Abstract
Nasal natural killer (NK) cell lymphoma is rare, so that its optimal therapy, long-term outcome, and prognostic factors are unclear. Data on 52 men and 15 women with well-characterized nasal NK cell lymphomas were analyzed retrospectively to define the impact of primary therapy on remission and long-term outcome and the validity of the International Prognostic Index (IPI). Most (84%) had stage I/II disease with an IPI score of 1 or less (52%). Seven patients received radiotherapy only; 47 patients received anthracycline-containing chemotherapy plus consolidation radiotherapy; and 12 patients received nonanthracycline-containing chemotherapy plus radiotherapy. The overall complete remission (CR) rate was 64.2%; the 20-year overall survival (OS) and disease-free survival (DFS) rates were 37.1% and 33.5%, respectively. Front-line radiotherapy was apparently better than chemotherapy for CR (100% versus 59%, P = .04) and OS (83.3% versus 32.0%, P = .03). Relapses occurred in 4 radiotherapy-treated (all local) and 14 chemotherapy-treated patients (9 local, 4 systemic). Among these, 5 late relapses (4 local, 1 systemic) occurred at 170 months (range, 92-348 months) from CR. The IPI score was of prognostic significance for the whole group (IPI ≤ 1 superior to IPI ≥ 2 for 20-year OS: 57.4% versus 27.6%, P = 0.012), as well as for patients treated with chemotherapy/radiotherapy (IPI ≤ 1 superior to IPI ≥ 2 for CR: 76.7% versus 35.7%, P = .017; and 10-year OS: 63.8% versus 26.8%, P = .003).
Introduction
Malignancies derived putatively from natural killer (NK) cells are a recently recognized distinct lymphoma subtype within the World Health Organization (WHO) classification of lymphoid tumors.1 Clinically, these lymphomas present most commonly as destructive lesions in the nasal cavity and other midline facial structures. Occasionally, the lymphoma occurs primarily in nonnasal areas, including the skin, gastrointestinal tract, salivary gland, and testis. Rarely, the disease runs a fulminant course with disseminated organ involvement, sometimes accompanied by a leukemic phase, and is rapidly fatal.1-4 Interestingly, these NK cell lymphomas show a geographic predilection, with patients reported mainly from Asian countries such as Hong Kong,5-9 Japan,10-16 and Korea,17,18 and South American countries including Mexico,19,20 Peru,21 and Guatemala.22 Some patients have been reported from other countries, although their ethnicity is usually poorly defined.23-26
Pathologically, NK cell malignancies show a broad morphologic spectrum. The lymphoma infiltrate is often angiocentric, with prominent necrosis and vascular destruction.1 Lymphoma cells may be small, medium to large in size, sometimes with an accompanying admixture of inflammatory cells. Cytologic examination shows that the lymphoma cells possess moderate pale cytoplasm often with azurophilic granules. Immunophentoypically, these cells characteristically express CD2, the NK cell marker CD56, the ϵ chain of CD3 (CD3ϵ) but not surface CD3, and other cytotoxic molecules such as granzyme B, TIA-1, or perforin.1 The CD3–CD3ϵ+CD56+ phenotype distinguishes these lymphomas from peripheral T-cell lymphomas that are CD3+. The T-cell receptor gene is in germline configuration, and there is an almost invariable association with Epstein-Barr virus (EBV) infection in the tumor cells. Molecular analysis has shown that the EBV is present in a clonal episomal form, suggesting that it might be involved in tumorigenesis.27 Therefore, the phenotype of CD3ϵ+CD56+EBV+ is an important clue to diagnosis. Exceptionally, lymphomas with almost identical cytologic features have been found to be T cell in origin.1 For this reason, the WHO classification has categorized NK cell lymphomas as NK/T-cell lymphomas. However, the fact remains that most of these tumors are of putative NK cell lineage.
Although the clinicopathologic features of nasal NK cell lymphomas are reasonably well defined, the optimal therapy and treatment outcome are unclear. Before immunophenotyping had become part of the lymphoma diagnosis, the term “polymorphic reticulosis” was coined to describe nasal lymphoma with a mixed cellular composition as opposed to conventional lymphomas with monotonous-appearing tumor cells.28 Other terms that had been used included “lethal midline granuloma” and “midline malignant reticulosis.” In the revised European-American classification of lymphoid malignancies, these tumors were classified as angiocentric T-cell lymphomas.29 Although many of the lymphomas reported as polymorphic reticulosis or angiocentric T-cell lymphomas were probably NK cell lymphomas,10,12,17,19,23,25,26 the lack of information on CD56 and EBV made it difficult to determine if that was in fact the case. Moreover, many clinical studies of nasal lymphomas actually included in their analysis other conventional B- and T-cell lymphomas, which were distinct from NK cell lymphomas.7,8,14,23,24 The heterogeneous case mixtures in these reports make interpretation of treatment results difficult. Furthermore, owing to the rarity of the lymphoma, many series had contained small numbers of patients.8,15,25,26 Because NK cell lymphoma is now a distinct clinicopathologic entity, there is a need to define its optimal treatment and the factors having an impact on outcome and prognosis.
To address these issues, we performed a retrospective study to analyze the clinical features, effect of treatment modalities, and long-term outcome of a consecutive cohort of uniformly diagnosed primary nasal NK cell lymphomas. Furthermore, we also investigated the applicability of the International Prognostic Index (IPI), which has been widely shown to be predictive of treatment outcome in malignant lymphomas of different grades and subtypes,30,31 to the prognostication of this lymphoma.
Patients, materials, and methods
Patients
Sixty-seven consecutive patients with primary nasal NK cell lymphoma were included in the analysis. The inclusion criteria were primary symptoms and major tumor bulk localized to the nasal cavity and adjacent tissues, histopathologic examination showing features typical of NK cell lymphoma, and tumor cells positive for CD3ϵ, CD56, and EBV. Patient demographics, clinical features, IPI scores, therapeutic modalities, treatment outcome, and site of relapses were analyzed. Staging investigations included complete blood count, serum biochemistry, serum lactate dehydrogenase (LDH), nasal panendoscopy, computed tomographic scan of the thorax and abdomen, and bilateral marrow trephine biopsies. The Ann Arbor staging system was used,32 with locally extensive disease staged as IE when the tumor spanned adjacent structures by contiguous spread.
Histologic and immunophenotypic diagnosis
Histopathologic examination was conducted on paraffin sections of formalin- or B5-fixed tissue after staining with hematoxylin-eosin. Immunohistochemical staining for CD3ϵ and CD56 and in situ hybridization for EBV-encoded RNA was performed as described.6
Treatment
There were 3 initial treatment modalities: (1) involved-field radiotherapy (RT) as the primary treatment to patients diagnosed before 1980; (2) an anthracycline-containing chemotherapeutic regimen followed by RT consolidation to patients diagnosed after 1980 and younger than 60 years; and (3) a nonanthracycline-containing chemotherapeutic regimen followed by RT consolidation to patients diagnosed after 1980 and older than 60 years. One patient was treated initially by surgery alone. Primary RT involved the delivery of 40 to 50 Gy equivalent in daily fractions of 1.8 to 2.0 Gy, using a lateral opposing field (from a cobalt 60 source until 1991, and a linear accelerator since 1991) covering the nasal, paranasal cavities, nasopharynx, and the Waldeyer ring. The field was extended to cover the cervical region if cervical nodes were present at diagnosis. The anthracycline-containing regimens included m-BACOD (methotrexate + leucovorin, bleomycin, doxorubicin, cyclophosphamide, vincristine, and dexamethasone), ProMACE-CytaBOM (prednisolone, doxorubicin, cyclophosphamide, etoposide, cytarabine, bleomycin, vincristine, and methotrexate + leucovorin), and CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone) given for 6 courses (Table 1).
Induction and salvage chemotherapy regimens
Regimen . | Dose and route . | Day . | Frequency . |
|---|---|---|---|
| CHOP | |||
| C - cyclophosphamide | 750 mg/m2 IV | 1 | Repeat every 21 d |
| H - doxorubicin (Adriamycin) | 50 mg/m2 IV | 1 | |
| O - vincristine (Oncovin) | 1.4 mg/m2 IV | 1 | |
| P - prednisolone | 100 mg PO | 1-5 | |
| m-BACOD | |||
| m - methotrexate (+ leucovorin) | 200 mg/m2 IV | 8, 15 | Repeat every 21 d |
| B - bleomycin | 4 mg/m2 IV | 1 | |
| A - doxorubicin (Adriamycin) | 45 mg/m2 IV | 1 | |
| C - cyclophosphamide | 600 mg/m2 IV | 1 | |
| O - vincristine (Oncovin) | 1.4 mg/m2 IV | 1 | |
| D - dexamethasone (Decadron) | 6 mg/m2 PO | 1-5 | |
| ProMACE-CytaBOM | |||
| Pro - prednisolone | 60 mg/m2 PO | 1-14 | Repeat every 21 d |
| A - doxorubicin (Adriamycin) | 25 mg/m2 IV | 1 | |
| C - cyclophosphamide | 650 mg/m2 IV | 1 | |
| E - etoposide | 120 mg/m2 IV | 1 | |
| Cyta - cytarabine | 300 mg/m2 IV | 8 | |
| B - bleomycin | 5 mg/m2 IV | 8 | |
| O - vincristine (Oncovin) | 1.4 mg/m2 IV | 8 | |
| M - methotrexate (+ leucovorin) | 120 mg/m2 IV | 8 | |
| COPP | |||
| C - cyclophosphamide | 450 mg/m2 IV | 1, 8 | Repeat every 28 d |
| O - vincristine (Oncovin) | 1.4 mg/m2 IV | 1, 8 | |
| P - procarbazine | 100 mg/m2 PO | 1-14 | |
| P - prednisolone | 40 mg/m2 PO | 1-14 | |
| IMVP-16 | |||
| I-ifosfamide | 1 gm/m2 IV | 1-5 | |
| M-methotrexate | 30 mg/m2 IM | 3, 10 | |
| VP-16 | 100 mg/m2 IV | 1-3 | |
| DHAP | |||
| Cisplatin | 100 mg/m2 IV | 1 | |
| Ara-C | 4 g/m2 IV | 1-2 | |
| Dexamethasone | 40 mg IV | 1-4 |
Regimen . | Dose and route . | Day . | Frequency . |
|---|---|---|---|
| CHOP | |||
| C - cyclophosphamide | 750 mg/m2 IV | 1 | Repeat every 21 d |
| H - doxorubicin (Adriamycin) | 50 mg/m2 IV | 1 | |
| O - vincristine (Oncovin) | 1.4 mg/m2 IV | 1 | |
| P - prednisolone | 100 mg PO | 1-5 | |
| m-BACOD | |||
| m - methotrexate (+ leucovorin) | 200 mg/m2 IV | 8, 15 | Repeat every 21 d |
| B - bleomycin | 4 mg/m2 IV | 1 | |
| A - doxorubicin (Adriamycin) | 45 mg/m2 IV | 1 | |
| C - cyclophosphamide | 600 mg/m2 IV | 1 | |
| O - vincristine (Oncovin) | 1.4 mg/m2 IV | 1 | |
| D - dexamethasone (Decadron) | 6 mg/m2 PO | 1-5 | |
| ProMACE-CytaBOM | |||
| Pro - prednisolone | 60 mg/m2 PO | 1-14 | Repeat every 21 d |
| A - doxorubicin (Adriamycin) | 25 mg/m2 IV | 1 | |
| C - cyclophosphamide | 650 mg/m2 IV | 1 | |
| E - etoposide | 120 mg/m2 IV | 1 | |
| Cyta - cytarabine | 300 mg/m2 IV | 8 | |
| B - bleomycin | 5 mg/m2 IV | 8 | |
| O - vincristine (Oncovin) | 1.4 mg/m2 IV | 8 | |
| M - methotrexate (+ leucovorin) | 120 mg/m2 IV | 8 | |
| COPP | |||
| C - cyclophosphamide | 450 mg/m2 IV | 1, 8 | Repeat every 28 d |
| O - vincristine (Oncovin) | 1.4 mg/m2 IV | 1, 8 | |
| P - procarbazine | 100 mg/m2 PO | 1-14 | |
| P - prednisolone | 40 mg/m2 PO | 1-14 | |
| IMVP-16 | |||
| I-ifosfamide | 1 gm/m2 IV | 1-5 | |
| M-methotrexate | 30 mg/m2 IM | 3, 10 | |
| VP-16 | 100 mg/m2 IV | 1-3 | |
| DHAP | |||
| Cisplatin | 100 mg/m2 IV | 1 | |
| Ara-C | 4 g/m2 IV | 1-2 | |
| Dexamethasone | 40 mg IV | 1-4 |
IV indicates intravenously, PO, orally, IM, intramuscularly.
The choice of regimen was at the discretion of the attending physician. This was followed by consolidation involved-field RT of 44 to 50 Gy, with a field size, daily fractions, and source similar to those given to patients receiving RT as primary treatment. However, RT was, in general, given 6 to 8 months after diagnosis in these patients. The nonanthracycline-containing regimen comprised COPP (cyclophosphamide, vincristine, procarbazine, and prednisolone), followed by consolidation involved-field RT as described. Salvage regimens of patients who were nonresponsive to the initial chemotherapy, or who had a relapse afterward, included m-BACOD, IMVP16 (ifosfamide, methotrexate, and VP-16), or DHAP (cisplatin, Ara-C, and dexamethasone) (Table 1). High-dose chemotherapy with autologous hematopoietic stem cell transplantation (HSCT) was offered to selected patients.33
Statistical analysis
Complete remission (CR) was defined as complete resolution of symptoms and signs and normalization of all imaging studies. Overall survival (OS) was measured from diagnosis to death or last follow-up. Disease-free survival (DFS) was measured from CR to the date of relapse. OS and DFS were computed by the Kaplan-Meier method.34 Survival curves were compared by the log-rank test. Eight patients underwent autologous bone marrow transplantation, and all survivals were censored at the time of transplantation. Demographic data between subgroups were compared by the χ2 test for categorical data (sex, stage, performance status, and IPI score), or the Student t test for continuous variables such as age and serum LDH level. All P values were 2-sided.
Results
Patients
There were 52 men and 15 women with a median age of 49 years (Table 2). Most of the patients presented with early disease (stage I/II, 84%; stage III/IV, 16%) that was localized to the nasal cavity and adjacent structures. B symptoms occurred in 23 (34.3%) patients. The median serum LDH level at diagnosis was 434 IU/mL (range, 144-2090 IU/mL). The Eastern Cooperative Oncology Group (ECOG) performance score was 0 to 1 for 52 (77.6%) patients. The IPI scores were 1 or less in 35 cases (52%) and 2 or more in 32 cases (48%).
Clinicopathologic features of 67 patients with nasal NK cell lymphomas
Clinicopathologic features . | n . |
|---|---|
| Male/female | 52/15 |
| Median age (range) | 49 y (17-84 y) |
| Initial sites of tumor | |
| Nasal cavity | 30 |
| Nasal cavity + nasopharynx | 11 |
| Nasal cavity + naso/oropharynx | 11 |
| Nasal cavity + naso/oropharynx + cervical lymph nodes | 4 |
| Nasal cavity + naso/oropharyngx + distant metastases | 11 |
| Stage | |
| I | 51 |
| II | 5 |
| III | 5 |
| IV | 6 |
| Median LDH,* IU/mL (range) | 434 (144-2090) |
| ECOG performance score | |
| 0 | 5 |
| 1 | 47 |
| 2 | 13 |
| 3 | 2 |
| 4 | 0 |
| IPI score | |
| 0 | 11 |
| 1 | 24 |
| 2 | 24 |
| 3 | 7 |
| 4 | 1 |
Clinicopathologic features . | n . |
|---|---|
| Male/female | 52/15 |
| Median age (range) | 49 y (17-84 y) |
| Initial sites of tumor | |
| Nasal cavity | 30 |
| Nasal cavity + nasopharynx | 11 |
| Nasal cavity + naso/oropharynx | 11 |
| Nasal cavity + naso/oropharynx + cervical lymph nodes | 4 |
| Nasal cavity + naso/oropharyngx + distant metastases | 11 |
| Stage | |
| I | 51 |
| II | 5 |
| III | 5 |
| IV | 6 |
| Median LDH,* IU/mL (range) | 434 (144-2090) |
| ECOG performance score | |
| 0 | 5 |
| 1 | 47 |
| 2 | 13 |
| 3 | 2 |
| 4 | 0 |
| IPI score | |
| 0 | 11 |
| 1 | 24 |
| 2 | 24 |
| 3 | 7 |
| 4 | 1 |
The reference range of LDH has changed over time, owing to differences in the assay methodologies. The reference ranges were: before 1977, 50-200 U/L; 1977-1981, male: 131-275 U/L, female: 137-269 U/L; 1982-1985, 130-275 U/L; 1986 onward, male: 197-401 U/L, female: 200-360 U/L.
Treatment modalities and outcome
The initial treatment was RT alone in 7 (10.6%) patients, an anthracycline-containing regimen followed by RT consolidation in 47 (70.1%) patients, and a nonanthracycline-containing regimen followed by RT consolidation in 12 (17.9%) patients. One patient was treated with surgery only. The treatment outcome is shown in Table 3. CR was achieved in 43 (64.2%) patients, of whom 19 subsequently had a relapse. At the time of analysis, 35 (52.2%) patients have died of disease. The median OS was 38 months, and the 10- and 20-year OS rates were 42.5% and 37.1%, respectively (Figure 1A). The 10- and 20-year DFS rates were 52.0% and 33.5%, respectively (Figure 1B). Interestingly, the DFS curve showed a rapid initial drop in survival rates followed by continual delayed relapses, resulting in a biphasic pattern.
Treatment and outcome of 66 patients with primary RT or primary chemotherapy*
Initial treatment, salvage therapy, and outcome . | n . |
|---|---|
| RT | 7 |
| Median age | 40 y |
| CR | 7 |
| NR | 0 |
| Relapse | |
| Local | 4 |
| Systemic | 0 |
| Median time from CR to relapse | 187 mo |
| Salvage treatment for patients with relapse | |
| CR | 2 |
| NR | 2 |
| Total no. of deaths | 2 |
| Anthracycline-containing regimen + RT consolidation | |
| CHOP | 25 |
| m-BACOD | 17 |
| ProMACE-CytaBOM | 5 |
| Median age | 42 y |
| CR | 31 |
| NR | 16 |
| Relapse | |
| Local | 8 |
| Systemic | 5 |
| Median time from CR to relapse | 100 mo |
| Salvage treatment for relapsed patients | |
| CR | 3 |
| PR | 1 |
| NR | 9 |
| Total no. of deaths | 23 |
| Nonanthracycline-containing regimen + RT consolidation | |
| COPP | 12 |
| Median age | 72 y |
| CR | 4 |
| NR | 8 |
| Relapse | |
| Local | 1 |
| Systemic | 0 |
| Median time from CR to relapse | 4 mo |
| Salvage treatment for patients with relapse | |
| CR | 0 |
| NR | 1 |
| Total no. of deaths | 9 |
Initial treatment, salvage therapy, and outcome . | n . |
|---|---|
| RT | 7 |
| Median age | 40 y |
| CR | 7 |
| NR | 0 |
| Relapse | |
| Local | 4 |
| Systemic | 0 |
| Median time from CR to relapse | 187 mo |
| Salvage treatment for patients with relapse | |
| CR | 2 |
| NR | 2 |
| Total no. of deaths | 2 |
| Anthracycline-containing regimen + RT consolidation | |
| CHOP | 25 |
| m-BACOD | 17 |
| ProMACE-CytaBOM | 5 |
| Median age | 42 y |
| CR | 31 |
| NR | 16 |
| Relapse | |
| Local | 8 |
| Systemic | 5 |
| Median time from CR to relapse | 100 mo |
| Salvage treatment for relapsed patients | |
| CR | 3 |
| PR | 1 |
| NR | 9 |
| Total no. of deaths | 23 |
| Nonanthracycline-containing regimen + RT consolidation | |
| COPP | 12 |
| Median age | 72 y |
| CR | 4 |
| NR | 8 |
| Relapse | |
| Local | 1 |
| Systemic | 0 |
| Median time from CR to relapse | 4 mo |
| Salvage treatment for patients with relapse | |
| CR | 0 |
| NR | 1 |
| Total no. of deaths | 9 |
CR indicates complete remission; PR, partial remission (> 50% reduction in tumor mass); NR, nonremission. The chemotherapy regimens are explained in Table 1.
Excluding one patient who achieved CR after initial treatment with surgery alone.
Overall treatment results of nasal NK cell lymphoma. (A) OS (n = 67). (B) DFS for CR patients (n = 43).
Overall treatment results of nasal NK cell lymphoma. (A) OS (n = 67). (B) DFS for CR patients (n = 43).
Outcome of primary treatment with RT compared with combination chemotherapy and RT
The outcome of primary treatment with RT alone was compared to that of chemotherapy (anthracycline- and nonanthracycline-containing) with RT consolidation. These patients were comparable with respect to age, sex, and IPI (Table 4). CR was achieved in all patients receiving RT (7 of 7, 100%), and only 35 of 59 (59.3%) of patients receiving chemotherapy/RT (P = .04). The 10-year OS was superior in the RT as compared with the chemotherapy/RT groups (83.3% versus 32.0%, P = .03; Figure 2). However, the 10-year DFS was comparable for the RT and chemotherapy/RT groups (83.3% versus 45.8%, P =. 22).
Clinical characteristics and IPI scores of patients who received RT or chemotherapy as primary treatment
. | RT* . | Chemotherapy* . | P† . |
|---|---|---|---|
| No. of patients | 7 | 59 | |
| Men | 5 (71.4%) | 46 (77.9%) | |
| Women | 2 (28.6%) | 13 (22.1%) | NS (.65) |
| Age older than 60 y | 3 (42.8%) | 20 (33.8%) | NS (.68) |
| Advanced stage (III/IV) | 0 | 11 (18.6%) | NS (.47) |
| B symptoms | 1 (14.3%) | 22 (37.3%) | NS (.27) |
| Elevated LDH level | 0 | 39 (66.7%) | NS (.12) |
| ECOG score 2 or higher | 2 (33.3%) | 13 (22.0%) | NS (.69) |
| IPI 2 or higher | 3 (33.3%) | 29 (45.7%) | NS (.99) |
. | RT* . | Chemotherapy* . | P† . |
|---|---|---|---|
| No. of patients | 7 | 59 | |
| Men | 5 (71.4%) | 46 (77.9%) | |
| Women | 2 (28.6%) | 13 (22.1%) | NS (.65) |
| Age older than 60 y | 3 (42.8%) | 20 (33.8%) | NS (.68) |
| Advanced stage (III/IV) | 0 | 11 (18.6%) | NS (.47) |
| B symptoms | 1 (14.3%) | 22 (37.3%) | NS (.27) |
| Elevated LDH level | 0 | 39 (66.7%) | NS (.12) |
| ECOG score 2 or higher | 2 (33.3%) | 13 (22.0%) | NS (.69) |
| IPI 2 or higher | 3 (33.3%) | 29 (45.7%) | NS (.99) |
NS indicates not significant.
The number in parenthesis represents the percentage of patients in either the RT or chemotherapy group.
P represents the comparison of each categorical variable of the RT with the chemotherapy group, using a 2 × 2 table by χ2 or Fisher exact test.
OS of patients showing a significance difference (P = .03) in favor of RT.
Patterns of treatment failure and salvage therapy
Twenty-four patients receiving combination chemotherapy failed to achieve CR, of whom 22 had persistence or progression of local disease, and 2 had systemic disease progression (one in liver and central nervous system and the other in cervical lymph nodes and the spleen). All of them received salvage chemotherapy, but only 1 patient managed to achieve CR, with the rest dying of refractory disease. Of the 19 patients who initially achieved CR but had subsequent relapses, 13 were local and 6 were systemic (one of each in the uterus, skin, muscle, parotid gland, scrotum, and bone marrow; Table 3). The median time from CR to relapse was 8 months (range, 1-348 months), with 13 patients (68.4%) having a relapse in the first 2 years. Local relapses occurred with similar frequencies in patients treated with RT or chemotherapy/RT (4 of 7 versus 9 of 14, P = .27). The outcome of these 19 patients with relapses is shown in Table 3. Eight patients underwent autologous HSCT at remission (CR1, n = 1; CR2, n = 3) or at relapsed or refractory disease (n = 4). Patients receiving transplants at CR1/CR2 were all surviving, at a median of 14 months (range, 6-96 months) after HSCT. However, patients receiving transplants at relapse or refractory disease had all died of progressive disease, with a medium survival of 4 months after HSCT.
Late relapses
Interestingly, 5 patients developed late relapses at more than 5 years from CR (range, 92-348 months; Table 3). The primary treatment was RT alone in 3 patients, who had relapses 15, 17, and 30 years afterward. At relapse, combination chemotherapy with m-BACOD led to a second CR in 2 of 3 patients. Both patients who remitted have remained in a second CR for 8 and 10 years, with the former patient having received an additional autologous HSCT. One patient, who received chemotherapy/RT as the primary treatment, had a relapse 9 years afterward and failed to respond to salvage chemotherapy. The last patient who had the primary tumor resected but without receiving further treatment had a relapse in his scrotum 10 years later. Although he responded initially to salvage chemotherapy, he ultimately had a systemic relapse and died.
IPI score in prognostication of patients treated with intensive chemotherapy
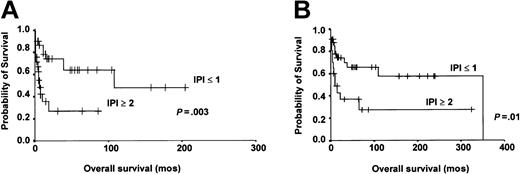
To assess the prognostic value of the IPI score, we initially analyzed the subgroup of patients who received anthracycline-containing chemotherapy, who constituted the majority (70%) of patients in this study. Patients with IPI less than or equal to 1 (low risk) were compared against those with IPI 2 or more (high risk) with respect to CR rate, OS, and DFS. The results showed that patients with low IPI were significantly superior to those with high IPI in CR (76.7% versus 35.7%, P = .017) and 10-year OS (63.8% versus 26.8%, P = .003; Figure 3A). However, the projected 10-year DFS was similar for patients with low or high IPI scores (51.8% versus 40%, P = .19). We then analyzed the prognostic impact of IPI in patients treated with either primary RT or aggressive chemotherapy. The projected 20-year OS for patients with low IPI score was still significantly superior to those with high IPI score (57.4% versus 27.6%, P = .012; Figure 3B).
Prognostic impact of IPI. (A) Treatment with intensive chemotherapy. Patients with an IPI less than or equal to 1 showed significantly better 10-year OS (P = .003). (B) Treatment with either RT or intensive chemotherapy. Patients with an IPI less than or equal to 1 still showed significantly better 20-year OS (P = .01).
Prognostic impact of IPI. (A) Treatment with intensive chemotherapy. Patients with an IPI less than or equal to 1 showed significantly better 10-year OS (P = .003). (B) Treatment with either RT or intensive chemotherapy. Patients with an IPI less than or equal to 1 still showed significantly better 20-year OS (P = .01).
Discussion
This study reported one of the largest series to date of nasal NK cell lymphoma. The clinicopathologic features of patients in this series were similar to other previous reported Asian studies that included nasal NK cell lymphomas as part of their cases,7,17 in that there was a male predominance (male-female = 3.3:1), a large proportion of patients with early disease (stage I/II = 84%), and a high frequency (about one third) of B symptoms despite apparently limited diseases. Interestingly, in non-Asian studies, the male-to-female ratio was less, ranging from 1.6:119 to a female predominance of 1:1.4.24 Most of these patients also had limited-stage disease,24 which was similar to Asian patients.
Because the majority of patients presented with early stage disease, a small proportion of patients with stage I/II were treated with RT alone. The chemotherapeutic regimen was heterogeneous, but could be divided essentially into anthracycline-containing regimens (each with comparable efficacies) for those younger than 60 years, and nonanthracycline-containing regimens for those older than 60 years. With this limitation, we showed that primary RT led to an apparent superior CR rate, which resulted in a better OS, as compared with primary chemotherapy. Data on nasal NK cell lymphoma treated with RT alone are scarce. Ribrag et al25 reported that in a series of early stage nasal NK/T-cell lymphoma, CR was achieved in all of 6 patients receiving RT alone, but only in 3 of 12 patients receiving combination chemotherapy. In another study of 15 patients with localized nasal NK/T-cell lymphomas, RT was planned after 4 courses of primary chemotherapy.18 However, 9 patients failed to respond to the primary combination chemotherapy. Seven patients received second-line chemotherapy, but all died of refractory disease. On the other hand, the remaining 2 patients received RT and both achieved CR and survived. These results seem to indicate that upfront RT might be beneficial to patients with early-stage nasal NK cell lymphomas. However, in view of the small number of patients receiving primary RT in this and other studies,18,25 a definite advantage of early or upfront RT should be validated in larger, prospective studies.
With different treatment modalities, we showed that an overall CR rate of 64.2% could be achieved, and that the projected 20-year OS and DFS were 37.1% and 33.5%, respectively. These results were similar to those reported in 2 previous studies. Kim et al17 reported that in a series of Korean patients with angiocentric lymphoma of the head and neck region (presumably containing a majority of NK/T-cell lymphomas) treated with RT alone, the CR rate was 66%, and the OS and DFS at 56 months were 40% and 37.5%, respectively. Cheung et al9 reported that in a series of Chinese patients with nasal NK/T-cell lymphomas treated with RT or chemotherapy or both, the CR rate was 68%, and the 5-year OS and DFS rates were 37.9% and 35.5%, respectively. Because NK cell lymphomas are rare in Western countries, similar data involving a large number of patients were not available. Ribrag et al25 reported an overall CR rate of 55% in 20 patients with nasal NK/T-cell lymphomas treated with RT or chemotherapy or both. However, the OS and DFS rates for the whole group were not reported, and the ethnicity of the patients was undefined. A Mexican study of nasal NK/T-cell lymphoma treated with RT followed by chemotherapy reported a CR rate of 90% and an 8-year OS and DFS of over 80%. Similarly, another Mexican team reported an OS rate of 73% with a median follow-up time of 10 years in 17 patients with nasal NK/T-cell lymphoma diagnosed at an American center.24 These results are widely disparate. One possibility is that there are ethnic differences in the response to treatment, although the extent of disparity in treatment outcome has not been observed in any lymphoma subtype. More likely, however, is the fact that many of the patients in the 2 Mexican studies were not fully characterized to be NK/T-cell lymphomas, owing to the lack of staining for EBV and CD56. Thus, the apparently better treatment outcome might have been due to the inclusion of lymphomas of other subtypes. Indeed, in a previous study that compared primary nasal lymphomas of B-cell, T-cell, and NK cell lineages,7 NK cell lymphomas had the worst prognosis with projected DFS and OS rates of 31% and 43%, respectively, as compared with those of 52% and 62% in T-cell lymphomas. These observations underscore the significance of careful histopathologic analysis of nasal lymphomas and the importance of confining the analysis to a homogeneous group of nasal NK cell lymphomas.
Prognostic factors in NK cell lymphomas have not been fully defined. The prognostic impact of IPI, initially shown in aggressive non-Hodgkin lymphomas,30 has been validated subsequently in lymphomas ranging from low to intermediate to high grades.31 Moreover, the IPI is also of prognostic significance in less conventional lymphomas, including extranodal gastric lymphomas,35 and peripheral T-cell lymphomas.26,36,37 However, in primary nasal NK cell lymphomas, 2 previous studies had failed to show a prognostic impact of IPI on the CR,19 OS, and DFS rates.7,9 On the contrary, we showed that in nasal NK cell lymphomas treated with primary RT or chemotherapy/RT, patients with IPI less than or equal to 1 were significantly better than those with IPI 2 or higher in the CR rate and long-term OS. Therefore, our study is the first to validate the use of the IPI in prognostication of nasal NK cell lymphomas. It is noteworthy that the IPI was apparently unrelated to the projected 10-year DFS, implying that other biomarkers prognostic of DFS need to be defined. We have reported recently that aberrant methylation of the p73 gene was found frequently in NK cell lymphoma.38 Because aberrant methylation has been shown to be prognostic in other malignancies, such as p15 methylation in acute promyelocytic leukemia,39 it will be interesting to define if methylation of p73 or other genes might be of prognostic significance in nasal NK cell lymphoma. Lastly, our results also showed the aggressive nature of NK cell lymphomas. More than 80% of our patients had stage I/II disease. In patients with diffuse large B-cell lymphomas and similar disease stages studied in the IPI project, the range of the expected CR rate was 85% to 94%, the projected OS was 68% to 79%, and the projected DFS was 68% to 75%.30 In contrast, our patients had much inferior CR (64%), OS (37%), and DFS (33%) rates.
Interestingly, the DFS curve in our study showed a biphasic pattern. The initial part portrayed the clinical course of an aggressive lymphoma with a sharp drop in survival rates, but the latter part showed a pattern of continual late relapses. Remarkably, in 5 patients delayed relapses occurred more than 5 years after an initial CR. In 4 patients the relapse was locally in the nasal region and in one patient in the scrotum. The reasons for these delayed relapses are unknown. Three of these patients had RT primarily, and because all had local relapses, the disease recurrence might be considered as in-field failures. This might suggest an inadequate irradiation dosage, and if so this could be potentially improved. Indeed, a recent study suggested that better RT planning with magnetic resonance imaging40 might reduce the risk of in-field failures.9 However, for minimal residual lymphoma cells to remain dormant to up to 30 years before relapsing was not a clinical course consistent with the aggressive nature of this lymphoma. Another possibility was a second and distinct lymphoma arising independently from a pool of chronically EBV-infected NK cells, after progressive accumulation of genetic mutations in the intervening years. Because EBV is present as a clonal episomal form in NK lymphoma cells,27 a comparison between the diagnostic and relapse tumors could address this issue. Essentially, the clonal EBV episomal form of the second tumor would be the same as the first one had it been a relapse, but different had it been a distinct lymphoma.41 Unfortunately, insufficient diagnostic tumor material precluded further investigations in our cases.
The rarity of this disease limits large-scale randomized studies. However, several of our findings have important implications on the treatment of nasal NK cell lymphomas. The high efficacy of radiotherapy in inducing CR, although observed in a relatively small number of patients, suggests that it might be advantageous to incorporate RT upfront in treatment protocols. The occurrence of failures at distant sites implies that systemic chemotherapy should also be used. Patients with high IPI scores and therefore a worse prognosis may benefit from additional therapy designed to prevent relapses. Accordingly, we have incorporated these objectives into a combined-modality regimen, in which patients will receive 3 courses of intensive chemotherapy, followed by involved-field RT, and then 3 more courses of chemotherapy. Patients with high-risk IPI will then be randomized to observation with frequent follow-up that includes molecular evaluation,42 or high-dose chemotherapy with HSCT.33 Life-long follow-up is recommended for all patients, in view of the possibility of delayed relapse.
Prepublished online as Blood First Edition Paper, August 21, 2003; DOI 10.1182/blood-2003-05-1401.
Supported by the Kadoorie Charitable Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal