Abstract
Gaucher disease is characterized by storage of glucosylceramide in lysosomes of tissue macrophages as the result of an autosomal recessively inherited deficiency in glucocerebrosidase. Progressive accumulation of these glycolipid-laden Gaucher cells causes a variety of debilitating symptoms. The disease can be effectively treated by costly intravenous infusions with recombinant glucocerebrosidase. Chitotriosidase is massively secreted by Gaucher cells and its plasma levels are used to monitor efficacy of enzyme therapy. Broad-scale application is hampered by the common genetic defect in this surrogate marker. We report that in plasma of symptomatic patients with Gaucher disease the chemokine CCL18 is on average 29-fold elevated, without overlap between patient and control values (median control plasma level is 33 ng/mL, range, 10-72 ng/mL; median Gaucher plasma level is 948 ng/mL, range, 237-2285 ng/mL). Plasma CCL18 concentrations decrease during therapy, comparably to chitotriosidase levels. Immunohistochemistry demonstrates that Gaucher cells are the prominent source of CCL18. Plasma CCL18 levels can serve as alternative surrogate marker for storage cells in patients with Gaucher disease and monitoring of plasma CCL18 levels proves to be useful in determination of therapeutic efficacy, especially in patients who are deficient in chitotriosidase activity. The potential physiologic consequences of chronically elevated CCL18 in patients with Gaucher disease are discussed.
Introduction
Gaucher disease is one of the most prevalent lysosomal storage disorders in humans. The disease is due to an autosomal recessively inherited deficiency in lysosomal glucocerebrosidase activity (EC 3.2.1.45), leading to accumulation of its substrate glucosylceramide in the lysosomes of macrophages.1 Progressive accumulation of these glycolipid-laden macrophages (Gaucher cells) in various locations in the body causes a spectrum of clinical symptoms. Three different clinical phenotypes are recognized, based on the age of onset of neurologic signs. The clinical manifestations of the most common nonneuronopathic variant (type I Gaucher disease) includes anemia and thrombocytopenia, hepatosplenomegaly, and skeletal deterioration.1 A frequent sign is the development of monoclonal or oligoclonal gammopathies.2
Noninvasive monitoring of Gaucher disease by determination of plasma factors that are exclusively secreted by Gaucher cells is of great importance for various reasons. The glucocerebrosidase genotype of individuals is not always predictive for the phenotypic expression of Gaucher disease.3 Even among monozygotic twins with abnormal glucocerebrosidase genotypes, a remarkable variability in disease manifestations can occur.4 The availability of an effective but very costly therapy has also urged the identification of surrogate markers for Gaucher cells that can guide decisions on initiation of therapy and dosing regimens. The identification of factors secreted by Gaucher cells is also of fundamental interest because it may lead to better understanding of the unique pathophysiology of the disorder.
A number of years ago we discovered that Gaucher cells massively secrete a hitherto unknown chitinase. The activity of the enzyme, named chitotriosidase, in plasma of symptomatic patients with Gaucher disease is elevated on average several hundred–fold. For example, in the initial chitotriosidase study, plasma activity was found to be elevated on average 641-fold (median control plasma, 20 nmol/mL/h; range, 4-76 nmol/mL/h; median Gaucher plasma, 12 824 nmol/mL/h; range, 3122-65 349 nmol/mL/h).5 Plasma chitotriosidase has proven to be a useful surrogate marker for Gaucher disease manifestations and is used for diagnosis, early determination of onset of disease, and monitoring of therapeutic efficacy.5-8 Plasma chitotriosidase levels do not reflect one particular clinical symptom, but rather are a reflection of the total body burden of Gaucher cells.9 The use of plasma chitotriosidase as a Gaucher cell marker is hampered by the fact that about 5% to 6% of the population, including those with Gaucher disease, are deficient in chitotriosidase activity due to a 24–base pair (bp) duplication in the chitotriosidase gene. Obviously these individuals cannot be monitored by the measurement of plasma chitotriosidase activity.5,10
To identify novel factors derived from Gaucher cells, we analyzed plasma samples of patients with Gaucher disease before therapeutic intervention and compared them with control plasma using surface-enhanced laser desorption/ionization (SELDI) mass spectrometry (MS). In plasma of a symptomatic patient a peptide of 7856 Da was found to be markedly increased in relation to Gaucher disease manifestation. Subtractive hybridization studies previously revealed that RNA encoding for a protein with identical mass is up-regulated in a Gaucher spleen.11 This protein, named pulmonary and activation-regulated chemokine (PARC, systematic name CCL18), is a member of the C-C chemokine family.12,13 Chemokines are a large family of low-molecular-weight (7-12 kDa) proteins. They are characterized by the presence of 4 conserved cysteines involved in forming essential disulfide bonds. CXC and CC chemokines are distinguished according to the position of the first 2 cysteines.14,15 Their main biologic role is found in mediating chemotaxis of leukocytes, a process mediated by G protein–coupled receptors of which most recognize more than 1 chemokine.14,15
We here report on CCL18 plasma levels in symptomatic patients with adult type I Gaucher disease. To test its value as surrogate disease marker, plasma CCL18 levels were analyzed in relation to parameters of disease severity, other surrogate markers, and the effect of therapy. The implications of chronically increased CCL18 for the pathophysiology of Gaucher disease are discussed.
Patients, materials, and methods
Patients
All patients with Gaucher disease (type I) studied (29 males and 26 females; 12-67 years old, at the initiation of therapy) were known to us either by contact with the Netherlands Gaucher Society or by referral to the Academic Medical Center. Of the 55 type I patients, 47 received enzyme replacement therapy (alglucerase, imiglucerase, [individualized dosing], Genzyme, Cambridge, MA), 2 patients received substrate reduction therapy (chronic oral administration of an imunosugar inhibitor of glucosylceramide synthesis (N-butyldeoxynojirimycin, Oxford Glycosciences, Abingdon, United Kingdom), and 6 patients were not treated. The controls consisted of 16 male and 20 female healthy volunteers. All adult type I Gaucher disease patients who were referred to the Academic Medical Center for the initiation of enzyme therapy were enrolled in a nationwide study that aims to monitor efficacy of therapy. Serum samples for the monitoring of disease parameters were obtained throughout this study. Approval was obtained from the Ethics Committee. Informed consent was provided according to the Declaration of Helsinki.
Assessment of disease severity
To assess the clinical severity of the Gaucher disease, the severity scoring index (SSI) was used. The score, accounting for a variety of clinical symptoms, is calculated according to Zimran et al.16 Several indicators of disease severity were assessed separately. They included hemoglobin level, platelet count, spleen size, liver size, and bone marrow fat fraction. Patients were also divided in 2 groups according to presence or absence of the spleen and presence or absence of skeletal symptoms.
SELDI TOF MS
Plasma samples were surveyed for basic proteins using surface-enhanced laser desorption/ionization (SELDI) time of flight (TOF) mass spectrometry (MS). Plasma samples were exposed to a weak cation exchange surface and bound proteins were subsequently analyzed on a PBSII reader (Ciphergen Biosystems, Fremont, CA). Samples (10 μL) were denatured in 9 M urea, 2% CHAPS (3[(3-cholamidopropyl)dimethylammonio]-propane-sulfonic acid), and 1% dithiothreitol (DTT) at room temperature (RT) for 60 minutes. An aliquot (10 μL) of this solution was mixed with 90 μL binding buffer (50 mM Tris [tris(hydroxymethyl)aminomethane] HCl, pH 8.0, 0.1% Triton X-100) and added to a WCX ProteinChip array (Ciphergen Biosystems). After 40 minutes at RT the ProteinChips were washed with binding buffer (2 times, 5 minutes) and 50 mM Tris HCl, pH 8 (2 times, 5 minutes). After a quick rinse with distilled water the ProteinChips were dried and matrix was added (sinapinic acid). ProteinChip arrays were read with laser intensity 200 and mass deflector set at 500 Da.
Immunocapture experiments were performed using a PS20 ProteinChip array precoated with anti-CCL18 or anti–tumor necrosis α (anti–TNF-α) monoclonal antibodies. Samples (10 μL) were incubated with 90 μL binding buffer and allowed to bind for 2 hours. After washing (as before) ProteinChip arrays were dried, matrix was applied, and arrays were read with laser intensity 180 and mass deflector set at 500 Da.
ELISA
Plasma CCL18 levels were measured by a sandwich enzyme-linked immunosorbent assay (ELISA) using a commercially available CytoSet (Biosource International, Camarillo, CA), consisting of a capture antibody, a biotinylated detection antibody, recombinant CCL18/PARC standard, and streptavidin-horseradish peroxidase (HRP) conjugate. Assay conditions were exactly as described by the manufacturer.
Enzyme activity assays
Serum angiotensin-converting enzyme (ACE) activity was measured using hippuryl-l-histidyl-l-leucine as substrate, as described.17 Activity of serum β-hexosaminidase was measured using 4-methylumbelliferyl-N-acetylglucosamine (Sigma, St Louis, MO) as substrate in citrate/phosphate buffer (0.1/0.2 M) at pH 4.0. The standard enzyme activity assay for chitotriosidase with 4 MU-chitotriose (4-methylumbelliferyl β-D-N,N′,N″-triacetylchitotriose; Sigma) as substrate was performed at pH 5.2, as previously described.5
Bone marrow fat fraction
Dixon quantitative chemical shift imaging (QCSI) was used to assess bone marrow fat fraction of the axial skeleton as described in detail by Maas et al18 and Hollak et al.19 In short, bone marrow fat fraction is used as a reflection of severity of bone marrow involvement because progressive infiltration of the bone marrow with Gaucher cells is associated with disappearance of normal adipocytes.
RNA isolation and Northern blot
Total spleen RNA was isolated using the RNAzol B (Biosolve, Barneveld, The Netherlands) RNA isolation kit according to the manufacturer's instructions. For Northern blot analysis, 15-μg samples of total RNA were run in 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2ethanesulfonic acid), 6% formaldehyde-agarose gels, transferred to Hybond N nylon membranes (Amersham, Buckinghamshire, United Kingdom) by the capillary method, and immobilized by UV cross-linking. The following probes were used: full-length CCL18 cDNA and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an RNA control. The probes were radiolabeled with 32P using the random priming method.20 Hybridization conditions were exactly as described before.10
Immunohistochemistry
Immunohistochemistry was performed on frozen sections of Gaucher spleen to detect expression patterns of CCL18. The methodology of immunocytochemical procedures used here has been reviewed in detail previously.21 In brief, frozen sections of 6 μm were cut and thaw-mounted on glass slides. Slides were kept overnight at RT in humidified atmosphere. After air-drying the slides for 1 hour, slides were fixed in fresh acetone containing 0.02% (vol/vol) H2O2. Slides were then air-dried for 10 minutes, washed with phosphate-buffered saline (PBS), and incubated with optimally diluted anti–CCL-18 antibody (AB60, mouse monoclonal; R&D Systems, Minneapolis, MN) overnight at 4°C in a humidified atmosphere. Incubations with secondary rabbit antimouse-Ig-biotin (Dako, Glostrup, Denmark) and tertiary HRP-labeled avidin-biotin-complex (ABC/HRP; Dako) were performed for 1 hour at RT. Between incubation steps slides were washed twice with PBS. HRP activity was revealed by incubation for 10 minutes at RT with 3-amino-9-ethyl-carbazole (AEC; Sigma), leading to a bright red precipitate. After washing, sections were counterstained with hematoxylin and embedded with glycerol-gelatin. Primary antibody reagent omission control staining was performed.
Statistical analysis
Results are given as median and range. The data were analyzed using the Mann-Whitney U test. Correlations were tested by the rank correlation test (Spearman coefficient, ρ). P values less than .05 were considered statistically significant.
Results
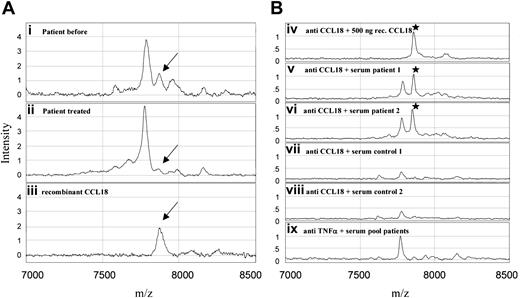
In an attempt to identify novel factors present in plasma of patients with Gaucher disease, we analyzed plasma samples of Gaucher patients before and after a few years of therapeutic intervention using SELDI MS. For this purpose Gaucher plasma was applied on a weak cation exchanger ProteinChip at different buffer conditions. A peptide of 7856 Da was virtually absent in control samples but prominent in a sample of an untreated symptomatic Gaucher patient (Figure 1A). The molecular mass and the basic isoelectric point of the peptide are remarkably similar to those of a member of the human C-C chemokine family named pulmonary and activation-regulated chemokine (PARC, systematic name CCL18), of which the mRNA was previously noted to be up-regulated in the spleen of a patient with Gaucher disease.11 Immunocapture experiments revealed that in serum of symptomatic patients elevated levels of CCL18 occur (Figure 1B).
SELDI-TOF mass spectra of profiling and antibody capture experiments. (A) Weak cation exchange (WCX2) ProteinChip array. Plasma samples of a Gaucher patient before (i) and after (ii) treatment and a control experiment with recombinant CCL18 in 0.5% (wt/vol) bovine serum albumin (iii). Mass range 7000 to 8500 m/z. (B) PS20 ProteinChip array coated with monoclonal antibodies. Anti-CCL18 (iv-viii) and (as a control) anti–TNF-α (ix). Recombinant CCL18 (iv), sera of Gaucher patients (v-vi), control sera (vii-viii) and a pool of patient samples (ix) were exposed to the antibody-coated arrays. Mass range 7000 to 8500 m/z.
SELDI-TOF mass spectra of profiling and antibody capture experiments. (A) Weak cation exchange (WCX2) ProteinChip array. Plasma samples of a Gaucher patient before (i) and after (ii) treatment and a control experiment with recombinant CCL18 in 0.5% (wt/vol) bovine serum albumin (iii). Mass range 7000 to 8500 m/z. (B) PS20 ProteinChip array coated with monoclonal antibodies. Anti-CCL18 (iv-viii) and (as a control) anti–TNF-α (ix). Recombinant CCL18 (iv), sera of Gaucher patients (v-vi), control sera (vii-viii) and a pool of patient samples (ix) were exposed to the antibody-coated arrays. Mass range 7000 to 8500 m/z.
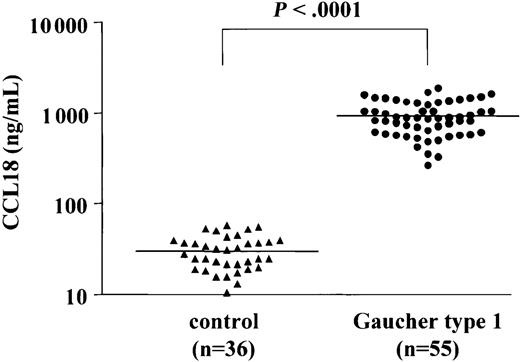
To accurately quantify the levels of CCL18/PARC in plasma of Gaucher patients a sandwich ELISA was used. Figure 2 shows the CCL18 levels in plasma samples from 36 controls and from 55 symptomatic Gaucher patients (prior to initiation of therapy). The median plasma CCL18 level in controls is 33 ng/mL (range, 10-72 ng/mL), whereas the median level in the patients' samples is 948 ng/mL (range, 237-2285 ng/mL). The levels of CCL18 in plasma of symptomatic Gaucher patients are thus on average 29-fold elevated. Importantly, no overlap is seen between values in symptomatic patients and controls. All patient plasma CCL18 values are considerably higher than the control mean ± 3SD (99.7% CI).
Plasma CCL18 levels in controls and patients with Gaucher disease. Plasma CCL18 levels in controls (n = 36) and Gaucher patients (n = 55). Chemokine concentrations were determined as described in “Patients, materials, and methods.”
Plasma CCL18 levels in controls and patients with Gaucher disease. Plasma CCL18 levels in controls (n = 36) and Gaucher patients (n = 55). Chemokine concentrations were determined as described in “Patients, materials, and methods.”
The relationship between plasma CCL18 levels and parameters of disease was examined. No strict correlation was noted with hematologic abnormalities (hemoglobin levels and platelet count) or degree of splenomegaly or hepatomegaly (data not shown). Moreover, we did not observe any difference between plasma CCL18 levels in patients with enlarged spleens or without spleens and no relationship between chemokine levels and occurrence of skeletal disease (data not shown). The bone marrow fat fraction, as assessed with QCSI,18 did not correlate with plasma CCL18 levels either. In patients with type I Gaucher disease with a mild degree of disease manifestation (individuals with an SSI < 5),16 plasma CCL18 levels tend to be lower than in more severely affected individuals (SSI ≥ 5), with a of median 722 ng/mL (range, 405-1160 ng/mL) versus median of 974 ng/mL (range, 303-2285 ng/mL), respectively (not statistically significant). Similar relationships between plasma chitotriosidase activity and other Gaucher disease parameters were demonstrated previously.9 Analysis of the present cohort of patients with type I Gaucher disease showed that plasma chitotriosidase, β-hexosaminidase, and ACE levels also tend to be lower in mildly affected individuals compared to severely affected patients; however, these were also not statistically significant (plasma chitotriosidase: [SSI < 5] median, 8085 nmol/mL/h [range, 4939-13358 nmol/mL/h] versus [SSI ≥ 5] median, 18 773 nmol/mL/h [range, 2393-80 074 nmol/mL/h]; plasma β-hexosaminidase: [SSI < 5] median, 1477 nmol/mL/h [range, 756-2678 nmoL/mL/h] versus [SSI ≥ 5] median, 2580 nmoL/mL/h [range, 1072-7486 nmol/mL/h]; plasma ACE: [SSI < 5] median, 140 U/L [range, 100-148 U/L] versus [SSI ≥ 5] median, 202 U/L [range, 116-324 U/L]). In the case of tartrate-resistant acid phosphatase (TRAP) a more limited correlation with disease severity was observed (plasma TRAP: [SSI < 5] median, 1053 nmol/mL/h [range, 768-2307 nmol/mL/h] versus [SSI ≥ 5] median, 2418 nmol/mL/h [range, 616-14 539 nmol/mL/h]). Plasma CCL18 values were weakly correlated to chitotriosidase, TRAP, and ACE levels: CCL18 versus chitotriosidase: n = 51, Spearman ρ 0.542, P < .0001; CCL18 versus chitotriosidase wild-type: n = 38, Spearman ρ 0.571, P = .0002; CCL18 versus TRAP: n = 36, Spearman ρ 0.558, P = .0004; CCL18 versus ACE: n = 29, Spearman ρ 0.493, P = .007. The relation with β-hexosaminidase levels was even less strict: n = 55, Spearman ρ 0.391, P = .003. The 4 chitotriosidase-deficient Gaucher patients showed high plasma CCL18 values (863, 1122, 951, 329 ng/mL), consistent with their severe disease manifestations.
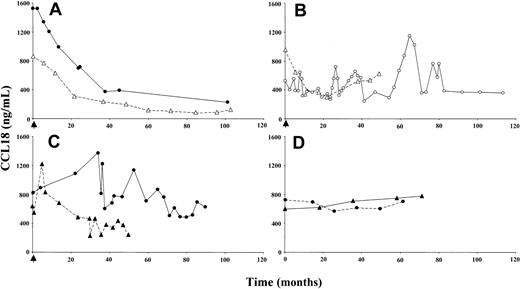
The effect of enzyme replacement therapy on plasma CCL18 in Gaucher patients was examined. Figure 3A shows the marked reduction in plasma CCL18 levels in 2 representative patients who responded clinically well to enzyme replacement therapy. As observed for chitotriosidase, there was an initial prominent reduction in plasma CCL18 concentration, followed by a slowly progressing decrease over the successive years. Patients responding poorly to this treatment showed no sustained reduction in plasma CCL18 concentration (Figure 3B). A decline in plasma CCL18 level was also observed for patients treated with substrate reduction therapy (Figure 3C). In sharp contrast, patients who did not receive enzyme replacement therapy showed a gradual increase of CCL18 plasma levels in time (Figure 3D).
Effect of therapeutic interventions on plasma CCL18 levels. (A) Plasma CCL18 levels in 2 individuals responding well to enzyme replacement therapy. (B) Plasma CCL18 levels in 2 individuals responding clinically poorly to enzyme replacement therapy. (C) Plasma CCL18 levels in 2 individuals on substrate deprivation therapy. (D) Plasma CCL18 levels in 2 untreated Gaucher patients. Closed symbols represent chitotriosidase wild-type individuals; open symbols, chitotriosidase-deficient individuals. The arrow indicates the initiation of therapy.
Effect of therapeutic interventions on plasma CCL18 levels. (A) Plasma CCL18 levels in 2 individuals responding well to enzyme replacement therapy. (B) Plasma CCL18 levels in 2 individuals responding clinically poorly to enzyme replacement therapy. (C) Plasma CCL18 levels in 2 individuals on substrate deprivation therapy. (D) Plasma CCL18 levels in 2 untreated Gaucher patients. Closed symbols represent chitotriosidase wild-type individuals; open symbols, chitotriosidase-deficient individuals. The arrow indicates the initiation of therapy.
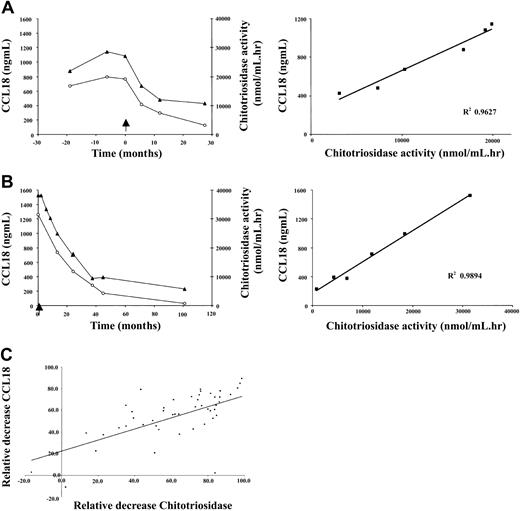
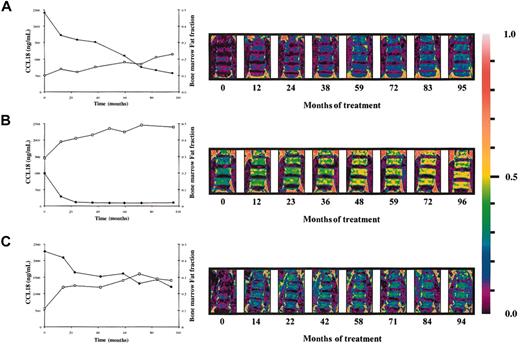
A weak correlation exists between the plasma levels of chitotriosidase and CCL18 in naive Gaucher patients. There is striking similarity in corrections in both parameters in individual Gaucher patients during therapy. Figure 4A-B shows that the reductions in plasma CCL18 and chitotriosidase levels on enzyme replacement therapy are completely proportional. The relative corrections in both markers correlate significantly as shown in Figure 4C (n = 47 individual Gaucher patients, Spearman ρ 0.706, P < .0001). The relative reduction in excess plasma ACE and excess plasma β-hexosaminidase levels during therapy also correlated to that in CCL18 (ACE: n = 40 individual Gaucher patients, Spearman ρ 0.579, P < .0001; plasma β-hexosaminidase: n = 39 individual Gaucher patients, Spearman ρ 0.6218, P < .0001). Interestingly, the relative reduction in plasma CCL18 level during therapy also closely correlated with the corrections in the fat fraction of the lumbar spine bone marrow (Figure 5). All the findings suggest that reductions in CCL18 reflect corrections in the presence of Gaucher cells.
Relationship between decrease in CCL18 plasma levels and chitotriosidase plasma activity. (A-B) Left panels show decrease in plasma CCL18 (▴) levels and plasma chitotriosidase activity (○) in individual patients on enzyme replacement therapy. Right panels show the relationship between the fractional changes in markers. (C) Relationship between fractional changes in markers following enzyme replacement therapy for at least 1 year. Excess activity at t = 0 was set at 100%. Depicted are results for 47 individual patients with Gaucher disease.
Relationship between decrease in CCL18 plasma levels and chitotriosidase plasma activity. (A-B) Left panels show decrease in plasma CCL18 (▴) levels and plasma chitotriosidase activity (○) in individual patients on enzyme replacement therapy. Right panels show the relationship between the fractional changes in markers. (C) Relationship between fractional changes in markers following enzyme replacement therapy for at least 1 year. Excess activity at t = 0 was set at 100%. Depicted are results for 47 individual patients with Gaucher disease.
Relationship between decrease in CCL18 plasma levels and lumbar spine bone marrow fat fraction. (A-C) Inverse relationship between the decrease in plasma CCL18 levels and increase in lumbar spine marrow fat fraction on enzyme replacement therapy. Patient A: Spearman ρ –0.952; P = .0011; patient B: Spearman ρ –0.802; P = .0218; patient C: Spearman ρ –0.815; P = .0108 (•, CCL18; □, bone marrow fat fraction). Right hand side shows changes in the bone marrow fat fraction of the lumbar spine during enzyme replacement therapy as visualized by QCSI.
Relationship between decrease in CCL18 plasma levels and lumbar spine bone marrow fat fraction. (A-C) Inverse relationship between the decrease in plasma CCL18 levels and increase in lumbar spine marrow fat fraction on enzyme replacement therapy. Patient A: Spearman ρ –0.952; P = .0011; patient B: Spearman ρ –0.802; P = .0218; patient C: Spearman ρ –0.815; P = .0108 (•, CCL18; □, bone marrow fat fraction). Right hand side shows changes in the bone marrow fat fraction of the lumbar spine during enzyme replacement therapy as visualized by QCSI.
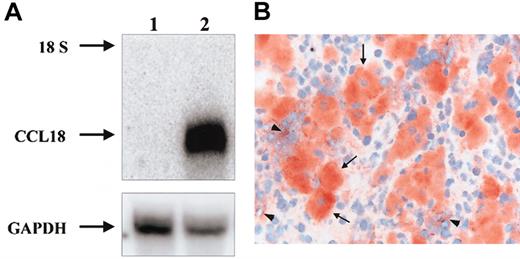
To investigate whether CCL18 is produced directly by Gaucher cells, the presence of CCL18 mRNA in Gaucher spleen was studied by Northern blot analysis. As can be seen in Figure 6A, CCL18 mRNA is highly expressed in the patient's spleen and almost absent in control tissue. Next, immunohistochemistry on sections from spleens of 2 Gaucher patients unequivocally revealed the specific expression of CCL18 protein in all Gaucher cells (Figure 6B-C). Expression of CCL18 was virtually absent in other cells in these spleen sections. Similar labeling was observed for chitotriosidase (not shown).
Expression of CCL18 in Gaucher spleen. (A) Detection of CCL18 mRNA in Gaucher spleen by Northern blot analysis. Control and Gaucher spleen total RNA was analyzed by Northern blotting as described in “Patients, materials, and methods.” Lane 1, control spleen; lane 2, Gaucher spleen. The probes used were full-length CCL18 cDNA and GAPDH as an RNA control. The 18S ribosomal band is indicated. (B) Detection of CCL18 protein by immunohistochemistry in Gaucher spleen. Clustered large swollen cells are Gaucher cells and label massively for CCL18 protein (arrows). Some surrounding spleen cells also show some labeling (arrowheads). Original magnification × 400.
Expression of CCL18 in Gaucher spleen. (A) Detection of CCL18 mRNA in Gaucher spleen by Northern blot analysis. Control and Gaucher spleen total RNA was analyzed by Northern blotting as described in “Patients, materials, and methods.” Lane 1, control spleen; lane 2, Gaucher spleen. The probes used were full-length CCL18 cDNA and GAPDH as an RNA control. The 18S ribosomal band is indicated. (B) Detection of CCL18 protein by immunohistochemistry in Gaucher spleen. Clustered large swollen cells are Gaucher cells and label massively for CCL18 protein (arrows). Some surrounding spleen cells also show some labeling (arrowheads). Original magnification × 400.
CCL18 is supposed to be involved in initiation of an adaptive immune response and recruitment of naïve T and B cells toward antigen-presenting cells, and as such may contribute to development of antibody-producing plasma cells. We therefore investigated whether the levels of CCL18 in plasma correlate with the presence of gammopathy. No obvious difference was noted between plasma CCL18 values in patients with or without detectable monoclonal gammopathy: mean, 1305 ng/mL (range, 525-2005 ng/mL; n = 9) versus mean, 932 ng/mL (range, 101-2285 ng/mL; n = 46), respectively. However, the plasma concentration of CCL18 (1878 ng/mL) was relatively high in one Gaucher patient who had developed multiple myeloma, a malignant B-cell disorder characterized by the uncontrolled proliferation of monoclonal plasma cells in the bone marrow.
Discussion
Our study describes a marked increase in plasma levels of the CC chemokine CCL18 in symptomatic patients with Gaucher disease. This chemokine, originally identified as a T-cell chemoattractant, also attracts CD38– mantle zone B lymphocytes. It has been speculated that CCL18 plays a role in the recruitment of T and B lymphocytes toward antigen-presenting cells, a crucial step in the initiation of adaptive immune responses.12,13,22,23 The chemotactic response of T cells to CCL18 is abolished by treatment of the cells with pertussis toxin, indicating a role for heterotrimeric G protein–coupled receptors. The exact receptor, however, remains unidentified.12 There have been several reports on elevated levels of CCL18 in human disease, for example, atherosclerosis, active hepatitis C infection, hypersensitive pneumonitis, allergic contact hypersensitivity, septic as well as rheumatoid arthritis, and ovarian carcinoma.24-30 Different detection methods have been used in the previous studies; in some investigations CCL18 mRNA was detected either by reverse transcription–polymerase chain reaction analysis or in situ hybridization24,25,27,29,30 and in other studies ELISAs were used to measure CCL18 protein concentration in synovial or ascitic fluid.26,28 To our knowledge this is the first report on chronically elevated plasma levels of CCL18 in a disease condition. Interestingly, the recurrent theme of all disease states in which CCL18 is overexpressed seems to be inflammation.
Our findings suggest that plasma CCL18 can act as a reliable surrogate disease marker that is useful in the case of Gaucher disease for further confirmation of diagnosis, demonstration of disease onset, and monitoring of efficacy of therapeutic intervention. The increase in plasma CCL18 is far more pronounced than that in ACE, β-hexosaminidase, and TRAP. Very mildly affected patients still show abnormal plasma CCL18 levels in contrast to the other markers. As compared to CCL18, chitotriosidase concentration is more spectacularly increased in symptomatic Gaucher patients, provided that they do not carry the chitotriosidase gene defect. In the case of Gaucher patients who are deficient in chitotriosidase activity, monitoring of plasma CCL18 seems a good and reliable alternative that can aid in the clinical management of Gaucher patients. Immunohistochemical analysis of spleen sections from 2 Gaucher patients have indeed revealed that Gaucher cells are the prominent source of CCL18 as well as chitotriosidase. Fractional corrections in plasma CCL18 levels and of other disease markers like chitotriosidase, ACE, and β-hexosaminidase are similar in Gaucher patients following therapy. Corrections in bone marrow fat fraction, an indirect assessment of Gaucher cell infiltration of the marrow, are also paralleled by corrections in plasma CCL18 (or chitotriosidase).
The CCL18 in plasma is derived from Gaucher cells in various body locations. This is suggested by the lack of correlation between the plasma CCL18 levels and splenic volume. As is the case for plasma chitotriosidase levels, the concentration of CCL18 in the circulation does not reflect one particular clinical sign of the disease but reflects the total body burden of Gaucher cells. Plasma CCL18 (or chitotriosidase) concentrations do not strictly correlate with the SSI. Several factors may contribute to this. First, the scoring index does not reflect the actual body burden on storage cells but rather scores the incidence of pathologic events. Second, Gaucher cells in different body locations may contribute differently to the chronic CCL18 concentration in the circulation. Third, the kinetics of elimination of CCL18 from the circulation by mechanisms such as receptor-mediated uptake and excretion via the kidney may vary among individual patients.
The potential role of chronically elevated CCL18 levels in the pathophysiology of Gaucher disease is of interest. Abnormalities concerning serum immunoglobulins and other manifestations of disturbed B-cell function, like monoclonal or oligoclonal gammopathies, occur frequently in patients with Gaucher disease. However, we did not observe any correlation between the plasma levels of CCL18 and the presence of a monoclonal gammopathy. Because all symptomatic Gaucher patients show at least 10-fold increased plasma levels of CCL18, it can be envisioned that this constitutes a risk factor for the development of disturbed B-cell function with other factors influencing the eventual outcome.
It has been reported that CCL18 can function as a natural antagonist of the CCR3 chemokine receptor.31,32 CCR3 is expressed by eosinophils, Th2 cell subsets, basophils, mast cells, neural tissue, some epithelia, and CD34+ progenitor cells.33,34 Eosinophil chemotaxis induced by the most potent CCR3 agonists, such as eotaxin and macrophage chemoattractant protein 4 (MCP-4), can be inhibited by CCL18 at concentrations as low as 10 nM.31,32 The CCL18 plasma levels in symptomatic Gaucher patients exceed these inhibitory concentrations considerably (3-27 times). It seems likely that tissues rich in Gaucher cells contain even higher CCL18 concentrations. At this moment it is not clear whether Gaucher patients show abnormalities in CCR3-mediated chemotaxis of eosinophils. Moreover, it cannot be excluded that the high concentrations of CCL18 in plasma and tissues block also other chemokine receptors, explaining neutrophil chemotaxis abnormalities in Gaucher disease.35,36
Increased plasma chitotriosidase levels have been extremely useful as surrogate markers for Gaucher cell burden in Gaucher patients. The extent of elevation in plasma CCL18 level in symptomatic patients is far less compared to the elevation in chitotriosidase concentration. The application of plasma CCL18 level is therefore of particular interest for those Gaucher patients in whom chitotriosidase is lacking due to a homozygosity for the common gene defect. At present it cannot be excluded that plasma CCL18 levels can also be markedly increased due to other pathologies. It should be clear that measurement of the plasma CCL18 level for primary diagnosis of Gaucher disease should therefore not be advocated. Only in those cases in which the disease has been confirmed by demonstration of glucocerebrosidase deficiency or gene defects is the monitoring of plasma CCL18 level useful to obtain an impression of Gaucher cell burden.
In conclusion, our finding of markedly elevated plasma CCL18 levels in symptomatic Gaucher patients warrants further investigations regarding its applicability in the clinical management of Gaucher disease as well as its role in the peculiar pathophysiology of the disorder.
Prepublished online as Blood First Edition Paper, September 11, 2003; DOI 10.1182/blood-2003-05-1612.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to acknowledge Jaap Jansen (Ciphergen Biosystems) for assistance using the PBSII reader (Ciphergen Biosystems) and Astrid Huiberts for technical assistance. We also would like to thank Ans Groener and Gabor Linthorst for useful suggestions. We are very grateful to the patient members of the Dutch Gaucher Society and to all other patients with Gaucher disease for their cooperation.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal