Abstract
Adeno-associated viral (AAV) vectors (serotype 2) efficiently transduce skeletal muscle, and have been used as gene delivery vehicles for hemophilia B and for muscular dystrophies in experimental animals and humans. Recent reports suggest that AAV vectors based on serotypes 1, 5, and 7 transduce murine skeletal muscle much more efficiently than AAV-2, with reported increases in expression ranging from 2-fold to 1000-fold. We sought to determine whether this increased efficacy could be observed in species other than mice. In immunodeficient mice we saw 10- to 20-fold higher levels of human factor IX (hF.IX) expression at a range of doses, and in hemophilic dogs we observed approximately 50-fold higher levels of expression. The increase in transgene expression was due partly to higher gene copy number and a larger number of cells transduced at each injection site. In all immunocompetent animals injected with AAV-1, inhibitory antibodies to F.IX developed, but in immunocompetent mice treated with high doses of vector, inhibitory antibodies eventually disappeared. These studies emphasize that the increased efficacy of AAV-1 vectors carries a risk of inhibitor formation, and that further studies will be required to define doses and treatment regimens that result in tolerance rather than immunity to F.IX.
Introduction
Recombinant adeno-associated viral (AAV) vectors efficiently transduce skeletal muscle, liver, and other cell types. AAV vectors derived from serotype 2 have been used in early-phase clinical studies in patients with cystic fibrosis,1 hemophilia B,2 and limb-girdle muscular dystrophy.3 Several groups have shown that other naturally occurring AAV serotypes exhibit distinct profiles in terms of tissue tropisms, and it is now well-established that AAV-1, AAV-5, and AAV-7 transduce murine skeletal muscle more efficiently than the more widely used serotype AAV-2,4-7 although there is disagreement in the literature about the fold improvement in transgene expression with AAV-1. In studies in humans with hemophilia B, we had previously shown that intramuscular injection of AAV-2 based, factor IX (F.IX)–expressing vectors at doses up to 2 × 1012 vector genome (vg)/kg was safe and well-tolerated. Biopsies of injected muscle provided clear evidence of gene transfer and expression, but at the doses tested did not generally result in circulating levels of F.IX more than 1%.2 Based on the studies demonstrating superior efficacy in mice,4-7 it has been suggested that use of an AAV-1–based vector would improve efficacy of this approach.
We therefore carried out a series of studies in tissue culture, in mice, and in hemophilic dogs to assess the safety and efficacy of AAV-1–mediated gene transfer for hemophilia B. We first undertook in vitro and murine studies to establish that AAV-1– and AAV-6–based vectors yield higher levels of F.IX, and to identify factors that account for this finding. AAV-6 is a naturally occurring recombinant between AAV-1 and AAV-26,8 and its efficiency in transducing skeletal muscle is not known. We next prepared AAV-1 vectors expressing canine F.IX (cF.IX) and injected these at intramuscular sites in dogs with hemophilia B arising from a missense mutation.9 At vector doses lower than those used in previous studies with AAV-2,10 we saw circulating cF.IX levels in the range of 87 ng/mL to 104 ng/mL in the first few weeks after injection, but levels fell to zero as inhibitory antibodies developed. Previous work by our group had shown that there is a dose-dependent increase in the likelihood of inhibitory antibody formation after intramuscular injection of AAV-2–cytomegalovirus (CMV)–F.IX in hemophilic dogs.11 The current studies show that these antibodies occur at considerably lower doses with AAV-1 vectors and suggest that the local levels of F.IX antigen produced are a major determinant of the likelihood of a harmful immune response. Additional studies in hemophilic mice demonstrate that injection of very high doses of AAV-1–CMV–human (h) F.IX triggers inhibitory antibody formation initially, followed by disappearance of inhibitors and long-term expression of hF.IX. Thus, in animals that are not tolerant to the transgene product, the superior efficacy of AAV-1 in skeletal muscle poses an increased risk of inhibitory antibody formation. Dose of vector and level of transgene expression may determine whether antibodies are transient or persistent.
Materials and methods
AAV vector construction and production
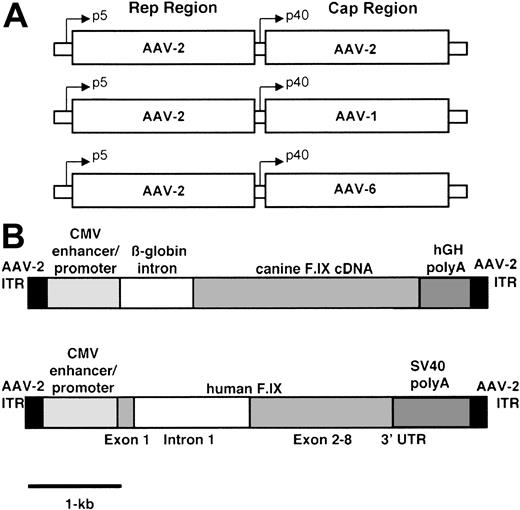
Recombinant AAV vectors were produced by triple transfection as previously described.12 The plasmids expressing canine or human F.IX under the control of the CMV promoter/enhancer and a second plasmid supplying adenovirus helper functions were identical to those described.10,13 A third plasmid containing the AAV-2 rep and cap genes was used to produce AAV-2 vectors, whereas a plasmid containing either AAV-1 or AAV-6 cap genes and AAV-2 rep gene and inverted terminal repeats was used to produce AAV-1 and AAV-6, respectively (Figure 1). AAV vectors were purified by repeated cesium chloride (CsCl) density gradient centrifugation and the titer of purified vectors was determined by quantitative dot-blot hybridization.12
Constructs used in vector preparation. (A) Helper plasmids contain rep gene sequences from AAV-2 and cap gene sequences from AAV-2, AAV-1, and AAV-6 respectively. (B) The canine transgene cassette contains the cF.IX cDNA, the human β-globin intron 1, the CMV enhancer/promoter, and the human growth hormone polyadenylation signal, flanked by the AAV-2 inverted terminal repeats. The human transgene cassette contains the human F.IX cDNA interrupted by a 1.4-kb fragment of hF.IX intron 1, the CMV enhancer/promoter, and the SV40 polyadenylation signal, also flanked by the AAV-2 inverted terminal repeats.
Constructs used in vector preparation. (A) Helper plasmids contain rep gene sequences from AAV-2 and cap gene sequences from AAV-2, AAV-1, and AAV-6 respectively. (B) The canine transgene cassette contains the cF.IX cDNA, the human β-globin intron 1, the CMV enhancer/promoter, and the human growth hormone polyadenylation signal, flanked by the AAV-2 inverted terminal repeats. The human transgene cassette contains the human F.IX cDNA interrupted by a 1.4-kb fragment of hF.IX intron 1, the CMV enhancer/promoter, and the SV40 polyadenylation signal, also flanked by the AAV-2 inverted terminal repeats.
Cell culture experiments
Human myoblasts were isolated from muscle biopsy samples obtained from healthy volunteers and then differentiated to mature myotubes by incubation in culture media containing 10% horse serum. A murine myoblast cell line (American Tissue Culture Collection, Manassas, VA) was kept undifferentiated by incubating with 10% fetal bovine serum or alternatively, was incubated with 10% horse serum to differentiate to mature myotubes. Cells were transduced with AAV–CMV-hF.IX vector at multiplicities of infection (MOIs) ranging from 1000 to 40 000 in serum-free media (Opti-MEMI; Life Technologies, Gaithersburg, MD), as previously described.14
Animal experiments
All procedures involving mice and dogs were approved by the Institutional Animal Care and Use Committee at The Children's Hospital of Philadelphia or at the University of North Carolina at Chapel Hill.
Twenty-four immunodeficient CD4 knockout mice on a C57Bl/6 background (The Jackson Laboratories, Bar Harbor, ME) were injected with AAV-1 or AAV-2 vectors encoding hF.IX under the control of the CMV promoter/enhancer. Cohorts of 4 mice were injected at 3 vector doses of AAV-1 or AAV-2: 2 × 1011, 1.2 × 1012, or 4 × 1012 vg/kg. Intramuscular injections were distributed into 4 skeletal muscle sites (tibialis anterior and quadriceps muscle). Circulating hF.IX antigen levels were measured in plasma samples collected every 2 weeks from the retro-orbital space using heparinized capillary tubes.
In addition, hemophilia B mice (C57Bl6, n = 10) were injected with AAV1–CMV-hF.IX vectors at doses ranging from 6.5 × 1011 vg/kg to 1.6 × 1013 vg/kg. In these experiments, F.IX antigen levels, clotting activity, and antibody formation to F.IX were simultaneously determined.
To assess T-cell response to skeletal muscle fibers transduced by AAV-1 vectors, additional immunocompetent mice (n = 4) were injected with AAV-1–hF.IX at a dose of 4 × 1012 vg/kg. Mice were killed at day 14 or day 30 following vector administration, and muscle tissue was harvested and fixed and stained by hematoxylin and eosin.
Hemophilia B dogs from University of North Carolina at Chapel Hill have a missense mutation in the F.IX gene, which results in F.IX antigen and clotting activity levels less than 1% of normal.9,15-17 The first animal received an intramuscular injection of AAV-1–CMV-cF.IX at a dose of 1 × 1012 vg/kg distributed into 36 sites (5 × 1011 vg per site). The second animal received an intramuscular injection of AAV-1–CMV-cF.IX at a dose of 2.4 × 1011 vg/kg, distributed in 28 sites of 6.8 × 1010 vg per site. Dog 1 received an intravenous infusion of cyclophosphamide, 200 mg/m2 to 250 mg/m2, beginning on day 15 after vector infusion and continuing weekly for 6 weeks. Dog 2 received cyclophosphamide 1 day prior to the day of injection and weekly thereafter up to 6 doses. No plasma infusion was required prior to injection.
Serial blood cell counts and biochemical analysis of serum samples for liver and kidney function tests, and muscle enzymes were performed as described before.10
Clotting assays, F.IX antigen, and antibody to F.IX
hF.IX concentration was determined using an enzyme-linked immunosorbent assay (ELISA) in which a monoclonal antibody FXC008 (Boehringer Mannheim, Indianapolis, IN) was used as a capture antibody at a dilution of 1:500; peroxidase conjugated polyclonal goat antihuman F.IX (Affinity Biologicals, Hamilton, ON, Canada) was used as a detecting antibody. Canine F.IX concentration was determined using FXC008 as a capture antibody as well. A rabbit anti–cF.IX antibody (Affinity Biolabs, Ontario, ON, Canada) was used at a dilution of 1:1000 as a secondary antibody, a swine antirabbit immunoglobulin G (IgG) peroxidase labeled at a dilution of 1:2000 (Dako, Carpinteria, CA) was used for detection. Whole-blood clotting time (WBCT) and the activated partial thromboplastin time (aPTT) were determined as previously described.10,18 The WBCT in hemostatically normal dogs ranges from 8 to 12 minutes and the aPTT ranges from 18 to 20 seconds. For hemophilic dogs the WBCT ranges from 30 to 60 minutes and the aPTT from 50 to 80 seconds. Shortening of the WBCT begins with increases in the F.IX levels above 2 ng/mL. Inhibitory antibodies to human or canine F.IX were assayed by Bethesda assay. In addition, inhibitory or noninhibitory antibodies to F.IX were measured by both a specific ELISA to IgG subclasses or by Western blot analysis as described.18,19
Histochemical analysis
Tissue obtained by muscle biopsy was frozen initially in cooled isopentane followed by liquid nitrogen. Muscle sections were stained with hematoxylin and eosin for histology. Muscle serial cryosections (5 μm-10 μm) were stained for F.IX expression by immunofluorescence using a 1:400 dilution of goat anti–hF.IX antibody (Affinity Biologicals) followed by antigoat IgG fluorescein–conjugated antibody at a dilution of 1:10 000 (Dako).
DNA analysis
Genomic DNA was extracted from injected murine muscle, digested with BglII (New England Biolabs, Beverly, MA), separated in a 1% agarose gel, and transferred to nitrocellulose membrane. Southern blot hybridization was performed using as probe a 0.7-kilobase (kb) fragment of the CMV enhancer/promoter. Intensity of bands on autoradiographs was quantitated by densitometric scanning of the exposed x-ray film (http://rsb.info.nih.gov/nih-image/).
Results
In vitro transduction of murine and human skeletal muscle cells by AAV-2–F.IX vectors yields higher levels of F.IX than transduction by AAV-1 vectors
Results from triplicate experiments demonstrated that F.IX levels are consistently 2- to 8.9-fold higher after differentiation to myotubes compared with undifferentiated myoblasts (Table 1). In both differentiated and undifferentiated murine C2C12 cells, AAV-2–F.IX yielded higher levels of F.IX (2- to 4-fold) compared with AAV-1–F.IX. Similar results were observed with normal human myoblasts. In a typical experiment, following differentiation to myotubes, cells were transduced at an MOI of 20 000. After 5 days, F.IX levels in conditioned medium averaged 212 ± 28 ng/mL per 48 hours for AAV-2–transduced cells and 111 ± 45 ng/mL per 48 hours for AAV-1–transduced cells.
F.IX levels in the conditioned medium from murine C2C12 cells transduced with AAV-CMV-F.IX vectors at a range of multiplicity of infection
Multiplicity of Infection (MOI) . | AAV-1, F.IX ng/mL per 48 h . | AAV-2, F.IX ng/mL per 48 h . | Fold difference . |
|---|---|---|---|
| Undifferentiated C2C12 cells | |||
| 1 000 | 3 ± 1 | 12 ± 2.6 | 4 |
| 5 000 | 19 ± 4 | 60 ± 13 | 3 |
| 20 000 | 29 ± 10 | 108 ± 37 | 3.7 |
| 40 000 | 36 ± 4.5 | 129 ± 40 | 3.5 |
| Differentiated C2C12 cells | |||
| 1 000 | 33 ± 10 | 107 ± 47 | 3.2 |
| 5 000 | 63 ± 29 | 126 ± 29.4 | 2 |
| 20 000 | 103 ± 12 | 208 ± 30 | 2 |
| 40 000 | 115 ± 8 | 247 ± 58 | 2 |
| Fold range difference | 3 to 10 | 2 to 15 |
Multiplicity of Infection (MOI) . | AAV-1, F.IX ng/mL per 48 h . | AAV-2, F.IX ng/mL per 48 h . | Fold difference . |
|---|---|---|---|
| Undifferentiated C2C12 cells | |||
| 1 000 | 3 ± 1 | 12 ± 2.6 | 4 |
| 5 000 | 19 ± 4 | 60 ± 13 | 3 |
| 20 000 | 29 ± 10 | 108 ± 37 | 3.7 |
| 40 000 | 36 ± 4.5 | 129 ± 40 | 3.5 |
| Differentiated C2C12 cells | |||
| 1 000 | 33 ± 10 | 107 ± 47 | 3.2 |
| 5 000 | 63 ± 29 | 126 ± 29.4 | 2 |
| 20 000 | 103 ± 12 | 208 ± 30 | 2 |
| 40 000 | 115 ± 8 | 247 ± 58 | 2 |
| Fold range difference | 3 to 10 | 2 to 15 |
Over prolonged periods and over a broad dose range, AAV-1—and AAV-6—based vectors result in higher levels of expression of a F.IX transgene compared with AAV-2 vectors in immunodeficient mice.
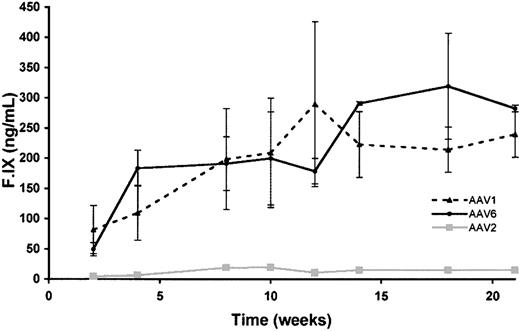
C57Bl/6/CD4 knockout mice were injected at doses ranging from 2 × 1011 vg/kg to 4 × 1012 vg/kg (n = 4 per group) with AAV vectors. AAV-1 and AAV-6 vectors resulted in 10- to 20-fold higher levels of circulating F.IX at each dose tested (Figure 2). F.IX levels in AAV-2–injected mice were generally reproducible (Table 2) with variation among mice being less than or equal to 20% at a dose of 1 × 1012 vg/kg, whereas variation was higher among mice at low doses of AAV-1 (as much as 50%) and less at higher doses (± 10%). A dose-dependent increase in levels of F.IX was seen for all doses of AAV-1 and AAV-6 tested (Table 2 [AAV-1]; AAV-6, data not shown).
Time course of hF.IX expression in C57Bl/6 CD4 knockout mice. Mice were injected at 2 × 1011 vg/kg at 4 intramuscular sites in the hindlimbs. Each line represents average values for the cohort (n = 4 mice). Circles represent mice injected with AAV-6–F.IX vector; triangles, mice injected with AAV-1–F.IX; squares, mice injected with AAV-2–F.IX.
Time course of hF.IX expression in C57Bl/6 CD4 knockout mice. Mice were injected at 2 × 1011 vg/kg at 4 intramuscular sites in the hindlimbs. Each line represents average values for the cohort (n = 4 mice). Circles represent mice injected with AAV-6–F.IX vector; triangles, mice injected with AAV-1–F.IX; squares, mice injected with AAV-2–F.IX.
Circulating F.IX levels 8 weeks after intramuscular injection of AAV vectors in mice
Vector dose, vg/kg . | AAV-1 . | AAV-2 . | Fold difference . |
|---|---|---|---|
| 2 × 1011 | 198 ± 96 | 19 ± 3 | 10.4 |
| 1 × 1012 | 436 ± 79 | 20 ± 5 | 21.8 |
| 4 × 1012 | 1115 ± 120 | 53 ± 10 | 21.0 |
Vector dose, vg/kg . | AAV-1 . | AAV-2 . | Fold difference . |
|---|---|---|---|
| 2 × 1011 | 198 ± 96 | 19 ± 3 | 10.4 |
| 1 × 1012 | 436 ± 79 | 20 ± 5 | 21.8 |
| 4 × 1012 | 1115 ± 120 | 53 ± 10 | 21.0 |
Superior expression from AAV-1 vectors is partly due to higher gene copy number at injected sites
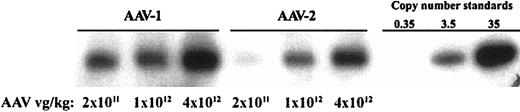
Samples of murine muscle tissue injected with a range of doses of AAV-1 or AAV-2 vectors were excised 12 weeks after injection and DNA was extracted. Southern blots were performed to determine DNA copy number (Figure 3). In these experiments, the dose per site is proportional to the dose per kilogram. A copy number standard was prepared by spiking AAV vector plasmid into murine DNA. Uncut DNA showed a diffuse high-molecular-weight signal (data not shown). Restriction digest with BglII produced a fragment of approximately 0.7 kb, as expected. Comparison of signal strength showed a higher gene copy number in AAV-1–transduced tissue compared with AAV-2–transduced tissue at every dose tested. The fold increase in gene copy number is greater at low doses than at higher doses (Table 3).
Gene copy number in AAV-injected muscle tissue. (A) Southern blot analysis of genomic DNA extracted from murine skeletal muscle injected with AAV vectors. The first 3 lanes are from mice injected with AAV-1–F.IX at the doses listed beneath each lane; the next 3 lanes are from mice injected with AAV-2–F.IX. Copy number standards were prepared by adding 10, 100, or 1000 pg plasmid to 20 μg murine genomic DNA.
Gene copy number in AAV-injected muscle tissue. (A) Southern blot analysis of genomic DNA extracted from murine skeletal muscle injected with AAV vectors. The first 3 lanes are from mice injected with AAV-1–F.IX at the doses listed beneath each lane; the next 3 lanes are from mice injected with AAV-2–F.IX. Copy number standards were prepared by adding 10, 100, or 1000 pg plasmid to 20 μg murine genomic DNA.
Densitometric analysis of Southern blot yields gene copy no. in injected muscle
. | . | Actual gene copy no. . | . | Relative gene copy no. . | . | Relative circulating F.IX, ng/mL . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Vector dose, vg/kg . | Fold increase in dose . | AAV-2 . | AAV-1 . | AAV-2 . | AAV-1 . | AAV-2 . | AAV-1 . | |||
| 2 × 1011 | 1 | 0.4 | 14.5 | 1 | 36 | 1.0 | 10 | |||
| 1 × 1012 | 5 | 3.0 | 20.0 | 8 | 50 | 1.0 | 20 | |||
| 4 × 1012 | 20 | 15.0 | 33.0 | 38 | 82 | 2.5 | 56 | |||
. | . | Actual gene copy no. . | . | Relative gene copy no. . | . | Relative circulating F.IX, ng/mL . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Vector dose, vg/kg . | Fold increase in dose . | AAV-2 . | AAV-1 . | AAV-2 . | AAV-1 . | AAV-2 . | AAV-1 . | |||
| 2 × 1011 | 1 | 0.4 | 14.5 | 1 | 36 | 1.0 | 10 | |||
| 1 × 1012 | 5 | 3.0 | 20.0 | 8 | 50 | 1.0 | 20 | |||
| 4 × 1012 | 20 | 15.0 | 33.0 | 38 | 82 | 2.5 | 56 | |||
Relative gene copy number values obtained by normalizing to AAV-2—injected value at lowest dose. Note that the difference in gene copy number between AAV-1—and AAV-2—injected tissue is most marked at low doses. Relative circulating F.IX levels were determined by normalizing to levels obtained with the lowest dose (2 × 1011 vg/kg) of AAV-2.
At an equivalent dose per injection site, larger numbers of muscle cells are transduced by AAV-1 vectors compared with AAV-2
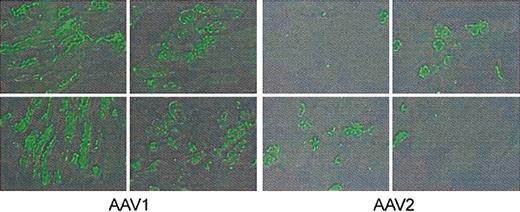
Mice injected at a dose of approximately 4 × 1012 vg/kg were killed 8 weeks after injection, and muscle tissue was excised and stained by immunofluorescence for hF.IX. Two technologists blinded to vector type scored positive cells by counting 26 fields in 6 sections. Scoring sections from the quadriceps and tibialis anterior, in AAV-1–injected tissue, 54% of cells were positive on average (range 38%-67%) and in AAV-2–injected cells, 22% (range 17%-32%) were positive on average. Representative sections are shown in Figure 4; typically, the number of F.IX-expressing cells was approximately 2- to 3-fold higher in the AAV-1–injected tissue.
Immunofluorescent staining for hF.IX in AAV-injected mouse muscle. Animals were injected in the hindlimbs with 4 × 1012 vg/kg AAV-1 or AAV-2, divided equally among 4 injection sites. Twelve weeks later mice were killed and injected muscle was resected for immunofluorescent staining. Positive cells were scored by observers blinded to vector type. Representative sections from the quadriceps are shown for AAV-1– and AAV-2–injected muscle; average percent of positive cells was 54% for AAV-1, 22% for AAV-2.
Immunofluorescent staining for hF.IX in AAV-injected mouse muscle. Animals were injected in the hindlimbs with 4 × 1012 vg/kg AAV-1 or AAV-2, divided equally among 4 injection sites. Twelve weeks later mice were killed and injected muscle was resected for immunofluorescent staining. Positive cells were scored by observers blinded to vector type. Representative sections from the quadriceps are shown for AAV-1– and AAV-2–injected muscle; average percent of positive cells was 54% for AAV-1, 22% for AAV-2.
Intramuscular injection of AAV-1 in immunocompetent mice triggers formation of antibody to the transgene product but not destruction of the transduced cells
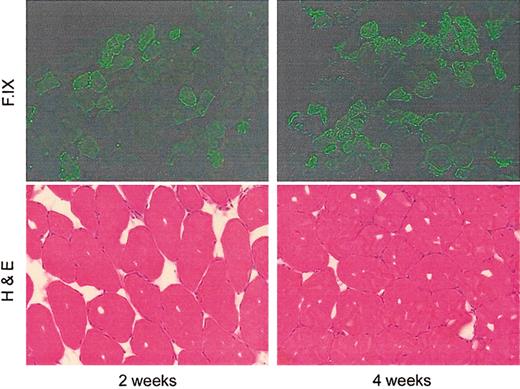
Previously we have shown that intramuscular injection of AAV-2 vectors in immunocompetent mice results in formation of antibodies to hF.IX,13 but long-term persistence of and expression from the transduced cells and no evidence of cellular infiltrate at the injected site.19 This is consistent with a primarily T helper cell 2 (Th2)–driven response. Although the capsid sequences of AAV-1, AAV-2, and AAV-6 are highly conserved, one cannot assume that the characteristics of the immune response will be identical. We therefore examined immune responses to vector injection/transgene expression in immunocompetent mice. In the first experiment, C57Bl/6 mice received an intramuscular injection of AAV-1–F.IX at a dose of 2 × 1010 vg per site (∼4 × 1012 vg/kg). Animals were killed at 2 weeks and 4 weeks after injection, and injected muscle was excised and stained with hematoxylin and eosin (Figure 5). Sections showed no evidence of inflammatory infiltrate or destruction of muscle architecture. Examination of injected tissue at time points out to 12 weeks showed no evidence for loss of transduced cells.
Paired immunofluorescent and hemotoxylin and eosin staining of AAV-1–injected murine muscle at early time points after injection.
Paired immunofluorescent and hemotoxylin and eosin staining of AAV-1–injected murine muscle at early time points after injection.
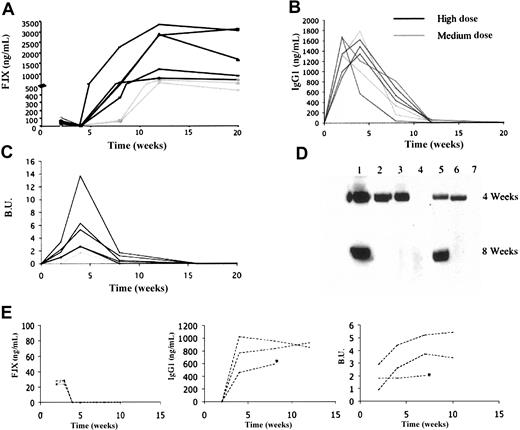
In the immunofluorescent stains, the number of positive cells increases from week 2 to week 4. Hematoxylin and eosin staining is remarkable for the absence of cellular infiltrates and the preservation of normal muscle architecture. In another experiment, C57Bl/6 mice with hemophilia B due to a large deletion in the F.IX gene20 were injected with AAV-1 at doses ranging from 4 × 1012 vg/kg to 1.6 × 1013 vg/kg. These doses resulted in circulating levels of F.IX ranging from 20 ng/mL to 110 ng/mL at 2 weeks following by undetectable levels at week 4 due to antibody formation to F.IX documented in all mice by Western blot and by ELISA for F.IX-specific IgG (Figure 6B,D). These were inhibitory antibodies as documented by Bethesda titers ranging from 0.5 to 13.7 Bethesda units (BU) (Figure 6C). Antibodies generally appeared approximately 2 weeks after injection and persisted for periods up to 8 weeks after injection. Following this, antibodies disappeared and F.IX was again detectable in the circulation at levels ranging from 200 ng/mL to 3000 ng/mL (Figure 6A).
Human F.IX expression and anti–F.IX anti-body measurements as a function of dose and time in AAV-1–injected immunocompetent hemophilic mice. C57Bl/6 hemophilia B mice were injected at a high (1.6 × 1013 vg/kg, n = 5), medium (4 × 1012 vg/kg, n = 2), or low (6.5 × 1011 vg/kg, n = 3) dose at intramuscular sites. Panels A-D refer to medium- and high-dose cohorts, panel E to low-dose cohort. Each line represents one mouse. (A) Human F.IX levels were determined by ELISA. Levels in high-dose animals range from 300 ng/mL to 2200 ng/mL at 8 weeks, with levels of approximately 100 ng/mL at the same time point in medium-dose animals. After the initial lag, F.IX levels remain stable for the duration of the experiment. (B) Anti–F.IX-specific IgG1 antibodies. These were detected in all animals, peaked at 2 to 4 weeks after injection, markedly decreased by 8 weeks, and disappeared by 12 weeks after injection. (C) Bethesda titers. Inhibitory antibody is first detected at 2 weeks after injection, reaches a maximum at 4 weeks, then gradually diminishes. (D) Western blot to detect anti–F.IX antibody. Lanes 1 to 5 contain serum from the mice treated at a high dose, lanes 6 to 7 conatin serum from mice treated at a lower dose. At the 4-week time point, antibody was detected in 5 of 7 mice. At the 8-week time point it was detected in only 2 of 7 mice. (E) Left panel represents hF.IX levels determined by ELISA. Center and right panels represent antibodies to F.IX detected in all 3 mice by specific IgG to F.IX or by Bethesda assay, respectively. *Denotes the death of one animal after week 8.
Human F.IX expression and anti–F.IX anti-body measurements as a function of dose and time in AAV-1–injected immunocompetent hemophilic mice. C57Bl/6 hemophilia B mice were injected at a high (1.6 × 1013 vg/kg, n = 5), medium (4 × 1012 vg/kg, n = 2), or low (6.5 × 1011 vg/kg, n = 3) dose at intramuscular sites. Panels A-D refer to medium- and high-dose cohorts, panel E to low-dose cohort. Each line represents one mouse. (A) Human F.IX levels were determined by ELISA. Levels in high-dose animals range from 300 ng/mL to 2200 ng/mL at 8 weeks, with levels of approximately 100 ng/mL at the same time point in medium-dose animals. After the initial lag, F.IX levels remain stable for the duration of the experiment. (B) Anti–F.IX-specific IgG1 antibodies. These were detected in all animals, peaked at 2 to 4 weeks after injection, markedly decreased by 8 weeks, and disappeared by 12 weeks after injection. (C) Bethesda titers. Inhibitory antibody is first detected at 2 weeks after injection, reaches a maximum at 4 weeks, then gradually diminishes. (D) Western blot to detect anti–F.IX antibody. Lanes 1 to 5 contain serum from the mice treated at a high dose, lanes 6 to 7 conatin serum from mice treated at a lower dose. At the 4-week time point, antibody was detected in 5 of 7 mice. At the 8-week time point it was detected in only 2 of 7 mice. (E) Left panel represents hF.IX levels determined by ELISA. Center and right panels represent antibodies to F.IX detected in all 3 mice by specific IgG to F.IX or by Bethesda assay, respectively. *Denotes the death of one animal after week 8.
A third group of animals (n = 3) was injected with a lower vector dose (6.5 × 1011 vg/kg). In this group, transient circulating F.IX levels of 20 ng/mL to 25 ng/mL were detected up to week 3. By week 4, antibody to F.IX was detected in all animals. In contrast to animals injected with higher vector doses, where antibodies to F.IX were decreasing by week 8 and had disappeared altogether by week 12 (Figure 6B), in the low-dose group, levels of antibodies to F.IX remained stable for the duration of the experiment (12 weeks) with no evidence of diminution (Figure 6E).
Intramuscular injection of AAV-1–cF.IX in hemophilic dogs results in inhibitor formation at lower doses than those that trigger inhibitor formation following AAV-2 intramuscular injection
Previous studies in a hemophilia B model showed that risk of inhibitor formation following intramuscular injection of AAV-2 vector rises as the dose of vector rises.11 Animals that received doses up to 3.4 × 1012 vg/kg did not form inhibitory antibodies, whereas 2 out of 3 animals injected at doses equal to or higher than 8.5 × 1012 vg/kg developed inhibitors (Table 4). Moreover, the vector dose per site also seemed to play a role, since, of 3 animals that received comparable doses (8 × 1012 vg/kg), the dog (B14) that received a high dose per site (1.2 × 1013 vg/site) formed a long-lasting high titer inhibitory antibody, whereas the 2 dogs that received a lower dose per site (2 × 1012 vg/site) either failed to form an inhibitor or developed only a transient low titer inhibitor. Analysis of immunoglobulin subclasses in the earlier study showed that noninhibitory antibodies consisted of IgG2 only, whereas animals with inhibitory antibodies showed both IgG1 and IgG2. Whether the dose dependence of risk of inhibitor formation resulted from increasing levels of F.IX antigen, higher numbers of viral particles, higher levels of a contaminant that acted as an adjuvant, or some combination of these factors, was not clear.
Summary of hemophilia B dogs injected intramuscularly with AAV-1 or AAV-2 vector
Dog . | Age, mos. . | Sex . | AAV serotype . | Total vector genome . | Vector genome per kg . | Vector dose per site . | Maximum cF.IX level, ng/mL . | Anti-cF.IX, Bethesda units . |
|---|---|---|---|---|---|---|---|---|
| E35 | 10 | female | AAV-1 | 1.6 × 1013 | 1.0 × 1012 | 5.0 × 1011 | 87* | 8-15 BU |
| E57† | 3 | male | AAV-1 | 2.0 × 1012 | 2.4 × 1011 | 6.8 × 1010 | 104 | 2-8.5 BU |
| B45 | 2 | male | AAV-2 | 7.4 × 1011 | 1.3 × 1011 | 4.1 × 1010 | 2.6 | none |
| B48 | 8 | female | AAV-2 | 6.8 × 1013 | 3.4 × 1012 | 1.1 × 1012 | 20 | none |
| D32† | 2.5 | male | AAV-2 | 2.5 × 1013 | 5.6 × 1012 | 2.0 × 1012 | 40 | none |
| D31 | 1.5 | male | AAV-2 | 3.2 × 1013 | 8.5 × 1012 | 2.0 × 1012 | 39 | none |
| B85 | 3 | female | AAV-2 | 5.0 × 1013 | 8.5 × 1012 | 2.1 × 1012 | 70 | 6.8 BU, t-ransient |
| B14 | 23 | male | AAV-2 | 2.3 × 1014 | 1.1 × 1013 | 1.2 × 1013 | 30* | ≤ 24.5 BU |
Dog . | Age, mos. . | Sex . | AAV serotype . | Total vector genome . | Vector genome per kg . | Vector dose per site . | Maximum cF.IX level, ng/mL . | Anti-cF.IX, Bethesda units . |
|---|---|---|---|---|---|---|---|---|
| E35 | 10 | female | AAV-1 | 1.6 × 1013 | 1.0 × 1012 | 5.0 × 1011 | 87* | 8-15 BU |
| E57† | 3 | male | AAV-1 | 2.0 × 1012 | 2.4 × 1011 | 6.8 × 1010 | 104 | 2-8.5 BU |
| B45 | 2 | male | AAV-2 | 7.4 × 1011 | 1.3 × 1011 | 4.1 × 1010 | 2.6 | none |
| B48 | 8 | female | AAV-2 | 6.8 × 1013 | 3.4 × 1012 | 1.1 × 1012 | 20 | none |
| D32† | 2.5 | male | AAV-2 | 2.5 × 1013 | 5.6 × 1012 | 2.0 × 1012 | 40 | none |
| D31 | 1.5 | male | AAV-2 | 3.2 × 1013 | 8.5 × 1012 | 2.0 × 1012 | 39 | none |
| B85 | 3 | female | AAV-2 | 5.0 × 1013 | 8.5 × 1012 | 2.1 × 1012 | 70 | 6.8 BU, t-ransient |
| B14 | 23 | male | AAV-2 | 2.3 × 1014 | 1.1 × 1013 | 1.2 × 1013 | 30* | ≤ 24.5 BU |
Maximum cF.IX levels detected before antibody formation to F.IX.
Dogs treated with cyclophosphamide, 1 dose per week for 6 weeks.
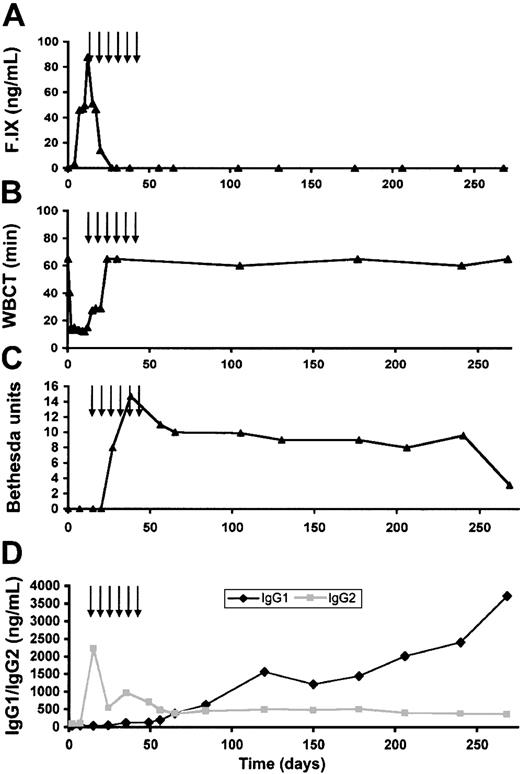
The higher expression levels of the AAV-1 vectors provided a means of examining which of these factors contributed to inhibitor formation. We injected a hemophilia B dog (E35) at intramuscular sites with AAV-1–cF.IX at a dose of 1 × 1012 vg/kg, 5 × 1011 vg per site. In terms of the dose per kilogram, this was 8-fold lower than the dose per kilogram associated with inhibitor formation with AAV-2 vectors, and 4-fold lower than the dose per site. However, as shown in Figure 7, even at these doses, which never trigger inhibitory antibody formation with AAV-2–F.IX, the injected animal formed inhibitory antibodies starting about 5 weeks after vector injection. As shown in Figure 7A, the cF.IX level peaked at 87 ng/mL (1.7% normal circulating level in humans) at 2 weeks after injection. This rise in F.IX levels was accompanied by a reduction in the WBCT (Figure 7B). As the inhibitor developed, though (Figure 7C), F.IX was no longer detectable in the circulation and the WBCT returned to baseline (> 60 minutes). This inhibitor rose to a peak of 15 BUs and persisted in the range of 8 BU to 10 BU for 250 days before falling to a lower titer. Determination of immunoglobulin subclasses again revealed high levels of IgG2 initially followed by development of IgG1 antibodies concomitant with the appearance of the inhibitor.
Coagulation testing and antibody studies in dog E35. (A) Canine F.IX antigen levels as a function of time after injection. F.IX level peaked 2 weeks after injection and rapidly declined to undetectable. Arrows denote time points at which cyclophosphamide was administered. (B) WBCT as a function of time after injection. The WBCT fell into the normal range by day 6 and began to prolong again by day 15. By day 23 it had returned to the baseline of more than 60 minutes. (C) Bethesda titer as a function of time. An inhibitory antibody of 8.2 BU was first detected at day 27, and persisted for the remainder of the animal's lifespan. (D) Anti–F.IX antibody measured by subclass as a function of time. IgG2 was first detected at high levels (2225 ng/mL) on day 15 after vector injection, prior to appearance of inhibitory antibody or to infusion of canine plasma. IgG1 rose gradually beginning about 35 days after vector injection and continued to rise throughout the course of the experiment.
Coagulation testing and antibody studies in dog E35. (A) Canine F.IX antigen levels as a function of time after injection. F.IX level peaked 2 weeks after injection and rapidly declined to undetectable. Arrows denote time points at which cyclophosphamide was administered. (B) WBCT as a function of time after injection. The WBCT fell into the normal range by day 6 and began to prolong again by day 15. By day 23 it had returned to the baseline of more than 60 minutes. (C) Bethesda titer as a function of time. An inhibitory antibody of 8.2 BU was first detected at day 27, and persisted for the remainder of the animal's lifespan. (D) Anti–F.IX antibody measured by subclass as a function of time. IgG2 was first detected at high levels (2225 ng/mL) on day 15 after vector injection, prior to appearance of inhibitory antibody or to infusion of canine plasma. IgG1 rose gradually beginning about 35 days after vector injection and continued to rise throughout the course of the experiment.
Clinical laboratory studies including muscle enzymes and liver function tests showed that intramuscular injection of AAV-1 was not associated with systemic or local toxicity at early times after administration. The development of an inhibitory antibody was heralded by a rise in the WBCT (from 12 minutes at day 10 to 27.5 minutes at day 15). We attempted to address this by instituting immunosuppressive therapy with intravenous cyclophosphamide (200 mg/m2-250 mg/m2) administered weekly from day 15 to day 50 after vector injection. However, this maneuver did not result in any significant diminution of inhibitor titer, and the animal experienced a life-threatening retropharyngeal bleed 10 months after injection, which required euthanasia.
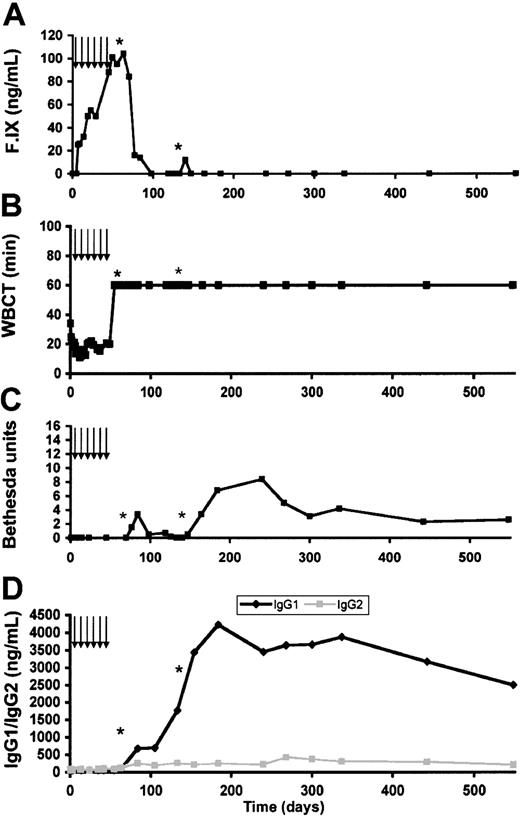
Dose reduction and transient immunosuppression at the time of vector administration delay but do not block the formation of inhibitory antibodies
Because our previous studies in dogs with hemophilia B have shown that the risk of inhibitor formation increased with dose per kilogram and dose per site, we wished to determine whether a reduction in dose would prevent the formation of inhibitory antibodies. Our earlier studies had also shown that transient immunosuppression with cyclophosphamide could prevent inhibitor formation, both in the missense mutation dogs treated at higher doses11 and in nonsense mutation dogs.21 We therefore transiently immunosuppressed a second animal, E57, (cyclophosphamide 200 mg/m2-250 mg/m2 one day before injection and weekly thereafter for a total of 6 infusions) treated at a vector dose of 2.4 × 1011 vg/kg (4-fold reduction compared with the first dog) and a dose per site of 6.7 × 1010 vg/site (8-fold reduction compared with E35). Initial results showed a gradual increase in cF.IX levels to a maximum of 104 ng/mL (Figure 8A). For comparison, a similar dose of AAV-2–F.IX (1.3 × 1011 vg/kg) in dog B45 resulted in circulating levels of only 2 ng/mL to 3 ng/mL, so that at low doses, AAV-1 appeared approximately 20-fold better than AAV-2 (Table 4). The combination of dose reduction and transient immunosuppression initially appeared successful, since there was no evidence of inhibitory antibody formation. Three weeks after cyclophosphamide was stopped, the animal developed a bleed in the hindlimb and was treated with canine plasma. After the plasma transfusion, an inhibitor was detected, initially at a titer of 2 BU and rising to approximately 4 BU. The inhibitor titer subsequently fell but rose again after a second plasma transfusion (Figure 8B-D). Timecourse analysis of the appearance of antibodies to F.IX shows that both IgG1 and IgG2 had appeared before the first plasma transfusion (Figure 8D). Of note, both dogs were never exposed to plasma-derived cF.IX prior to AAV vector injection.
Coagulation testing and antibody studies in dog E57. (A) Canine F.IX antigen levels as a function of time after injection. Arrows denote time points at which cyclophosphamide was administered; asterisks denote infusions of canine plasma. F.IX level peaked 55 days after injection and fell rapidly after cyclophosphamide was discontinued. (B) WBCT as a function of time after injection. The WBCT fell to the normal range about 1 week after vector injection. Levels remained reduced into or near the normal range until approximately day 70 when they were again prolonged. (C) Bethesda titer as a function of time after injection. Bethesda titer was undetectable until day 77. The titer was initially low but subsequently rose to approximately 8 BU. (D) Anti–F.IX antibody subclasses as a function of time. Both subclasses remain at baseline through the duration of cyclophosphamide therapy, but IgG1 rises rapidly, and IgG2 more slowly, after cyclophosphamide discontinuation.
Coagulation testing and antibody studies in dog E57. (A) Canine F.IX antigen levels as a function of time after injection. Arrows denote time points at which cyclophosphamide was administered; asterisks denote infusions of canine plasma. F.IX level peaked 55 days after injection and fell rapidly after cyclophosphamide was discontinued. (B) WBCT as a function of time after injection. The WBCT fell to the normal range about 1 week after vector injection. Levels remained reduced into or near the normal range until approximately day 70 when they were again prolonged. (C) Bethesda titer as a function of time after injection. Bethesda titer was undetectable until day 77. The titer was initially low but subsequently rose to approximately 8 BU. (D) Anti–F.IX antibody subclasses as a function of time. Both subclasses remain at baseline through the duration of cyclophosphamide therapy, but IgG1 rises rapidly, and IgG2 more slowly, after cyclophosphamide discontinuation.
Discussion
AAV-2–mediated gene transfer to murine skeletal muscle resulted in circulating F.IX levels of 250 ng/mL to 350 ng/mL at doses of approximately 1 × 1013 vg/kg13; a similar study in hemophilic dogs yielded circulating levels of 70 ng/mL at a comparable dose.10 A phase 1 safety study of intramuscular injection of an AAV-2 vector in humans with hemophilia B showed an excellent safety profile and strong evidence for gene transfer and expression on muscle biopsy2 but circulating levels of F.IX that were generally subtherapeutic (< 1%) at the doses tested (up to ∼2 × 1012 vg/kg). Studies in hemophilic dogs had shown that the risk of inhibitory antibody formation rose with increasing doses of vector per site, so that dose escalation in the human study required ever-increasing numbers of injection sites. To scale up 3- to 5-fold in dose from 2 × 1012 vg/kg (to reach doses that were therapeutic in mice and dogs) would have required about 300 injection sites, which did not seem clinically feasible. Since published studies4,6 documented higher levels of transgene expression using an AAV-1 vector in murine skeletal muscle, we sought to determine whether these results could be extended to dogs with hemophilia B. We recognized at the outset that immune responses to the transgene product were likely to be a major consideration and we therefore assessed these responses as a part of our analysis.
Our study shows that, in both small and large animal models, AAV-1 results in higher levels of transgene expression compared with AAV-2 vectors. Although experiments in tissue culture documented that F.IX expression was generally higher with AAV-2 vectors compared with AAV-1, the opposite result was seen in immunodeficient mice, where F.IX levels were 10- to 20-fold higher with AAV-1 and AAV-6 vectors injected intramuscularly. This failure of experiments in tissue culture to predict relative levels of expression in vivo has been documented for a number of viral vectors, and the observation underscores the fact that tissue-culture data must be interpreted with caution in this setting. Our data on the relative efficacy of AAV-1–compared with AAV-2–derived vectors in mice addresses a point of controversy in the field. An initial report by Xiao et al6 documented 2- to 10-fold higher circulating levels of transgene products (erythropoietin and α1-antitrypsin) in mice injected with 2 × 1012 vg/kg of AAV-1 versus AAV-2 vectors, whereas a subsequent report by Chao et al4 reported 500- to 1000-fold higher levels of cF.IX in mice injected with AAV-1 versus AAV-2 at doses of approximately 1 × 1013 vg/kg. Our results were much closer to those of Xiao et al with a 10- to 20-fold increase in circulating levels of hF.IX in mice over a range of doses from 2 × 1011 vg/kg to 4 × 1012 vg/kg. Our results are also similar across species, since we observed a comparable dose advantage for AAV-1 in the hemophilic dog model. This is best appreciated by comparing doses and circulating levels in E57 to results in animals injected with AAV-2 cF.IX vectors that used the same gene expression cassette and differed only in the capsid gene sequences in the AAV helper plasmid. Thus, compared with previously published results in animal D32,11 injected with 5.6 × 1012 vg/kg AAV-2 resulting in circulating levels of 40 ng/mL, AAV-1 was 58-fold better, whereas compared with dog B85,10 which received 8.5 × 1012 vg/kg and achieved circulating levels of 70 ng/mL, AAV-1 was 53-fold better (Table 4). One can argue that the comparison to D32 is more valid, since both D32 and E57 were treated with identical regimens of cyclophosphamide, but the similarity of results with either D32 or B85 suggests that the effect of the cyclophosphamide at the doses administered had a minimal effect on transduction levels. (Note that comparisons to the dog that received a higher dose of AAV-1 [E35] are not possible, since this animal developed an inhibitory antibody before reaching a plateau level of F.IX).
The superior performance of AAV-1 compared with AAV-2 in skeletal muscle is at least partly due to improved transduction as reflected by a higher gene copy number and higher numbers of cells transduced. Thus, analysis of mice injected with equivalent doses of AAV-1 and AAV-2 (dose of ∼4 × 1012 vg/kg, 2 × 1010 vg per site) showed that both gene copy number and total number of cells transduced were approximately 2- to 3-fold higher for AAV-1. If the vector gains entry to twice as many cells, then the increase in gene copy number is predictable assuming similar efficiency of conversion to a double-stranded form and stabilization of vector sequences. Yet a higher number of transduced cells cannot alone account for the improved performance of AAV-1, since at these doses circulating levels of F.IX are 20-fold higher in the AAV-1–injected mice. This suggests that there is a higher level of expression per transduced cell, or that there is production of F.IX in many more cells at levels sufficiently low that they are not readily identified as positive on immunofluoresence staining. More data are required to resolve this point, since it is not clear what fraction of vector DNA detected on Southern blot is transcriptionally active.
The major complication of the current therapy for hemophilia (intravenous infusion of clotting factors) is development of inhibitory antibodies. A wealth of data in hemophilic mouse and dog models demonstrates that inhibitors also arise in the context of gene transfer for hemophilia.10,11,21 Some of the factors that influence the risk of inhibitor formation in the setting of gene transfer include, in addition to the underlying mutation, the vector itself,19 the route of administration, the presence or absence of a tissue-specific promoter,22 and the dose. A complex interplay among these factors may control the eventual outcome, so that, for example, an innate immune response to a virally derived vector may promote an adaptive immune response to the transgene product.
Our previous studies have shown that, in the setting of an F.IX-expressing AAV-2 vector delivered to skeletal muscle in hemophilic dogs, as the dose of vector increases the likelihood of inhibitor formation also increases, with the dose per site being one of the strongest predictors of inhibitor formation.11 One of the complicating factors in analysis of the immune response in gene transfer is the multicomponent nature of viral vectors. Thus, as the dose per site is raised, the host is responding to at least 3 factors: (1) an increase in the total number of viral particles; (2) an increase in the total amount of transgene product synthesized locally; and (3) an increase in the total amount of any contaminant in the preparation. The higher efficacy of AAV-1 allowed us to distinguish among these possibilities experimentally, since one can produce similar amounts of circulating F.IX using a much lower dose of vector. If one compares dog D32 (injected with AAV-2) with E57 (injected with AAV-1), E57 received a 30-fold lower dose per site and a 20-fold lower dose per kilogram. Thus the total number of vector particles and the total amount of any contaminant were considerably reduced in E57, yet E57 developed a long-lasting inhibitory antibody, whereas D32 did not. (Note that both dogs were treated with identical transient immunosuppressive regimens at the time of vector injection.) Similarly, if E57 is compared with D31, a dog that also received AAV-2, the dose per site was again 30-fold lower in E57 and the dose per kilogram was 30- to 40-fold lower, yet E57 developed a long-lasting inhibitor whereas D31 did not. These data suggest that it is the amount of transgene product (antigen) synthesized locally that is the major factor promoting development of a harmful immune response. This conclusion is consistent with a body of data in the vaccine literature that correlates amount of antigen delivered to an intramuscular site with strength of immune response.23
The experiments in immunocompetent hemophilic mice are of particular interest. These mice were injected with AAV-1 at doses ranging from 6.5 × 1011 vg/kg to 1.6 × 1013 vg/kg. At a dose of 6.5 × 1011 vg/kg, mice developed antibodies to F.IX that persisted for the duration of the experiment. At higher doses, mice initially developed inhibitory antibodies, but these disappeared over time, yielding very high plateau circulating levels of F.IX, in the range of 200 ng/mL to 3000 ng/mL. These findings are consistent with a model proposed by Zinkernagel,23 in which antigens that enter the secondary lymphoid organs in large amounts for a prolonged period induce and then delete reactive T cells. In this model, the localization, dose, and duration of antigen persistence are the crucial determinants of whether immune reactivity develops, with low doses of antigen being ignored immunologically, very high doses resulting in induction and then deletion of reactive T cells, and doses between these inducing an effective immune response. Zinkernagel's model provides a framework for interpreting the experiments presented here as well as our earlier experiments in which AAV-2 was used to effect gene transfer into skeletal muscle in hemophilic dogs and humans. Thus, AAV-2–mediated gene transfer to skeletal muscle failed to trigger an immune response to the transgene product at low doses (as seen in the first group of dogs injected,10 and in the human subjects injected2 ), whereas injection of hemophilic mice or dogs with AAV-1, resulting in higher circulating levels of F.IX (antigen), allows development of an immune response (mice injected at 6.5 × 1011 vg/kg; dogs injected at 2.4 × 1011 vg/kg-10 × 1011 vg/kg). At still higher doses (as seen in the hemophilic mice reported here), resulting in very high antigen levels, an immune response is induced and then lost, presumably because the reactive T cells have been deleted. Although the model is consistent with the data in hand, additional experimental data will be required to confirm or refute this hypothesis.
In summary, AAV-1 results in superior transduction of skeletal muscle compared with AAV-2, in dogs as well as in mice. In this initial report of results with AAV-1 in large animals, we noted a similar fold increase in circulating levels of the transgene product, F.IX, in mice and in hemophilic dogs. This increase, on the order of 10- to 50-fold, is consistent with the first report in mice,6 but is considerably less than a later report in mice.4 For AAV-2, experiments in a wide range of animals, including mice, rats, rabbits, dogs, nonhuman primates, and humans showed that the vector had similar transduction characteristics in skeletal muscle regardless of species. More work will be required to determine whether the same is true for AAV-1, but these initial results suggest that this vector too may have similar characteristics in a variety of species. The higher efficacy of AAV-1 is associated with an increase in gene copy number and a higher number of transduced cells, although these factors alone are not adequate to account for the higher levels of expression. Finally, the data presented here in immunocompetent hemophilic mice and in hemophilic dogs demonstrate that gene delivery by AAV-1 to skeletal muscle can evoke an immune response to the transgene product. The data in mice, where the inhibitory antibodies to F.IX ultimately disappeared, suggest that higher doses of vector, resulting in higher circulating levels of F.IX, may eventually tolerize the animal to the transgene product, but more extensive experimental analysis will be required to determine if this observation in mice can be extended to larger, outbred models of hemophilia.
Prepublished online as Blood First Edition Paper, September 11, 2003; DOI 10.1182/blood-2003-05-1446.
Supported by National Institutes of Health grants KO1 DK60580-01 (V.R.A.), R24 HL63098 (T.C.N.) and PO1 HL64190 (K.A.H.), Hemophilia of Georgia, and the Howard Hughes Medical Institute.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dazhou Ni and Jianhua Liu for excellent technical assistance. The authors acknowledge Dr Terri Heiman-Patterson and Dr Jeffrey Deitch (Hahnemann University Hospital) for providing human myoblast cells in culture.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal