Abstract
The Kaposi sarcoma–associated herpesvirus (KSHV)–encoded latency-associated nuclear antigen (LANA) modulates viral and cellular gene expression, including interleukin 6 (IL-6), a growth factor for KSHV-associated diseases. LANA-driven IL-6 expression is dependent on the activator protein 1 (AP1) response element (RE) within the IL-6 promoter. We show that LANA activates the AP1 RE in a Jun-dependent fashion and that LANA enhances the transcriptional activity of a GAL4-Jun fusion protein. Coimmunoprecipitation studies documented a physical interaction between LANA and c-Jun in transiently transfected 293 cells as well as the KSHV-infected BCBL-1 primary effusion lymphoma (PEL) cell line. Taken together, these data indicate that LANA is a transcriptional coactivator of c-Jun. In addition, electrophoretic mobility shift assays demonstrated that LANA induces binding of a c-Jun-Fos heterodimer to the AP1 RE, but does not itself bind to the AP1 RE. RNA interference experiments confirmed that LANA activates the AP1 RE, stimulates binding of a c-Jun-Fos heterodimer to the AP1 RE, and induces expression of IL-6. These data indicate that LANA is a transcriptional coactivator of c-Jun, a function that may have implications for the pathogenesis of KSHV-associated diseases.
Introduction
The Kaposi sarcoma-associated herpesvirus (KSHV), also known as human herpesvirus 8 (HHV-8), has been associated with HIV-related and -unrelated cases of Kaposi sarcoma (KS), primary effusion lymphoma (PEL), and multicentric Castleman disease (MCD).1-4 Seroepidemiologic studies suggest that KSHV plays a causative role in KS.5 Furthermore, KSHV induces transformation of primary human endothelial cells.6 Because of the rarity of PEL and MCD, these diseases are not amenable to seroepidemiologic studies to establish KSHV as an etiologic agent. However, KSHV infection is unique among B-cell lymphomas, and the severity of MCD correlates with KSHV viral load.7
The majority of KSHV-infected cells harbor the virus in a latent form.8,9 During viral latency, a small cohort of the more than 80 viral open reading frames (ORFs) is expressed.9,10 One of these latent genes, latency-associated nuclear antigen (LANA), is critical to the persistence of KSHV episomes and functions in this capacity by tethering viral episomes to chromosomes during mitosis, which allows for efficient segregation of viral episomes to daughter cells.11 In addition, LANA physically interacts with cellular proteins, including p53,12 pRB,13 RING3,14 histone H1,15 ATF4/CREB2,16 and members of the mSin3 corepressor complex.17 Functional consequences of these interactions include inhibition of p53-mediated apoptosis,12 inhibition of the transcriptional activity of ATF4/CREB2,16 dysregulation of β-catenin and the Wnt signaling pathway,18 and, in conjunction with Hras, transformation of primary rat embryonic fibroblasts.13
We have recently shown that LANA specifically activates the activator protein 1 (AP1) response element (RE) and induces expression of cellular interleukin 6 (IL-6) in an AP1-dependent fashion.19 This finding may have particular importance to the pathogenesis of KSHV-associated neoplasms because IL-6 has been reported to be a crucial growth factor for these diseases.20-22 TheAP1 proteins represent a family of transcription factors, including Jun and Fos family proteins that bind to the AP1 RE within the regulatory regions of target genes and induce gene transcription.23,24 DNA binding and transcriptional activation by AP1 family members dependent on the formation of homodimers and heterodimers mediated by their leucine zipper structures.24 Phosphorylation of serine residues 63 and 73 within the transactivation domain of c-Jun by the Jun N-terminal kinase (JNK) results in transcriptional activation.25 In fact, JNK is the only mitogen-activated protein (MAP) kinase that phosphorylates the transactivation domain of c-Jun.26,27 Transcriptional activity of c-Jun and JunB is increased by p300, a transcriptional coactivator that physically binds to c-Jun and JunB.28 In addition, DNA binding of c-Jun dimers is enhanced by dephosphorylation at carboxy-terminal threonine and serine residues, whereas phosphorylation at these sites inhibits DNA binding.29,30
In the current study, we explored the mechanism of LANA-mediated activation of the AP1 RE. We considered 3 possible mechanisms: (1) LANA activates a signal transduction pathway that leads to activation of members of the AP1 transcription factor family; (2) LANA functions as a transcription factor that directly binds to the AP1 RE and induces transcription; and (3) LANA is a transcriptional coactivator of an AP1 family member.
Materials and methods
Cells and reagents
Human embryonal kidney 293 cells were obtained from the American Type Culture Collection (Manassas, VA) and maintained in modified Eagle medium (MEM; Omega Scientific, Thousand Oaks, CA) supplemented with 10% fetal bovine serum (FBS; Omega Scientific) and penicillin (100 U/mL) and streptomycin (100 μg/mL). A 293 tetracycline-inducible cell line (293-TetOff-EGFP-LANA), in which tetracycline suppresses expression of an enhanced green fluorescent protein (EGFP)–LANA fusion, was generated as previously described.19 The BCBL-1 PEL cell line, which is infected with KSHV but not other herpesviruses, was maintained in RPMI plus 20% FBS and antibiotics.
The c-Jun and Fos antibodies for electrophoretic mobility supershift assays and the actin antibody were obtained from Santa Cruz Biotechnology (Santa Cruz, CA); the EGFP antibody was from Clontech (Palo Alto, CA). The JNK, phospho-JNK, c-Jun, and phospho-c-Jun antibodies used in Western blotting were from Cell Signaling Technology (Beverly, MA). The c-Jun antibody used for immunoprecipitation was from Upstate Biotechnology (Lake Placid, NY). The LANA monoclonal antibody was from Novacastra Laboratories (Newcastle upon Tyne, United Kingdom), and the FLAG antibody (anti-FLAG M2 monoclonal antibody) was from Sigma-Aldrich (St Louis, MO).
The pAP1-luc, pκB-luc, and pGRE-luc reporter plasmids, which contain tandem copies of the AP1, κB, and glucocorticoid response elements (GREs) that drive firefly luciferase expression, were purchased from Clontech, as was the EGFP-C2 plasmid, in which the cytomegalovirus (CMV) promoter drives constitutive expression of EGFP. The pRL-SV40 reporter plasmid (Promega, Madison, WI) expresses Renilla luciferase under control of the SV40 promoter/enhancer and was used for normalization of transfection efficiency in BCBL-1 cells. The plasmids for the JNK in vivo signal transduction trans-reporting system were purchased from Stratagene (La Jolla, CA; see “Results”). We generated an expression construct for EGFP-LANA, in which LANA is expressed with an N-terminal EGFP tag, as previously described.19 The LANA ORF was subcloned into the p3 × FLAG-CMV-10 expression vector (Sigma-Aldrich) to enable expression of an N-terminal 3 × FLAG-LANA fusion protein. A JNK-dominant negative (JNK-DN) construct, a gift from Dr Genhong Cheng (UCLA School of Medicine), contains mutations at the phosphorylation sites (Thr183Ala and Tyr185Phe).31 A Jun-DN construct, a gift from Dr Charles Sawyers (UCLA School of Medicine), contains an insertion at nucleotide 282 in the dimerization domain of c-Jun, which results in a frame-shift downstream of the insertion.32 The Jun-DN ORF was subcloned into the pcDNA3.1 mammalian expression vector (Invitrogen, Carlsbad, CA).
Transient transfections
The 293 cells were plated at 105 cells/well in 24-well plates the day prior to transfection. All plasmids were transfected with Lipofectamine Plus (Invitrogen) in serum-free medium according to the manufacturer's instructions. Total DNA was held constant by transfecting relevant control vectors, when appropriate. Transfection efficiency in 293 cells was determined by the percentage of EGFP-expressing cells as measured by counting the number of fluorescing cells in 3 separate, representative fields at × 100 magnification with an inverted phase-contrast UV microscope (TS100-F; Nikon, Melville, NY) and dividing by the number of nonfluorescing cells in the same fields. Cell lysates were harvested at 48 hours for reporter gene expression.
Reporter gene assays
Luciferase assays were performed on cell lysates 48 hours after transfection with a luciferase assay kit (Promega) according to the manufacturer's instructions. For 293 cells, results of reporter assays were normalized for transfection efficiency based on percentage of EGFP+ cells (“Transient transfections”). Firefly luciferase expression in BCBL-1 cells was normalized to that of Renilla luciferase using the Dual Luciferase Assay System (Clontech). Luminescence was measured on the TD20/20 tube luminometer (Turner Designs, Sunnyvale, CA).
Immunoprecipitation of JNK and JNK in vitro kinase assay
Immunoprecipitation of JNK and the JNK in vitro kinase assay were performed with a kit according to the manufacturer's instructions (Cell Signaling Technology). Briefly, total cellular protein was immunoprecipitated on glutathione-S-transferase (GST)–jun-agarose beads. The immunoprecipitated protein was then either subjected to immunoblotting with an anti-JNK antibody or a kinase assay followed by immunoblotting with an antiphosphorylated-jun antibody.
Western blot
Protein was resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Protein was transferred to a nitrocellulose membrane with the Trans Blot system (Bio-Rad, Hercules, CA). The membrane was blocked with triethanolamine-buffered saline (TBS) with 1% bovine serum albumin (BSA) and 1% nonfat powdered milk. Membranes were immunoblotted with relevant primary antibodies. Horseradish peroxidase–conjugated secondary antibodies (Santa Cruz) used for detection of bands were by chemiluminescence (ECL Western blotting detection reagents; Amersham Biosciences, Piscataway, NJ).
EMSA
For the electrophoretic mobility shift assay (EMSA), cells were washed with cold phosphate-buffered saline (PBS), and buffer A (10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.9, 1.5 mM MgCl2, 10 mM KCl, and 1 mM dithiothreitol [DTT]) with proteinase inhibitors (200 μM phenylmethylsulfonyl fluoride [PMSF], 1 μM leupeptin, 1 μM aprotinin, and 100 μM EDTA [ethylenediaminetetraacetic acid]) was then added prior to pulverization with a tissue grinder. Subsequently, nuclei were pelleted, lysed with buffer C (20 mM, pH 7.9, HEPES, 0.42 M NaCl, 1.5 mM MgCl2, 0.2 M EDTA, 25% glycerol, 1 mM DTT, and 200 μM PMSF), and then passed several times through a 25-gauge needle. Debris was removed by centrifugation.
Wild-type and mutant AP1 oligonucleotide probes were purchased from Santa Cruz Biotechnology. Nuclear protein (15 μg) was combined with end-labeled, double-stranded oligonucleotide probe, 1 μg poly-dIdC (Amersham Pharmacia Biotech, Piscataway, NJ), 1 μg BSA, and 5 mM spermidine in a final reaction volume of 20 μL for 20 minutes at room temperature. The DNA protein complex was run on a 4% nondenaturing polyacrylamide gel with 0.4 × tris-borate EDTA (TBE) running buffer prior to subsequent autoradiography. Cold-competition experiments were performed with a 100-fold molar excess of cold wild-type or cold mutant κB or AP1 oligonucleotides. For supershift assays, nuclear protein was preincubated with 2μL antibody (p65 and p50 TransCruz Gel Supershift antibodies, Santa Cruz Biotechnology) for 20 minutes at room temperature.
Coimmunoprecipitation
Nuclear protein was extracted (see the protocol in “EMSA”) from BCBL-1 cells and 293 cells transiently transfected with 3 × FLAG-LANA and c-Jun expression vectors or control vectors. Nuclear protein was incubated with antibodies (c-Jun = 10 μL; LANA = 100 μL; FLAG = 5 μL) at 4°C overnight with continuous rotation. Sixty microliters of protein G/protein A agarose beads (Calbiochem, San Diego, CA) were added for 3 hours at 4°C with continuous rotation. The immunoprecipitate was then washed 5 times with 0.5 mL immunoprecipitation buffer (25 mM HEPES, pH 7.5, 150 mM NaCl, 2 mM EDTA, 10% glycerol, 1% Triton-100) containing proteinase inhibitors (200 μM PMSF, 1 μM leupeptin, 1 μM pepstatin, and 0.2 M aprotinin). After the final wash and centrifugation, 50 μL of 4 × SDS loading dye was added, and the protein was resolved on a 4% to 12% Tris (tris(hydroxymethyl)aminomethane)–glycine gel (Invitrogen) for subsequent Western blotting.
RNA interference
Small interfering RNA (siRNA; Dharmacon Research, Lafayette, CO) for LANA with the following target sequence was used: 5′-AAACAGGUCUCCGGAAAGAUG-3′. A control siRNA termed Scramble II (target sequence: 5′-AAGCGCGCUUUGUAGGAUUCG-3′) was also purchased from Dharmacon and has no significant homology to any known human or KSHV sequence. For reporter gene assays, 6 × 105 BCBL-1 cells were plated in 24-well plates in 500μL Opti-MEM (Invitrogen) without serum and antibiotics. Cells were cotransfected with reporter plasmids (1 μg), including the pRLSV40 plasmid (1 ng) for normalization for transfection, and siRNA (60 pmol) with 2 μL Lipofectamine 2000 according to the manufacturer's instructions. Five hours after transfection, medium was removed and replaced with Opti-MEM plus 10% FBS. Forty-eight hours after transfection, protein was extracted for reporter gene assays.
For the EMSAs and Westerns blots, 1 × 107 BCBL-1 cells were plated in 4 mL Opti-MEM without antibiotics or serum in a 10-cm dish. siRNA (1800 pmol) was transfected with 15 μL Oligofectamine. Six hours after transfection, medium was changed to Opti-MEM plus 10% FBS. Forty-eight hours after transfection, nuclear protein was extracted.
For cytokine measurements, siRNA (150 pmol) was transfected into BCBL-1 cells (2.5 × 105 cells/well) with Oligofectamine (1.5 μL; Invitrogen) according to the manufacturer's instructions, and supernatant was harvested at 48 hours.
Cytokine assays
IL-6 and tumor necrosis factor α (TNF-α), were measured on ELISA plates (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions.
Results
LANA-mediated activation of the AP1 RE is Jun dependent
We have previously demonstrated that LANA activates reporter gene expression driven by the AP1 RE.19 Thus we investigated the mechanisms that underlie LANA-mediated activation of the AP1 RE. We first tested whether LANA-mediated activation of the AP1 RE was dependent on Jun. We performed transient transfection experiments in 293 cells; we transfected an EGFP-LANA construct or the EGFP control vector with or without a Jun-DN construct. LANA potently induced reporter gene expression from the pAP1-luc plasmid (Figure 1), which contains 4 copies of the AP1 RE that drives expression of firefly luciferase. In the presence of the Jun-DN construct, LANA-induced reporter gene expression was completely abrogated (Figure 1). The Jun-DN construct had no effect on reporter gene expression driven by the pGRE-luc plasmid (data not shown) or a κB-driven reporter.19
LANA-mediated activation of the AP1 RE is Jun dependent. The EGFP-LANA or EGFP control vector and the pAP1-luc reporter were cotransfected with the Jun-DN expression construct or the empty vector control. Concentration of all vectors was 0.1 μg. Firefly luciferase was assayed at 48 hours. The results, which were normalized to that of the negative control group (EGFP control + control vector for Jun-DN), are the means of 3 experiments and are reported ± SD. RLU indicates relative luminescence units.
LANA-mediated activation of the AP1 RE is Jun dependent. The EGFP-LANA or EGFP control vector and the pAP1-luc reporter were cotransfected with the Jun-DN expression construct or the empty vector control. Concentration of all vectors was 0.1 μg. Firefly luciferase was assayed at 48 hours. The results, which were normalized to that of the negative control group (EGFP control + control vector for Jun-DN), are the means of 3 experiments and are reported ± SD. RLU indicates relative luminescence units.
The specific Jun family member that mediates LANA-induced AP1 activation cannot be determined by these experiments because the Jun-DN will inhibit the function of all Jun family members. Because LANA has been shown to modulate the activity of regulatory elements in reporter gene constructs that are commonly used as internal standards,33 we normalized for transfection efficiency based on the percentage of green fluorescing cells. In addition, ORF73 of KSHV, which encodes the LANA protein, is quite large, and, therefore, for the same quantity of DNA, the actual number of plasmid copies of the EGFP-LANA construct is about half that of the EGFP control vector. This correlates with our observation that the frequency of EGFP-LANA fluorescing cells is approximately half that of the EGFP-expressing cells in transiently transfected 293 cells.
The fact that LANA-mediated activation of the AP1 RE is dependent on a Jun family member does not necessarily indicate that LANA functions directly through Jun. Thus, it is possible that LANA exerts its effects through potential dimerization partners of Jun, in which case LANA-mediated activation of the AP1 RE would still require Jun activity. To address this issue, we used a system that involves a GAL4-driven heterologous promoter driven by a GAL4-Jun fusion protein. In this system, the GAL4 DNA-binding domain is fused in-frame with the c-Jun transactivation domain. Activation of the c-Jun transactivation domain results in activation of a reporter that contains 5 copies of the GAL4 RE that drives expression of the firefly luciferase gene (pGAL4-luc). In transient transfection experiments, EGFP-LANA activated the GAL4-c-Jun fusion compared to the EGFP control vector (Figure 2). In addition, EGFP-LANA enhanced the activation of the GAL4-c-Jun fusion by MEKK, a MAP kinase kinase that is an upstream activator of JNK (Figure 2). Dose responsiveness of the MEKK and LANA effects were also demonstrated in the GAL4-c-Jun fusion system (data not shown).
LANA enhances the transcriptional activity of a GAL4-c-Jun transactivation domain fusion. The EGFP-LANA expression plasmid (50 ng) or the vector control (50 ng) was cotransfected with either MEKK or vFLIP expression plasmids (0.1 μg) or relevant control vectors. The GAL4-c-Jun transactivation domain fusion construct and pGAL4-luc reporter plasmid (0.1 μg each) were included in all transfections. The results, which were normalized to that of the negative control group (EGFP control + control vector for MEKK/vFLIP), are the means of 3 experiments and are reported ± SD.
LANA enhances the transcriptional activity of a GAL4-c-Jun transactivation domain fusion. The EGFP-LANA expression plasmid (50 ng) or the vector control (50 ng) was cotransfected with either MEKK or vFLIP expression plasmids (0.1 μg) or relevant control vectors. The GAL4-c-Jun transactivation domain fusion construct and pGAL4-luc reporter plasmid (0.1 μg each) were included in all transfections. The results, which were normalized to that of the negative control group (EGFP control + control vector for MEKK/vFLIP), are the means of 3 experiments and are reported ± SD.
We recently demonstrated that another KSHV latent protein, known as vFLIP (FADD-like interferon-converting enzyme [FLICE] inhibitory protein), is an upstream activator of the JNK/AP1 pathway and that LANA potentiates vFLIP-induced cellular IL-6 production.34 Consequently, we tested the ability of LANA to enhance vFLIP-mediated activation of the JNK/AP1 pathway. In transient transfections in 293 cells, LANA potentiated the effects of vFLIP on activation of the GAL4-c-Jun fusion (Figure 2).
LANA does not activate JNK
The fact that LANA-mediated activation of the AP1 RE is Jun dependent along with the results of the GAL4 system suggests that LANA is a transcriptional coactivator of c-Jun, although these results could be explained by the possibility that LANA functions as an upstream activator of the JNK/AP1 pathway. Thus, we wanted to exclude this latter possibility. Because JNK is the only MAP kinase that phosphorylates the transactivation domain of c-Jun,26,27 we focused our studies on JNK and its possible activation by LANA. For this purpose, we used a tetracycline-inducible system to express EGFP-LANA in 293 cells (293-TetOff-EGFP-LANA), in which the antibiotic tetracycline (or its derivative, doxycycline) suppresses transgene expression. We have previously demonstrated the efficacy of this system to induce expression of EGFP-LANA.19 By Western blot, EGFP-LANA expression did not affect the level of expression of total or phosphorylated JNK (Figure 3A). In vitro kinase assays for JNK did not reveal any difference in JNK activity on EGFP-LANA expression (Figure 3B). Activation of JNK by tetradecanoyl phorbol acetate (TPA) served as a positive control for the assay and demonstrated that the JNK signal transduction pathway is intact in 293-TetOff-EGFP-LANA cells (Figure 3B). Thus, LANA does not directly or indirectly function as an upstream activator of the JNK/AP1 pathway.
LANA is not an upstream activator of JNK. (A) Western blot for total and phospho-JNK on protein extracts (20 μg/lane) from 293 TetOff-EGFP-LANA cells were maintained with or without doxycycline (dox, 1 μg/mL, to suppress EGFP-LANA expression). Top panels: Western blot for phospho-JNK (P-JNK) and total JNK; middle panel: Western blot for actin to confirm equivalent protein loading; bottom panel: Western blot for EGFP-LANA. (B) 293 TetOff-EGFP-LANA cells were maintained with or without dox (1 μg/mL) and treated with TPA (20 ng/mL) or vehicle control for 20 minutes. Total cellular protein (250 μg) was subjected to a JNK in vitro kinase assay. Bottom panel: Western blot for total JNK on immunoprecipitated protein; top panel: Western blot for phospho-c-Jun (P-Jun).
LANA is not an upstream activator of JNK. (A) Western blot for total and phospho-JNK on protein extracts (20 μg/lane) from 293 TetOff-EGFP-LANA cells were maintained with or without doxycycline (dox, 1 μg/mL, to suppress EGFP-LANA expression). Top panels: Western blot for phospho-JNK (P-JNK) and total JNK; middle panel: Western blot for actin to confirm equivalent protein loading; bottom panel: Western blot for EGFP-LANA. (B) 293 TetOff-EGFP-LANA cells were maintained with or without dox (1 μg/mL) and treated with TPA (20 ng/mL) or vehicle control for 20 minutes. Total cellular protein (250 μg) was subjected to a JNK in vitro kinase assay. Bottom panel: Western blot for total JNK on immunoprecipitated protein; top panel: Western blot for phospho-c-Jun (P-Jun).
LANA physically interacts with c-Jun
Because our results indicate that LANA is a transcriptional coactivator of c-Jun, we postulated that LANA ought to physically interact with c-Jun. To demonstrate in vivo binding of LANA to c-Jun, we performed coimmunoprecipitation studies in 293 cells that were transiently transfected with 3 × FLAG-LANA and c-Jun expression constructs. Cellular extracts were subjected to immunoprecipitation with an anti–c-Jun antibody, and immunoblotting of the resulting immunocomplexes with an anti-FLAG antibody successfully detected the presence of the 3 × FLAG-LANA fusion protein (Figure 4A, top panel). Successful expression of the full-length c-Jun and 3 × FLAG-LANA proteins was demonstrated (Figure 4A, bottom panels). To establish the specificity of the interaction, we immunoblotted with an antibody against IκBα, which did not identify a band in the immunoprecipitate (data not shown). Furthermore, when the same cellular extract was immunoprecipitated with an immunoglobulin control antibody, c-Jun was not detected (Figure 4A, top panel). Similar coimmunoprecipitation experiments with extracts from 293 cells transiently transfected with various combinations of the empty control vectors served as additional negative controls and further confirmed the specificity of the LANA/c-Jun interaction (Figure 4A). Reciprocal coimmunoprecipitation experiments in which immunoprecipitation was performed with an antibody to FLAG followed by immunoblotting for c-Jun also demonstrated the interaction between LANA and c-Jun in 293 cells (Figure 4B).
LANA physically interacts with c-Jun. (A) The indicated plasmids were transiently transfected into 293 cells, and total protein was extracted at 48 hours for coimmunoprecipitation. The protein from the first 2 lanes is the same. Top panel is coimmunoprecipitation. The arrow indicates a specific LANA band; the arrowhead represents a nonspecific band that is also faintly detected in lanes that were not transfected with 3 × FLAG-LANA. Middle and bottom panels: Western blots with Jun and FLAG antibodies to demonstrate expression of c-Jun and 3 × FLAG-LANA. (B) Same as panel A, but reciprocal coimmunoprecipitation; 2.5 μg of the 3 × FLAG-LANA and the c-Jun expression vectors or appropriate controls were used. (C) Coimmunoprecipitation of LANA/c-Jun in BCBL-1 cells. Nuclear protein was extracted from BCBL-1 or Raji cells and immunoprecipitated with an anti-LANA monoclonal antibody (or Ig control) followed by Western for c-Jun. (D) Same as panel C, but reciprocal coimmunoprecipitation.
LANA physically interacts with c-Jun. (A) The indicated plasmids were transiently transfected into 293 cells, and total protein was extracted at 48 hours for coimmunoprecipitation. The protein from the first 2 lanes is the same. Top panel is coimmunoprecipitation. The arrow indicates a specific LANA band; the arrowhead represents a nonspecific band that is also faintly detected in lanes that were not transfected with 3 × FLAG-LANA. Middle and bottom panels: Western blots with Jun and FLAG antibodies to demonstrate expression of c-Jun and 3 × FLAG-LANA. (B) Same as panel A, but reciprocal coimmunoprecipitation; 2.5 μg of the 3 × FLAG-LANA and the c-Jun expression vectors or appropriate controls were used. (C) Coimmunoprecipitation of LANA/c-Jun in BCBL-1 cells. Nuclear protein was extracted from BCBL-1 or Raji cells and immunoprecipitated with an anti-LANA monoclonal antibody (or Ig control) followed by Western for c-Jun. (D) Same as panel C, but reciprocal coimmunoprecipitation.
To further establish the relevance of the interaction between LANA and c-Jun, we performed coimmunoprecipitation studies on the BCBL-1 PEL cell line, which is endogenously infected with KSHV. Immunoprecipitation of c-Jun successfully “pulled down” LANA as demonstrated by Western blotting with an anti-LANA monoclonal antibody (Figure 4C). Similar immunoprecipitation studies on Raji cells, which are not infected with KSHV, as well as immunoprecipitation with an IgG control antibody, served as negative controls (Figure 4C). The reciprocal coimmunoprecipitation confirmed the presence of a LANA/c-Jun complex in BCBL-1 cells (Figure 4D). When the membranes used for Figure 4C-D were stripped, probing with an antibody against IκBα did not detect a specific band (data not shown).
LANA does not directly bind to the AP1 RE but induces binding of Jun-Fos heterodimers
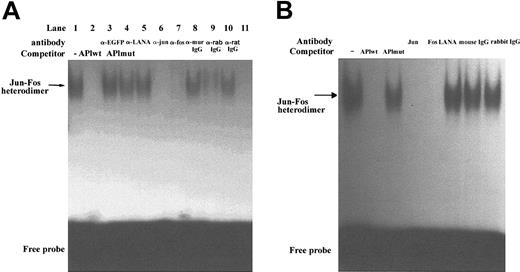
Although our results indicate that LANA is a transcriptional coactivator of c-Jun, we also wanted to exclude the possibility that LANA functions as a transcription factor that directly binds to the AP1 RE to induce gene transcription. To examine the potential of LANA to function in this regard, we performed EMSAs with an AP1 consensus probe and various sources of LANA protein. We performed EMSAs with nuclear extracts from 293-TetOff-EGFP-LANA cells. In the absence of EGFP-LANA expression, there was no gel retardation of the AP1 probe (Figure 5A, lane 11). On induction of EGFP-LANA expression, a single, intense band was visualized (Figure 5A, lane 1). The specificity of the band was demonstrated by cold-competition experiments with a molar excess of either cold wild-type or cold mutant AP1 probes (Figure 5A, lanes 2-3). Supershift experiments demonstrated that this band was composed of a Jun-Fos heterodimer and did not contain LANA (Figure 5A, lanes 4-10). Antibodies to c-Jun and Fos led to abrogation of the shifted band (Figure 5A, lanes 6-7; the c-Jun and Fos antibodies target the DNA-binding domain of c-Jun and Fos and inhibit DNA binding rather than actually inducing a supershift). Antibodies to EGFP, LANA, and isotype controls failed to affect the gel shift pattern (Figure 5A, lanes 8-10). To confirm that the induction of the Jun-Fos heterodimer was not due to expression of the EGFP moiety, we showed that expression of EGFP alone did not induce a gel shift of the AP1 probe (data not shown). EMSAs performed with nuclear extracts from endogenously KSHV-infected BCBL-1 cell lines, which are not coinfected with Epstein-Barr virus (EBV) or other herpesviruses, confirmed the presence of a shifted band that contained a Jun-Fos heterodimer (Figure 5B).
LANA induces DNA binding of a Jun-Fos heterodimer to the AP1 RE but does not directly bind to the AP1 RE. (A) AP1 EMSA using nuclear extracts from 293-TetOff-EGFP-LANA cells treated without (lanes 1-10) or with (lane 11) dox (1 μg/mL). Cold competition with excess wild-type AP1 probe abrogated the gel-shifted band (lane 2), whereas cold mutant probe had no effect (lane 3). Supershift experiments are shown in lanes 4 to 10. (B) EMSAs on nuclear extracts from BCBL-1 cells. Experiments were similar to those in panel A, including cold competition and supershift experiments.
LANA induces DNA binding of a Jun-Fos heterodimer to the AP1 RE but does not directly bind to the AP1 RE. (A) AP1 EMSA using nuclear extracts from 293-TetOff-EGFP-LANA cells treated without (lanes 1-10) or with (lane 11) dox (1 μg/mL). Cold competition with excess wild-type AP1 probe abrogated the gel-shifted band (lane 2), whereas cold mutant probe had no effect (lane 3). Supershift experiments are shown in lanes 4 to 10. (B) EMSAs on nuclear extracts from BCBL-1 cells. Experiments were similar to those in panel A, including cold competition and supershift experiments.
To further exclude that LANA was present in the protein complex visualized on the gel shift, we demonstrated that the gel retardation of the AP1 probe in 293 cells and BCBL-1 was identical in TPA-stimulated HeLa cells, which are not infected with KSHV and do not express LANA (data not shown). In addition, the supershift assay on HeLa cell nuclear extracts demonstrated that the AP1 complex was also composed of a Jun-Fos heterodimer (data not shown). Thus, these results indicate that LANA does not mediate AP1-driven gene expression by directly binding to the AP1 RE. Rather, in addition to its ability to function as a transcriptional coactivator of c-Jun, LANA also induces binding of a Jun-Fos heterodimer to the AP1 RE.
LANA induces c-Jun protein expression
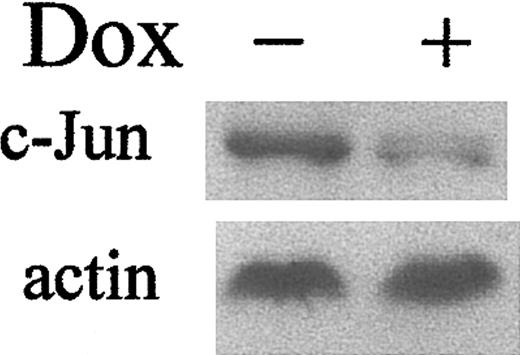
Transcription of the c-Jun gene is positively autoregulated by c-Jun itself.35,36 Thus, increased Jun transcriptional activity mediated by LANA could induce transcription of the c-Jun gene and thereby result in increased c-Jun protein expression. In fact, Western blotting confirmed that LANA does induce c-Jun protein expression (Figure 6).
LANA induces c-Jun expression. The 293 TetOff-EGFP-LANA cells were maintained with or without dox (1 μg/mL). Top panel: Western blot for c-Jun; bottom panel: Western blot for actin to demonstrate equivalent protein loading.
LANA induces c-Jun expression. The 293 TetOff-EGFP-LANA cells were maintained with or without dox (1 μg/mL). Top panel: Western blot for c-Jun; bottom panel: Western blot for actin to demonstrate equivalent protein loading.
Effects of gene silencing of LANA by RNAi in KSHV-infected PEL cells
To extend the biologic context of the effects of LANA on transcriptional coactivation of c-Jun to a more relevant system, we investigated the biochemical effects of gene silencing of LANA by RNA interference (RNAi) in BCBL-1 cells. We generated an siRNA duplex that targets a 21-mer region in the 5′ region of the LANA ORF and compared the effects of LANA siRNA to that of a scrambled control siRNA. Transfection of LANA siRNA abrogated LANA protein expression as assayed by Western blotting (Figure 7A, top panel); no effect on LANA expression was seen with the control siRNA or transfection reagent alone (Figure 7A, top panel). Equal protein loading and the specificity of gene silencing effect of the LANA siRNA were demonstrated by probing with an anti–actin antibody (Figure 7A, bottom panel).
Effects of gene silencing of LANA by RNAi in BCBL-1 cells. (A) BCBL-1 cells were transfected with LANA siRNA or control siRNA or transfection reagent alone. Experiments were performed in duplicate. Forty-eight hours later, nuclear protein (20 μg) was subjected to Western blotting. Top panel: Western blot for LANA; bottom panel: Western blot for actin to demonstrate equivalent protein loading and the specificity of the LANA siRNA effects. (B) BCBL-1 cells were transfected with LANA or control siRNA and the indicated reporter plasmids. Protein was extracted after 48 hours for luciferase assays. The results are the means of 3 experiments and are reported as the relative reporter gene expression (in RLUs) of groups treated with LANA siRNA normalized to values for control siRNA ± SD. (C) The same protein used in panel A was used for Western blotting for c-Jun (top panel) and actin (bottom panel) to confirm equal protein loading. (D) EMSA with AP1 probe. The same nuclear protein was used as in panel A. Cold competition experiments demonstrated the specificity of the band. (E) BCBL-1 cells were transfected with LANA or control siRNA, and supernatants were harvested after 48 hours for IL-6 ELISA. The results are the means of 3 experiments ± SD.
Effects of gene silencing of LANA by RNAi in BCBL-1 cells. (A) BCBL-1 cells were transfected with LANA siRNA or control siRNA or transfection reagent alone. Experiments were performed in duplicate. Forty-eight hours later, nuclear protein (20 μg) was subjected to Western blotting. Top panel: Western blot for LANA; bottom panel: Western blot for actin to demonstrate equivalent protein loading and the specificity of the LANA siRNA effects. (B) BCBL-1 cells were transfected with LANA or control siRNA and the indicated reporter plasmids. Protein was extracted after 48 hours for luciferase assays. The results are the means of 3 experiments and are reported as the relative reporter gene expression (in RLUs) of groups treated with LANA siRNA normalized to values for control siRNA ± SD. (C) The same protein used in panel A was used for Western blotting for c-Jun (top panel) and actin (bottom panel) to confirm equal protein loading. (D) EMSA with AP1 probe. The same nuclear protein was used as in panel A. Cold competition experiments demonstrated the specificity of the band. (E) BCBL-1 cells were transfected with LANA or control siRNA, and supernatants were harvested after 48 hours for IL-6 ELISA. The results are the means of 3 experiments ± SD.
To determine the effects of LANA gene silencing on AP1-driven reporter activity, we cotransfected BCBL-1 cells with an AP1, κB, GRE, or basal promoter driven reporter with the LANA or control siRNA and assessed reporter gene expression. As shown in Figure 7B, the LANA siRNA markedly reduced the reporter gene expression from the AP1 construct compared to the control siRNA, but no significant effects were seen with the κB, GRE, or basal promoter driven reporters.
We also wanted to confirm the effects of LANA on c-Jun expression and induction of a Jun-Fos heterodimer that binds to the AP1 RE as demonstrated in 293 cells. Using nuclear extracts from the same experiments shown in Figure 7A, we performed Western blots for c-Jun, which showed abrogation of c-Jun protein expression with LANA siRNA, but not with control siRNA (Figure 7C). In addition, our gel shift experiments demonstrated that LANA gene silencing led to loss of the AP1 complex (Figure 7D).
To further establish the biologic significance of LANA, we examined the effects of LANA gene silencing on cellular IL-6 expression in BCBL-1 supernatants. Cellular IL-6 has been implicated as a growth factor for PEL cells,20 as well as the neoplastic cells of KS and MCD.21,22 LANA siRNA reduced expression of cellular IL-6 expression by about 50% compared to control siRNA (Figure 7E); the specificity of this observation was confirmed by the fact that no effect of LANA siRNA on TNF-α levels was observed (data not shown).
Discussion
The transcriptional activity of c-Jun is primarily mediated through phosphorylation of serines 63 and 73 in the transactivation domain.25 Transcriptional coactivators and repressors and regulation of DNA binding, which is in part mediated by phosphorylation of the carboxy-terminus of c-Jun, provide additional layers of regulation of c-Jun activity.23,29 We have identified LANA as a transcriptional coactivator of c-Jun as evidenced by the fact that: (1) LANA activates the AP1 RE in a Jun-dependent fashion, (2) LANA enhances the transcriptional activity of a GAL4-c-Jun transactivation domain fusion protein, (3) LANA does not function as an upstream activator of JNK, and (4) LANA physically interacts with c-Jun. In addition, LANA induces c-Jun protein expression and DNA binding of a Jun-Fos heterodimer to the AP1 consensus RE. The biologic significance of these results was underscored by gene-silencing experiments in PEL cells, in which abrogation of LANA expression was associated with loss of AP1-driven reporter gene expression, decreased c-Jun expression, loss of DNA binding of Jun-Fos heterodimers, and reduction in cellular IL-6 expression. The latter result is of particular importance because IL-6 is a growth factor for KS cells,21 is required for the development of MCD in a mouse model,22 and is implicated in PEL pathogenesis,20 although recent reports identify the virally encoded IL-6 as a more important cytokine as a PEL growth factor.37
As a transcriptional coactivator of c-Jun that is capable of enhancing the transcriptional activity of a GAL4 fusion that contains the c-Jun transactivation domain only, LANA likely physically interacts with the transactivation domain of c-Jun. Precedent for such an interaction comes from studies of p300, which is a transcriptional coactivator of c-Jun and JunB that binds to the transactivation domain of these Jun family members.28 It is quite possible that LANA also binds to and regulates the transcriptional activity of other AP1 family members, and this is an area that requires further study. In addition to its function as a transcriptional activator of c-Jun, LANA may enhance AP1 activity by inducing DNA binding of c-Jun-Fos heterodimers to the AP1 RE, as demonstrated in 293 and BCBL-1 cells. The latter function may in part be attributed to the up-regulation of c-Jun expression that occurs in the presence of LANA. In addition, posttranslational modification of AP1 factors is likely to play an important role in determining DNA binding. For example, dephosphorylation of C-terminal serine and threonine residues promotes DNA binding, whereas phosphorylation impedes it.29 In support of this notion, a recent report documents the ability of LANA to bind to inhibit glycogen synthase kinase 3β (GSK3β),18 which is known to phosphorylate carboxy-terminal residues of c-Jun and thereby inhibit c-Jun DNA binding. By inactivating GSK3β, LANA could promote c-Jun DNA binding. Thus, we postulate that LANA modulates, either directly or indirectly, the intrinsic DNA-binding properties of AP1 family members. Elucidation of the mechanisms responsible for enhanced DNA binding of AP1 in the presence of LANA will require additional investigations. Moreover, LANA may have similar effects on other Jun family members as well as Fos proteins, a possibility that will also involve further studies.
Several mechanisms may account for the ability of LANA to up-regulate expression of c-Jun. Because the c-Jun promoter contains AP1-binding sites,35,36 LANA, in its capacity as a transcriptional coactivator of c-Jun, may augment c-Jun transcription. In addition, the abundance of c-Jun is also regulated by modulation of c-Jun protein stability,38 and it is possible that LANA may function in this regard. Effects of LANA on c-Jun mRNA stability and translation represent other considerations, although c-Jun protein expression is not significantly regulated in this fashion. A recent study demonstrated that LANA stabilizes β-catenin, which, in turn, leads to activation of the Tcf/Lef transcription factors.18 These transcription factors can activate transcription of genes containing Tcf- and Lef-binding sites, including c-JUN.
Although KSHV can transform primary endothelial cells and KSHV appears to be a causative agent in KSHV-associated neoplasms, the mechanisms and specific viral genes responsible for the transformation process remain to be fully elucidated. Viral genes that are capable of inducing transformation include kaposin,39 viral interferon regulatory factor,40 ORF K1,41 and the viral G protein–coupled receptor.42,43 Recently, it was reported that LANA enhances the ability of Hras to induce transformation of rat embryonic fibroblasts and drives entry into the S phase.13,18 LANA serves as a particularly attractive candidate to be involved in transformation by KSHV because it is an abundantly expressed latent protein and the vast majority of neoplastic cells in KSHV-associated diseases harbor the virus in a latent state.8-10,44,45 Interestingly, transformation induced by Hras, an upstream activator of MAP kinase signaling pathways including the JNK pathway, is c-Jun dependent,46 and coexpression of c-Jun is required for oncogenic Hras-induced transformation of primary rat embryonic fibroblasts.47 Moreover, c-Jun itself, without cooperation from other genes, is oncogenic under certain conditions.48,49 Thus, the ability of LANA to function as a transcriptional coactivator of c-Jun has implications for the transformation of KSHV-infected cells.
Whereas KS tumors frequently harbor mutations and amplification of the ras gene,50 PEL cells typically lack ras mutations.51 During KS oncogenesis, LANA may cooperate with oncogenic ras to activate the JNK pathway. In contrast, in PEL cells, alternative mechanisms of JNK activation may operate to compensate for the absence of ras mutations. For instance, the KSHV-encoded vFLIP and G protein–coupled receptor also activate the JNK pathway.34,42 Interactions between vFLIP and LANA seem to be particularly plausible because both proteins are expressed as latent proteins from the same message,44,52,53 whereas the G protein–coupled receptor is a lytic protein that is expressed in a minority of KSHV-infected cells.54 Thus, heightened transcriptional activity of c-Jun by LANA in cooperation with other viral proteins, such as vFLIP, may represent an important biochemical function in KSHV-induced transformation.
In addition to the role that LANA may play in cellular transformation, LANA-mediated transcriptional coactivation of c-Jun may function in maintenance of the transformed state. AP1-regulated genes affect both proliferation and apoptosis.55 For example, c-Jun positively regulates expression of cyclin D1, which promotes the G1-S phase transition56 ; c-Jun is a direct repressor of transcription of p53 and thereby inhibits apoptosis.57 Thus, LANA, by enhancing the transcriptional activity of c-Jun and through its many previously reported functions, may promote proliferation and inhibit apoptosis. Future studies of the cooperation between latent viral proteins in the initiation and promotion of the transformed state will provide insight into the pathogenesis of KSHV-associated diseases.
Prepublished online as Blood First Edition Paper, September 11, 2003; DOI 10.1182/blood-2003-05-1538.
Supported by grants from the National Institutes of Health (5R01CA80004) and American Cancer Society (RPG-00-305-01-MBC), and an Interdisciplinary Research Grant from the Jonsson Comprehensive Cancer Center at UCLA (M.B.R.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal