ICN, Santa Ana, CA). Samples were collected from cell lysates after 2 hours of S35 metabolic labeling, and from supernatants after 24 hours of S35 metabolic labeling, and proteins were separated by size using sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE).48
Isolation and culture of alveolar macrophages
Alveolar macrophages were collected from naive C57BL/6 mice by bronchial-alveolar lavage (BAL) as previously described.45-49 BAL cells were collected by centrifugation, resuspended in RPMI media containing 1% l-glutamine and 2% fetal bovine serum (FBS), and counted for viability on a hemacytometer with trypan blue exclusion staining. Alveolar macrophages were cultured in the 96-well plate in 300 μL RPMI media containing 1% l-glutamine and 2% FBS at 37°C, 5% CO2.
In vitro neutralization of LPS by transgene protein BPI produced either in vitro or in vivo
A549 cell supernatant was derived by infecting 2 × 106 A549 cells in a tissue-culture flask without media, with 2 × 108 pfu AdhBPI or AdDl70.3 in 2 mL PBS++ for one hour at 37°C, 5% CO2. Media (RPMI containing 1% l-glutamine, 2% FBS) were added to the cells and allowed to incubate for 18 hours. Cells were washed 3 times with PBS to remove virus, and cells were incubated with media for 3 days to allow maximal protein secretion. The supernatant was collected and stored at –20°C until further use. To generate BPI-containing biologic fluids in vivo, AdhBPI or AdDl70.3 (5 × 108 pfu) was delivered intranasally to C57BL/6 mice and the lungs were lavaged at day 4 after gene transfer.
A549 cell supernatant from AdhBPI- or control vector–infected cells or lung lavage fluids isolated from AdhBPI- or control vector–infected lungs were incubated with 7 ng/mL LPS (Sigma, St Louis, MO) for 30 minutes at room temperature prior to in vitro stimulation of freshly isolated alveolar macrophages (0.1 × 106 cells/well) in a 96-well plate at 37°C, 5% CO2. Following 24 hours of incubation, culture supernatants were collected and stored at –20°C until cytokine analysis.
To demonstrate the LPS-neutralizing activity of AdhBPI-A549 supernatant or AdhBPI–lung lavage, fluid was mediated specifically by BPI protein, 0.25 μL BPI-containing A549 supernatant, or 25 μL AdhBPI–lung lavage fluid was incubated with 1 μL goat antihuman BPI serum or control serum50 (kindly provided by Dr Jerrold Weiss, Iowa University) at 37°C for one hour. These samples were then incubated with LPS as previously described, before being introduced to macrophage culture.
Intraperitoneal BPI gene transfer by AdhBPI
AdhBPI was administered intraperitoneally 24 hours prior to intraperitoneal LPS challenge in female C57BL/6 mice, aged 8 to 10 weeks (Charles River, Montreal, QC, Canada). AdhBPI or AdDl70.3 was diluted in PBS resulting in a total volume of 200 μL containing 5 × 108 pfu of virus. Intraperitoneal gene transfer was performed using a 25-gauge needle, on mice anesthetized with isofluorine, as previously described.47 Following injection, the abdominal area was gently massaged to aid in the distribution of virus in the peritoneal cavity.
Mouse models of endotoxemia
LPS was diluted to 0.8 mg/kg with PBS in a total volume of 200 μL, which was injected with a 25-gauge needle intraperitoneally into anesthetized mice.45,47 Blood was collected retro-orbitally in sterile, nonheparinized capillary tubes at 1, 1.5, and 2.5 hours after endotoxin challenge. Blood was processed for serum and stored at –20°C until cytokine detection.
To set up a mouse model of lethal sepsis, d-galactosamine (Sigma) was dissolved in PBS at 40 mg/mL and prepared fresh before each injection. d-galactosamine was then mixed with LPS and intraperitoneally injected into mice at a dose of 300 mg/kg d-galactosamine and 500 ng LPS/mouse.51,52 Mice were carefully monitored and killed when end-point conditions were met (severely decreased mobility, ruffled fur, and loss of > 20% original body weight). Additionally, for cytokine measurements, blood was collected retro-orbitally in sterile, nonheparinized capillary tubes at 1.5 and 2.5 hours after endotoxin challenge. Blood was processed for serum and stored at –20°C until cytokine detection.
Cytokine assay
TNF-α and macrophage inflammatory protein 2 (MIP-2) cytokines were detected in the serum and culture supernatant using sandwich enzyme-linked immunosorbent assay (ELISA) kits (R&D, Minneapolis, MN). The sensitivity of detection for these kits was 5 pg/mL or less.
Statistical analysis
The data were statistically analyzed by a Student t test using Microsoft Excel software (Seattle, WA), and the difference was considered to be statistically significant when P values were .05 or less.
Results
Construction of adenoviral vector expressing human BPI
To prepare human BPI cDNA, total cellular RNA was extracted from a human leukocyte sample enriched for CD34+ cells, and RT-PCR was performed to generate and amplify BPI cDNA. The BPI cDNA was separated on a 1% agarose electrophoresis gel, positively identified as a 1.5-kb–sized product as predicted (Figure 1A). The sequence of BPI cDNA was verified compared with the sequence of human BPI in GENBANK. BPI cDNA was inserted into the shuttle plasmid pDC104 and cotransfection with pDC104hBPI, 293 cells, and pBHG10loxCreL plasmid was performed to generate a recombinant replication-deficient adenoviral vector expressing human BPI (Figure 1B). This adenoviral vector was then amplified on a large scale, purified, titrated, and stored at –70°C as previously described.46
Molecular characterization of adenoviral vector expressing human BPI
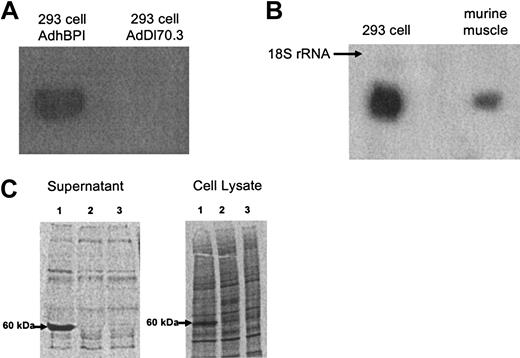
To verify the presence of hBPI cDNA in AdhBPI, AdhBPI was amplified in infected 293 cells. Total viral DNA was extracted from viral-infected cells and analyzed by Southern hybridization. A correct-sized BPI DNA signal was detected in 293 cells infected with AdhBPI, but not AdDl70.3 (Figure 2A). To examine mRNA expression of hBPI in cells infected with AdhBPI, RNA was isolated from AdhBPI-infected 293 cells. Total cellular RNA was isolated from each flask of 293 cells following an 18-hour infection, and the presence of BPI mRNA was examined by Northern hybridization. A correct 1623-bp sized BPI mRNA was detected in 293 cells (Figure 2B), thus demonstrating the ability of AdhBPI to infect 293 cells and led to subsequent transcription of BPI cDNA in these cells. Furthermore, the ability of AdhBPI to infect cells and express transgene in vivo was examined. Murine hind leg muscle tissue was infected with AdhBPI, and 12 hours after gene transfer, RNA was extracted from the homogenized muscle tissue as previously described.47 The correct-sized BPI mRNA was detected by Northern hybridization (Figure 2B). These results thus indicate the ability of AdhBPI to effectively transfer and express BPI transgene in vivo.
Molecular characterization of adenoviral vector expressing human BPI. (A) Verification of the BPI transgene in AdhBPI viral genomic DNA. 293 cells were infected with AdhBPI or AdDl70.3 as control. Total cellular DNA was extracted and subject to Southern hybridization. (B) BPI mRNA expression in vitro and in vivo. 293 cells or mouse hind leg muscle were infected with AdhBPI in vitro and in vivo, respectively. Total cellular or tissue RNA was extracted and subject to Northern hybridization. BPI mRNA was identified as a 1623-bp product. Samples from control vector–infected cells or tissue gave rise to no signals (not shown). (C) Demonstration of secreted and cell-associated BPI protein in A549 cells infected with AdhBPI (lane 1), control vector AdDl70.3 (lane 2), or without infection (lane 3). S35-labeled newly synthesized protein was separated in SDS-PAGE gel and BPI protein was identified as a 60-kDa product.
Molecular characterization of adenoviral vector expressing human BPI. (A) Verification of the BPI transgene in AdhBPI viral genomic DNA. 293 cells were infected with AdhBPI or AdDl70.3 as control. Total cellular DNA was extracted and subject to Southern hybridization. (B) BPI mRNA expression in vitro and in vivo. 293 cells or mouse hind leg muscle were infected with AdhBPI in vitro and in vivo, respectively. Total cellular or tissue RNA was extracted and subject to Northern hybridization. BPI mRNA was identified as a 1623-bp product. Samples from control vector–infected cells or tissue gave rise to no signals (not shown). (C) Demonstration of secreted and cell-associated BPI protein in A549 cells infected with AdhBPI (lane 1), control vector AdDl70.3 (lane 2), or without infection (lane 3). S35-labeled newly synthesized protein was separated in SDS-PAGE gel and BPI protein was identified as a 60-kDa product.
Since BPI protein is primarily stored within human neutrophil granules under normal physiologic conditions, and actively secreted BPI will be required for achieving therapeutic purposes, we examined whether AdhBPI gene transfer would lead to the secretion of BPI protein from cells of nonneutrophil nature. To this end, both culture supernatant and cellular lysates of AdhBPI-infected A549 cells were evaluated using S35 metabolic protein labeling techniques. Supernatants from A549 cells infected with AdhBPI contained a large amount of 60-kDa protein, consistent with the size of human BPI (Figure 2C). In contrast, in the control samples, neither AdDl70.3 control vector–infected nor noninfected produced any such protein. In comparison, the cell lysate of A549 cells infected with AdhBPI, but not with AdDl70.3 control vector, also contained the 60-kDa BPI protein, the amount of which appeared much less than that detected in the supernatant (Figure 2C). These results suggest that AdhBPI gene transfer leads to a significant secretion of BPI protein from transduced cells.
In vitro demonstration of bioactivity of transgene protein BPI
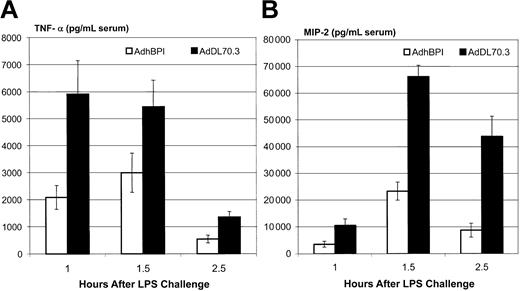
BPI protein has a potent endotoxin-neutralizing ability and it binds to LPS with an even higher affinity than LBP (natural endogenous LPS-binding protein).24-26 To assess the bioactivity of AdhBPI-derived transgene protein, we used an in vitro assay that involved the initial incubation of BPI-containing culture supernatant with LPS and subsequent stimulation of freshly isolated alveolar macrophages by such LPS-containing preparation. The readout was the amount of proinflammatory cytokines TNF-α and MIP-2 (a murine neutrophil chemoattractant) released into macrophage culture supernatant. These 2 cytokines play an important role in orchestrating acute inflammatory responses during sepsis and endotoxin-induced tissue injury.8,9,45 Since from an LPS-dose response experiment it was discovered that a concentration of 7 ng/mL LPS represented a minimal dose of LPS that gave rise to marked production of cytokines in freshly isolated macrophages, this dose of LPS was used for BPI LPS–neutralization studies. We found that BPI-containing, but not the control, supernatant potently neutralized LPS and abrogated LPS-stimulated release of both TNF-α and MIP-2 in a dose-dependent manner (Figure 3A-B). These results indicate that AdhBPI-derived transgene protein is biologically active.
Neutralization of LPS in vitro by BPI-containing A549 cell supernatant and lung lavage fluid. LPS was preincubated with supernatant derived from AdhBPI- or AdDl70.3-infected A549 cells (A-B) or lung lavage fluids from AdhBPI- or AdDl70.3-infected C57BL/6 mice (C) and was then added to alveolar macrophage culture. In some experiments, A549 cell supernatant or lung lavage fluid was treated with goat anti-BPI or control serum before incubation with LPS and macrophages (D). Concentration of TNF-α (A,C-D) and MIP-2 (B) in supernatant was determined using ELISA. Results are expressed as means ± SEM from triplicate wells, representative of 2 independent experiments. The difference between AdhBPI and AdDl70.3 is statistically significant (P ≤ .012, P ≤ .000 004, and P ≤ .000 007 for .0025, 0.25, and 25 μL, respectively, for TNF-α in panel A; and P ≤ .02, P ≤ .0001, and P ≤ .000 003 for .0025, 0.25, and 25 μL, respectively, for MIP-2 in panel B; P ≤ .000 0006 for 25 μL in panel C).
Neutralization of LPS in vitro by BPI-containing A549 cell supernatant and lung lavage fluid. LPS was preincubated with supernatant derived from AdhBPI- or AdDl70.3-infected A549 cells (A-B) or lung lavage fluids from AdhBPI- or AdDl70.3-infected C57BL/6 mice (C) and was then added to alveolar macrophage culture. In some experiments, A549 cell supernatant or lung lavage fluid was treated with goat anti-BPI or control serum before incubation with LPS and macrophages (D). Concentration of TNF-α (A,C-D) and MIP-2 (B) in supernatant was determined using ELISA. Results are expressed as means ± SEM from triplicate wells, representative of 2 independent experiments. The difference between AdhBPI and AdDl70.3 is statistically significant (P ≤ .012, P ≤ .000 004, and P ≤ .000 007 for .0025, 0.25, and 25 μL, respectively, for TNF-α in panel A; and P ≤ .02, P ≤ .0001, and P ≤ .000 003 for .0025, 0.25, and 25 μL, respectively, for MIP-2 in panel B; P ≤ .000 0006 for 25 μL in panel C).
To demonstrate the bioactivity of in vivo–derived hBPI transgene protein, an ex vivo approach was undertaken. The bronchoalveolar lavage fluid (BAL) recovered from the lungs of mice infected intranasally with AdhBPI or control vector was preincubated with 7 ng/mL LPS and then added to a culture of freshly isolated alveolar macrophages for 24 hours. Pretreatment of LPS with the BAL of AdhBPI-infected, but not AdDl70.3-infected, mice triggered much lower levels of TNF-α production by macrophages (Figure 3C), indicating that the human BPI product generated in vivo upon AdhBPI gene transfer is also secreted and biologically active. It is of note that the minimal effective dose of BPI-containing BAL fluid (25 μL) was larger than that of the culture supernatant (0.25 μL; Figure 3A), suggesting different concentrations of BPI present in these samples.
To demonstrate that the LPS-neutralizing activity of BPI-containing samples was indeed mediated by BPI itself, either BPI-containing A549 cell supernatant or BPI-containing lung lavage fluid was treated with antihuman BPI or control serum before being incubated with LPS. We found that the BPI-containing samples pretreated with anti-BPI antibodies, but not those pretreated with control antibodies, almost completely lost their LPS-neutralizing capacity as shown by restoration of LPS-stimulated TNF-α production in macrophages (Figure 3D). These indicate the BPI specificity of LPS neutralization by these biologic BPI-containing samples generated both in vitro and in vivo following BPI gene transfer.
Inhibition of proinflammatory cytokine responses during endotoxemia by intraperitoneal BPI gene transfer
To begin the evaluation of the therapeutic potential of AdhBPI gene transfer vector, we tested the effect of intraperitoneal BPI gene transfer on cytokine responses in vivo by using a previously established mouse model of nonlethal endotoxemia (0.8 mg LPS/kg).45,47 We have previously demonstrated that upon intraperitoneal LPS challenge, there was a quick surge of TNF-α around 1 hour after LPS followed by other proinflammatory cytokine responses.45,47 Thus, we examined the cytokine profile in the peripheral blood at 1, 1.5, and 2.5 hours after LPS challenge. We found that in control mice (mice treated intraperitoneally with the control adenoviral vector, AdDl70.3) the circulating level of TNF-α peaked at one hour and markedly declined by 2.5 hours (Figure 4A). In comparison, the level of MIP-2 peaked at 1.5 hours and declined but remained relatively high at 2.5 hours. In contrast, the overall levels of these 2 cytokines in mice receiving BPI gene transfer were markedly reduced, although the kinetics of cytokine responses were unaltered (Figure 4B). These results demonstrate that AdhBPI-mediated gene transfer could neutralize LPS and thus reduce LPS-induced proinflammatory responses in vivo.
Reduction of proinflammatory cytokines by adenoviral BPI gene transfer during endotoxemia. Mice injected intraperitoneally with either AdhBPI or AdDl70.3 (5 × 108 pfu) were challenged with LPS (0.8 mg/kg). Serum samples were taken at 1, 1.5, and 2.5 hours after LPS challenge and measured by ELISA for TNF-α (A) and MIP-2 (B). Results are expressed as means ± SEM from 5 to 11 mice/group. The difference between AdhBPI and AdDl70.3 control is statistically significant (P ≤ .009, P ≤ .02, and P ≤ .002 for 1, 1.5, and 2.5 hours, respectively, for TNF-α; P ≤ .01, P ≤ .000 005, and P ≤ .0006 for 1, 1.5, and 2.5 hours, respectively, for MIP-2).
Reduction of proinflammatory cytokines by adenoviral BPI gene transfer during endotoxemia. Mice injected intraperitoneally with either AdhBPI or AdDl70.3 (5 × 108 pfu) were challenged with LPS (0.8 mg/kg). Serum samples were taken at 1, 1.5, and 2.5 hours after LPS challenge and measured by ELISA for TNF-α (A) and MIP-2 (B). Results are expressed as means ± SEM from 5 to 11 mice/group. The difference between AdhBPI and AdDl70.3 control is statistically significant (P ≤ .009, P ≤ .02, and P ≤ .002 for 1, 1.5, and 2.5 hours, respectively, for TNF-α; P ≤ .01, P ≤ .000 005, and P ≤ .0006 for 1, 1.5, and 2.5 hours, respectively, for MIP-2).
Protection from lethal endotoxemia by intraperitoneal BPI gene transfer
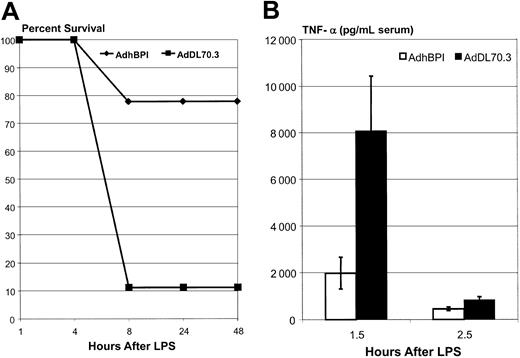
To investigate whether BPI gene transfer could also improve the survival of endotoxic hosts, we used a mouse model of lethal endotoxemia elicited by coinjection of d-galactosamine and LPS.51,52 Galactosamine treatment increases the sensitivity of hosts to LPS challenge, thus helping to overcome the insensitive nature of C57BL/6 mice, in terms of mortality, in response to even extremely high LPS dosing. The majority of control mice that received only the treatment with a control adenoviral vector succumbed to LPS challenge within 8 hours (Figure 5A). In sharp contrast, about 80% of mice receiving BPI gene transfer were protected. AdhBPI-treated mice also exhibited many fewer signs of sickness during end-point monitoring compared with control mice. Accompanied with improved survival were significantly lower circulating levels of TNF-α both at 1.5 and 2.5 hours after LPS (Figure 5B). These results demonstrate that adenoviral vector–mediated BPI gene transfer provides protection to mice sensitized to a lethal dose of LPS challenge.
Protection of mice from lethal sepsis. Mice injected intraperitoneally with either AdhBPI or AdDl70.3 (5 × 108 pfu) were challenged with a preparation containing both LPS (500 ng/mouse) and d-galactosamine (8 mg/kg). Mortality of mice (9 mice/group) was followed over a period of 2 days (A). In separate experiments, serum samples were taken from AdhBPI- or AdDl70.3-treated mice (4-5 mice/group) at 1.5 and 2.5 hours after LPS/d-galactosamine challenge and measured for TNF-α (B). Results are expressed as means ± SEM and are representative of 2 independent experiments. The difference in panel B between AdhBPI and AdDl70.3 is statistically significant at 2 time points (P ≤ .02).
Protection of mice from lethal sepsis. Mice injected intraperitoneally with either AdhBPI or AdDl70.3 (5 × 108 pfu) were challenged with a preparation containing both LPS (500 ng/mouse) and d-galactosamine (8 mg/kg). Mortality of mice (9 mice/group) was followed over a period of 2 days (A). In separate experiments, serum samples were taken from AdhBPI- or AdDl70.3-treated mice (4-5 mice/group) at 1.5 and 2.5 hours after LPS/d-galactosamine challenge and measured for TNF-α (B). Results are expressed as means ± SEM and are representative of 2 independent experiments. The difference in panel B between AdhBPI and AdDl70.3 is statistically significant at 2 time points (P ≤ .02).
Discussion
Our studies consider the rationale behind using recombinant BPI as an adjunct therapeutic to sepsis management, but investigate the transgene delivery method using an adenoviral vector as a vehicle. A recombinant, replication-deficient adenoviral vector expressing full-length human BPI was developed. AdhBPI-mediated gene transfer both in vitro and in vivo resulted in the generation of secreted BPI protein capable of potent neutralization of LPS and subsequent blockade of LPS-induced cytokine production in macrophages. Of importance, we show that mice receiving AdhBPI had a markedly reduced circulating level of TNF-α and MIP-2 in a model of endotoxemia. Furthermore we demonstrate that BPI gene transfer protected mice from lethal endotoxic shock. These results coincide with the observed reduction of proinflammatory mediators in published models of endotoxemia or Gram-negative sepsis using recombinant N-terminal human BPI protein,24-35 and therefore support the use of BPI transgene as an adjunct therapeutic agent for such conditions. However, it should be pointed out that in our current study, we have evaluated only the therapeutic effect of AdhBPI in endotoxic models, and whether AdhBPI vector is effective in models of Gram-negative bacterial sepsis still remains to be determined. It is of importance to bear in mind that while BPI is able to effectively neutralize LPS from all tested Gram-negative bacterial strains,25 its Gram-negative bactericidal activities are variable between strains.25,26 Moreover, often the therapeutic effect of BPI in bacterial septic models was not readily appreciable unless it was used in conjunction with antibiotics.31,53,54
While the majority of therapeutic strategies designed for treating sepsis have failed,18 recent studies suggest that recombinant BPI protein is a promising therapeutic agent.33-40 However, the production and purification of recombinant BPI have proved uneasy tasks, since BPI cannot be produced in the conventional Gram-negative bacterial strains and has to be produced in mammalian cell lines.24 It is also difficult to maintain the integrity of purified full-length BPI protein due to its large molecular weight and complex structure. Thus, the recombinant BPI currently in use for clinical trials is a 23-kDa fragment of BPI N-terminal region, which does not have the bacterial phagocytosis opsonizing ability.24-26 Furthermore, due to its high cost and short half-life, it is difficult to maintain the sustained in vivo levels of BPI and accomplish the most desirable therapeutic results. And the nature of recombinant protein also restricts its future application to targeting tissue sites because of its poor tissue-penetrating ability. In this regard, transient gene transfer represents an attractive approach that may ameliorate these weaknesses. Transient gene transfer aims to accomplish sustained therapeutic levels of protein either in the circulation or at tissue sites by delivering a single dose of transgene into a tissue site of choice, thus turning the host's own cells into a pump that manufactures and constantly releases the therapeutic protein for a transient but prolonged period of time.
Unfortunately, BPI transgene-based therapeutic modality has not been previously developed. Since we have had a long-standing strength in recombinant adenoviral vectorology, we set out to develop an adenoviral gene transfer vector for expressing a full length of human BPI protein. Compared with the use of recombinant N-terminal BPI protein, expression of full-length human BPI will allow us to preserve the full range of biologic activities of BPI, including LPS neutralization, bacterial killing, and facilitation of bacterial phagocytosis.24-26 Human adenovirus has been widely used as a gene delivery vehicle in preclinical studies and as well in a number of clinical trials. While there has been concern about its usefulness and safety for treating genetic diseases and its direct delivery into internal organs, its great potential for vaccination and treatment of acute acquired diseases is still well recognized. Adenoviral gene transfer does not lead to the incorporation of viral gene into the host genome and proves the most effective of all of the current gene transfer vectors. It also has the advantage of being able to efficiently transduce the gene into many cell types, which allows gene delivery at multiple tissue sites. Indeed, we have demonstrated its application in tissue sites including the lung, muscle, peritoneal cavity, joints, and skin.45,47,55-57 A single dose of adenoviral gene delivery results in elevated levels of transgene products for a period of 10 to 14 days. And a second repeat administration at an alternate tissue site of the same host allows further sustained levels.47 Since it takes about 8 hours after adenoviral gene transfer for transgene products to be markedly raised, the potential of using adenoviral BPI vector as an adjunct therapeutic agent to treat the patients already diagnosed with sepsis could be limited. However, adenoviral BPI gene transfer vector holds the potential to be used as a prophylactic therapeutic in patients at high risk to develop sepsis or Gram-negative bacterial infection or patients who suffer Gram-negative bacterial infection but have not yet developed sepsis. Further appreciation of the potential therapeutic effect of AdhBPI in Gram-negative infection models will likely involve the use of antibiotics since successful application of recombinant BPI protein both in experimental Gram-negative infection models and clinical studies was demonstrated only in conjunction with the concurrent use of antibiotics.24,38,31,34,53,54
Prepublished online as Blood First Edition Paper, September 22, 2003; DOI 10.1182/blood-2003-02-0660.
Supported by a grant from the Ontario Thoracic Society.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to the invaluable technical assistance by Jennifer Hall, Jin Ni, Tom Havey, Duncan Chong, and Xueya Feng, and to the helpful scientific discussion with Michael Santosuosso. We also acknowledge the kind provision of goat anti-BPI sera from Dr Jerrold Weiss at Iowa University.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal