Abstract
Autologous peripheral blood stem cell (PBSC)–supported high-dose melphalan is now considered standard therapy for myeloma, at least for younger patients. The markedly reduced toxicity of allotransplants using nonmyeloablative regimens (mini-allotransplantations) may hold promise for more widely exploiting the well-documented graft-versus-myeloma (GVM) effect. New active drugs include immunomodulatory agents, such as thalidomide and CC-5013 (Revimid; Celgene, Warren, NJ), and the proteasome inhibitor, PS 341 (Velcade; Millenium, Cambridge, MA), all of which not only target myeloma cells directly but also exert an indirect effect by suppressing growth and survival signals elaborated by the bone marrow microenvironment's interaction with myeloma cells. Among the prognostic factors evaluated, cytogenetic abnormalities (CAs), which are present in one third of patients with newly diagnosed disease, identify a particularly poor prognosis subgroup with a median survival not exceeding 2 to 3 years. By contrast, in the absence of CAs, 4-year survival rates of 80% to 90% can be obtained with tandem autotransplantations. Fundamental and clinical research should, therefore, focus on the molecular and biologic mechanisms of treatment failure in the high-risk subgroup.
Introduction
Since the last reviews on this topic,1,2 further insight has been gained into the biology and genetics of multiple myeloma.3,4 Therapeutic options have increased,5,6 and patient outcomes have improved.7,8
Regarding the fundamental biology of myeloma, the presence of somatic hypermutations of immunoglobulin variable region genes is consistent with an immortalizing event during plasma cell generation in germinal centers of lymph nodes.4 The self-renewal capacity of CD38+, CD45–, and CD19– plasma cells in the severe combined immunodeficiency-human (SCID-hu) mouse, an animal model of myeloma growth, suggests that these apparently terminally differentiated B cells retain self-renewal capacity.9
Genomic instability, a hallmark of multiple myeloma, is already present at the stage of monoclonal gammopathy of undetermined significance (MGUS),10-12 but it is not clear whether MGUS represents a prerequisite precursor stage for all cases of myeloma. At the gene expression level, MGUS cannot be distinguished from myeloma, although plasma cells of both MGUS and myeloma can be clearly distinguished from healthy bone marrow plasma cells.13 The recent demonstration of short telomeres in myeloma cells and long telomeres in healthy plasma cells may help distinguish, when applied to MGUS, subsets with different propensities for progression to overt disease.14
In the treatment arena, autologous peripheral blood stem cell (PBSC)–supported high-dose melphalan is now considered standard therapy for myeloma, at least for younger patients.8,15,16 New active drugs include immunomodulatory agents, such as thalidomide17 and CC-5013 (Revimid; Celgene, Warren, NJ),18 and the proteasome inhibitor, PS 341 (Velcade; Millenium, Cambridge, MA).19 Finally, the markedly reduced toxicity of allotransplantations using nonmyeloablative regimens (“mini-allotransplantations”) may hold promise for more widely exploiting the well-documented graft-versus-myeloma (GVM) effect.20-22
Historical review of myeloma therapy: the first 30 years
Emergence of high-dose melphalan as standard therapy
The addition of glucocorticoids to oral melphalan, introduced by Bergsagel et al in 1962,23 became the mainstay of therapy (melphalan-prednisone [MP] regimen),24 although complete remission (CR) was rare (< 5%) and median survival did not exceed 3 years. The addition of other alkylating agents, anthracyclines, and vinca alkaloids to the MP regimen did not improve patient survival.25 However, the observation of a high response rate among 9 patients with high-risk myeloma, including 3 CRs among 5 previously treated patients, after a single high dose (100-140 mg/m2) of melphalan administered intravenously suggested a steep dose-response effect for melphalan.26 Excessive morbidity and treatment-related mortality because of prolonged myelosuppression could be markedly reduced by infusion of autologous bone marrow collected previously from patients in remission.27,28 Chemotherapy-moblilized and, subsequently, growth factor–mobilized PBSCs shortened the duration of bone marrow aplasia further so that even myeloablative dose regimens could be applied safely, with a current mortality of 1% to 2% in patients up to age 65.29,30
Many phase 2 autotransplantation studies in patients with newly diagnosed myeloma have reported CR rates of 30% to 40% and median survivals of 4 to 5 years.31-34 A high 10-year survival rate of 43% among 167 patients younger than 55 years of age and with low beta-2-microglobulin (B2M < 3 mg/L) was recently reported by the Royal Marsden group.35 Among all their 451 patients receiving a single melphalan 200 mg/m2-based transplant between 1985 and 2001, 8-year event-free survival (EFS) and overall survival (OS) rates were 16% and 34%, respectively.36 Similar results have been observed by the European Blood and Marrow Transplant Registry.37
In contrast to a single cycle of high-dose melphalan, repeated administrations of melphalan at low doses inflict only sublethal tumor cell damage and, therefore, may promote additional mutations, increasing genomic instability in tumor cells. Because of its hematopoietic stem cell toxicity, chronic application of low-dose melphalan also increases the risk of secondary myelodysplasia (t-MDS).38 Indeed, the risk of t-MDS is much higher when patients have received prolonged standard therapy prior to autotransplants. In a retrospective analysis, all 7 cases of t-MDS were identified in a group of 117 patients with more extensive standard therapy prior to transplantation, whereas no such cases were observed in the 71 patients with minimal prior therapy.38 Moreover, using interphase fluorescence in situ hybridization (FISH) technology, the signature chromosomal abnormalities of MDS were already detectable in the collected stem cells, lending further support to the contribution of chronic low-dose alkylating agent therapy to the development of MDS after autotransplantation.39
PBSC procurement is compromised by prolonged exposure to stem cell–toxic standard-dose alkylating agent regimens, including melphalan and nitrosoureas, and by local irradiation to bone marrow–containing sites.40,41 The nonmyelotoxic VAD regimen (4-day continuous infusion of vincristine and doxorubicin hydrochloride [Adriamycin; Pharmacia & Upjohn, Kalamazoo, MI] plus dexamethasone pulsing) has emerged as the induction therapy of choice prior to hematopoietic stem cell procurement.42
Although assuring prompt hematopoietic recovery in the majority of patients receiving an adequate number of CD34 cells (≥ 2 × 106/kg, but preferably ≥ 5 × 106/kg), autografts do not protect against damage to other organ sites. Mucositis has emerged as the dose-limiting toxicity of most autotransplantation trials using melphalan. To achieve further tumor cytoreduction in more patients and beyond the clinical CR threshold (corresponding to 109-1010 tumor cells), tandem autotransplantations were introduced as a means to increase CR rate and to extend the duration of disease control and survival. When spaced by 3 to 4 months to allow for sufficient mucous membrane recovery, repeated applications of melphalan 200 mg/m2 can be administered safely with a cumulative mortality rate of 2% to 3%. Thus, a phase 2 pilot trial of Total Therapy I effected stringently defined CR in 40% of patients.43 An update of an historically controlled comparison of 152 untreated patients receiving Total Therapy I and Southwest Oncology Group (SWOG) standard therapies (S8624, S9028, S9210) revealed significant prolongation of both EFS and OS in the high-dose therapy group (Figure 1, Table 1). Patients were matched by age and prestudy levels of B2M and albumin.
Comparison of tandem autotransplants with melphalan 200 mg/m2 (as part of Total Therapy I) versus standard alkylating agent therapy administered under the auspices of SWOG.43 Patients were matched by age (within 10 years), B2M (within 2 mg/L), and albumin (within 1 g/dL), major prognostic factors in SWOG trials. In a comparison of patients enrolled on Total Therapy I (TT I) and SWOG trials, no statistically significant differences were noted for the following characteristics: age 60 years or older (TT I, 26%; SWOG, 26%; P = .3); B2M 3 mg/L or more (TT I, 50%; SWOG, 47%; P = .3); albumin less than 3.5 g/dL (TT I, 24%; SWOG, 22%; P = .1); IgA isotype (TT I, 17%; SWOG, 18%; P = .9); creatinine 2 mg/L or more (TT I, 8%; SWOG, 11%; P = .2). TT I consisted of induction with the VAD regimen (vincristine, Adriamycin, dexamethasone) times 3 cycles; high-dose cyclophosphamide 6 g/m2 plus granulocyte-macrophage colony-stimulating factor (GM-CSF) with subsequent peripheral blood stem cell collection, tandem autotransplantations with melphalan 200 mg/m2 3 to 6 months apart (in cases in which a partial response was not achieved after the first transplantation, melphalan 140 mg/m2 plus total body irradiation [TBI] 8.5 Gy was administered), and interferon maintenance (median follow-up, 10 years). SWOG trials S8624, S9028, and S9210 were carried out from 1986 to 1997, contemporaneously with TT I (median follow-up, 9 years). Significantly superior overall survival (A) and event-free survival (B) were observed with TT I.
Comparison of tandem autotransplants with melphalan 200 mg/m2 (as part of Total Therapy I) versus standard alkylating agent therapy administered under the auspices of SWOG.43 Patients were matched by age (within 10 years), B2M (within 2 mg/L), and albumin (within 1 g/dL), major prognostic factors in SWOG trials. In a comparison of patients enrolled on Total Therapy I (TT I) and SWOG trials, no statistically significant differences were noted for the following characteristics: age 60 years or older (TT I, 26%; SWOG, 26%; P = .3); B2M 3 mg/L or more (TT I, 50%; SWOG, 47%; P = .3); albumin less than 3.5 g/dL (TT I, 24%; SWOG, 22%; P = .1); IgA isotype (TT I, 17%; SWOG, 18%; P = .9); creatinine 2 mg/L or more (TT I, 8%; SWOG, 11%; P = .2). TT I consisted of induction with the VAD regimen (vincristine, Adriamycin, dexamethasone) times 3 cycles; high-dose cyclophosphamide 6 g/m2 plus granulocyte-macrophage colony-stimulating factor (GM-CSF) with subsequent peripheral blood stem cell collection, tandem autotransplantations with melphalan 200 mg/m2 3 to 6 months apart (in cases in which a partial response was not achieved after the first transplantation, melphalan 140 mg/m2 plus total body irradiation [TBI] 8.5 Gy was administered), and interferon maintenance (median follow-up, 10 years). SWOG trials S8624, S9028, and S9210 were carried out from 1986 to 1997, contemporaneously with TT I (median follow-up, 9 years). Significantly superior overall survival (A) and event-free survival (B) were observed with TT I.
Summary of high-dose therapy trials in multiple myeloma
Study . | Randomization . | Regimens . | N . | Mean age, y . | Median FU, mo . | % CR (P) . | EFS, median mo. (P) . | OS, median mo. (P) . |
|---|---|---|---|---|---|---|---|---|
| Standard-dose therapy vs high-dose therapy | ||||||||
| Attal et al8 IFM 90* | Before treatment | VMCP/BVAP × 18 vs VMCP/BVAP × 4-6 → CTX + MEL 140 + TBI 8 Gy | 100 vs 100 | 58 vs 57 | 108 | 14 vs 38 (< .001) | 18 vs 28 (.01) | 44 vs 57 (20% vs 35% @ 7 y) (.03) |
| Child et al16 MRC VII* | Before treatment | ABCM × 4-12 vs CVAMP × 3 → CTX + MEL 200 | 200 vs 201 | 56 vs 55 | 42 | 8 vs 44 (< .001) | 20 vs 32 (16% vs 36% @ 4 y) (< .001) | 42 vs 54 (46% vs 55% @ 4 y) (.04) |
| Blade et al50 PETHEMA† | Responders to induction | ABCM/VBAD × 12 vs ABCM/VBAD × 4 → MEL 200 | 83 vs 81 | 56 vs 56 | 66 | 11 vs 30 (< .002) | 34 vs 43 NA | 67 vs 65 NA |
| Single transplantation vs tandem transplantation | ||||||||
| Attal et al15 IFM 94* | Before treatment | VAD × 3-4 → G-CSF→ MEL 140 + TBI 8 Gy vs VAD × 3-4 → G-CSF→ MEL 140; MEL 140 + TBI 8 Gy | 199 vs 200 | 52 vs 52 | 75 | 42 vs 50 ≥ n-CR (< .1) | 25 vs 30 (10% vs 20% @ 7 y) (< .03) | 48 vs 58 (21% vs 42% @ 7 y) (.01) |
| Cavo et al51 BOLOGNA 96 | Before treatment | VAD × 4 → CTX → MEL 200 vs VAD × 4 → CTX → MEL 200; MEL 120 + busulfan | 110 vs 110 | 53 vs 53 | 38 | 21 vs 24 NS | 25 vs 34 NA (< .05) | 56 vs 60 NS |
| Fermand et al53 MAG 95 | Before treatment | DEX × 2 → CTX → VAD × 3-4 → MEL 140 + VP16 + CTX + TBI 12 Gy vs DEX × 2 → CTX → VAD × 3-4 → MEL 140; MEL 140 + VP16 + TBI 12 Gy | 97 vs 96 | 50 vs 50 | 53 | 39 vs 37 NS | 31 vs 33 NS | 49 vs 73 NA (.14) |
| Segeren et al52 HOVON* (intermediate dose therapy) | After VAD ± response | VAD × 3-4 → CTX → MEL 70 × 2 vs VAD × 3-4 → CTX → MEL 70 × 2 → CTX + TBI 9 Gy | 129 vs 132 | 55 vs 56 | 40 | 14 vs 28 (.004) | NA (15% vs 29% @ 4 y) (< .03) | NA (55% vs 50% @ 4 y) (< .3) |
| Standard-dose therapy vs tandem transplantation | ||||||||
| Barlogie et al43 SWOG vs TT I* | Historical controls | VMCB (P)/VBAP (P)/VAD vs VAD × 2-3 → CTX → EDAP → MEL 200 × 2 (< PR, MEL 140 + TBI 8.5 Gy) | 152 vs 152 | 52 vs 52 | 114 | NA vs 41 | 16 vs 37 (5% vs 15% @ 10 y) (< .0001) | 43 vs 79 (15% vs 33% @ 10 y) (< .0001) |
Study . | Randomization . | Regimens . | N . | Mean age, y . | Median FU, mo . | % CR (P) . | EFS, median mo. (P) . | OS, median mo. (P) . |
|---|---|---|---|---|---|---|---|---|
| Standard-dose therapy vs high-dose therapy | ||||||||
| Attal et al8 IFM 90* | Before treatment | VMCP/BVAP × 18 vs VMCP/BVAP × 4-6 → CTX + MEL 140 + TBI 8 Gy | 100 vs 100 | 58 vs 57 | 108 | 14 vs 38 (< .001) | 18 vs 28 (.01) | 44 vs 57 (20% vs 35% @ 7 y) (.03) |
| Child et al16 MRC VII* | Before treatment | ABCM × 4-12 vs CVAMP × 3 → CTX + MEL 200 | 200 vs 201 | 56 vs 55 | 42 | 8 vs 44 (< .001) | 20 vs 32 (16% vs 36% @ 4 y) (< .001) | 42 vs 54 (46% vs 55% @ 4 y) (.04) |
| Blade et al50 PETHEMA† | Responders to induction | ABCM/VBAD × 12 vs ABCM/VBAD × 4 → MEL 200 | 83 vs 81 | 56 vs 56 | 66 | 11 vs 30 (< .002) | 34 vs 43 NA | 67 vs 65 NA |
| Single transplantation vs tandem transplantation | ||||||||
| Attal et al15 IFM 94* | Before treatment | VAD × 3-4 → G-CSF→ MEL 140 + TBI 8 Gy vs VAD × 3-4 → G-CSF→ MEL 140; MEL 140 + TBI 8 Gy | 199 vs 200 | 52 vs 52 | 75 | 42 vs 50 ≥ n-CR (< .1) | 25 vs 30 (10% vs 20% @ 7 y) (< .03) | 48 vs 58 (21% vs 42% @ 7 y) (.01) |
| Cavo et al51 BOLOGNA 96 | Before treatment | VAD × 4 → CTX → MEL 200 vs VAD × 4 → CTX → MEL 200; MEL 120 + busulfan | 110 vs 110 | 53 vs 53 | 38 | 21 vs 24 NS | 25 vs 34 NA (< .05) | 56 vs 60 NS |
| Fermand et al53 MAG 95 | Before treatment | DEX × 2 → CTX → VAD × 3-4 → MEL 140 + VP16 + CTX + TBI 12 Gy vs DEX × 2 → CTX → VAD × 3-4 → MEL 140; MEL 140 + VP16 + TBI 12 Gy | 97 vs 96 | 50 vs 50 | 53 | 39 vs 37 NS | 31 vs 33 NS | 49 vs 73 NA (.14) |
| Segeren et al52 HOVON* (intermediate dose therapy) | After VAD ± response | VAD × 3-4 → CTX → MEL 70 × 2 vs VAD × 3-4 → CTX → MEL 70 × 2 → CTX + TBI 9 Gy | 129 vs 132 | 55 vs 56 | 40 | 14 vs 28 (.004) | NA (15% vs 29% @ 4 y) (< .03) | NA (55% vs 50% @ 4 y) (< .3) |
| Standard-dose therapy vs tandem transplantation | ||||||||
| Barlogie et al43 SWOG vs TT I* | Historical controls | VMCB (P)/VBAP (P)/VAD vs VAD × 2-3 → CTX → EDAP → MEL 200 × 2 (< PR, MEL 140 + TBI 8.5 Gy) | 152 vs 152 | 52 vs 52 | 114 | NA vs 41 | 16 vs 37 (5% vs 15% @ 10 y) (< .0001) | 43 vs 79 (15% vs 33% @ 10 y) (< .0001) |
FU indicates follow-up; CTX, cyclophosphamide; PETHEMA, Programa para el Estudio de la Therapéutica en Hemopatía Maligna; G-CSF, granulocyte colony-stimulating factor; MAG, Myélomo Autogreffe; HOVON, Stichting Hemato-Oncologie voor Volwassen Nederland; MEL, melphalan; DEX, dexamethasone; and MRC, Medical Research Council.
IFN maintenance therapy.
IFN and DEX maintenance therapy.
Salvage treatment
The clinical importance of distinguishing primary resistance and resistant relapse has been well established.1,44 Other potentially relevant features to consider in planning salvage therapies include duration of response, circumstances of relapse while on or off maintenance therapy, prior application (once or twice) of autotransplantations, and hematopoietic reserve, as reflected best by a normal platelet count. We recommend procurement of sufficient CD34 quantities (10-20 × 106/kg) early in the disease course so that, under appropriate clinical circumstances, sufficient PBSCs will be available for management of myeloma relapse (including further transplantations), treatment-induced cytopenia, or t-MDS/acute myelogenous leukemia (AML).
Traditional salvage therapies include the VAD regimen, the first effective treatment for MP-resistant myeloma42 and, subsequently, dexamethasone pulsing, emphasizing the efficacy of repeated high doses of glucocorticoids.44 Other drug combinations (eg, DCEP, comprising dexamethasone pulsing and 4-day continuous infusions of cyclophosphamide, etoposide, and cisplatin45 ; or DT-PACE, with added thalidomide and Adriamycin) have shown efficacy, especially in myeloma with high-proliferative features.46,47 In the past 10 years, several new agents have been identified that exhibit remarkable activity in refractory myeloma (thalidomide, PS 341, CC-5013, arsenic trioxide; described in more detail in “New agents”).
The salvage setting is the typical clinical scenario in which novel treatments are discovered. In the case of autologous transplantation, remarkable activity was observed in patients with primary refractory myeloma, an observation since confirmed in a number of phase 2 and randomized trials.1,15,43,48 Hence, in contrast to current Centers for Medicare and Medicaid Services (CMS) directions, such patients should not be excluded from consideration of PBSC collection and high-dose therapy.
Advances in myeloma treatment: the past 10 years
Controlled clinical trials of dose intensity versus standard therapy
In pivotal prospective randomized trials, the Intergroupe Francophone du Myélome (IFM) demonstrated superiority of high-dose therapy (melphalan 140 mg/m2 + TBI 8 Gy) over standard therapy (VMCP/VBAP [vincristine, melphalan, cyclophosphamide, and prednisone; vincristine, BCNU, Adriamycin, and prednisone]) (IFM 90 trial)8 and, recently, of tandem over single autotransplantations (IFM 94 trial).15 The IFM 95 trial established that single-agent melphalan (200 mg/m2) afforded better tolerance and superior survival than did melphalan 140 mg/m2 plus TBI 8 Gy.49 The Medical Research Council (MRC) of the United Kingdom recently reported, in its Myeloma VII prospective randomized clinical trial of 401 patients, a superior CR rate (44% versus 8%, P < .001) and a 1-year extension of both OS (54 versus 42 months, P = .04) and progression-free survival (32 versus 20 months, P < .001) after autotransplantation (chiefly using melphalan 200 mg/m2) compared with standard therapy with the ABCM regimen (doxorubicin, carmustine, cyclophosphamide, melphalan).16
The late divergence of survival curves in IFM 90 and IFM 94 trials after only 3 to 4 years should caution against premature publication of other randomized clinical trials addressing single autotransplantations versus standard chemotherapy50 and tandem versus single transplantation procedures.51-53 Trial designs frequently differ with regard to patient age, timing of randomization, inclusion of nonresponders, and maintenance therapies, as well as the frequency of salvage transplantations.54 (Table 1 gives a comprehensive summary). In the case of the MRC Myeloma VII trial, the superior outcome of patients treated with high-dose therapy may in part be related to a more intensive induction regimen (C-VAMP [cyclophosphamide, vincristine, Adriamycin, methylprednisolone, and prednisone] and high-dose cyclophosphamide for PBSC mobilization) than was applied in the standard treatment arm (ABCM). However, the posttransplantation effect of the quality of pretransplantation response is controversial.1,48 Furthermore, a higher proportion of patients on the transplantation arm received interferon maintenance (78% versus 48%).
Table 1 provides a summary of randomized clinical trials, recently updated at the IX International Myeloma Workshop convened in June 2003 in Salamanca, Spain.
Prognostic factors with high-dose therapy
Adverse implications of elevations of B2M, C-reactive protein (CRP), lactic dehydrogenase (LDH), and creatinine and of decreased levels of albumin, hemoglobin, and platelets also apply to high-dose therapy trials.54 Tumor cell proliferative activity, as captured by the plasma cell labeling index, is low at diagnosis (median, 1.0%) and, when increased, adversely affects outcome with both standard- and high-dose therapy regimens.55 Poor prognosis has also been linked to the detection of abnormal metaphases, present in only one third of patients with newly diagnosed disease.56,57 In contrast, aneuploidy can be detected in virtually all patients by DNA flow cytometry58 and FISH.11 FISH-defined chromosome 13 deletions, present in 50% of patients, portend a poor outcome.59 However, a recent head-on comparison with standard cytogenetics, as part of the Arkansas Total Therapy II trial, traced the greatest risk to metaphase-defined chromosome 13 deletion, present in approximately 15% of patients60 (Figure 2). Together with hypodiploidy,61 deletion 13 accounts for approximately one half of all karyotypic abnormalities and confers a uniformly poor prognosis.62 This increased risk probably reflects a greater degree of stromal cell independence, in terms of myeloma cell proliferation and survival, in addition to greater drug resistance. Long-term follow-up of the good-risk group, lacking cytogenetic abnormalities, is anxiously being awaited to determine the fraction of patients remaining disease-free beyond 10 years. In a comparison of CR duration among patients receiving Total Therapy II and its predecessor Total Therapy I, the 4-year estimate for those patients without cytogenetic abnormalities was 76% (confidence interval [CI], 65%-86%) with Total Therapy II compared to 36% (CI, 25%-48%) with Total Therapy I (P < .0001) (data not shown).
Adverse prognostic implications of chromosome 13 deletion.60 Overall survival (A) and event-free survival (B) are portrayed for 363 patients receiving Total Therapy II (up-front randomization to thalidomide; intensive induction chemotherapy with VAD, DCEP, CAD [cyclophosphamide, Adriamycin, dexamethasone with PBSC collection, DCEP; tandem autotransplantations with melphalan 200 mg/m2; consolidation chemotherapy every 3 months times 4 cycles; and interferon maintenance). Prior to therapy, standard cytogenetics were performed on at least 20 metaphase samples by using standard Giemsa banding. Another bone marrow sample was subjected to interphase FISH for the detection of deletion 13 among 500 cells, counterstained with the disease-concordant antilight chain antibody. Survival was similar in the absence of cytogenetic abnormalities (no CAs) whether or not deletion 13 was detectable by FISH. Survival was shortest in the presence of metaphase-defined deletion 13 (FISH 13, CA); an intermediate outcome was observed in patients with other metaphase CA (no FISH 13, CA).
Adverse prognostic implications of chromosome 13 deletion.60 Overall survival (A) and event-free survival (B) are portrayed for 363 patients receiving Total Therapy II (up-front randomization to thalidomide; intensive induction chemotherapy with VAD, DCEP, CAD [cyclophosphamide, Adriamycin, dexamethasone with PBSC collection, DCEP; tandem autotransplantations with melphalan 200 mg/m2; consolidation chemotherapy every 3 months times 4 cycles; and interferon maintenance). Prior to therapy, standard cytogenetics were performed on at least 20 metaphase samples by using standard Giemsa banding. Another bone marrow sample was subjected to interphase FISH for the detection of deletion 13 among 500 cells, counterstained with the disease-concordant antilight chain antibody. Survival was similar in the absence of cytogenetic abnormalities (no CAs) whether or not deletion 13 was detectable by FISH. Survival was shortest in the presence of metaphase-defined deletion 13 (FISH 13, CA); an intermediate outcome was observed in patients with other metaphase CA (no FISH 13, CA).
The ongoing IFM 99 trial applies a risk-based approach according to B2M status and FISH-defined chromosome 13 deletions.63 Low-risk patients (< 2 unfavorable factors) are randomly assigned, after tandem autotransplantations with melphalan 140 mg/m2 followed by melphalan 200 mg/m,2 to no maintenance, bisphosphonate maintenance, or bisphosphonate plus thalidomide maintenance. High-risk patients (2 risk factors) receive tandem autotransplantations with melphalan 200 mg/m2 followed by further dose-increased melphalan 220 mg/m2 plus anti–interleukin-6 monoclonal antibody. Those with HLA-compatible sibling donors are offered mini-allotransplantations after a single autotransplantation (described in “Allogeneic transplantations”).
New agents
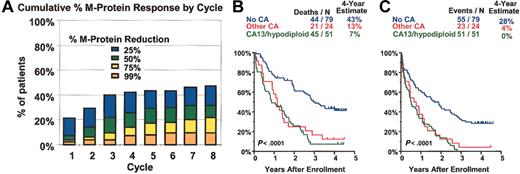
Thalidomide. When thalidomide'sefficacy in advanced and refractory myeloma was first reported in 1999,17 it represented only the third independently active compound for treating multiple myeloma. Long-term follow-up of 169 patients enrolled in a phase 2 clinical trial using incremental dosing of thalidomide up to 800 mg per day confirmed the original observation of a partial response rate of approximately one third and extended EFS and OS. Cytogenetic abnormalities conferred a poor prognosis64 also with thalidomide treatment (Figure 3; Table 2).
Thalidomide in advanced and refractory myeloma. Thalidomide was administered to 169 patients; 76% had received 1 cycle and 53% at least 2 cycles of high-dose therapy; 67% had exhibited cytogenetic abnormalities, including 37% with abnormalities of chromosome 13. Thalidomide was administered at a starting dose of 200 mg daily, with escalation by 200 mg every 2 weeks, according to tolerance, for a maximum of 800 mg. (A) Time to different levels of myeloma protein response (reduction by at least 25%, 50%, 75%, and > 99%). At 8 months, 31% were estimated to have achieved a partial response (≥ 50% myeloma protein reduction). Most responders could be identified within 2 months using 25% or more myeloma protein reduction criteria. Superior survival (B) and event-free survival (C) were noted among 79 patients presenting without cytogenetic abnormalities (no CA). Survival was inferior in the presence of cytogenetic abnormalities (CAs), either involving chromosome 13 deletions and hypodiploidy (CA 13/hypodiploid, 51 patients) or other chromosomes (other CA, 24 patients). Fifteen patients lacked cytogenetic information.
Thalidomide in advanced and refractory myeloma. Thalidomide was administered to 169 patients; 76% had received 1 cycle and 53% at least 2 cycles of high-dose therapy; 67% had exhibited cytogenetic abnormalities, including 37% with abnormalities of chromosome 13. Thalidomide was administered at a starting dose of 200 mg daily, with escalation by 200 mg every 2 weeks, according to tolerance, for a maximum of 800 mg. (A) Time to different levels of myeloma protein response (reduction by at least 25%, 50%, 75%, and > 99%). At 8 months, 31% were estimated to have achieved a partial response (≥ 50% myeloma protein reduction). Most responders could be identified within 2 months using 25% or more myeloma protein reduction criteria. Superior survival (B) and event-free survival (C) were noted among 79 patients presenting without cytogenetic abnormalities (no CA). Survival was inferior in the presence of cytogenetic abnormalities (CAs), either involving chromosome 13 deletions and hypodiploidy (CA 13/hypodiploid, 51 patients) or other chromosomes (other CA, 24 patients). Fifteen patients lacked cytogenetic information.
Patient characteristics for thalidomide phase 2 trial for advanced and refractory myeloma
Parameter . | % . |
|---|---|
| Age greater than 60 y | 44 |
| B2M greater than 6 mg/L | 22 |
| Abnormal cytogenetics | 67 |
| Deletion 13 | 37 |
| Prior therapy longer than 60 mo | 20 |
| Prior high-dose therapy | 76 |
| More than 1 cycle | 53 |
Parameter . | % . |
|---|---|
| Age greater than 60 y | 44 |
| B2M greater than 6 mg/L | 22 |
| Abnormal cytogenetics | 67 |
| Deletion 13 | 37 |
| Prior therapy longer than 60 mo | 20 |
| Prior high-dose therapy | 76 |
| More than 1 cycle | 53 |
Many thousand myeloma patients worldwide have since benefited from thalidomide, which can be conveniently combined with chemotherapy because of its virtual lack of myelosuppression.5,65 However, peripheral neuropathy is a major treatment-limiting toxicity that affects 50% to 80% of patients, the severity and reversibility of which are related to both dose and duration of drug administration.66 In case of grade 2 neuropathy, dose reduction or suspension of therapy frequently results in symptom improvement or resolution, an important consideration for patients with recently diagnosed disease with a good prognosis. In the setting of advanced and refractory disease, higher thalidomide doses of 400 mg or more are often required, which are associated with grade 3 neurotoxicity in approximately one third of patients, which is not readily reversible. As part of an up-front randomized trial of Total Therapy II for patients with newly diagnosed disease (described earlier), time course and severity of neuropathy were carefully monitored. Among 550 patients currently enrolled, the 4-year estimate of grade 3 or greater sensory neuropathy is 16% among 263 patients receiving thalidomide and only 5% among the 280 patients randomly assigned to no thalidomide (Figure 4A). Excessive thromboembolic complications could be traced to combination therapies that included doxorubicin67,68 and were effectively eliminated by therapeutic anticoagulation with low molecular weight heparin (Figure 4B-C).69 A marked synergy between thalidomide and dexamethasone70 has prompted evaluation of this combination for response induction prior to and for maintenance therapy after autotransplantations.71,72 Much is still to be learned regarding thalidomide dosing and scheduling in different clinical scenarios73 and the purported mechanisms of its antimyeloma effects.6
Thalidomide-related toxicities experienced in a phase 3 trial of Total Therapy II. (A) Significantly higher incidence of grades 2 and 3 sensory neuropathy on thalidomide-containing treatment arm. (B) Significantly higher incidence of deep venous thrombosis (DVT) on thalidomide arm, which could be eliminated by prophylactic use of low-molecular-weight heparin (Lovenox; Aventis, Strasbourg, France) (C).
Thalidomide-related toxicities experienced in a phase 3 trial of Total Therapy II. (A) Significantly higher incidence of grades 2 and 3 sensory neuropathy on thalidomide-containing treatment arm. (B) Significantly higher incidence of deep venous thrombosis (DVT) on thalidomide arm, which could be eliminated by prophylactic use of low-molecular-weight heparin (Lovenox; Aventis, Strasbourg, France) (C).
CC-5013 (Revimid). CC-5013 is another immunomodulatory agent that exhibits virtually no sedative and only occasionally neurotoxic side effects. Responses have been reported in one third of patients with advanced and refractory myeloma.17,74 Many of these patients had been previously exposed to thalidomide, although true thalidomide resistance was infrequently established. Unlike thalidomide, CC-5013 causes myelosuppression which, in the setting of compromised bone marrow reserve because of extensive prior cytotoxic drug exposure, may not be fully reversible. In a phase 3 trial for advanced myeloma that compared 2 different schedules of administration (50 mg × 10 doses and 25 mg × 20 doses every 28 days) (Figure 5A; Table 3), we observed higher response rates with the more prolonged 25 mg dose schedule (Figure 5B). Greater than grade 2 thrombocytopenia was linked to pretreatment platelet count less than 100 000/μL (100 × 109/L) as a reflection of impaired hematopoietic reserve (Figure 5C). Trials have been initiated to evaluate CC-5013 alone and in combination with dexamethasone for response induction and maintenance in the context of autotransplantations.
CC-5013 phase 3 trial for advanced and refractory myeloma. (A) Dosing schedule. (B) Kinetics of response according to dosing schedule. M-protein responses were significantly higher with the 25 mg than with the 50 mg CC-5013 dose, although total doses per cycle were identical (500 mg). (C) Grade greater than 2 thrombocytopenia (< 50 000/μL[< 50 × 109/L]) was significantly more frequent and of earlier onset when platelet levels before CC-5013 were less than 100 000/μL (100 × 109/L).
CC-5013 phase 3 trial for advanced and refractory myeloma. (A) Dosing schedule. (B) Kinetics of response according to dosing schedule. M-protein responses were significantly higher with the 25 mg than with the 50 mg CC-5013 dose, although total doses per cycle were identical (500 mg). (C) Grade greater than 2 thrombocytopenia (< 50 000/μL[< 50 × 109/L]) was significantly more frequent and of earlier onset when platelet levels before CC-5013 were less than 100 000/μL (100 × 109/L).
Patient characteristics for CC-5013 phase 3 trial for advanced and refractory myeloma
. | % of patients . | . | |
|---|---|---|---|
| Parameter . | 25 mg, n = 27 . | 50 mg, n = 28 . | |
| Age 60 y or older | 78 | 54 | |
| B2M 4 mg/L or greater | 46 | 39 | |
| LDH 190 IU/L or greater | 31 | 41 | |
| Abnormal cytogenetics | 58 | 47 | |
| Deletion 13 | 35 | 27 | |
| Prior therapy 60 mo or more | 58 | 41 | |
| Prior high-dose therapy | 85 | 86 | |
| More than 1 cycle | 46 | 48 | |
| Prior THAL | 92 | 93 | |
. | % of patients . | . | |
|---|---|---|---|
| Parameter . | 25 mg, n = 27 . | 50 mg, n = 28 . | |
| Age 60 y or older | 78 | 54 | |
| B2M 4 mg/L or greater | 46 | 39 | |
| LDH 190 IU/L or greater | 31 | 41 | |
| Abnormal cytogenetics | 58 | 47 | |
| Deletion 13 | 35 | 27 | |
| Prior therapy 60 mo or more | 58 | 41 | |
| Prior high-dose therapy | 85 | 86 | |
| More than 1 cycle | 46 | 48 | |
| Prior THAL | 92 | 93 | |
Patient characteristics were comparable between the 2 treatment arms, including almost universal exposure to prior thalidomide (THAL); more patients 60 years or older were randomly assigned to CC-5013 25 mg (P = .08).
PS 341 (Velcade). The proteasome inhibitor PS 341 represents an entirely new class of agents,75,76 sometimes with remarkable activity in myeloma that is refractory to multiple lines of standard- and high-dose regimens (including thalidomide).19,77 Combination trials of PS 341 with melphalan78,79 and doxorubicin (Doxil; ALZA Pharmaceuticals, Mountain View, CA)80 are currently in progress. At our institution, a combination of PS 341 plus thalidomide for posttransplantation relapse has revealed remarkable activity without aggravating preexisting thalidomide-related neuropathy (Table 4; Figure 6A). Thus, 60% of 46 patients with sufficient follow-up achieved at least partial response (> 50% M-protein reduction) at the end of the third cycle. OS and EFS were superior in patients presenting without cytogenetic abnormalities (Figure 6B-C).81 No difference is yet apparent with regard to a thalidomide dose effect.
Patient characteristics for PS 341 + thalidomide phase 2 trial for advanced and refractory myeloma
Parameter . | % of patients . |
|---|---|
| Age 60 y or older | 61 |
| B2M 4 mg/L or greater | 61 |
| LDH 190 IU/L or greater | 43 |
| Abnormal cytogenetics | 76 |
| Deletion 13 | 52 |
| Prior therapy 60 mo or more | 37 |
| Prior autotransplantation | 100 |
| More than 1 cycle | 72 |
| Prior THAL | 78 |
| Prior VEL | 0 |
Parameter . | % of patients . |
|---|---|
| Age 60 y or older | 61 |
| B2M 4 mg/L or greater | 61 |
| LDH 190 IU/L or greater | 43 |
| Abnormal cytogenetics | 76 |
| Deletion 13 | 52 |
| Prior therapy 60 mo or more | 37 |
| Prior autotransplantation | 100 |
| More than 1 cycle | 72 |
| Prior THAL | 78 |
| Prior VEL | 0 |
All patients had relapsed from a prior autotransplantation; 78% had been treated with and were resistant to thalidomide (THAL); none had received prior PS 341 (VEL). Cytogenetic abnormalities were present in 76%, including deletion 13 in 52%.
PS 341 (Velcade) plus thalidomide for posttransplantation relapse in 46 patients. PS 341 was administered at a dose of 1 mg/m2 on days 1, 4, 8, and 11 of each cycle, repeated every 21 days. Thalidomide was added with the start of the second cycle of PS 341 at a daily starting dose of 50 mg (12 patients) with increases to 100 mg (10 patients), 150 mg (11 patients), and 200 mg (13 patients) in the subsequent cohorts. Thalidomide dose increments were implemented whenever at least 7 patients had completed cycle number 2 at the lower thalidomide dose level without neuropathy greater than grade 2. (A) Cumulative incidence, on an intent-to-treat basis, by different levels of myeloma protein reduction. Response ensued so quickly that 60% had achieved a partial response (PR; ≥ 50% M-protein reduction) at the end of cycle 3. Near-CR (> 99% reduction; only immunofixation revealed monoclonal protein) was noted in 20% to 30%. Overall survival (B) and event-free survival (C) are portrayed by using Kaplan-Meier plots, related to presence of cytogenetic abnormalities (CA). Superior survival was observed in the absence of CA (no CA), whereas patients with CA 13/hypodiploid or other CA had a poor prognosis (Figure 3 legend).
PS 341 (Velcade) plus thalidomide for posttransplantation relapse in 46 patients. PS 341 was administered at a dose of 1 mg/m2 on days 1, 4, 8, and 11 of each cycle, repeated every 21 days. Thalidomide was added with the start of the second cycle of PS 341 at a daily starting dose of 50 mg (12 patients) with increases to 100 mg (10 patients), 150 mg (11 patients), and 200 mg (13 patients) in the subsequent cohorts. Thalidomide dose increments were implemented whenever at least 7 patients had completed cycle number 2 at the lower thalidomide dose level without neuropathy greater than grade 2. (A) Cumulative incidence, on an intent-to-treat basis, by different levels of myeloma protein reduction. Response ensued so quickly that 60% had achieved a partial response (PR; ≥ 50% M-protein reduction) at the end of cycle 3. Near-CR (> 99% reduction; only immunofixation revealed monoclonal protein) was noted in 20% to 30%. Overall survival (B) and event-free survival (C) are portrayed by using Kaplan-Meier plots, related to presence of cytogenetic abnormalities (CA). Superior survival was observed in the absence of CA (no CA), whereas patients with CA 13/hypodiploid or other CA had a poor prognosis (Figure 3 legend).
Other agents. Arsenic trioxide targets mitochondria, which play a major role in programmed cell death.82,83 Monoclonal antibodies to interleukin-6 (IL-6)84 and CD2085 are also under investigation. The frequent presence of RAS mutations has motivated studies of farnesyl transferase inhibitors.86-88 High expression of KIT in 20% to 40% of myeloma patients (J.S., unpublished observations, May 2000) justifies studies with STI 571 (Gleevec). Research is in progress to also target unique 14q32 translocations, such as rearrangements that involve the fibroblast growth factor receptor-3 gene (FGF-R3), often associated with chromosome 13 deletions and associated with poor prognosis.89
Gene expression profiling after in vivo drug exposure
Although initially applied to exploit its antiangiogenic properties,90 mechanisms of thalidomide action in patients with myeloma include several other indirect effects (eg, interference with myeloma cell-stromal cell adhesion, cytokine down-regulation, and immunomodulatory effects on T and natural killer [NK] cells) as well as direct proapoptotic effects.6 Similar mechanisms have also been demonstrated for CC-5013, PS 341, and dexamethasone.6 Serial investigations of gene expression profiling prior to and after drug administration in vivo have already revealed drug-unique alterations that should clarify mechanistic pathways underlying response or resistance of myeloma to established and new agents (Figure 7A).91 Similarly, responsiveness to PS 341 could be predicted with a high level of accuracy (Figure 7B). The use of such sensitivity profiles may allow selection of patients for specific therapies and, thus, avoid administration of ineffective and often toxic drugs.
Gene expression patterns can be used to evaluate the consequences of, and predict response to, chemotherapy. (A) Gene expression changes after short-term in vivo drug exposure can potentially reveal molecular mechanisms of action. RNA isolated from purified plasma cells before and after 48 hours in vivo treatment with PS 341 (VEL, n = 15), CC-5013 (REV, n = 13), dexamethasone (DEX, n = 20), or thalidomide (THAL, n = 18) was applied to U95Av2 microarrays (Affymetrix, Santa Clara, CA). Significant induction or suppression of gene expression after drug treatment was calculated on the basis of the percentage of change from baseline ([follow-up – baseline]/baseline). The top 15 genes in each drug group were selected according to rank P value and plotted on the basis of the percentage of change (red = increase in expression; green = decrease in expression). Drug treatment samples are grouped in columns, and genes are in rows. Note that genes up-regulated by dexamethasone (first 15 genes from the top) tend to be unique to that drug. Also note that most of the genes up-regulated by CC-5013 are also up-regulated by thalidomide, thus indicating possible common molecular targets for these 2 drug analogs. (B) Gene cluster of normalized expression values of a group of genes demonstrating differences between myeloma cases exhibiting response or no response to PS 341 proteasome inhibitor therapy. Plasma cells were purified from bone marrow aspirates from 40 patients before the initiation of therapy and after informed consent. Total RNA was isolated, and gene expression levels of approximately 12 000 genes were analyzed using the Affymetrix U95Av2 GeneChip microarray. After sufficient follow-up, 21 responders (exhibiting at least a 50% reduction in serum M protein) and 19 nonresponders (exhibiting progression, stabilization, or no > 25% reduction in M protein) were identified. With the use of a combination of chi square, Wilcoxon rank, and discriminant analysis, 40 genes were identified as being expressed higher in the nonresponder group and 42 higher in the responder group. Genes are in rows, and patient samples are in columns. The nonresponders are grouped under the green bar and responders under the red bar. The table below panel B shows the correlation of results from a gene expression–based prediction model of response and actual response to PS 341 therapy by using the same 40 patients analyzed in panel B. The results presented in the matrix were derived by first assessing the mean difference for all 12 625 probe sets between responders and nonresponders, and then obtaining P values and adjusted P values based on permutation tests (20 000 samples). The 40 samples were then randomly grouped into 5 strata of n = 8. Using 30 of the most significantly differentially expressed genes, a prediction model was developed by using stepwise logistic regression on data from 4 of the 5 strata (80% of total data, n = 32). The model developed on 80% of the data was applied to the 20% (n = 8) left out as a validation group. With 5 strata we developed 5 separate models and validated them on 5 separate validation samples. We averaged over the 5 validation samples to get an estimate of 88% (35 of 40 correctly classified).
Gene expression patterns can be used to evaluate the consequences of, and predict response to, chemotherapy. (A) Gene expression changes after short-term in vivo drug exposure can potentially reveal molecular mechanisms of action. RNA isolated from purified plasma cells before and after 48 hours in vivo treatment with PS 341 (VEL, n = 15), CC-5013 (REV, n = 13), dexamethasone (DEX, n = 20), or thalidomide (THAL, n = 18) was applied to U95Av2 microarrays (Affymetrix, Santa Clara, CA). Significant induction or suppression of gene expression after drug treatment was calculated on the basis of the percentage of change from baseline ([follow-up – baseline]/baseline). The top 15 genes in each drug group were selected according to rank P value and plotted on the basis of the percentage of change (red = increase in expression; green = decrease in expression). Drug treatment samples are grouped in columns, and genes are in rows. Note that genes up-regulated by dexamethasone (first 15 genes from the top) tend to be unique to that drug. Also note that most of the genes up-regulated by CC-5013 are also up-regulated by thalidomide, thus indicating possible common molecular targets for these 2 drug analogs. (B) Gene cluster of normalized expression values of a group of genes demonstrating differences between myeloma cases exhibiting response or no response to PS 341 proteasome inhibitor therapy. Plasma cells were purified from bone marrow aspirates from 40 patients before the initiation of therapy and after informed consent. Total RNA was isolated, and gene expression levels of approximately 12 000 genes were analyzed using the Affymetrix U95Av2 GeneChip microarray. After sufficient follow-up, 21 responders (exhibiting at least a 50% reduction in serum M protein) and 19 nonresponders (exhibiting progression, stabilization, or no > 25% reduction in M protein) were identified. With the use of a combination of chi square, Wilcoxon rank, and discriminant analysis, 40 genes were identified as being expressed higher in the nonresponder group and 42 higher in the responder group. Genes are in rows, and patient samples are in columns. The nonresponders are grouped under the green bar and responders under the red bar. The table below panel B shows the correlation of results from a gene expression–based prediction model of response and actual response to PS 341 therapy by using the same 40 patients analyzed in panel B. The results presented in the matrix were derived by first assessing the mean difference for all 12 625 probe sets between responders and nonresponders, and then obtaining P values and adjusted P values based on permutation tests (20 000 samples). The 40 samples were then randomly grouped into 5 strata of n = 8. Using 30 of the most significantly differentially expressed genes, a prediction model was developed by using stepwise logistic regression on data from 4 of the 5 strata (80% of total data, n = 32). The model developed on 80% of the data was applied to the 20% (n = 8) left out as a validation group. With 5 strata we developed 5 separate models and validated them on 5 separate validation samples. We averaged over the 5 validation samples to get an estimate of 88% (35 of 40 correctly classified).
Allogeneic transplantations
Because of histocompatibility and age restrictions, only 1 of 7 patients with myeloma potentially benefits from allogeneic sibling transplantations, exerting a profound GVM effect.92 As a result of severe graft-versus-host disease (GVHD) and opportunistic infections, first-year treatment-related mortality approaches 50% in cases of standard myeloablative conditioning regimens. Of 136 patients treated with allogeneic transplantations at the Fred Hutchinson Cancer Center between 1987 and 1999, 48% died within the first 100 days, explaining the low 5-year EFS and OS rates of only 14% and 22%, respectively.93 Of the 46 patients (34%) achieving CR, 48% were alive and 30% remained event-free at 5 years; 12 have remained disease-free at 5 to 13 years after transplantation. Most trials and registry data suggest that, after significantly faster patient attrition in the first 2 to 3 years, survivors of allogeneic transplantations may experience fewer late relapses than survivors of autotransplantations.37
Robust donor hematopoiesis can also be established after nonmyeloablative regimens (eg, TBI 2 Gy + fludarabine,21 melphalan 140 mg/m2 + fludarabine,94 or melphalan 100 mg/m2 with subsequent cyclosporin A-based immunosuppression).22,95 Because of usually mild extramedullary toxicity and only transient myelo-suppression (frequently without need for transfusion support), mini-allogeneic transplantations are much better tolerated than standard donor transplantations, resulting in a steep drop in 100-day mortality to 5% to 10%, although 25% to 35% still eventually succumb in the first year, mainly as the consequence of chronic GVHD.96,97 Unfortunately, an antitumor effect is typically only seen in the context of acute or chronic GVHD. A consensus is emerging that mini-allogeneic transplantations should be explored as consolidation therapy after a single melphalan-based autotransplantation, possibly providing the immunosuppression and disease control required for stable hematopoietic engraftment and a profound and lasting GVM effect.
Immunotherapy
Interferon was the first biologically based treatment for myeloma.98 Meta-analysis of randomized trials revealed that patients receiving interferon as maintenance therapy had slightly improved EFS and OS compared with patients receiving no maintenance therapy.99 Idiotype (Id) vaccination trials have been performed over the course of almost 10 years, demonstrating the feasibility of generating both T- and B-cell–based anti-Id responses.100,101 Although clinical benefit has not yet been established, evidence does exist for the ability of Id-specific cytotoxic T cells to lyse autologous primary myeloma cells.102 More recently, dendritic cells have been used in conjunction with Id protein and tumor lysate.103,104 Although immune responses appeared stronger than those after Id vaccination, information on clinical benefit is still needed. The frequent expression of cancer testis antigens by myeloma cells, especially by the highly proliferative variety, has recently been confirmed by gene expression profiling.105,106 (J.S., unpublished data, January 2002). Trials are, therefore, under way to determine whether vaccination with such peptides represents a more convenient and effective means of eliciting antimyeloma cytotoxic T-cell responses, which were associated with remarkable clinical activity in pilot studies of malignant melanoma.107
Bone-targeting modalities
The identification of signaling pathways in multiple myeloma has shed light on the need to target the bone marrow microenvironment as a means of disrupting the tumor–host interaction and self-supporting feedback loops. Myeloma cell adhesion to the bone marrow microenvironment and extracellular matrix triggers paracrine and autocrine growth loops involving, among other cytokines, IL-6, IL-15, and insulin-like growth factor-1 (IGF-1).4 The receptor associated factor/mitogen-activated protein kinase (RAF-MAPK), phosphatidylinositol 3-kinase (PI-3K)/Akt, nuclear factor κB (NF-κB), and signal transducer and activator of transcription 3 (STAT-3) pathways are activated, exerting signals on both myeloma and host cells that contribute to bone destruction, myeloma cell survival and proliferation, genomic instability, and drug resistance.4,6 The receptor activator of NF-κB and its ligand (RANK/RANK-L)/osteoprotegerin (OPG) axis, responsible for osteoclast activation,108-110 and the DKK-1/FRZB pathway, associated with osteoblast inactivation,111 have recently been recognized as additional important frameworks for myeloma–bone interaction and disease progression. Thus, disrupting these feedback loops has emerged as a new strategy of myeloma therapy. Indeed, beyond delaying the onset of skeletal events, the new generation of bisphosphonates, pamidronate,112 and zoledronic acid113 also exert antimyeloma effects indirectly by inducing osteoclast apoptosis, thus reducing a major source of the antiapoptotic IL-6 molecule,114,115 or directly by inducing myeloma cell apoptosis. Anecdotal clinical responses have been observed in smoldering multiple myeloma.116 The potentially beneficial antitumor effect elicited by bisphosphonates, however, has not been studied systematically. Thus, although frequently practiced, use of these agents in patients without bone disease is not based on prospective randomized trial results and needs to be viewed in the context of these agents' potential for nephrotoxicity. With appropriate monitoring of renal function and nonspecific proteinuria (sometimes the earliest sign of nephrotoxicity), pamidronate (90 mg intravenously over at least 2 hours monthly) or zoledronic acid (4 mg intravenously over at least 15 minutes monthly) can be given safely and indefinitely. In case of development of renal insufficiency related to myeloma progression or other causes, bisphosphonate dosing should be suspended until normalization or stabilization of renal function has been established. In case of severe bone disease, reduced doses and slower infusion times may be considered.
The emerging treatment paradigm of targeting healthy host cells may also pertain to immunomodulatory agents (thalidomide and CC-5013), dexamethasone, and PS 341, thus validating Paget's “seed and soil” hypothesis117 and Salmon's concept148 of treating the “soil” as a means of compromising the growth of the myeloma “seed.”
Special clinical scenarios
High host risk: the elderly and patients with renal failure
The rapid hematopoietic recovery afforded by mobilized PBSCs, also recently confirmed in the IFM 94 trial,15 was critical for the successful administration of melphalan to populations particularly vulnerable to high-dose therapy (high host-risk populations), such as those with renal failure118,119 or advanced age (> 70 years).120,121 The higher incidence of mucositis and other extramedullary toxicities encountered in patients receiving the standard high-dose melphalan regimen of 200 mg/m2 was virtually eliminated by dose reduction to 140 mg/m2 and, especially, to 100 mg/m2.122 Thus, advanced age and renal failure are no longer considered contraindications for dose-adjusted autotransplantations. These should be promptly applied once maximum cytoreduction has been achieved, preferably with noncytotoxic regimens such as high-dose dexamethasone pulsing either alone or in combination with thalidomide. In the absence of adequate tumor cytoreduction and persistent renal failure of recent onset because of presumed cast nephropathy, melphalan dose–adjusted autotransplantations should be instituted promptly by using growth factor–mobilized PBSCs. From 70% to 80% of such patients achieve at least 75% myeloma protein reduction (now best evaluated by the serum-free light chain assay), resulting in improvement or even normalization of renal function in 50%.123
Primary AL amyloidosis and immunoglobulin deposition disease
Even though their tumor load is very low, patients with primary AL amyloidosis and immunoglobulin deposition disease suffer from the consequences of myeloma secretory products, resulting in damage to kidneys, heart, gastrointestinal tract, liver, spleen, and peripheral and autonomic nerves. All current treatment targets the monoclonal plasma cell population. Whereas standard MP has only been marginally effective, high-dose dexamethasone pulsing plus interferon, effecting more rapid and profound responses in myeloma, has also shown encouraging results in primary amyloidosis.124,125 Although perhaps useful in noncardiac AL, thalidomide should be used with extreme caution in the setting of cardiac disease because of its well-recognized bradycardic effects. The Boston University group has pioneered the use of autologous stem cell–supported high-dose melphalan, demonstrating end-organ responses, especially in patients achieving CR defined by myeloma protein elimination.126 Cardiac amyloid remains the most challenging clinical condition and is currently addressed with dose-reduced melphalan (70-100 mg/m2) and stem cell support to avoid cardiac catastrophes possibly linked to arrhythmias, caused by fluid overload or cytokines.127
Solitary plasmacytomas of bone and extramedullary sites
Patients with soft tissue solitary plasmacytomas can often be cured with appropriately dosed local radiation (at least 4.5 Gy). By contrast, this local treatment approach fails in most patients with presumed solitary plasmacytomas of bone.128 The development of multiple myeloma in such patients probably reflects multifocal systemic disease present at the outset and heretofore not detectable by standard X-ray imaging but revealed by recently applied magnetic resonance imaging (MRI)129 and positron-emission tomography (PET) using 2-[fluorine-18]Fluoro-2-deoxy-D-glucose (FDG).130 Prospective trials are needed to determine whether, by applying appropriate staging tools, solitary plasmacytomas of bone truly exist, and, if so, if they can be cured by local irradiation only.
Local radiation, typically administered with high-dose dexamethasone, also has a palliative role for the treatment of focal lesions as part of disseminated myeloma. However, especially in patients with newly diagnosed disease, primary systemic therapy usually controls focal problems, including cord compression, as quickly and effectively as does local radiation (example, Figure 8). Avoiding radiation to major bone marrow–containing skeletal regions preserves the ability to procure adequate PBSC quantities. In advanced disease, it is critical to determine whether the source of pain is local tumor growth or vertebral collapse, with bone particles compressing the cord. In the latter case, surgical laminectomy is the preferred means of intervention. Vertebroplasty and kyphoplasty have both become important adjuncts of disease management, promptly alleviating pain because of vertebral collapse131,132 (example, Figure 9).
Cord compression detected by MRI. This is an example of rapid resolution of extensive spinal cord compression as a result of combination chemotherapy with DT PACE47 (dexamethasone 40 mg daily × 4, thalidomide 400 mg daily × 4, 4-day continuous intravenous infusions of cisplatin 10 mg/m2/d, Adriamycin 10 mg/m2/d, cyclophosphamide 400 mg/m2/d, and etoposide 40 mg/m2/d). Epidural disease from multiple myeloma responding to treatment: post-gadolinium T1-weighted MRI before (left) and after (right) treatment of epidural disease (arrows). Only a thin “stripe” of enhancing tissue remains after treatment.
Cord compression detected by MRI. This is an example of rapid resolution of extensive spinal cord compression as a result of combination chemotherapy with DT PACE47 (dexamethasone 40 mg daily × 4, thalidomide 400 mg daily × 4, 4-day continuous intravenous infusions of cisplatin 10 mg/m2/d, Adriamycin 10 mg/m2/d, cyclophosphamide 400 mg/m2/d, and etoposide 40 mg/m2/d). Epidural disease from multiple myeloma responding to treatment: post-gadolinium T1-weighted MRI before (left) and after (right) treatment of epidural disease (arrows). Only a thin “stripe” of enhancing tissue remains after treatment.
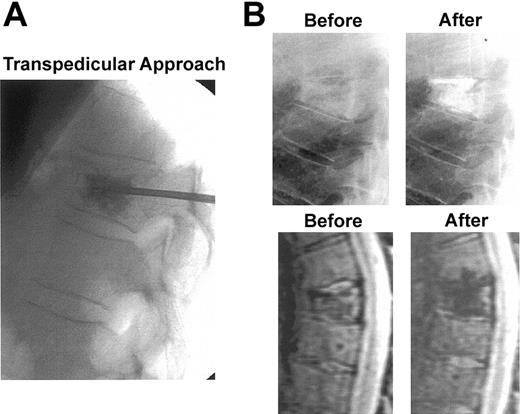
Compression fracture with vertebroplasty intervention. (A) X-ray procedure using standard transpedicular approach. (B) X-ray (top panels) and short inversion imaging (STIR)–weighted MRI sagittal images (bottom panels) of a compression fracture of T7 before and after vertebroplasty. Immediate and dramatic pain relief because of improved mechanical stability as well as the polymethylmethacrylate's neurotoxic effects.133
Compression fracture with vertebroplasty intervention. (A) X-ray procedure using standard transpedicular approach. (B) X-ray (top panels) and short inversion imaging (STIR)–weighted MRI sagittal images (bottom panels) of a compression fracture of T7 before and after vertebroplasty. Immediate and dramatic pain relief because of improved mechanical stability as well as the polymethylmethacrylate's neurotoxic effects.133
MGUS and smoldering multiple myeloma
The precursor lesion MGUS is usually not targeted for therapy. Smoldering multiple myeloma can be viewed as an advanced stage of MGUS that has not yet produced symptoms. Thalidomide alone,134 in combination with bisphosphonates or even with added dexamethasone, is now being explored as a means of preventing progression of smoldering to symptomatic myeloma. Indicators of high-risk smoldering myeloma include elevated levels of paraprotein, the presence of an immunoglobulin A (IgA) isotype, and a lytic bone lesion.135
Anemia
Anemia of variable severity affects more than two thirds of patients with myeloma. Most patients have an inappropriate erythropoietin response for the degree of their anemia. Overexpression of Fas-ligand, macrophage inflammatory protein 1α (MIP-1α), and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) by the myeloma cells triggers death signals in immature erythroblasts.136 In randomized, placebo-controlled and community-based studies, recombinant human erythropoietin has been shown to significantly decrease the frequency of transfusion requirements and improve the quality of life as well as performance status in anemic patients with myeloma.137 The effect of such treatment is greater in patients with limited prior therapy. Furthermore, patients with mild anemia (hemoglobin levels of 11-12 g/dL [110-120 g/L]) should not be excluded from erythropoietin therapy, because the greatest incremental improvement in quality of life appears to occur when hemoglobin increases to the 13 g/dL (130 g/L) range. Anemic patients should receive erythropoietin at doses of 40 000 U per week until hemoglobin levels exceed 13 g/dL (130 g/L). In intensive therapy–based programs, such an approach will decrease transfusion needs in addition to improving the quality of life. Darbepoietin, given at a dose of 200 μg every 2 weeks, is equally effective.
Hyperviscosity syndrome
Clinical manifestations of hyperviscosity are relatively infrequent and should be suspected in case of mental status changes, respiratory problems, renal insufficiency, or bleeding unexplained by other etiologies. The diagnosis is made by measuring serum viscosity or by funduscopic examination, showing a slow blood flow in often distorted blood vessels. Hyperviscosity can give the clinical and radiologic picture of pulmonary edema, but it will only worsen with the administration of diuretics. Plasmapheresis is the appropriate treatment for patients with symptomatic hyperviscosity, occurring more often with IgA and IgG3 isotypes. It should be continued until serum viscosity has normalized.
Immunosuppression and infections
Defects in B- and T-cell function are common among patients with symptomatic myeloma. With therapy, additional immune defects may develop, depending on the modality used (corticosteroids, cytotoxic chemotherapy, autologous, or allogeneic stem cell transplantation) as well as the presence of renal failure or progressive disease.138 The immune defects that increase the risk for serious infections are severe neutropenia and depressed CD4 counts. After effective cytoreduction, the levels of uninvolved immunoglobulins recover,139 whereas CD4+ T cells may remain suppressed for some time.138 Type and intensity of antineoplastic therapy used and the ensuing immunosuppression and end-organ toxicity (particularly mucositis) dictate the approach to managing infections.138 Preventing infection in the autologous transplantation setting relies on infusing sufficiently high doses of CD34+ cells (preferably ≥ 5 × 106/kg),140 avoiding TBI,49 adjusting melphalan dose intensity for renal function117,118 and age,119,120 and providing effective infection control and antimicrobial prophylaxis.138,141 Acyclovir reduces herpes simplex virus (HSV) reactivation among HSV-seropositive patients, whereas fluoroquinolones and fluconazole prevent bacterial and yeast infections in high-risk patients.138,141 The role of prophylactic hematopoietic growth factors in this setting is unclear. Following recovery, patients may develop herpes zoster virus or Pneumocystis carinii infections, particularly when CD4 and CD8 counts, 3 months after autotransplantation, remain low (< 400 and 800 cells/μL, respectively).142 Prophylaxis with acyclovir and trimethoprim-sulfamethoxazole (TMP-SMX; or alternatives) is recommended. The role of intravenous immunoglobulins remains controversial.
Patients undergoing allotransplantations are at the highest risk of severe, particularly fungal, infections even when nonmyeloablative regimens are used.141,143,144 Management of infection in these patients has been recently described.144
Myeloma patients exhibit a poor antibody response to pneumococcal and influenza vaccines. Whether vaccination will be effective after immune reconstitution among long-term myeloma survivors remains to be determined.
Current issues
Current issues with regard to autologous PBSC-supported high-dose therapy pertain to (1) need for maximum tumor cytoreduction prior to autotransplantation, (2) benefit from nonmyelotoxic peri-transplantation therapy (eg, dexamethasone, thalidomide, or anti–IL-6 monoclonal antibody) administered during the hematopoietic recovery phase to dampen the cytokine storm that may facilitate myeloma cell survival, (3) posttransplantation consolidation and maintenance strategies, (4) need for purging PBSCs to reduce the number of reinfused tumor cells, and (5) utilization of mini-allotransplantations. Higher glucocorticoid doses (prednisone 50 mg versus 10 mg on alternating days) have been shown, in a recent prospective randomized trial, to extend disease control and survival after standard therapies.145 Interferon-alpha maintenance marginally prolongs both EFS and OS in both standard- and high-dose therapy regimens.146 Newer agents, such as thalidomide and CC-5013, are now being evaluated for maintenance of disease control. Purging of autologous bone marrow or PBSCs has not improved clinical outcome,147 probably because of a high remaining tumor burden, minimizing a potential benefit of tumor cell-reduced autografts. The role of mini-allotransplantations is being evaluated after a preceding melphalan 200 mg/m2-based autotransplantation, mainly in high-risk patients (IFM 99). We also advocate the exploration of this tandem autologous/allogeneic transplantation approach only in high-risk myeloma patients, so that the potential risk of such intervention does not exceed the risk posed by the disease itself. Given the dire prognosis of metaphase-defined chromosome 13 deletions or hypodiploidy, the clinical benefit of this novel approach should be apparent within a few years.
Summary and conclusions
A consensus is emerging among myeloma investigators that stringently defined CR in myeloma is an important early objective toward achieving durable disease control. Because of its potential of inducing CR rates in the 40% to 50% range, autologous stem cell-supported high-dose melphalan has emerged as standard therapy for multiple myeloma. The feasibility of applying this approach in patients with renal failure (having a high probability of recovery if present for < 6 months) and in older patients (even beyond 70 years of age) has enabled clinicians to apply effective treatment (dose-reduced melphalan at 100-140 mg/m2) safely also to patients previously perceived to be at high risk because of comorbidities. Therefore, when discussing toxicity and efficacy of autotransplantations for myeloma, the composition of the conditioning regimen and dose intensities of applied agents, together with the quantity and quality of hematopoietic stem cells, determine procedure-related safety and long-term benefit. We recommend that all patients with newly diagnosed disease with symptomatic myeloma be considered for appropriately adjusted high-dose melphalan and autologous stem cell support as a safe and optimal means to achieve marked tumor cytoreduction with the attendant benefits of recovery from organ dysfunction and improvement in quality of life.
The discovery of entirely new classes of agents exhibiting activity in myeloma that has become refractory to even high doses of melphalan has stimulated their testing in various clinical scenarios, such as response induction and posttransplantation maintenance. Further refinements of mini-allogeneic transplantation procedures will hopefully make such an approach more widely available, especially once promising results are demonstrated in high-risk disease. New agent trials, whether targeting myeloma cells or the bone marrow microenvironment, should be combined with translational research to demonstrate that the intended target has indeed been altered.
Systematic application of cytogenetics and molecular genetics, especially gene expression profiling, should aid in a molecular classification of multiple myeloma. It readily distinguishes those patients prone to bone disease, amyloidosis, and other clinical manifestations. These applications should also provide more robust prognostication, aiding in mechanistic investigations of drug action and, finally, helping to identify new treatment targets in myeloma cells, the bone marrow microenvironment, and the immune system.
Major experimental challenges lie ahead to investigate the growth- and survival-promoting interplay of myeloma cells with their microenvironmental host partners, especially osteoclasts and osteoblasts and their precursors. Given the present state of our knowledge, treatment failure has to take into account the myeloma-sustaining influence of host cells, in addition to the traditional tumor cell targets, with multiple mechanisms of resistance and DNA repair. Traditional tumor cell inactivation should, therefore, be accompanied by strategies aimed at disrupting myeloma–host cell adhesion and its ensuing autocatalytic cytokine cascade. We expect that the use of tumor- and host-directed nongenocidal therapies (eg, glucocorticoids, bisphosphonates, anti–IL-6, immunomodulatory agents, proteasome inhibitors) will be important adjuncts toward either effecting a traditional cure or a return to a chronic benign disease state akin to MGUS or smoldering myeloma.
Supported by grant P01CA55 819-05A1S10 from the National Institutes of Health.
Prepublished online as Blood First Edition Paper, September 11, 2003; DOI 10.1182/blood-2003-04-1045.
The authors are grateful to the faculty and staff of the Myeloma Institute for insightful discussion and critical thinking that have fostered contributions to multiple myeloma research and clinical therapy over many years. The authors are also indebted to the support staff members for their caring, compassion, and skillful technical assistance.
This paper is dedicated to myeloma patients, their families, and caregivers who have given us the opportunity to serve their needs, search for answers, and seek a cure. It is further dedicated to the many physicians across the United States and around the world who have entrusted their patients' care to us.

![Figure 1. Comparison of tandem autotransplants with melphalan 200 mg/m2 (as part of Total Therapy I) versus standard alkylating agent therapy administered under the auspices of SWOG.43 Patients were matched by age (within 10 years), B2M (within 2 mg/L), and albumin (within 1 g/dL), major prognostic factors in SWOG trials. In a comparison of patients enrolled on Total Therapy I (TT I) and SWOG trials, no statistically significant differences were noted for the following characteristics: age 60 years or older (TT I, 26%; SWOG, 26%; P = .3); B2M 3 mg/L or more (TT I, 50%; SWOG, 47%; P = .3); albumin less than 3.5 g/dL (TT I, 24%; SWOG, 22%; P = .1); IgA isotype (TT I, 17%; SWOG, 18%; P = .9); creatinine 2 mg/L or more (TT I, 8%; SWOG, 11%; P = .2). TT I consisted of induction with the VAD regimen (vincristine, Adriamycin, dexamethasone) times 3 cycles; high-dose cyclophosphamide 6 g/m2 plus granulocyte-macrophage colony-stimulating factor (GM-CSF) with subsequent peripheral blood stem cell collection, tandem autotransplantations with melphalan 200 mg/m2 3 to 6 months apart (in cases in which a partial response was not achieved after the first transplantation, melphalan 140 mg/m2 plus total body irradiation [TBI] 8.5 Gy was administered), and interferon maintenance (median follow-up, 10 years). SWOG trials S8624, S9028, and S9210 were carried out from 1986 to 1997, contemporaneously with TT I (median follow-up, 9 years). Significantly superior overall survival (A) and event-free survival (B) were observed with TT I.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/1/10.1182_blood-2003-04-1045/6/m_h80145469001.jpeg?Expires=1767741983&Signature=oj-sUkj2H687aE~fpMyHNv1F-UOgc1TJfl4usq~V3YAT9668GStV6GW6LnDlA-Y4Wha~ml6rpU7fmGszS9wE-WISqhPofC7p45K2~3kK4BNbYZNWnjXt10-ZI2Wlxq4eMjJWvmWjJF8ZDchjnviH3ukCilAQz7lsXvzp55vxCR2CHKV9GVxQEz-6a4dT9pUq6rTVdCHxRt6nq-O4NIA8~x5WWyF0KdjEF3JYXcopyx9AxY0YqbTXzR0AJOQ87kKrTuZvEHEmXclzKpSk6DhUvU6nHi5wDLnFsG04jENh14s0hKpTwD5rbfnck~FoR1nBtmaPhhQbwqg1IrMUXZZ1lg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 5. CC-5013 phase 3 trial for advanced and refractory myeloma. (A) Dosing schedule. (B) Kinetics of response according to dosing schedule. M-protein responses were significantly higher with the 25 mg than with the 50 mg CC-5013 dose, although total doses per cycle were identical (500 mg). (C) Grade greater than 2 thrombocytopenia (< 50 000/μL[< 50 × 109/L]) was significantly more frequent and of earlier onset when platelet levels before CC-5013 were less than 100 000/μL (100 × 109/L).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/1/10.1182_blood-2003-04-1045/6/m_h80145469005.jpeg?Expires=1767741983&Signature=SfG0gVdV7rPtBCSzIydWdvj8jsHEIdvH-1H07DLAJGdHLqgWsPBYp-XTInhR0slwxhW6Rj5MO1Buq-f02hn2GyNQS4jeEqUX6DZquu76jGWymS-5h6uqB36mVZ3ffE~BzTYZjpK9V0n1z2kyD2gizUTrxheoZ8EjSgitu2TbKqtRHL1byU86nmYpz6MuhEuPmabTnaTjB0IeAZo35f1az41EcQkAwKRADb6n14kxQSPc35EWmkRs0wrSMWjm~WWPGQ6V9XDwt-lV6BW1OS9Gq1bAMhxr39Q9LhvLV3UnFotDub~KxBhuy55EGfa0e8Lhd12rqZeKpTkGrtT9xHjV5Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 7. Gene expression patterns can be used to evaluate the consequences of, and predict response to, chemotherapy. (A) Gene expression changes after short-term in vivo drug exposure can potentially reveal molecular mechanisms of action. RNA isolated from purified plasma cells before and after 48 hours in vivo treatment with PS 341 (VEL, n = 15), CC-5013 (REV, n = 13), dexamethasone (DEX, n = 20), or thalidomide (THAL, n = 18) was applied to U95Av2 microarrays (Affymetrix, Santa Clara, CA). Significant induction or suppression of gene expression after drug treatment was calculated on the basis of the percentage of change from baseline ([follow-up – baseline]/baseline). The top 15 genes in each drug group were selected according to rank P value and plotted on the basis of the percentage of change (red = increase in expression; green = decrease in expression). Drug treatment samples are grouped in columns, and genes are in rows. Note that genes up-regulated by dexamethasone (first 15 genes from the top) tend to be unique to that drug. Also note that most of the genes up-regulated by CC-5013 are also up-regulated by thalidomide, thus indicating possible common molecular targets for these 2 drug analogs. (B) Gene cluster of normalized expression values of a group of genes demonstrating differences between myeloma cases exhibiting response or no response to PS 341 proteasome inhibitor therapy. Plasma cells were purified from bone marrow aspirates from 40 patients before the initiation of therapy and after informed consent. Total RNA was isolated, and gene expression levels of approximately 12 000 genes were analyzed using the Affymetrix U95Av2 GeneChip microarray. After sufficient follow-up, 21 responders (exhibiting at least a 50% reduction in serum M protein) and 19 nonresponders (exhibiting progression, stabilization, or no > 25% reduction in M protein) were identified. With the use of a combination of chi square, Wilcoxon rank, and discriminant analysis, 40 genes were identified as being expressed higher in the nonresponder group and 42 higher in the responder group. Genes are in rows, and patient samples are in columns. The nonresponders are grouped under the green bar and responders under the red bar. The table below panel B shows the correlation of results from a gene expression–based prediction model of response and actual response to PS 341 therapy by using the same 40 patients analyzed in panel B. The results presented in the matrix were derived by first assessing the mean difference for all 12 625 probe sets between responders and nonresponders, and then obtaining P values and adjusted P values based on permutation tests (20 000 samples). The 40 samples were then randomly grouped into 5 strata of n = 8. Using 30 of the most significantly differentially expressed genes, a prediction model was developed by using stepwise logistic regression on data from 4 of the 5 strata (80% of total data, n = 32). The model developed on 80% of the data was applied to the 20% (n = 8) left out as a validation group. With 5 strata we developed 5 separate models and validated them on 5 separate validation samples. We averaged over the 5 validation samples to get an estimate of 88% (35 of 40 correctly classified).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/1/10.1182_blood-2003-04-1045/6/m_h80145469007.jpeg?Expires=1767741983&Signature=uFp3gnsS64LrLLjVGzBy0d-h5OczFuHI5mywpM7tUK5q~wYUb9dI1YZOxA2MELut6HlXsLXo3UepVKVr1KsBzmmQoSI0ozrKacTwpbza1IgQpDbAhqpxEsU79q0fz7IcVyebVAVgdFYU9oraZ3yjTedu0uIk1sd-Ug2t~UCDwbO~LjD2t3H46hliPGSP5LMsS6EcdXxYuJE8WdxuEyQDNwCykq-1V8DwZU5PgIHiDknercl3KhxfEVZ3lJap1vd5JFVTlA59-5~3VBOnkAosEhZ21yqZe0L9ZJtQjvebK2AJASSwpIPUSr4Aw0Jrfn8ETWhXfRql-8vwlL2YaU3K6w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal