Abstract
Besides regulating leukocyte trafficking in normal and injured tissues, several chemokines may positively or negatively regulate angiogenesis. Here we report that CCL16 activates an angiogenic program in vascular endothelial cells by activating CCR1. CCL16 induces dose-dependent random and directional migration of endothelial cells isolated from large vessels and liver capillaries without inducing their proliferation. It also promotes endothelial differentiation into capillary-like structures in an in vitro assay and is angiogenic in the chick chorionallantoic membrane. These angiogenic activities are neutralized by a specific antibody against CCL16. The direct angiogenic activity of CCL16 is further amplified by its ability to prime endothelium to a mitogen signal induced by vascular endothelial growth factor A and to raise their basal production of CXCL8 and CCL2, 2 other angiogenic chemokines. BX471 (R-N-[5-chloro-2-[2-[4(4-fluorophenyl) methyl]-2-methyl-1-piperazinyl]-2-oxoethoxy]phenyl] urea hydrochloric acid salt), a CCR1 antagonist, inhibits angiogenic properties of CCL16, whereas blocking of CCR8 or desensitizing CCR2, which are both well known receptors for CCL16, did not abolish endothelial activation. CCL16 may be specifically cross-linked to CCR1 expressed on endothelial cells. The largely restricted CCL16 expression in the liver suggests that this chemokine may play a role in hepatic vascular formation during development and in angiogenesis associated to hepatic diseases.
Introduction
Chemokines are polypeptide molecules mainly involved in the control of leukocyte functions and trafficking through the activation of receptors belonging to the 7-transmembrane spanning, G-protein–coupled receptor family. Near the NH2 end of the molecule, the presence of cysteine residues identifies 4 subfamilies. They are characterized by the presence of a unique C, or of 2 Cs separated by a single (CXC) or 3 amino acids (CXXXC) or adjacent (CC), and show largely overlapping biologic activities.1
CCL16, also known as liver-expressed chemokine or NCC-4 or lymphocyte and monocyte chemoattractant or HCC-4, is a CC chemokine that specifically attracts lymphocytes, dendritic cells, and monocytes; increases their adhesive properties; and has myelosuppressive activity.2-6 It is constitutively expressed in liver3,7 and is increased by interleukin 10 (IL-10) in activated monocytes.2 Interestingly, CCL16 is present in human plasma suggesting that it may be active outside hepatic tissue.7 CCR1, CCR2, CCR5, and CCR8 are the functional receptors of this chemokine.5,7 CCL16 released by engineered tumor cells elicits their rejection by a CD8+- and neutrophil-dependent mechanism and a rapid induction of a tumor-specific systemic immune response. This suggests that CCL16 improves recognition of poorly immunogenic cells by promoting a cross talk between T lymphocytes and antigen-presenting cells.8 The histochemical analysis of these tumors showed expression of adhesion molecules by endothelial cells (ECs), without signs of vascular necrosis or inhibition of angiogenesis, in spite of the abundant neutrophil infiltrate that usually characterizes other models of tumor rejection promoted by cytokines, where vasculature is severely affected.9-13
Chemokines play a role in embryo and adult vascular bed formation.14,15 Besides an indirect mechanism involving angiogenic inducers released by activated leukocytes,16,17 both CC and CXC chemokines may directly activate a proangiogenic18-28 or an angiostatic19,29-35 program in ECs.
In CXC chemokines, IL-8 (CXCL8), neutrophil-activating peptide-2 (CXCL7), epithelial cell–derived neutrophil activating protein-78 (CXCL5), stroma-derived factor-1α (CXCL12), and growth-related oncogenes α and β (CXCL1, CXCL3)18,19,22,23,28,36,37 have been shown to promote in vitro EC motility and to be angiogenic in cornea assays.
Interferon-γ–inducible protein-10 (CXCL10), monokine induced by interferon-γ (CXCL9), interferon-inducible T-cell α-chemoattractant (CXCL11), growth-related oncogene-β (CXCL2), and platelet factor-4 (CXCL4) are CXC chemokines with angiostatic effect and are potent inhibitors of tumor angiogenesis.11,13,19,29-32,34
CX3CL1, the sole member of the CX3C chemokine family, has been demonstrated to be a powerful mitogen of ECs and angiogenic too by activating CX3CR1.38
An emerging role of CC chemokines in the assembly of vascular patterns came from the study of the pathogenetic effect of human herpes virus-8.20 The viral macrophage inflammatory protein I (vMIP-I) and -II, which share extensive sequence homology with human protein (≈40%), and vMIP-III are angiogenic in the chorionallantoic assay.20,21 Monocyte-chemotactic peptide-1 (CCL2),25 I-309 (CCL1),24 and eotaxin (CCL11)27 are the 3 human CC chemokines demonstrated to be angiogenic by activating CCR2, CCR8, and CCR3, respectively. C6kine (CCL21) is the sole chemokine belonging to the CC subfamily showing angiostatic properties.33
To gain a better understanding of the putative activity of CCL16 on vessel walls that emerged in CCL16-mediated tumor rejection in mice,8 we studied biologic functions of human ECs stimulated by this chemokine. We found that CCL16 induces EC motility by interacting with CCR1 and has angiogenic activity in vitro and in vivo.
Materials and methods
CCL16 and other stimuli
Recombinant human CCL16 was expressed and purified from Escherichia coli (a kind gift from PeproTech Inc, Rocky Hill, NJ; endotoxin level < 0.1 ng/μg). CCL16 cDNA was expressed in mammalian cells and the released protein was purified on heparin-sepharose affinity chromatography on the basis of the heparin binding sequence.2-4 CCL16 cDNA was cloned by Human Genome Sciences (Rockville, MD) through an expressed sequence tags database search by a high degree of homology to the CC chemokine gene. The CCL16 open reading frame was amplified as a HindIII/XbaI fragment and subcloned into pcDNA3 vector. Stable transfectants were prepared in murine mammary adenocarcinoma cells trichostatin A (TSA) as described8 and conditioned medium was recovered after 72 hours of culture in Dulbecco modified Eagle medium (DMEM; Sigma-Aldrich, St Louis, MO) with 0.1% fetal calf serum (FCS; Life Technologies, Inc, Gaithersburg, MD). Alternatively the construct was used to transiently transfect Cos-7 cells (American Type Culture Collection, LGC Promochem, Teddington, United Kingdom) by Lipofectamine (Invitrogen Italia SRL, Milan, Italy). Typically, each subconfluent culture of Cos-7 cells in a 100-mm diameter Petri dish containing 6 mL of DMEM supplemented with 1 mM sodium butyrate was transfected with 10 μg of DNA, and after 48 hours the culture medium was recovered. Medium (50 mL) from TSA and Cos-7 cells carrying vector alone (vec-TSA; vec-Cos) or CCL16 cDNA (CCL16-TSA, CCL16-Cos) was diluted (1:1, vol/vol) with Tris-buffered saline (10 mM Tris hydroxymethyl aminomethane, 0.15 M NaCl, pH 7.2) and applied to a HiTrap Heparin HP column (Amersham Pharmacia Biotech, Uppsala, Sweden) maintained at 4°C. The column was washed with 20 mL of buffer and then CCL16 was eluted with 5 mL Tris-buffered saline containing 0.6 M NaCl. In preliminary experiments in which a linear gradient of NaCl (0-2 M) was applied to the column, this salt concentration was demonstrated optimal to elute CCL16. CCL16 purification was checked by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE; 10%) and immunoblotting. Fifty microliters of samples were denatured in 100 μL of 50 mM Tris/HCl (pH 6.8) containing 10% glycerol, 1% SDS, and 5% β-mercaptoetanol; fractionated by SDS-PAGE; and electroblotted onto Immobilon-P membrane (Millipore Co, Bedford, MA). The membrane was incubated with a goat polyclonal anti-CCL16 antibody (Ab) (1:500) raised against recombinant CCL16 (rCCL16; PeproTech) and staining was visualized by enhanced chemiluminescence technique (Amersham Pharmacia Biotech). In the experiments shown in this work we used 8 different batches of CCL16-Cos and vec-Cos and 2 batches of CCL16-TSA and vec-TSA. All batches used gave similar results in chemotaxis assay.
The quantification of purified CCL16-Cos or CCL16-TSA was performed by coating an 8-well strip (Falcon, BD Biosciences, Milan, Italy) with 50 μL of scalar dilutions of recombinant protein (from 1000 to 1 ng/mL) or sample overnight at 4°C. After washing with Dulbecco phosphate buffer solution (Sigma-Aldrich) containing 0.05% Tween 20 (Sigma-Aldrich), the strips were incubated with purified with anti-CCL16 Ab (1:2000) for 2 hours. After repeated washes with Dulbecco phosphate buffer solution containing 0.05% Tween (Sigma-Aldrich), the strips were incubated with a biotin-conjugated goat antirabbit immunoglobulin (Ig; Dako, Milan, Italy) for 1 hour at room temperature and subsequently with avidin-peroxidase for 30 minutes. A peroxidase substrate (2,2′ azino bis 3-ethylbenzen-thiazoline-6-sulphonic acid; Sigma-Aldrich) was used as for the colorimetric reaction. Optical density was determined using a microplate reader (BioRad, Milan, Italy) set to 405 nm. CCL16 preparations were neutralized by overnight incubation with anti-CCL16 Ab (1:100) or goat IgG (Sigma-Aldrich).Vascular endothelial growth factor A165 (VEGF-A165) and CCL11 were purchased from R&D Systems (Minneapolis, MN).
Cells
Human ECs were isolated from umbilical cord veins (HUVECs) characterized and grown in M199 medium (Sigma-Aldrich) containing 20% FCS as previously described.39 They were used at early passages (I-IV). Human liver capillary ECs from patients undergoing liver surgery were isolated, purified, and characterized as previously described from normal hepatic tissue.40,41 Written informed consent was obtained from the donors. After collagenase digestion of liver fragments (2 hours at 37°C in M199 medium containing 0.25% of collagenase D [Boehringer-Mannheim, Mannheim, Germany] and 0.25% bovine serum albumin [BSA]), the cell suspension was plated on culture dishes previously coated with human oncofetal fibronectin.40 This step allowed us to obtain an enrichment of the EC population after 5 to 7 days of incubation. To purify ECs, 5 × 106 magnetic beads (Dynal, Oslo, Norway) covalently bound with Ulex Europaeus 1 lectin (Sigma-Aldrich) were added to 106 cells (ratio of cells to beads = 1: 5) and incubated for 30 minutes at 4°C, and ECs bound to the beads were separated from the unbound cells using the magnetic particles concentrator (Dynal). Liver ECs were plated onto collagen type I–(Sigma-Aldrich; 10 μg/mL) and oncofetal fibronectin–coated (10 μg/mL) dishes at a density of 2 × 104/cm2. Cells were maintained in endothelial basal medium supplemented with 10% FCS, 100 μg/mL heparin, 1 μg/mL hydrocortisone, 10 ng/mL epidermal growth factor, and 10 μg/mL bovine brain extract (all purchased from Clonetics, San Diego, CA).
Kaposi sarcoma (KS) cells (Prof A. Albini, Istituto Tumori [IST], Genova Italy) were derived from a non-AIDS patient and are immortalized without signs of senescence after more than 120 in vitro passages. This cell line shares common markers and similar biologic behavior with typical KS “spindle cells.”42 They were grown in M199 with 20% FCS.
Flow cytometric analysis
Indirect immunofluorescence was performed on liver ECs by exposing cells to saturating amounts of monoclonal Ab to human CCR1 (R&D Systems). Fluorescein-conjugated F(ab′)2 fragments of goat antimouse diluted (Sigma-Aldrich) 1:100 were used as the secondary Ab. After staining, cells were analyzed using a FACScan flow cytometer (BD Biosciences).
Reverse transcriptase–polymerase chain reaction (RT-PCR)
Total cellular RNA was isolated by RNAzol (Tel-Test, Friendswood, TX) according to manufacturer's instruction. Ten micrograms of total RNA were reverse transcribed using an Rnase H-RT (Promega, Madison, WI) according to the manufacturer's protocol. Resultant cDNA was amplified for the following primers: CCR1: 5′-GCA GCC TTC ACT TTC CTC AC (sense) and 5′-AGG CGT AGA TCA CTG GGT TG (antisense); CCR2: 5′-CTC GTC GGT GTT CAT CTT TG (sense) and 5′-TCT CAC TGC CCT ATG CCT CT (antisense); CCR8: 5′-CAT CCT GGT CCT TGT GGT CT (sense) and 5′-GGC TTG GTC TTG TTG TGG TT (antisense). Amplifications were performed in a thermocycler (PerkinElmer Life Sciences, Boston, MA) by using 1.25 U of Taq polymerase (Roche Applied Science, Monza, Italy) after heating at 94°C for 3 minutes, followed by 30 amplification cycles: 45 seconds at 94°C, 45 seconds at 60°C, and 120 seconds at 72°C.43
Cross-linking experiment
rCCL16 (2 μg) was dissolved in 200 μL of sodium phosphate buffer 20 mM (pH 7.4) and transferred in iodogen-coated tubes (50 μg/mL; Pierce Chemicals, Rockford, IL) where it was iodinated (5 minutes, 4°C) with 1 mCi (37 MBq) [125I] (Amersham Pharmacia Biotech). Twenty microliters of phosphate buffer 20 mM (pH 7.2) containing 1% BSA, 0.4 M NaCl, 0.1% CHAPS (3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid; Pierce) was added and the reaction products were separated on Sephadex-G10. The specific activity of the tracer was 74 000 counts per minute (cpm)/ng. [125I]CCL16 retained its biologic activity as measured by migration of HUVECs. For cross-linking, experiments on confluent HUVECs (2 × 107) were incubated in DMEM containing 20 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; pH 7.4), 0.1% BSA, 100 μg/mL soybean trypsin inhibitor, and bacitracin binding medium with 0.5 nM of [125I]CCL16 at room temperature for 10 minutes and then 1 mM BS3 (bis[sulfosuccinamide]suberate; Pierce) was added. The cross-linking reaction was allowed to proceed at 4°C for 30 minutes. Binding specificity was evaluated by adding 0.5 μM CCL16. After 2 washes with phosphate-buffered saline, cells were lysed (20 minutes, 4°C) in NaCl 150 mM, 50 mM (pH 7.4) containing 1% Triton X-100, phosphatase, and protease inhibitors (pepstatin, 50 μg/mL; leupeptin, 50 μg/mL; aprotinin, 10 μg/mL; 2 mM phenyl-methyl-sulfonil fluoride; soybean trypsin inhibitor, 500 μg/mL; 5 mM ethylendiaminetetraacetate). After preclearing with protein A–Sepharose, CCR1 was immunoprecipitated by a specific polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) (1 h, 4°C) and proteins were separated by SDS-PAGE (10%). Bands were visualized by autoradiography.
Cell motility
Chemotaxis assays were performed as previously described44 by the Boyden chamber technique (Neuroprobe, Pleasanton, CA; AP48 micro chemotaxis chamber) using a 5-μm pore size polycarbonate filter (Neuroprobe) coated on both sides with gelatin (0.1% for 6 hours at room temperature). CCL16 preparations in medium supplemented with 0.25% BSA were seeded in the bottom compartment of the chamber, and 2 × 105 suspended cells in medium 199 containing 1% FCS were then seeded in the top compartment. After 6 hours of incubation at 37°C, the top surface of the filter was scraped with a rubber policeman. The filters were fixed and stained with Diff-Quick (Baxter Spa, Rome, Italy) and 10 fields were counted after coding samples at × 400 magnification. Checkerboard analysis of the chemotactic response was performed by varying the concentrations of CCL16 preparations in the top and bottom compartment of the Boyden Chamber.39
Cell motility was further analyzed by time-lapse videomicroscopy. HUVECs (150 cells/mm2) were plated on fibronectin-coated (2 μg/mL) Petri dishes for 1 hour and then stimulated in M199 with CCL16-Cos (50 ng/mL) or equal volume of vec-Cos. Cells were observed with an inverted photomicroscope (model DM IRB HC; Leica Microsystem, Heerbrugg, Switzerland) and their motility was recorded. Constant temperature (37°C) and CO2 (5%) were maintained throughout the experiment by means of heatable stage and a climate chamber. Phase contrast snap photographs and 5-hour long movies (one frame every 5 minutes) were taken with a cooled digital CCD Hamamatsu ORCA camera (Hamamatsu Photonics Deutschland, Herrsching, Germany), digitally recorded, and analyzed with ImageProPlus 4.0 imaging software (Media Cybernetics, Leiden, The Netherlands). Cell paths were generated from centroid positions and migration parameters were computed with DIAS software (Solltech Inc, Oakdale, IA). The x and y coordinates of the cell centroids were recorded every 10 minutes.
The reported cell speed for each condition is an average of 100 cells. Directional persistence was calculated by determining the ratio between the net path length and the total path length.45 Single cell trajectories (n = 10) were plotted using Excel software and displayed in windrose graphs.
EC proliferation
The evaluation of EC proliferation was performed with Biotrak cell proliferation enzyme-linked immunosorbent assay (ELISA) system (Amersham Pharmacia Biotech). Cells (2.5 × 103) were plated in 96-well plates coated with human fibronectin (Sigma-Aldrich) and grown for 12 hours in M199 containing 5% FCS. Then, they were washed and maintained for 12 hours at 37°C in serum-free M199 containing 5% human serum albumin and subsequently stimulated with CCL16 or VEGF-A165 for 24 hours. BrdU (5-bromo-2′-deoxyuridine) was added to the cells for an additional time of 3 hours. In some experiments, ECs were preincubated for 1 hour with CCL16-Cos or VEGF-A165, washed, and then stimulated with VEGF-A165 or with CCL-16, respectively. Fixed cells were treated according to the manufacturer's instructions and the resultant color developed by the immune-complexes peroxidase-labeled anti-BrdU was read at 450 nm in a plate spectrophotometer (PerkinElmer Life Sciences).
Cytosolic Ca2+ measurement
HUVECs (8 × 105) were seeded on a glass coverslip (12-mm diameter) coated with fibronectin (Sigma-Aldrich, 10 μg/mL) and grown for 24 hours as described above. [Ca2+] transients were measured by loading HUVECs with 5 μM Fura-2/AM (Molecular Probes, Eugene, OR) for 45 minutes at 37°C in culture medium. After loading, coverslips were washed with culture medium and preincubated for 15 minutes at 37°C with BX471 (R-N-[5-chloro-2-[2-[4(4-fluorophenyl) methyl]-2-methyl-1-piperazinyl]-2-oxoethoxy]phenyl] urea hydrochloric acid salt; Berlex Biosciences, Richmond, CA) or vehicle and then stimulated with CCL16-Cos or CCL11. Fluorescence of the intracellular Fura 2 was measured in a PerkinElmer LS-50B spectrofluorimeter equipped with FL-Winlab software (PerkinElmer). The standard monochromator settings were 340-nm excitation and 510-nm emission. For the test, the coverslip was firmly positioned in a quartz cuvette (1 cm) containing 1 mL of Hanks balanced salt solution so that emission radiation reached the coverslip surface at a 45° angle.46
In vitro morphogenesis assay
Matrigel (Collaborative Biomedical Products, BD Biosciences; growth factor-free) was added to each well of a 48-well plate and incubated at 37°C for 30 minutes to allow gel formation. HUVECs (2 × 104/well) were plated onto matrigel in M199 containing 1% FCS and stimulated with CCL16-Cos, vec-Cos, or VEGF-A165. After 14 hours of incubation in a 5% CO2 humidified atmosphere at 37°C, the cell 3-dimensional organization was examined under DM-IBM inverted phase contrast photomicroscope (Leica, Milano, Italy) and then photographed.47 Capillary-like structures were quantitated48 by automatic counting in triplicate of low-power fields (× 40) using the ImageProPlus 4.0 imaging software, and percentage variation was expressed assuming VEGF-A165–treated ECs as 100%.
Chick embryo chorioallantoic membrane (CAM) assay
Fertilized White Leghorn chick eggs were incubated under conditions of constant humidity at 37°C. On the third day of incubation, a square window was opened in the eggshell after removal of 2 to 3 mL of albumen so as to detach the developing CAM from the shell. The window was sealed with a glass of the same size and the eggs were returned to the incubator. At day 8, 1-mm3 sterilized gelatin sponges (Gelfoam; Upjohn Co, Kalamazoo, MI) adsorbed with CCL16-Cos or vec-Cos dissolved in 3 μL of phosphate-buffered saline were implanted on the top of growing CAMs under sterile conditions within a laminar flow hood.49 Sponges containing vehicle alone or 50 ng of VEGF-A165 were used as negative and positive controls, respectively. In some experiments sponges were loaded with both CCL16-Cos and BX471. CAMs were examined daily until day 12 and photographed in ovo under a Zeiss stereomicroscope SR equipped with an MC 63 Camera System (Zeiss, Oberkochen, Germany). The number of vessels was quantified with ImageProPlus 4.0 imaging software in 3 randomly selected areas (1 mm2) as previously described.50
EC treatment with chemokine receptor antagonists
In some experiments, ECs were preincubated with BX471 urea hydrochloric acid salt for 15 minutes at 37°C or with goat Ab anti-CCR8 (Alexis Biochemicals, San Diego, CA; 1:200) or nonimmune goat IgG (Sigma-Aldrich) for 2 hours at 22°C before adding agonists.
Cytokine production
Confluent HUVECs (3 × 105) were starved overnight in M199 supplemented with 2% FCS and 3% human lipopolysaccharide-free serum albumin (Farma Biagini, Lucca, Italy). Cells were stimulated with different concentrations of CCL16-Cos or vec-Cos for the indicated times in M199 containing 5% FCS. Medium was recovered, centrifuged (13 000g, 5 minutes at 4°C), and stored at –80°C. The amount of CXCL8 and CCL2 released was evaluated with cytokine-specific ELISA kits (Amersham Pharmacia Biotech; sensitivity, < 10 pg/mL).
Results
CCL16 stimulates directional and random motility of ECs and KS cells
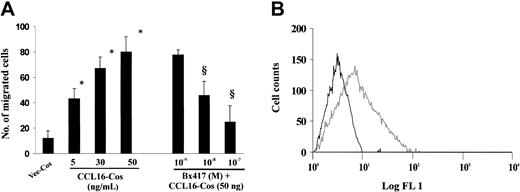
Stimulation of EC motility is a common feature of angiogenic inducers, some of which may promote cell proliferation.16 To assess whether CCL16 could initiate an angiogenic program, its ability to induce cell movement was studied in Boyden chambers by using human ECs isolated from large vessels or hepatic tissue. CCL16-Cos and rLEC showed a dose-dependent chemotactic activity on HUVECs, the former being more active (Figure 1). The maximal chemotactic activity observed at 50 ng/mL of CCL16-Cos was completely abrogated by pretreating the chemokine with a neutralizing Ab anti-CCL16 (Figure 1). Similarly, Ab anti-CCL16 neutralized the chemotactic effect of rCCL16 (50 ng/mL) (rCCL16 + IgG: 43 ± 5 migrated ECs; rCCL16 + Ab anti-CCL16: 14 ± 6; Ab anti-CCL16: 12 ± 7; mean ± SD, n = 4). Also CCL16-TSA activated HUVEC chemotaxis but its action was only partially reduced by the specific Ab (60% of inhibition) suggesting that other chemotactic molecules were released by TSA cells and coeluted with CCL16 from the heparin-affinity chromatography (Figure 1).
HUVECs motility triggered by CCL16. Chemotaxis of HUVECs (2 × 105/50 μL) was studied by adding the indicated preparations of CCL16 in the bottom compartment of a Boyden chamber. After 6 hours, migrated cells in 10 microscopic fields at an original magnification of × 400 were counted. Mean ± SD of at least 4 experiments. Data were analyzed by one-way analysis of variance (F = 83.80) and Student-Newman-Keuls test. * indicates P < .05 versus Vec-Cos; §, P < .05 versus CCL16 + Ab anti-CCL16; **, P < .05 versus unstimulated cells; §§, P < .05 versus Vec-TSA.
HUVECs motility triggered by CCL16. Chemotaxis of HUVECs (2 × 105/50 μL) was studied by adding the indicated preparations of CCL16 in the bottom compartment of a Boyden chamber. After 6 hours, migrated cells in 10 microscopic fields at an original magnification of × 400 were counted. Mean ± SD of at least 4 experiments. Data were analyzed by one-way analysis of variance (F = 83.80) and Student-Newman-Keuls test. * indicates P < .05 versus Vec-Cos; §, P < .05 versus CCL16 + Ab anti-CCL16; **, P < .05 versus unstimulated cells; §§, P < .05 versus Vec-TSA.
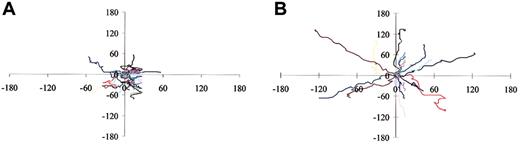
Migration speeds of HUVECs were quantified using time-lapse videomicroscopy of individual cells (Figure 2). HUVECs exhibited a baseline mean migration speed of approximately 16 μm/hour that rose to approximately 25 μm/hour after challenge with CCL16-Cos. CCL16-Cos stimulation resulted in higher directional persistence of cell migration as visualized by windrose plot; while unstimulated HUVECs moved in random directions, stimulated cells tended to continue to migrate in a particular direction. Differences in migration patterns were quantified by comparing the ratios of the shortest direct distance from the starting point of each recording to the end point (D), to the total distance run by the cell (T). The directional persistence (D/T ratio)45 of CCL-16-Cos–stimulated cells was about 2-fold higher than that of unstimulated cells (0.193 ± 0.07 versus 0.101 ± 0.05; P < .003; Figure 2).
Tracks of HUVECs stimulated with CCL16. Panels A and B respectively represent paths of cells tracked by video-lapse microscopy in presence of Vec-Cos or CCL16-Cos (50 ng/mL) over a period of 5 hours. Cell paths are replotted such that all paths start from the origin.
Tracks of HUVECs stimulated with CCL16. Panels A and B respectively represent paths of cells tracked by video-lapse microscopy in presence of Vec-Cos or CCL16-Cos (50 ng/mL) over a period of 5 hours. Cell paths are replotted such that all paths start from the origin.
To distinguish the chemokinetic component of HUVEC motility, the chemotactic gradient was abrogated by adding increasing amounts of CCL16-Cos in the top chamber of the Boyden apparatus. The checkerboard analysis of the results obtained demonstrated the capacity of CCL16 to increase random motility of the cells (Table 1).
CCL 16-induced HUVEC chemotaxis
. | Top chamber . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| Bottom chamber . | No. of migrated cells . | . | . | . | |||
| CCL 16-Cos, ng/mL . | 0 . | 5 . | 25 . | 50 . | |||
| 0 | 21 ± 4 | 22 ± 3 | 23 ± 3 | 24 ± 5 | |||
| 5 | 30 ± 5 | 35 ± 5 | 31 ± 3 | 30 ± 6 | |||
| 25 | 47 ± 5 | 43 ± 7 | 56 ± 4 | 50 ± 5 | |||
| 50 | 58 ± 3 | 55 ± 5 | 63 ± 4 | 68 ± 4 | |||
. | Top chamber . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| Bottom chamber . | No. of migrated cells . | . | . | . | |||
| CCL 16-Cos, ng/mL . | 0 . | 5 . | 25 . | 50 . | |||
| 0 | 21 ± 4 | 22 ± 3 | 23 ± 3 | 24 ± 5 | |||
| 5 | 30 ± 5 | 35 ± 5 | 31 ± 3 | 30 ± 6 | |||
| 25 | 47 ± 5 | 43 ± 7 | 56 ± 4 | 50 ± 5 | |||
| 50 | 58 ± 3 | 55 ± 5 | 63 ± 4 | 68 ± 4 | |||
Checkerboard analysis of CCL 16-induced HUVEC chemotaxis was evaluated by adding CCL 16-Cos at specified concentrations in the bottom compartment of the Boyden chamber in the absence or presence of specified concentrations of the chemokine added in the top compartment of the apparatus. Mean ± SD of 3 experiments.
We extended our observations to include capillary ECs isolated from human liver, the tissue in which CCL16 is mainly produced.2-4 Figure 3 and Table 2 show that CCL16 promoted both chemotaxis and chemokinesis of hepatic ECs, indicating that this chemokine is also active on microvascular ECs.
Hepatic ECs motility triggered by CCL16. (A) Chemotaxis of liver ECs (2 × 105/50 μL) was evaluated as described in Figure 1. In some experiments cells were preincubated for 15 minutes with BX417. Mean ± SD of 3 experiments. Data were analyzed by one-way analysis of variance (F = 35.61) and Student-Newman-Keuls test. * indicates P < .05 versus Vec-Cos; §, P < .05 versus CCL16-Cos (50 ng). (B) CCR1 expression on the cell surface of liver ECs. Cells were incubated with either IgG control (black line) or with CCR1 mAb (gray line) and analyzed by flow cytometry. A representative experiment of 2 is shown.
Hepatic ECs motility triggered by CCL16. (A) Chemotaxis of liver ECs (2 × 105/50 μL) was evaluated as described in Figure 1. In some experiments cells were preincubated for 15 minutes with BX417. Mean ± SD of 3 experiments. Data were analyzed by one-way analysis of variance (F = 35.61) and Student-Newman-Keuls test. * indicates P < .05 versus Vec-Cos; §, P < .05 versus CCL16-Cos (50 ng). (B) CCR1 expression on the cell surface of liver ECs. Cells were incubated with either IgG control (black line) or with CCR1 mAb (gray line) and analyzed by flow cytometry. A representative experiment of 2 is shown.
CCL 16-induced chemotaxis of hepatic ECs
. | Top chamber . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| Bottom chamber . | No. of migrated cells . | . | . | . | |||
| CCL 16-Cos, ng/mL . | 0 . | 5 . | 25 . | 50 . | |||
| 0 | 11 ± 6 | 14 ± 7 | 12 ± 6 | 10 ± 5 | |||
| 5 | 44 ± 8 | 39 ± 11 | 41 ± 5 | 38 ± 5 | |||
| 25 | 66 ± 5 | 53 ± 12 | 73 ± 9 | 54 ± 8 | |||
| 50 | 78 ± 9 | 85 ± 7 | 79 ± 6 | 88 ± 6 | |||
. | Top chamber . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| Bottom chamber . | No. of migrated cells . | . | . | . | |||
| CCL 16-Cos, ng/mL . | 0 . | 5 . | 25 . | 50 . | |||
| 0 | 11 ± 6 | 14 ± 7 | 12 ± 6 | 10 ± 5 | |||
| 5 | 44 ± 8 | 39 ± 11 | 41 ± 5 | 38 ± 5 | |||
| 25 | 66 ± 5 | 53 ± 12 | 73 ± 9 | 54 ± 8 | |||
| 50 | 78 ± 9 | 85 ± 7 | 79 ± 6 | 88 ± 6 | |||
Checkerboard analysis of CCL 16-induced ECs chemotaxis was performed as detailed in Table 1. Mean ± SD of 4 samples in one experiment of 2 with a similar result.
Finally we evaluated the motility effect of CCL16 on KS “spindle cells” isolated from an epidemic lesion. These cells are a heterogeneous population with 3 distinct phenotypes: one reminiscent of activated vascular and lymphatic ECs, the second of macrophagic and dendritic cells, and the last with mixed markers of macrophage and ECs.51 Figure 4 shows that CCL16-Cos induced KS chemotaxis in a dose-dependent manner.
KS cell chemotaxis triggered by CCL16. Chemotaxis of KS cells (1.5 × 105/50 μL) was evaluated as detailed in Figure 1. In some experiments, KS cells were preincubated respectively for 15 minutes and 2 hours with BX417 or with Ab anti-CCR8 or nonimmune IgG. Mean ± SD of 3 experiments. Data were analyzed by one-way analysis of variance (F = 13.70) and Student-Newman-Keuls test. * indicates P < .05 versus Vec-Cos; §, P < .05 versus CCL16-Cos (50 ng).
KS cell chemotaxis triggered by CCL16. Chemotaxis of KS cells (1.5 × 105/50 μL) was evaluated as detailed in Figure 1. In some experiments, KS cells were preincubated respectively for 15 minutes and 2 hours with BX417 or with Ab anti-CCR8 or nonimmune IgG. Mean ± SD of 3 experiments. Data were analyzed by one-way analysis of variance (F = 13.70) and Student-Newman-Keuls test. * indicates P < .05 versus Vec-Cos; §, P < .05 versus CCL16-Cos (50 ng).
CCL16 primes HUVECs to the mitogen activity of VEGF-A165
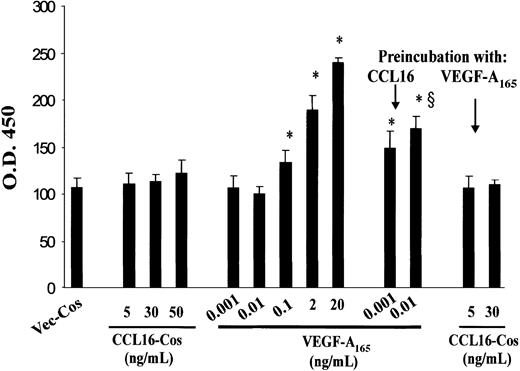
CCL16-Cos (Figure 5) and rLEC (not shown) did not induce in vitro proliferation of HUVECs or the hepatic capillary ECs (not shown), while VEGF-A165 significantly increased the incorporation of BrdU in a dose-dependent way. However, CCL16-Cos exhibited a positive modulator effect on VEGF-A165 activity. The preincubation of HUVECs with CCL16-Cos allowed an ineffective dose of VEGF-A165 (0.001 and 0.01 ng/mL) to be mitogenic. In contrast, HUVECs preincubated with nonmitogenic concentrations of VEGF-A165 did not become responsive to CCL16-Cos (Figure 5), indicating that in this context the priming is not reciprocally shared by the 2 molecules.
Effects of CCL16 on HUVEC proliferation. HUVECs (2.5 × 103) were plated on fibronectin and grown for 12 hours in Medium 199 containing 5% FCS. Then, cells were starved for 12 hours in Medium 199 containing 5% BSA and then stimulated with CCL16-Cos or VEGF-A165 for 24 hours and during the last 3 hours in the presence of BrdU that was detected by an ELISA assay. Where indicated, HUVECs were preincubated for 1 hour with CCL16-Cos (30 ng/mL) or VEGF-A165 (0.01 ng/mL), washed, and then respectively stimulated with VEGF-A165 or CCL-16. Mean ± SD of 3 experiments. Data were analyzed by one-way analysis of variance (F = 106.1) and Student-Newman-Keuls test. * indicates P < .05 versus VEGF-A165 (0.001 ng/mL); §, P < .05 versus VEGF-A165 (0.01 ng/mL).
Effects of CCL16 on HUVEC proliferation. HUVECs (2.5 × 103) were plated on fibronectin and grown for 12 hours in Medium 199 containing 5% FCS. Then, cells were starved for 12 hours in Medium 199 containing 5% BSA and then stimulated with CCL16-Cos or VEGF-A165 for 24 hours and during the last 3 hours in the presence of BrdU that was detected by an ELISA assay. Where indicated, HUVECs were preincubated for 1 hour with CCL16-Cos (30 ng/mL) or VEGF-A165 (0.01 ng/mL), washed, and then respectively stimulated with VEGF-A165 or CCL-16. Mean ± SD of 3 experiments. Data were analyzed by one-way analysis of variance (F = 106.1) and Student-Newman-Keuls test. * indicates P < .05 versus VEGF-A165 (0.001 ng/mL); §, P < .05 versus VEGF-A165 (0.01 ng/mL).
CCL16 activates CCR1
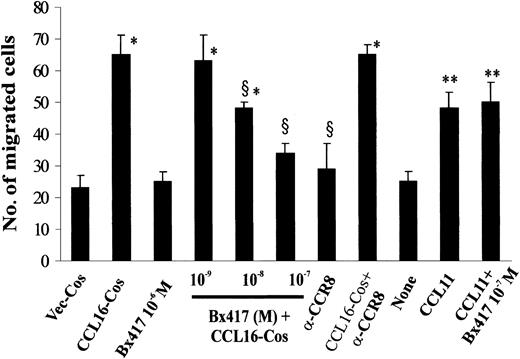
CCL16 exerts its functions on leukocytes mainly through CCR1, CCR2, and CCR8.5,7 Figure 6 shows that in our experimental conditions HUVECs expressed transcripts of all 3 receptors, as already reported.22,25,26,52-55 BX47156 and a CCR8-neutralizing Ab26 were used to evaluate the role of CCR8 and CCR1, respectively, in EC activation by CCL16. BX471, a 4-hydroxypiperidine derivative inhibitor of CCR1,56 inhibited in a dose-dependent manner the chemotaxis of HUVECs (Figure 6), liver capillary ECs that express CCR1 (Figure 3B) and KS cells (Figure 4) triggered by CCL16-Cos. In HUVECs, this CCR1 inhibitor did not block the chemotaxis induced by CCL11 (Figure 7), a ligand of CCR3 in ECs,27 indicating the specificity of the compound. CCR8 neutralization by a specific Ab did not block the activity of CCL16-Cos on the 3 cell types examined here (Figure 7).
Analysis of CCR1, CCR2, and CCR8 mRNA expression in HUVECs. RT-PCR was performed on RNA of HUVECs grown in complete medium by using specific primers and conditions indicated in “Materials and methods.” In the control sample, the reverse transcription reaction was not performed and RNA was used instead of cDNA. The size of amplification products of CCR1, CCR2, and CCR8 were respectively 360, 583, and 540 bp. The figure is representative of 3 experiments performed.
Analysis of CCR1, CCR2, and CCR8 mRNA expression in HUVECs. RT-PCR was performed on RNA of HUVECs grown in complete medium by using specific primers and conditions indicated in “Materials and methods.” In the control sample, the reverse transcription reaction was not performed and RNA was used instead of cDNA. The size of amplification products of CCR1, CCR2, and CCR8 were respectively 360, 583, and 540 bp. The figure is representative of 3 experiments performed.
Inhibition of CCL16-mediated HUVEC chemotaxis by BX471 compound. HUVECs were preincubated with BX471 or Ab anti-CCR8 or nonimmune IgG as detailed in Figure 2 and then stimulated as indicated. Mean ± SD of 3 experiments. Data were analyzed by one-way ANOVA (F = 27.83) and Student-Newman-Keuls test. * indicates P < .05 versus Vec-Cos; §, P < .05 versus CCL16-Cos (50 ng); **, P < .05 versus none.
Inhibition of CCL16-mediated HUVEC chemotaxis by BX471 compound. HUVECs were preincubated with BX471 or Ab anti-CCR8 or nonimmune IgG as detailed in Figure 2 and then stimulated as indicated. Mean ± SD of 3 experiments. Data were analyzed by one-way ANOVA (F = 27.83) and Student-Newman-Keuls test. * indicates P < .05 versus Vec-Cos; §, P < .05 versus CCL16-Cos (50 ng); **, P < .05 versus none.
As can be seen in Figure 8A and C, CCL16-Cos induced a rapid raise in the cytosolic Ca2+ level in HUVECs and KS cells. BX471 (10–7 M) completely abrogated this flux in stimulated HUVECs (Figure 8A) but was unable to antagonize the calcium mobilization induced by CCL11 (Figure 8B). HUVECs challenged with a first pulse of CCL16-Cos were unable to respond to a second dose of the chemokine, suggesting the receptor desensitization (Figure 8D). In contrast, HUVECs were not desensitized to CCL16-Cos when previously challenged with CCL2 (Figure 8D), suggesting that CCL16 did not use CCR2 in HUVECs. As control, cells did not respond to a second stimulation by CCL2 (50 ng/mL; not shown).
Cytosolic Ca2+ flux in triggered by CCL16 and modulation by BX471 compound. Cells were loaded with Fura2 and changes in fluorescence was monitored by a Perkin-Elmer LS-50B spectrofluorimeter. Panels A and B show Ca2+ transient stimulated by CCL16-Cos (Vec-Cos was negative; not shown) (A) or CCL11 (B) in HUVECs alone or preincubated with BX471 (15 minutes at 37°C). Panel C shows the effect of CCL16-Cos on KS cells (Vec-Cos was negative; not shown). Panel D shows the desensitization of HUVECs to a second stimulation with CCL16-Cos (50 ng/mL; black line) and the lack of desensitizing effect of CCL2 (50 ng/mL) on CCL16-Cos (50 ng/mL; orange line). Green lines indicate CCL16 + BX471 × 107 M; purple lines, CCL16 + BX471 × 108 M; and red lines, CCL16 + BX471 × 109 M. The line is interrupted exclusively to reduce the figure space. This is a representative experiment of 3 performed.
Cytosolic Ca2+ flux in triggered by CCL16 and modulation by BX471 compound. Cells were loaded with Fura2 and changes in fluorescence was monitored by a Perkin-Elmer LS-50B spectrofluorimeter. Panels A and B show Ca2+ transient stimulated by CCL16-Cos (Vec-Cos was negative; not shown) (A) or CCL11 (B) in HUVECs alone or preincubated with BX471 (15 minutes at 37°C). Panel C shows the effect of CCL16-Cos on KS cells (Vec-Cos was negative; not shown). Panel D shows the desensitization of HUVECs to a second stimulation with CCL16-Cos (50 ng/mL; black line) and the lack of desensitizing effect of CCL2 (50 ng/mL) on CCL16-Cos (50 ng/mL; orange line). Green lines indicate CCL16 + BX471 × 107 M; purple lines, CCL16 + BX471 × 108 M; and red lines, CCL16 + BX471 × 109 M. The line is interrupted exclusively to reduce the figure space. This is a representative experiment of 3 performed.
To reach further evidences on the role of CCR1, [125I]CCL16 was cross-linked to HUVECs and the cell lysate was immunoprecipitated with anti-CCR1 Ab. The [125I]CCL16 formed 2 complexes with apparent molecular masses of 46 kDa, which corresponds to the size of the complex between the receptor and the ligand, and 91 kDa, which is compatible with a dimeric complex (Figure 9). Altogether, these data indicate that CCR1 is activated in ECs by CCL16.
Cross-linking of [125I]CCL16 to HUVECs. HUVECs (2 × 107) were cross-linked with 0.5 nM [125I]CCL16 in presence or absence of 100-fold unlabeled ligand as detailed in “Materials and methods.” At the end of incubation, cell lysate was immunoprecipitated by a polyclonal Ab anti-CCR1 and protein separated by SDS-PAGE (10%). The figure is representative of 3 experiments performed.
Cross-linking of [125I]CCL16 to HUVECs. HUVECs (2 × 107) were cross-linked with 0.5 nM [125I]CCL16 in presence or absence of 100-fold unlabeled ligand as detailed in “Materials and methods.” At the end of incubation, cell lysate was immunoprecipitated by a polyclonal Ab anti-CCR1 and protein separated by SDS-PAGE (10%). The figure is representative of 3 experiments performed.
CCL16 induces EC morphogenesis
ECs plated on matrigel, an extracellular matrix preparation, assemble into capillary-like structures,57 mainly through directed cell migration governed by the presence of a chemoattractant.58 HUVECs plated on matrigel preparation purified from growth factor are isolated or aggregated in clumps of rounded cells after challenge with Vec-Cos (Figure 10A). The addition of CCL16-Cos promoted a continuous multicellular network (Figure 10Aii) similar to that observed in the presence of VEGF-A165 (Figure 10Aviii). The effect of CCL16-Cos was specifically neutralized by anti-CCL16 Ab (Figure 10Aiii) but not by irrelevant immunoglobulins (Figure 10Aiv). BX471 used at 10–7 M completely inhibited the effect of CCL16-Cos (Figure 10Av), while at 10–8 M it reduced the morphogenetic effect by about 40% (Figure 10Avi). This failure of HUVECs to undergo characteristic morphogenic changes was not attributable to an interference of these compounds to cell adhesion to matrigel (not shown). Neutralizing Ab against CCR8 added to HUVECs did not prevent the effect of CCL16-Cos (Figure 10Aviii).
HUVEC morphogenesis induced by CCL16. Human HUVECs (2 × 104) were plated onto matrigel deprived of growth factors and stimulated as indicated. After 14 hours of incubation the cell's 3-dimensional organization was recorded (original magnification × 10). (Ai) HUVECs stimulated with Vec-Cos; (ii) ECs stimulated with CCL16-Cos (50 ng/mL); (iii) HUVECs stimulated with CCL16-Cos (50 ng/mL) neutralized by Ab anti-CCL16; (iv) HUVECs stimulated with CCL16-Cos (50 ng/mL) treated with goat IgG; (v) HUVECs preincubated with BX417 (10–7 M) and then stimulated with CCL16-Cos; (vi) HUVECs preincubated with BX417 (10–8 M) for 15 minutes and then stimulated with CCL16-Cos (50 ng/mL); (vii) HUVECs preincubated with Ab anti-CCR8 then stimulated with CCL16-Cos (50 ng/mL); (viii) HUVECs stimulated with VEGF-A165 (20 ng/mL). Results representative of 3 independent experiments. (B) Morphometric analysis of the above-indicated conditions expressed as % of variation assuming VEGF-A165 treated HUVECs as 100% (Aviii). Mean ± SD of 4 independent experiments.
HUVEC morphogenesis induced by CCL16. Human HUVECs (2 × 104) were plated onto matrigel deprived of growth factors and stimulated as indicated. After 14 hours of incubation the cell's 3-dimensional organization was recorded (original magnification × 10). (Ai) HUVECs stimulated with Vec-Cos; (ii) ECs stimulated with CCL16-Cos (50 ng/mL); (iii) HUVECs stimulated with CCL16-Cos (50 ng/mL) neutralized by Ab anti-CCL16; (iv) HUVECs stimulated with CCL16-Cos (50 ng/mL) treated with goat IgG; (v) HUVECs preincubated with BX417 (10–7 M) and then stimulated with CCL16-Cos; (vi) HUVECs preincubated with BX417 (10–8 M) for 15 minutes and then stimulated with CCL16-Cos (50 ng/mL); (vii) HUVECs preincubated with Ab anti-CCR8 then stimulated with CCL16-Cos (50 ng/mL); (viii) HUVECs stimulated with VEGF-A165 (20 ng/mL). Results representative of 3 independent experiments. (B) Morphometric analysis of the above-indicated conditions expressed as % of variation assuming VEGF-A165 treated HUVECs as 100% (Aviii). Mean ± SD of 4 independent experiments.
CCL16 promotes the release of CXCL8 and CCL2 from HUVECs
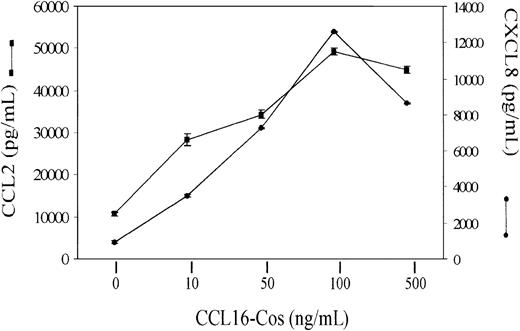
Since ECs are a source of chemokines with proangiogenic and proinflammatory activity,1 we investigated the production of CXCL8 and CCL2 after stimulation with CCL16-Cos. While Vec-Cos did not modify the basal production of both chemokines (not shown), 48 hours of stimulation of HUVECs with CCL16-Cos resulted in remarkable production of CXCL8 and CCL2 (Figure 11). The effect of CCL16 was dose dependent reaching the peak at 100 ng/mL.
Chemokine production by HUVECs stimulated with CCL16-Cos. Starved HUVECs (3 × 105) were stimulated for 48 hours with different concentrations of CCL16-Cos as indicated. The amount of CXCL8 and CCL2 released was evaluated by ELISA kits. Mean ± SD of 3 replicates in one representative experiment of 3 performed.
Chemokine production by HUVECs stimulated with CCL16-Cos. Starved HUVECs (3 × 105) were stimulated for 48 hours with different concentrations of CCL16-Cos as indicated. The amount of CXCL8 and CCL2 released was evaluated by ELISA kits. Mean ± SD of 3 replicates in one representative experiment of 3 performed.
CCL16 induces an angiogenic response in the CAM assay
To gain insights into the angiogenic activity of CCL16, we studied the vascularization of CAM carrying a gelatin sponge treated with CCL16-Cos. On incubation day 12, macroscopically, the gelatin sponges treated with the chemokine were surrounded by allantoic vessels radiating toward the implant in a “spoked-wheel” pattern (Figure 12B-C). In the specimens treated with Vec-Cos, a minimal vascular reaction was detectable around the sponges (Figure 12A). Morphometric analysis demonstrated that the number of vessels was 2.5-fold higher in CAM stimulated with the highest CCL16-Cos concentration (Vec-Cos: 4.93 ± 1.56 mm2; CCL16-Cos, 100 ng/sponge: 12.56 ± 1.98 mm2; P < .005; and CCL16-Cos, 50 ng/sponge: 8.67 ± 1.34; P < .05; n = 6). The implantation of a sponge with CCL16-Cos (100 ng) neutralized by the specific neutralizing Ab against CCL16 dramatically reduced the CAM vascularization (anti-CCL16–treated CCL16-Cos: 5.86 ± 2.03 vessels/mm2; IgG-treated CCL16-Cos: 13.20 ± 1.12 vessels/mm2; n = 5; P < .001). Nonimmune IgG did not inhibit CCL16-mediated vascularization (not shown).
Angiogenesis in CAMs induced by CCL16-Cos. CAMs were recorded at day 12 of incubation, 96 hours after the implant of a gelatin sponge soaked with Vec-Cos (A), CCL16-Cos at 100 ng (B) or 50 ng (C), or with CCL16-Cos (100 ng) neutralized by an Ab anti-CCL16 (D). Panels E and F show the CAM vascularization induced by BX471 (1 μmol/sponge) alone (E) or combined with CCL16-Cos (100 ng/sponge). Arrows in B and in C indicate the new capillaries attracted by the sponge containing CCL16. Original magnifications × 250. The picture is representative of one experiment of at least 4 performed.
Angiogenesis in CAMs induced by CCL16-Cos. CAMs were recorded at day 12 of incubation, 96 hours after the implant of a gelatin sponge soaked with Vec-Cos (A), CCL16-Cos at 100 ng (B) or 50 ng (C), or with CCL16-Cos (100 ng) neutralized by an Ab anti-CCL16 (D). Panels E and F show the CAM vascularization induced by BX471 (1 μmol/sponge) alone (E) or combined with CCL16-Cos (100 ng/sponge). Arrows in B and in C indicate the new capillaries attracted by the sponge containing CCL16. Original magnifications × 250. The picture is representative of one experiment of at least 4 performed.
To correlate the role of CCR1 with the angiogenic activity of CCL16, we studied the chemokine-induced angiogenesis in the presence of BX471. We observed that the presence of BX471 (1 μmol/sponge) with CCL16 significantly inhibited the angiogenic effect of the chemokine alone (P < .002), while the CCR1 antagonist had no significant effect in the absence of the chemokine (Figure 12E-F; BX471 + CCL16-Cos [100 ng/sponge]: 7.89 ± 1.69 vessels/mm2; BX471 + Vec-Cos: 4.47 ± 0.89 vessels/mm2; n = 4).
Discussion
Here, using in vitro and in vivo assays we demonstrate that CCL16, a CC chemokine, can positively influence angiogenesis in 3 different ways: (1) by modulating EC motility, which is pivotal in vessel formation16 ; (2) by inducing the release of CXCL8 and CCL2, 2 proinflammatory1 and proangiogenic chemokines,18,25,37,59 from stimulated ECs; and (3) by sensitizing ECs to ineffective concentrations of VEGF-A, a known powerful angiogenic inducer.16 These effects are specific because an Ab anti-CCL16 abolished these angiogenic responses.
CCL16 induces in a dose-dependent manner random and directional motility of ECs isolated from human large vessels and hepatic capillaries. Furthermore it promotes EC morphogenesis, a differentiative process strictly dependent on cell movement under the control of a chemoattractant.58 By using the Boyden chamber and time-lapse videomicroscopy we have clearly demonstrated that ECs tend to move more quickly in a particular direction in the presence of CCL16. However, checkerboard analysis obtained by abolishing the CCL16 gradient points out the ability of this chemokine to also increase nondirectional motility.
The migratory effect of CCL16 was obtained with recombinant molecules expressed in eukaryotic and in prokaryotic cells. Semipurified CCL16-Cos was more active than CCL16 expressed in E coli, suggesting that posttranslational modifications are important for CCL16-mediated EC motility. Actually, CCL16 contains one potential N-glycosylation signal (100Asn-Leu-Ser102) and it has been demonstrated that glycosylation,60 processing at NH2-terminus,34,61,62 or proteolytic cleavages63 may alter the specific activities and receptor usage of chemokines.
The ability to form networking capillary tubes is a cellautonomous property of ECs, which do need permissive but not instructive signals from the microenvironment.57 When plated on matrigel, a natural basal membrane matrix, CCL16 induces ECs to differentiate in geometric tubular networks, which are almost identical to vascular networks observed in vivo.64 This process lasts about 14 hours and is characterized by a highly directional cell movement allowing formation of bidimensional clusters and cords followed by the folding up of the cells into capillary-like tubes.64 This phenomenon is reminiscent of the formation of primitive plexus occurring in embryo life when angioblasts either coalesce at the location where they emerge from the mesoderm or they migrate through tissues and form blood vessels at distant sites and is mainly regulated by a chemoattractant gradient.65-68
Similar to other angiogenic chemokines,19,23-25,27,28,38 CCL16 did not promote EC proliferation. However it primes ECs to be responsive to ineffective doses of VEGF-A165. The mechanism of this effect is not explained here. However, emerging data suggest cooperation between chemokine receptors and tyrosine kinase receptors. For instance, chemokine receptors and VEGF receptor-2 share transcriptional pathways regulated by STAT (signal transducers and activators of transcription) molecules69-71 and require cytosolic Ca2+ mobilization to signal.1,72 Finally, it has been demonstrated that stimulation of a G-protein receptor may transactivate VEGF receptor-273 and that CXCL12 has an additive effect in capillary network formation in vitro with fibroblast growth factor-2 and angiopoietin-2, 2 known ligands of tyrosine kinase receptors.74
The direct effect of CCL16 on angiogenic functions of ECs may be amplified by the production of CXCL8 and CCL2 by ECs themselves. CXCL8 is able to activate a whole angiogenic program characterized by the stimulation of proliferation, survival, and migration of ECs as well as the production of metalloproteinases,18,37 whereas the in vivo angiogenic effect of CCL2 is only related to its ability to stimulate EC motility.25
ECs express CCR1, CCR2, and CCR8,22,25,26,52-55 known to be the most important receptors of CCL16.5,7 In our experimental conditions, CCR1 seems to be the key receptor in angiogenesis functions induced by CCL16. Actually, CCL16 was specifically cross-linked to CCR1 expressed by ECs and formed both monomers and dimers, as already described for CCR2, CCR5, CXCR2, and CXCR4.75-77 BX417, a specific inhibitor of CCR1,56 abrogated migration, Ca2+ fluxes, and morphogenesis of ECs challenged with CCL16 as well as CAM vascularization. To rule out the role of CCR2 and CCR8 we used 2 different approaches. Since receptor desensitization is a common feature of chemokine receptors, we looked at Ca2+ flux, which is an immediate downstream event after receptor ligand occupancy.1 The raise of cytosolic Ca2+ occurred in ECs challenged with CCL16, even if cells were previously stimulated with CCL2, a ligand of CCR2,25 suggesting that CCL2 and CCL16 use different receptors. The use of a neutralizing Ab anti-CCR8 allows us to exclude that this receptor is used by CCL16. In fact it did not inhibit the migratory effect of CCL16 on ECs and KS cells, while it was effective in inhibiting EC migration by CCL1.26 Therefore ECs express the 3 putative receptors of CCL16, but only binding with CCR1 seems to be efficient in inducing an angiogenic program. This event is well recognized in the biology of chemokines and may depend on several possibilities including the uncoupling of the receptor from its signaling machinery,78 the number of receptors on the cell surface, the ligand affinity,79 and the cooperation with other chemokine receptors80 with formation of heterodimers.76
The physiopathology role of CCL16 is largely unknown. CCL16 may cooperate with other angiogenic inducers in tumor vascularization because recent observations indicate that CCR1 expression is detected on ECs in hepatoma tissues but not in normal liver tissues54 and its expression is increased on ECs cocultured with cancer cells.55 Because CCL16 is mainly produced by the liver3,81 and may activate hepatic ECs, it should be interesting to investigate its role in angiogenesis associated to hepatic diseases, including carcinomas and cirrhosis or in hepatic vasculature remodeling occurring during embryo life. In this regard, the concept of the existence of angiogenic inducers exclusively active on distinct anatomical districts is emerging.82 Other evidences allow the conclusion that CCL16 is involved in pathologic angiogenesis. Here we demonstrate that it is chemoattractant for spindle cells isolated from KS. The hallmarks of KS are intense angiogenesis, proliferation of endothelial-derived spindle, inflammation, and casual linkage to human herpes virus 8, which encodes also 3 chemokine genes.20,51 Furthermore CCL16 is produced by mononuclear cells stimulated by IL-10,2 a cytokine that positively regulates angiogenesis in some settings83,84 while it is antiangiogenic in others.85,86
In conclusion, this paper adds new insights into the biologic activities triggered by CCL16 showing that it is an inducer of angiogenesis, potentially relevant in several diseases. Further studies will be required to define the contribution of inflammatory cells recruited by CCL16 in the angiogenic response and the possible selective effect in hepatic diseases based on its profile expression in human tissues.
Supported by Associazione Italiana per la Ricerca sul Cancro, Istituto Superiore di Sanità (IV Programma Nazionale di Ricerca sull'AIDS-2001 and Progetto “Tumor therapy”); Ministero dell' Uiversità e della Ricerca (MIUR) (60% and PRIN 2002 projects); Centro Nazionale delle Ricerche-MIUR (Progetto Strategico Oncologia and Agenzia 2000); and Fondi incentivazione della Ricerca di Base (Progetto Nuova Ingegneria Medica).
P.M. is employed by Human Genome Sciences.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Prepublished online as Blood First Edition Paper, September 4, 2003; DOI 10.1182/blood-2003-05-1387.
We are indebted to Dr R. Horuk (Berlex Biosciences, Richmond, CA) for providing us with the BX471 compound, Prof A. Albini (IST, Genova, Italy) for KS cells, and Prof A. Mantovani (University of Milan, Italy) for helpful discussions.





![Figure 9. Cross-linking of [125I]CCL16 to HUVECs. HUVECs (2 × 107) were cross-linked with 0.5 nM [125I]CCL16 in presence or absence of 100-fold unlabeled ligand as detailed in “Materials and methods.” At the end of incubation, cell lysate was immunoprecipitated by a polyclonal Ab anti-CCR1 and protein separated by SDS-PAGE (10%). The figure is representative of 3 experiments performed.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/1/10.1182_blood-2003-05-1387/6/m_h80145410009.jpeg?Expires=1769083264&Signature=ODPQvIugmr9cMSngWuEaOq-5OOzujs~lHOJVruE8tlLObj1c6ijx8AkEbi3IsexzFG61vdoJBDzEGA-aHeHI9UJ6IFckkXWdOko245sb7hNamEpiUG~aatNqIss2Wi3A6TlonoBC42NdKRMDUUpd9ZBj3ZQZBKhs8ClmaQrwHhDOiTP7d~X2K2Dw5-Sw8RLL943~J5l0W7c6WJKaaVqENH71a5UCw5jiINAYmJYoc-Rxc32uwMZ~FMjEAFJ6lm3GW1NPK2K4iRLL9AqqW2E4MVuKq43DmNecZXuDTZzYUfZ2l500~Y39swIkNy-FqCB5c1naXslZX4180zTtd2vHig__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal