Abstract
T-cell anergy is a tolerance mechanism defined as a hyporesponsive status of antigen-specific T cells upon prior antigen encounter and is believed to play a critical role in the evasion of tumor immunity and the amelioration of allogeneic transplant rejection. Molecular mechanisms in controlling T-cell anergy are less known. We show here that administration of an agonistic monoclonal antibody (mAb) to CD137, a member of the tumor necrosis factor receptor superfamily, prevents the induction of CD8+ cytolytic T-lymphocyte (CTL) anergy by soluble antigens. More importantly, CD137 mAb restores the functions of established anergic CTLs upon reencountering their cognate antigen. As a result, infusion of CD137 mAb inhibits progressive tumor growth that is caused by soluble tumor antigen-induced tolerance in a P815R model. CD137 mAb also restores proliferation and effector functions of anergic alloreactive 2C T cells in a bone marrow transplantation model. Our results indicate that ligation of CD137 receptor delivers a regulatory signal for T-cell anergy and implicate manipulation of the CD137 pathway as a new approach to break T-cell tolerance.
Introduction
T-cell anergy is a persistent status of unresponsiveness on T cells upon prior exposure to the same antigen and could be distinguished from other tolerance mechanisms including deletion, suppression, cytokine deviation, and ignorance. Anergy can often be induced in vitro by the stimulation of T cells in the absence of appropriate costimulation.1,2 There is ample evidence that T-cell anergy can be induced in vivo by cross-presentation of a large number of soluble antigens or direct presentation of cellular antigens in an environment without appropriate costimulation.3-5 By providing interleukin-2 (IL-2) or CD28 costimulation in vitro, CD4+ T-cell anergy may be prevented or even broken, although these methods are ineffective in vivo.6-8 Recent studies showed that, by enforcing signaling through OX40 or CD40 simultaneously with T-cell receptor (TCR) engagement, anergy induction on CD4+ T cells could be prevented in several animal models.9,10 It was also shown that anergy in CD4+ T cells by soluble antigens could also be reversed by OX40 signaling in vivo.9
CD8+ cytolytic T lymphocytes (CTLs) play a prominent role in the control of cancers and transplant rejection. Anergy in the CD8+ T-cell compartment, therefore, may allow tumors to escape immune attack.11 On the other hand, CD8+ T-cell anergy facilitates acceptance of allogeneic cells, tissues, and organs. Unlike classical anergy described in CD4+ T cells, induction of CD8+ T-cell anergy may be independent of B7-1 and B7-2 costimulation.12 CD8+ T cells may even become anergic following antigen presentation by dendritic cells, which express a rich array of costimulatory molecules.13 While proliferation of anergic CD8+ T cells is impaired, it has been shown that anergic CD8+ CTLs continue to release IL-10 after exposure to antigen, suggesting a regulatory function for anergic CD8+ T cells.14 In addition, anergic CD8+ CTLs may retain cytolytic activity against target cells, a phenomenon termed “split anergy.”15 Whereas preventing the formation of T-cell anergy is possible, the methods and approaches to break or to reverse established anergy in CD8+ CTLs have not been reported.
CD137 (4-1BB, ILA) is an inducible molecule found on activated T cells, natural killer (NK) cells, dendritic cells, and monocytes, and engagement by its natural ligand (CD137L) or agonistic monoclonal antibody (mAb) leads to growth, cytokine production, and functional maturation.16-19 In the presence of TCR signaling, CD137 mAb costimulates T-cell growth in vitro.20-23 Administration of agonistic CD137 mAb either alone or following peptide vaccination is capable of stimulating a potent tumor-specific CD8+ CTL response, leading to regression of established tumors in various mouse models.24,25 CD137 stimulation also prevents activation-induced cell death by superantigen in CD8+ T cells.26 The importance of CD137 in the generation of a fully competent CD8+ T-cell response was shown in both graft versus host disease (GVHD) and in viral infection models using CD137- or CD137L-deficient mice.27-31 Collectively, these data support the roles of CD137 signaling in the regulation of growth and death of CD8+ T cells. In this report, we present evidence that CD137 engagement can prevent and even reverse established anergy in CD8+ T cells. These findings reveal a new mechanism for the effect of CD137 in the stimulation of T-cell responses in vivo.
Materials and methods
Mice and cell lines
Female C57BL/6 (B6), DBA/2, and BDF1 (B6 × DBA/2) mice were purchased from the National Cancer Institute (Frederick, MD). OT-1 and 2C mice express transgenic T-cell receptor specific to an H-2Kb–restricted chicken ovalbumin (OVA) and to an H-2Ld–restricted murine α-ketoglutarate dehydrogenase, respectively.5 The EL4 T-cell lymphoma was purchased from American Type Culture Collection (ATCC) (Rockville, MD). P815R is a subclone of P815 mastocytoma and regresses spontaneously after inoculation into syngeneic DBA/2 mice.32 Cells were maintained in a complete medium of RPMI 1640 (Mediatech, Herndon, VA) supplemented with 10% fetal bovine serum (FBS) (HyClone, Logan, UT), 25 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 2 mM glutamine, 100 U/mL penicillin G, and 100 μg/mL streptomycin sulfate. For tumor challenge experiments, 1.5 × 104 P815R was injected intradermally into the shaved flanks of DBA/2 mice.
Peptide, tetramer, and antibodies
The OVA (257-264) peptide (SIINFEKL) is an H-2Kb–restricted CTL epitope derived from chicken ovalbumin. The OVA (55-62) peptide (KVVRFDKL), which is not recognized by OT-1 TCR transgenic T cells, was used as a control peptide. The P1A (35-43) peptide (LPYLGWLVF) is an H-2Ld–restricted CTL epitope. All peptides were synthesized by the Mayo Molecular Biology Core Facility, and the purity of the peptide was more than 90% as determined by reverse-phase high-performance liquid chromatography (HPLC) purification. The peptides were dissolved in dimethyl sulfoxide (DMSO) and reconstituted in phosphate-buffered saline (PBS) to a final concentration of 1 mg/mL (5% DMSO) for administration to mice.
The H-2Kb/OVA tetramer conjugated to phycoerythrin (PE) was obtained from the National Institutes of Health (NIH) tetramer core facility (Atlanta, GA). Tetramer staining was performed as previously described.24
A rat mAb against mouse CD137 was generated and purified as previously described.24 The rat immunoglobulin G (IgG) control antibody was purchased from Sigma (Gilbertsville, PA). PE-conjugated 1B2, a clonotypic mAb against 2C T cells, was a generous gift from Dr Larry R. Pease (Mayo Clinic, Rochester, MN). Purified fluorescein isothiocyanate (FITC)–conjugated anti-CD69, anti-CD25, anti-CD49d, anti-CD8, and isotype control mAb were purchased from PharMingen (San Diego, CA). CyChrome-conjugated anti-CD8 mAb was purchased from PharMingen.
Induction of T-cell anergy
A total of 3 × 106 to 7 × 106 lymph node and spleen cells from OT-1 mice were tail vein–injected into wild-type B6 mice in 0.5 mL Hanks balanced salt solution (HBSS) (Cellgro, Herndon, VA). Twenty-four hours later, experimental mice were given 0.5 mg OVA (257-264) peptide, an antigenic epitope to OT-1 cells, intravenously in 0.5 mL total volume, while control mice were given OVA (55-62) peptide (or PBS alone in some experiments) in a similar fashion. On the day of peptide administration, and again 3 days later, mice were given 100 μg of either rat IgG or anti-CD137 mAb intraperitoneally. Mice were killed at various time points following peptide administration, and the total number of OT-1 cells present in the spleen and lymph nodes of each mouse was determined by H-2Kb/OVA (257-264) tetramer analysis. For restimulation experiments, 10 days following the administration of peptide, spleens were harvested from the mice. After lysing red blood cells in ACK lysis buffer, the spleen cells were resuspended in RPMI and plated in triplicate onto a 96-well plate at a density of 5.5 × 105 cells per well in 200 μL total volume. One group of cells was unstimulated while a second group was restimulated with 1 ng/mL OVA peptide. On the same day that splenocytes were restimulated, the frequency of OVA-specific T cells was determined using the H-2Kb/OVA (257-264) tetramer, as previously described. Therefore, the absolute number of OT-1 cells added to each well could be calculated prior to restimulation in vitro. Supernatants were collected from the wells in each group 48 and 72 hours after restimulation, and IL-2 (48 hours) and interferon-γ (IFN-γ) (72 hours) production was measured by sandwich enzyme-linked immunosorbent assay (ELISA) following the manufacturer's instructions (PharMingen). The proliferation of T cells was assessed by the addition of 1 μCi (0.037 MBq) per well 3H-thymidine (3H-TdR) during the last 15 hours of the 3-day culture. 3H-TdR incorporation was measured in a MicroBeta TriLux liquid scintillation counter (Wallac, Turku, Finland). Antigen-specific proliferation or cytokine production per OT-1 cell was calculated by subtracting any nonspecific proliferation (or cytokine production) observed in the unstimulated groups from the proliferation (or cytokine production) observed in the peptide-stimulated groups. This figure was then divided by the number of OT-1 cells (103) initially present in the well prior to restimulation in order to derive the net change in counts per minute (Δcpm) per 103 OT-1 cells.
To measure the ability of anti-CD137 mAb to reverse anergy, OT-1 cells were adoptively transferred into naive B6 recipients. Anergy was induced by the intravenous administration of 0.5 mg OVA peptide as described in the previous paragraph. Ten days later, mice were given 0.5 mg OVA peptide or control peptide intravenously. On the same day, mice intraperitoneally received 100 μg of either rat IgG or anti-CD137 mAb. Mice were killed at various time points following rechallenge with the OVA peptide, and the number of OT-1 cells present in the spleen and lymph nodes of each mouse was determined by tetramer analysis, as before.
Flow cytometry sorting and cytotoxicity assay
For flow cytometry sorting experiments, T cells were purified from spleens and lymph nodes of mice using Thy1.2 microbeads, according to the manufacturer's instructions. Purified Thy1.2+ cells were subsequently stained with H-2Kb/OVA tetramer and anti-CD8 mAb. Cells that were positive for both tetramer and CD8 were sorted using a FACSVantage Flow Cytometry System (BD Immunocytometry Systems, San Jose, CA). At least 90% of the sorted cells were tetramer-positive and CD8+ (data not shown). A 4-hour 51chromium (51Cr)–release assay was performed, as previously described,24 using OVA-pulsed EL4 or unpulsed EL4 as target cells.
Bone marrow transplantation
BDF1 mice were first exposed to lethal-dose irradiation (11 Gy) and subsequently intravenously given 1 × 107 spleen cells together with 5 × 106 bone marrow cells from 2C TCR transgenic mice. Ten days later, CD8+ T cells were purified from spleen cells using anti-CD8 microbeads (Miltenyi Biotec, Auburn, CA) and subjected to in vitro analyses. Alternatively, BDF1 mice, which had undergone bone marrow transplantation (BMT) from 2C mice 10 days before, further received intravenous carboxyfluorescein diacetate succinimidyl ester (CFSE)–labeled (1 μM; Molecular Probes, Eugene, OR) BDF1 spleen cells. On the same day, the mice were also intraperitoneally given 100 μg of either control rat IgG or anti-CD137 mAb. Five days later, the number of 2C T cells and transferred BDF1 cells was assessed by FACSCalibur flow cytometry with CellQuest software (BD Biosciences, Mountain View, CA).
Results
CD137 mAb prevents and reverses tumor antigen peptide-induced tolerance
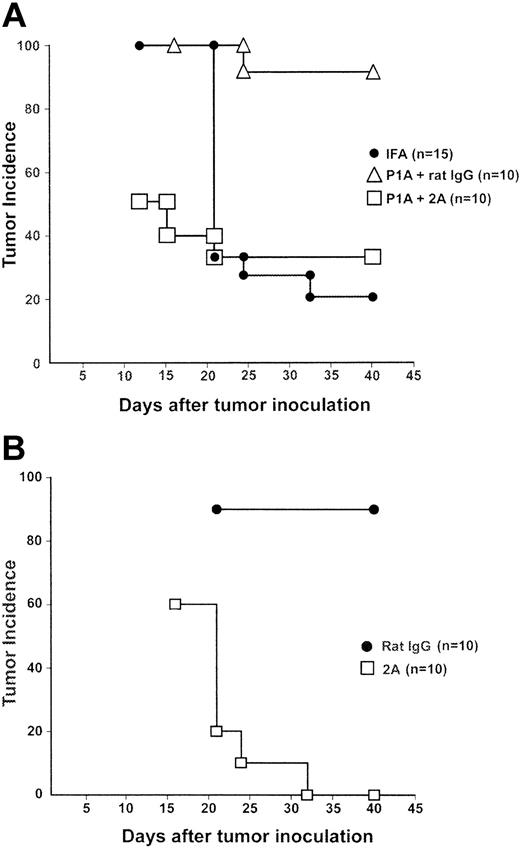
We showed previously that immunization with the P1A peptide, which encodes a CD8+ CTL epitope for a dominant tumor antigen,33 induced specific T-cell tolerance and led to progression of a regressor P815 cell line (P815R) in syngeneic DBA/2 mice.32 We utilized this system to evaluate the effect of CD137 ligation in T-cell tolerance. Subcutaneous injection of P815R cells into DBA/2 mice led to the transient growth of tumors in all mice and subsequent regression. At day 40 after tumor inoculation, 80% of mice that were treated with incomplete Freund adjuvant (IFA) alone were tumor free. In contrast, only 10% of mice were tumor free following the administration of P1A peptide with a control rat IgG 10 days prior to tumor challenge. Interestingly, injection of CD137 mAb, when giving tolerogenic P1A peptide, prevented tumor outgrowth and protected 70% of mice from tumors (Figure 1A). This result indicates that CD137 mAb could prevent the P1A peptide from inducing tumor tolerance.
CD137 mAb inhibited progressive growth of P815R tumors that were induced by tolerogenic P1A peptide. (A) DBA/2 mice were immunized subcutaneously with 50 μg P1A peptide emulsified in IFA at 2 sites. Control mice received only IFA. On the day of peptide immunization, and again 3 days later, mice were given 100 μg rat IgG or anti-CD137 (2A). Ten days following peptide immunization, mice were challenged with 1.5 × 104 P815R cells in the right flank. Tumor incidence of each group is plotted after the point when the maximal number of mice developed tumor. The difference between the rat IgG- and CD137-treated groups is statistically significant according to the log-rank test (P = .0038). (B) DBA/2 mice were immunized with P1A peptide emulsified in IFA as in panel A. Ten days later, the mice were challenged with 1.5 × 104 P815R cells. Three days after tumor challenge, and again 3 days later, the mice were given rat IgG or anti-CD137 (2A). Mice were monitored closely for both tumor development and regression following challenge. The difference between the rat IgG- and CD137-treated groups is statistically significant according to the log-rank test (P < .0001).
CD137 mAb inhibited progressive growth of P815R tumors that were induced by tolerogenic P1A peptide. (A) DBA/2 mice were immunized subcutaneously with 50 μg P1A peptide emulsified in IFA at 2 sites. Control mice received only IFA. On the day of peptide immunization, and again 3 days later, mice were given 100 μg rat IgG or anti-CD137 (2A). Ten days following peptide immunization, mice were challenged with 1.5 × 104 P815R cells in the right flank. Tumor incidence of each group is plotted after the point when the maximal number of mice developed tumor. The difference between the rat IgG- and CD137-treated groups is statistically significant according to the log-rank test (P = .0038). (B) DBA/2 mice were immunized with P1A peptide emulsified in IFA as in panel A. Ten days later, the mice were challenged with 1.5 × 104 P815R cells. Three days after tumor challenge, and again 3 days later, the mice were given rat IgG or anti-CD137 (2A). Mice were monitored closely for both tumor development and regression following challenge. The difference between the rat IgG- and CD137-treated groups is statistically significant according to the log-rank test (P < .0001).
We further determined the role of CD137 mAb in mice with preestablished tolerance. Mice were first inoculated with P1A peptide in the same protocol as described in the previous paragraph. Ten days later, the mice were challenged with P815R cells. As shown in Figure 1B, 90% of mice developed progressively growing tumors after the challenge, indicating an establishment of tolerance in the mice. Three days following tumor challenge, mice were given CD137 mAb. This treatment led to tumor regression in 100% of mice. Our results thus suggest CD137 ligation could also reverse established CD8+ T-cell tolerance.
Anergy induction in OT-1 transgenic T cells
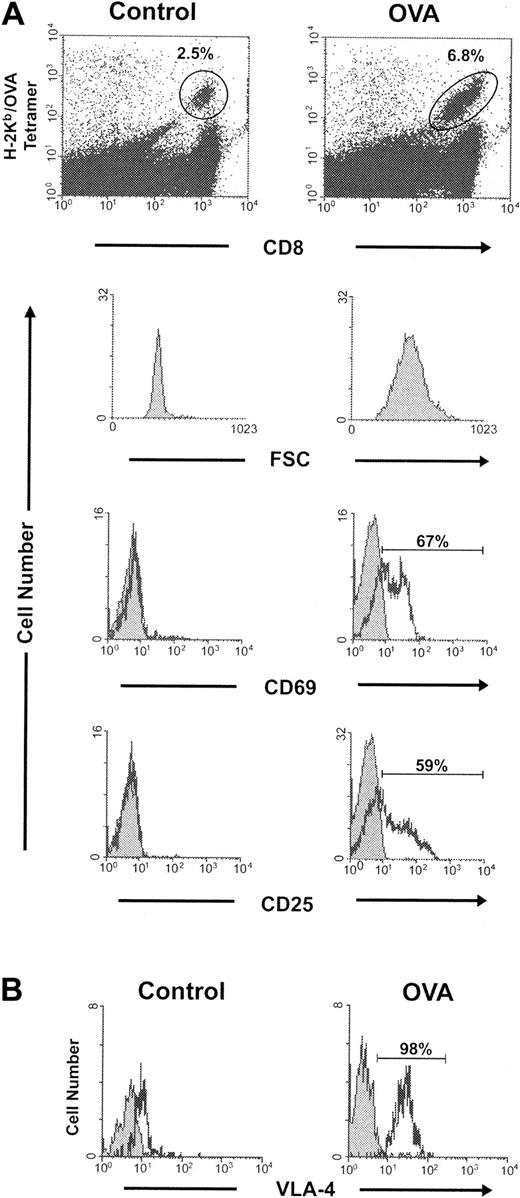
We utilized a well-studied OT-1 system to further analyze the effect of CD137 ligation in T-cell tolerance. In this model, soluble OVA peptide encoding an H-2Kb–restricted epitope or a control peptide was administered intravenously into the mice accommodating OT-1 TCR transgenic T cells. Two days later, pooled lymph node and spleen cells were stained with both anti-CD8 and tetrameric H-2Kb/OVA (OVA tetramer). Whereas only 2.5% of the CD8+ cells were OVA tetramer–positive in the mice treated with control peptide, OT-1 T cells in the OVA-treated mice increased to 6.8%. OT-1 cell blastogenesis was also observed in the OVA-treated mice as demonstrated by the increase in forward scatter by fluorescence-activated cell sorter (FACS) analysis. Furthermore, more than 50% of the OT-1 cells in the OVA-treated mice expressed the T-cell activation markers, CD69 and CD25 (Figure 2A). In addition, 10 days after peptide injection, virtually 100% of the OT-1 T cells in the mice exposed to OVA peptide expressed the late activation marker VLA-4 (very late antigen-4) (Figure 2B), indicating an unexceptional antigen encounter of OT-1 T cells in this system.
OT-1 activation following intravenous administration of OVA peptide. C57BL/6 mice were given OT-1 cells intravenously. Twenty-four hours later, the mice were given 0.5 mg of either a control peptide or OVA peptide intravenously. (A) Two days following the administration of peptide, 2 mice in each group were killed and the pooled spleen and lymph node cells were stained with both H-2Kb/OVA tetramer and anti-CD8. The percent of CD8+ cells that were tetramer-positive (gates as indicated) is shown. Cells were subsequently stained with anti-CD69 or anti-CD25 (black) and an isotype control (gray). Forward scatter (FSC), CD69, and CD25 expression was analyzed in the gated cells. (B) Ten days following the control or OVA peptide injection, spleen cells harvested from the recipient mice were stained with anti–VLA-4 (black) or an isotype control (gray) in conjunction with both H-2Kb/OVA tetramer and anti-CD8. The expression of VLA-4 was analyzed in the gate of tetramer-positive CD8+ OT-1 T cells.
OT-1 activation following intravenous administration of OVA peptide. C57BL/6 mice were given OT-1 cells intravenously. Twenty-four hours later, the mice were given 0.5 mg of either a control peptide or OVA peptide intravenously. (A) Two days following the administration of peptide, 2 mice in each group were killed and the pooled spleen and lymph node cells were stained with both H-2Kb/OVA tetramer and anti-CD8. The percent of CD8+ cells that were tetramer-positive (gates as indicated) is shown. Cells were subsequently stained with anti-CD69 or anti-CD25 (black) and an isotype control (gray). Forward scatter (FSC), CD69, and CD25 expression was analyzed in the gated cells. (B) Ten days following the control or OVA peptide injection, spleen cells harvested from the recipient mice were stained with anti–VLA-4 (black) or an isotype control (gray) in conjunction with both H-2Kb/OVA tetramer and anti-CD8. The expression of VLA-4 was analyzed in the gate of tetramer-positive CD8+ OT-1 T cells.
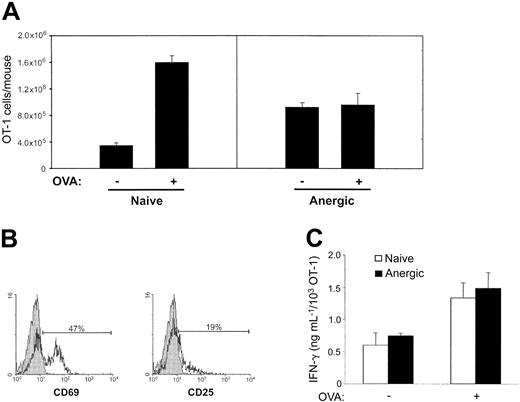
To determine the fate of OT-1 T cells after exposure to OVA peptide, the mice were rechallenged intravenously 10 days later with a control peptide or OVA peptide. For comparison, naive mice that had not received the OVA peptide were also given the control peptide or OVA peptide. Two days following peptide administration, spleens and lymph nodes were harvested and the total number of OT-1 cells was calculated by OVA tetramer staining. A more than 4-fold expansion was observed in the naive mice treated with OVA peptide. In contrast, no significant expansion of OT-1 cells was observed in those mice that had received OVA peptide 10 days previously (Figure 3A). These results demonstrate the induction of OT-1 T-cell unresponsiveness without significant deletion in OVA-treated mice and support a role of OVA peptide in the induction of OT-1 anergy. Unlike naive OT-1 T cells, in which the expression of CD69 and CD25 could be induced following antigenic challenge, most anergic OT-1 T cells failed to express significant levels of CD25 following antigenic rechallenge, although expression of CD69 was observed (Figure 3B). Interestingly, although anergic OT-1 cells were incapable of proliferating in vivo, these cells secrete IFN-γ upon restimulation to a level similar to naive OT-1 T cells (Figure 3C). Taken together, our results demonstrate administration of OVA peptide induces anergy of OT-1 T cells.
Induction of OT-1 anergy by OVA peptide in vivo. C57BL/6 mice that had been received OT-1 T cells were given PBS (naive) or 0.5 mg OVA (anergic) intravenously. Ten days later, the mice were rechallenged with either the control (–) or OVA (+) peptide. Two days following the rechallenge, the mice were killed and pooled spleen and lymph node cells were stained with H-2Kb/OVA tetramer and anti-CD8. (A) The total number of OT-1 cells present in each mouse was calculated and is shown as the mean (± SD) of 3 mice in each group. (B) Tetramer-positive and CD8+ cells in the tolerized mice that were positive for both tetramer and CD8 were stained with isotype control (gray) and anti-CD69 or anti-CD25 (black). (C) Ten days following the administration of PBS or OVA, spleens were harvested and restimulated in triplicate in the absence or presence of OVA peptide (1 ng/mL) in 96-well plates. At the time of restimulation, the frequency of OVA-specific T cells was determined by tetramer analysis. OVA-specific IFN-γ production was measured 72 hours later by ELISA. IFN-γ production was determined on a per cell basis as described in “Materials and methods.” Data shown are representative of at least 3 independently performed experiments.
Induction of OT-1 anergy by OVA peptide in vivo. C57BL/6 mice that had been received OT-1 T cells were given PBS (naive) or 0.5 mg OVA (anergic) intravenously. Ten days later, the mice were rechallenged with either the control (–) or OVA (+) peptide. Two days following the rechallenge, the mice were killed and pooled spleen and lymph node cells were stained with H-2Kb/OVA tetramer and anti-CD8. (A) The total number of OT-1 cells present in each mouse was calculated and is shown as the mean (± SD) of 3 mice in each group. (B) Tetramer-positive and CD8+ cells in the tolerized mice that were positive for both tetramer and CD8 were stained with isotype control (gray) and anti-CD69 or anti-CD25 (black). (C) Ten days following the administration of PBS or OVA, spleens were harvested and restimulated in triplicate in the absence or presence of OVA peptide (1 ng/mL) in 96-well plates. At the time of restimulation, the frequency of OVA-specific T cells was determined by tetramer analysis. OVA-specific IFN-γ production was measured 72 hours later by ELISA. IFN-γ production was determined on a per cell basis as described in “Materials and methods.” Data shown are representative of at least 3 independently performed experiments.
CD137 mAb prevents OT-1 T-cell anergy in vivo
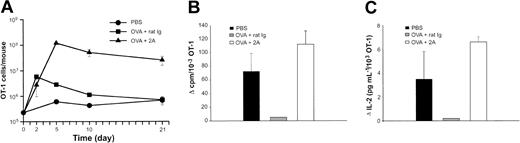
We first tested the effect of CD137 mAb in the prevention of T-cell anergy. OT-1–reconstituted mice were first infused with OVA peptide and subsequently treated with either a CD137 mAb or control rat IgG. Mice were killed at various time points up to 21 days following antigenic challenge, and the total number of OT-1 cells present in the spleens and lymph nodes in each group of mice was determined by tetramer staining. As shown in Figure 4A, treatment with CD137 mAb led to an approximately 10-fold increase in the expansion of OT-1 cells compared with those mice that received the control rat IgG. The robust T-cell response observed following CD137 mAb injection led to significant splenomegaly in the CD137 mAb–treated mice (data not shown). In contrast, the T-cell response in the mice given the control rat IgG peaked 2 days following OVA administration and rapidly declined, reaching baseline by day 21. OT-1 cells in the CD137 mAb–treated mice persisted for at least 21 days following antigenic stimulation. Our results thus demonstrate the ability of CD137 stimulation to promote the expansion of OT-1 T cells in vivo.
CD137 stimulation prevents the induction of anergy on OT-1 cells in vivo. (A) B6 mice were given OT-1 cells prior to the intravenous administration of PBS or OVA peptide. On the day of peptide administration and again 3 days later, mice were given 100 μg of either control rat IgG or anti-CD137 (2A). Mice were killed at the time points indicated, and the total number of OT-1 cells present in each mouse was calculated following tetramer analysis. The data shown are the mean (± SD) of 3 mice in each group. (B-C) Ten days following the administration of PBS or OVA, spleens were harvested and restimulated in triplicate in the absence or presence of OVA peptide (1 ng/mL) in 96-well plates. At the time of restimulation, the frequency of OVA-specific T cells was determined by tetramer analysis. OVA-specific proliferation and IL-2 production were determined 72 and 48 hours later, respectively. Thymidine incorporation and IL-2 production were determined on a per cell basis as described in “Materials and methods.” Data shown are representative of at least 3 independently performed experiments.
CD137 stimulation prevents the induction of anergy on OT-1 cells in vivo. (A) B6 mice were given OT-1 cells prior to the intravenous administration of PBS or OVA peptide. On the day of peptide administration and again 3 days later, mice were given 100 μg of either control rat IgG or anti-CD137 (2A). Mice were killed at the time points indicated, and the total number of OT-1 cells present in each mouse was calculated following tetramer analysis. The data shown are the mean (± SD) of 3 mice in each group. (B-C) Ten days following the administration of PBS or OVA, spleens were harvested and restimulated in triplicate in the absence or presence of OVA peptide (1 ng/mL) in 96-well plates. At the time of restimulation, the frequency of OVA-specific T cells was determined by tetramer analysis. OVA-specific proliferation and IL-2 production were determined 72 and 48 hours later, respectively. Thymidine incorporation and IL-2 production were determined on a per cell basis as described in “Materials and methods.” Data shown are representative of at least 3 independently performed experiments.
To determine the consequence of CD137 mAb–mediated stimulation of T cells in the presence of tolerogenic OVA peptide, the 3 groups of mice shown in Figure 4A were killed 10 days following peptide administration. The number of OT-1 cells present in the pooled splenocytes from each group of mice was determined following tetramer staining. The splenocytes were subsequently restimulated in vitro with an optimal concentration of OVA peptide. OT-1 proliferation and IL-2 secretion were measured 72 or 48 hours later, respectively, and determined on a per cell basis. Unlike the naive T cells from mice that had received PBS, OT-1 cells from mice that were given OVA and the control rat IgG failed to proliferate (Figure 4B) and to secrete IL-2 (Figure 4C) following in vitro restimulation. In sharp contrast, OT-1 proliferation and IL-2 secretion were observed in those mice that received CD137 mAb. In addition, intracellular IL-2 staining by specific mAb in flow cytometry analysis showed that only activated OT-1 T cells had detectable intracellular IL-2 whereas non–OT-1 T cells do not (data not shown). Our results thus provide further evidence that CD137 ligation prevents the induction of T-cell anergy.
CD137 mAb reverses established OT-1 T-cell anergy in vivo
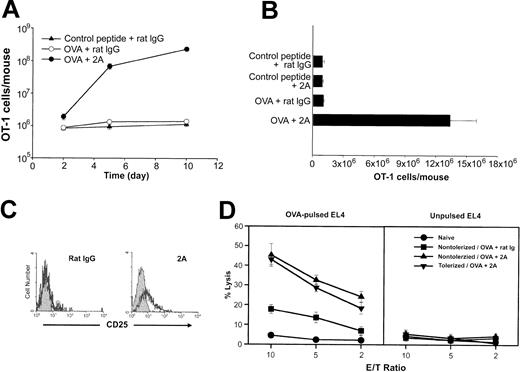
We next sought to determine the effect of CD137 mAb in OT-1 cells with established anergy. To address this, the mice were given OVA peptide to induce anergy in OT-1 T cells. Ten days later, the mice were treated with CD137 mAb together with OVA peptide. Mice were killed 2, 5, and 10 days following treatment, and the total number of OT-1 T cells was determined following tetramer staining. As shown in Figure 5A, a marked expansion of the OT-1 cells was observed in OVA-tolerized mice that were treated with anti-CD137 and OVA peptide. The anergic OT-1 cells, however, failed to expand following a subsequent challenge with either the OVA peptide or control peptide. Our results indicate that CD137 signaling, in addition to preventing the induction of T-cell anergy, breaks anergy in OT-1 T cells that had been previously established.
CD137 stimulation reverses OT-1 T-cell anergy in vivo. (A) Anergy was established in OT-1 recipients upon the intravenous administration of OVA peptide as described before. Ten days later, mice were rechallenged with either a control peptide or OVA peptide. The rechallenged mice that were given OVA received either a rat IgG control or anti-CD137 (2A). Mice were killed at the time points indicated, and the total number of OT-1 cells present in each mouse was determined by tetramer analysis, as before. (B) Following the establishment of anergy, mice were rechallenged with various combinations of either the control or OVA peptides and the control rat IgG or anti-CD137 (2A), as indicated. The total number of OT-1 cells present in each mouse 3 days later is shown. Data shown in panels A and B are the mean (± SD) of 3 mice in each group. (C) Two days following OVA challenge and antibody administration (rat IgG or 2A, as indicated), the anergic mice were killed. Cells that were positive for both tetramer and CD8 were stained with either an isotype control (gray) or anti-CD25 (black), as before. (D) OT-1 cells were adoptively transferred into B6 mice. A group of mice was left untreated (nontolerized) while anergy was established as described before in an additional group of mice (tolerized). Ten days later, these mice were challenged with OVA and treated with either control rat IgG or anti-CD137 (2A). Five days following the OVA rechallenge, H-2Kb/OVA-positive OT-1 cells were sorted by FACS and used as effectors in a 4-hour 51Cr-release assay for cytotoxicity against OVA-pulsed EL4 and unpulsed EL4 at the indicated E/T ratios. Naive OT-1 cells, freshly purified from an OT-1 mouse, were also used as effector cells.
CD137 stimulation reverses OT-1 T-cell anergy in vivo. (A) Anergy was established in OT-1 recipients upon the intravenous administration of OVA peptide as described before. Ten days later, mice were rechallenged with either a control peptide or OVA peptide. The rechallenged mice that were given OVA received either a rat IgG control or anti-CD137 (2A). Mice were killed at the time points indicated, and the total number of OT-1 cells present in each mouse was determined by tetramer analysis, as before. (B) Following the establishment of anergy, mice were rechallenged with various combinations of either the control or OVA peptides and the control rat IgG or anti-CD137 (2A), as indicated. The total number of OT-1 cells present in each mouse 3 days later is shown. Data shown in panels A and B are the mean (± SD) of 3 mice in each group. (C) Two days following OVA challenge and antibody administration (rat IgG or 2A, as indicated), the anergic mice were killed. Cells that were positive for both tetramer and CD8 were stained with either an isotype control (gray) or anti-CD25 (black), as before. (D) OT-1 cells were adoptively transferred into B6 mice. A group of mice was left untreated (nontolerized) while anergy was established as described before in an additional group of mice (tolerized). Ten days later, these mice were challenged with OVA and treated with either control rat IgG or anti-CD137 (2A). Five days following the OVA rechallenge, H-2Kb/OVA-positive OT-1 cells were sorted by FACS and used as effectors in a 4-hour 51Cr-release assay for cytotoxicity against OVA-pulsed EL4 and unpulsed EL4 at the indicated E/T ratios. Naive OT-1 cells, freshly purified from an OT-1 mouse, were also used as effector cells.
While our results indicate that CD137 mAb together with OVA peptide can reverse anergy in OT-1 T cells, it is unclear whether T-cell receptor engagement is required for CD137 mAb to break anergy. To test this, the tolerized mice were prepared as described previously. Ten days after the induction of tolerance, the mice were given various combinations of control peptide or OVA peptide and control rat IgG or CD137 mAb. Three days later, the mice were killed and the total number of OT-1 cells determined. OT-1 cell expansion was only observed in the group of mice that received both OVA peptide and CD137 mAb but not in those treated by OVA peptide or CD137 mAb alone (Figure 5B). As shown in Figure 3B, anergic OT-1 cells failed to express CD25 following encounter with antigen. However, CD137 signaling restored, at least in part, the ability of these cells to express CD25 (Figure 5C). Our results thus suggest that reversal of anergy in CD8+ T cells by CD137 mAb requires TCR engagement.
We next sought to test whether or not the cytolytic activity of OT-1 T cells is retained following CD137 mAb injection. OT-1 cells were sorted from the OVA-tolerized mice 5 days after challenge with OVA peptide plus CD137 mAb and were used as effectors in a 4-hour 51Cr-release assay for cytotoxicity against OVA-pulsed EL4 and control EL4 cells. As a control, OT-1 cells were identically sorted from the nontolerized mice 5 days after receiving OVA peptide plus either rat IgG or CD137 mAb. As shown in Figure 5D, approximately 20% specific lysis was observed at an effector-target (E/T) ratio of 10 to 1 in those mice that had received the control mAb. However, a more than 2-fold increase in cytotoxicity was observed in OT-1 cells isolated from those mice that had received CD137 mAb. Importantly, a similar level of cytotoxicity was observed in OT-1 cells isolated from the nontolerized and the tolerized mice when they received CD137 mAb, suggesting that CD137 mAb not only restores their proliferative capacity, but also promotes cytotoxicity, in previously anergized OT-1 cells.
CD137 mAb reverses anergy of alloreactive CTLs in bone marrow chimera
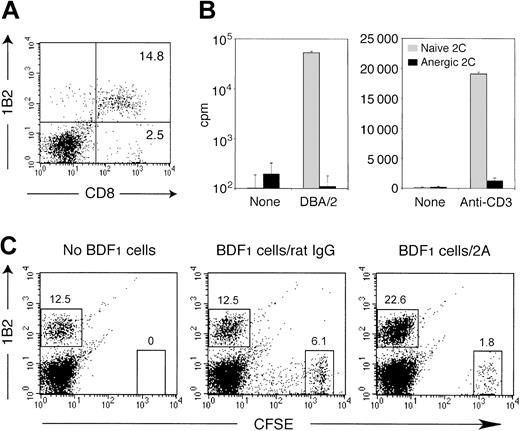
We also determined whether CD137 receptor ligation is capable of regulating cell-associated antigens in addition to soluble antigens. Constant exposure of T cells to allogeneic host antigens in the setting of bone marrow transplantation often leads to formation of bone marrow chimera with complete anergy of alloreactive T cells. We evaluated the regulatory effect of CD137 mAb in the established anergy of allogeneic T cells. In this model, bone marrow and spleen cells from 2C TCR transgenic mice were transplanted into lethally irradiated BDF1 mice. 2C T cells promptly became vigorously activated and expanded following transfer in response to host H-2Ld antigen. However, most cells were subsequently deleted and a small number of 2C T cells became anergic and persisted in the recipient mice.34,35 With a clonotypic mAb 1B2 for 2C TCR, we demonstrated that approximately 85% of CD8+ T cells were 2C T cells in the reconstituted BDF1 mice 10 days after BMT (Figure 6A). The persistent 2C T cells were unresponsive to stimulation by Ld-expressing spleen cells or immobilized anti-CD3 mAb (Figure 6B), indicating an establishment of peripheral tolerance on 2C T cells. To determine the effect of CD137 mAb, the recipient mice carrying anergized 2C T cells were given CFSE-labeled BDF1 spleen cells as an antigen source in conjunction with either CD137 mAb or control rat IgG. On day 5 after treatment, the activation status of anergic 2C T cells was determined. Upon infusion of CD137 mAb, the number of anergic 2C T cells increased, accompanied by a marked reduction of transferred BDF1 spleen cells, indicating a recovery of proliferation and effector function of anergic 2C T cells. No significant expansion of anergic 2C T cells was observed in rat IgG-treated mice in spite of the transfer of BDF1 spleen cells (Figure 6C). A small population of transferred BDF1 cells showed diluted CFSE contents, suggesting a spontaneous proliferation (Figure 6C). This observation, however, could be interpreted as hybrid resistance caused by NK cell activation. Taken together, our data demonstrate that CD137 mAb also breaks established tolerance of anergic T cells to allogeneic antigens.
CD137 agonistic mAb reverses established anergy of alloreactive 2C T cells in bone marrow chimera. BDF1 mice were exposed to a lethal dose of irradiation (11 Gy) followed by an intravenous transfer of 5 × 106 bone marrow cells and 1 × 107 spleen cells from 2C TCR transgenic mice. (A) Ten days after BMT, spleen cells were prepared from the recipient mice and subjected to analysis by a flow cytometry. The percentage of 1B2-positive CD8+ 2C T cells and 1B2-negative CD8+ T cells is shown in the corresponding quadrants. (B) Ten days after BMT, 2C T cells were purified from the recipient spleen cells by an isolation of CD8+ T cells. The cells (1 × 105 cells per well) were subsequently cultured with irradiated (30 Gy) DBA/2 spleen cells (5 × 105 cells per well) or alone (left panel), and in the presence or absence of immobilized 1 μg/mL anti-CD3 mAb (right panel). CD8+ T cells isolated from naive 2C mice were cultured similarly as control. Proliferative activity was determined by 3H-thymidine incorporation on day 3. The data are shown as an average ± SD of triplicate wells. (C) Ten days after BMT, the recipient mice were further transferred intravenously with CFSE-labeled BDF1 spleen cells (2.5 × 107 cells) or were untreated. In the groups with cell transfer, 100 μg of either control rat IgG or CD137 mAb (2A) was injected intraperitoneally on the same day. After 5 days, spleen cells from the recipient mice were analyzed by a flow cytometry. The percentage of 1B2-positive 2C T cells and the transferred BDF1 cells (CFSEhigh cells) are indicated.
CD137 agonistic mAb reverses established anergy of alloreactive 2C T cells in bone marrow chimera. BDF1 mice were exposed to a lethal dose of irradiation (11 Gy) followed by an intravenous transfer of 5 × 106 bone marrow cells and 1 × 107 spleen cells from 2C TCR transgenic mice. (A) Ten days after BMT, spleen cells were prepared from the recipient mice and subjected to analysis by a flow cytometry. The percentage of 1B2-positive CD8+ 2C T cells and 1B2-negative CD8+ T cells is shown in the corresponding quadrants. (B) Ten days after BMT, 2C T cells were purified from the recipient spleen cells by an isolation of CD8+ T cells. The cells (1 × 105 cells per well) were subsequently cultured with irradiated (30 Gy) DBA/2 spleen cells (5 × 105 cells per well) or alone (left panel), and in the presence or absence of immobilized 1 μg/mL anti-CD3 mAb (right panel). CD8+ T cells isolated from naive 2C mice were cultured similarly as control. Proliferative activity was determined by 3H-thymidine incorporation on day 3. The data are shown as an average ± SD of triplicate wells. (C) Ten days after BMT, the recipient mice were further transferred intravenously with CFSE-labeled BDF1 spleen cells (2.5 × 107 cells) or were untreated. In the groups with cell transfer, 100 μg of either control rat IgG or CD137 mAb (2A) was injected intraperitoneally on the same day. After 5 days, spleen cells from the recipient mice were analyzed by a flow cytometry. The percentage of 1B2-positive 2C T cells and the transferred BDF1 cells (CFSEhigh cells) are indicated.
Discussion
The main finding presented in this report is that agonistic mAb against CD137 could prevent and reverse established anergy of CD8+ CTLs in vivo. This is demonstrated in 3 animal models, including CTL anergy induction by a soluble model antigen, a tumor antigen, and an allogeneic antigen. Our observation that established anergy of CTLs in vivo could be restored functionally by CD137 mAb is particularly interesting because this may have direct implications in the manipulation of T-cell responses in diseases such as cancer and transplant rejection. Intravenous infusion of soluble antigens is a classical method to induce tolerance of antigen-specific T cells. We found that infusion of a high dose of OVA CTL peptide led to complete unresponsiveness in adoptively transferred OT-1 cells. The method appears to be very efficient, because more than 95% of the OT-1 T cells expressed the late activation marker VLA-4 following peptide administration. This suggests that nearly all of the OT-1 T cells had encountered antigen. The inability of these OT-1 T cells to proliferate was verified both in vitro and in vivo, thus demonstrating the efficient induction of anergy. Importantly, CD137 ligation not only prevented the induction of anergy in OT-1 T cells following peptide administration but also restored the ability of these anergic T cells to proliferate upon antigenic restimulation. Similarly, infusion of a P1A peptide also induced tolerance of endogenous CTL responses specific for P1A, although P1A is a nonmutated self-tumor antigen.33 As a result, the regressive tumor P815R was converted to become progressive in tolerant mice. Complete anergy of H-2Ld–specific 2C T cells was also established in the mice that received 2C bone marrow transplantation. In all 3 systems, CD137 mAb induced T-cell activation as shown by recovery of proliferation and effector function or cytokine production. These experiments thus firmly establish the role of CD137 ligation in the regulation of T-cell tolerance/anergy.
Direct triggering of CD137 on CD8+ T cells may be responsible for the effect of CD137 mAb on the prevention and reversal of anergy whereas the effect of other cell subsets could not be excluded at this time. In the OT-1 adoptive transfer system, interestingly, either injection of antigen in soluble form or administration of CD137 mAb alone did not affect the anergy whereas a combination of both stimuli was required to have the effect (Figure 5B). These results suggest that appropriate antigen presentation is required for the effect of CD137 mAb, although we cannot conclude whether these stimuli directly recover T-cell anergy or do so by indirect mechanisms such as increased cytokine production. In this regard, activated CD4+ T cells also express CD137 and, upon triggering, release cytokines such as IL-2 that may provide helper signals to reverse CD8+ T-cell anergy. However, CD137 mAb could reverse CD8+ 2C T-cell anergy associated with BMT in the presence of a very low percentage of CD4+ T cells (data not shown). In addition, the effect of CD137 mAb in the stimulation of CTL responses in vivo is often independent of CD4+ T cells.36 These results do not support a central role of CD4+ T cells in CD137 mAb–mediated reversal of T-cell anergy. The recent observation by Maus et al showing that CD137 signaling on human CD8+ T cells is required for in vitro survival is consistent with our studies, and the effect could be, at least in part, explained by prevention of anergy.37 Other possible mechanisms include triggering of NK cells18 and dendritic cells (DCs)17 because these cells express CD137 and their immunoregulatory functions after triggering of CD137 have been implicated in vitro and in vivo. Our preliminary data showed that depletion of NK1.1-positive cells did not affect the effect of CD137 mAb in the reversal of OVA-induced T-cell anergy in vivo. Furthermore, adoptive transfer of CD137 mAb–activated DCs into OVA-tolerized mice did not stimulate proliferation of OT-1 T cells (K.T. et al, unpublished data, January 2003). Taken together, our results support a predominant effect of CD137 mAb on CD8+ T cells in anergy regulation.
Although anergic OT-1 T cells are deficient in secreting IL-2, their ability to produce IFN-γ remains intact, a finding consistent with previous reports.38 The biochemical mechanisms of CD137 signaling on CD8+ T cells in the prevention and reversal of anergy are unknown. A block in the Ras/mitogen-activated protein (Ras/MAP) kinase pathway has been identified in anergic CD4+ T cells and may be responsible for the formation of anergy.39,40 CD137 triggering up-regulates the MAP kinases Jun N-terminal kinase (JNK) and p38.41,42 Therefore, regulation of MAP kinases may be at least partially responsible for this effect. CD137 may stimulate T-cell proliferation in both an IL-2–dependent and an IL-2–independent fashion.43 The ability of CD137 signaling to reverse anergy may also be attributed to its ability to stimulate IL-2 secretion. However, the expression of the high-affinity IL-2 receptor CD25 by only a minority of anergic OT-1 cells following CD137 stimulation may suggest that T-cell expansion observed in these mice after CD137 mAb treatment is not entirely dependent on IL-2.
Numerous studies have demonstrated that blockade of costimulatory signals efficiently inhibits allogeneic T-cell responses leading to GVHD prevention and allograft rejection in association with major histocompatibility complex (MHC)–mismatched hematopoietic cell and organ transplantation. Mechanisms responsible for such effects include deletion, anergy, and active suppression of alloreactive T cells.5,44,45 In our present study, CD137 stimulation reverses BMT-associated T-cell anergy leading to 2C T-cell expansion and killing of host-derived cells, supporting the effects of CD137 mAb on both the proliferative and effector phases as shown in the OT-1 system. These findings propose a potential application of CD137 mAb to generate graft versus leukemia (GVL) effects against leukemic relapse following BMT, although it would be critical to successfully sequester GVHD from GVL effects. It has been reported that cytokines such as IL-2 and IL-12 preserve GVL effects mediated by alloreactive CD8+ T cells while inhibiting GVHD associated with CD4+ T cells.46 CD137 signaling predominantly activates CD8+ T cells and provides weaker or rather suppressive effects on CD4+ T-cell activation,47,48 indicating its potential for GVL effects. Administration of CD137 mAb together with the immunization of tumor antigens could be another approach because the effect of CD137 mAb may be guided by TCR signaling.
Because most of the tumor antigens that have been identified to date are self-antigens, the imposition of self-tolerance in peripheral T cells represents a significant challenge for efficient tumor immunotherapy. The induction of self-tolerance following tumor antigen-based therapy is an additional concern, because the administration of some tumor-specific peptides has been shown to induce T-cell anergy, thus promoting tumor outgrowth in animal models.49,50 This is also the case for the nonmutated self-tumor antigen, P1A, in which vaccination of P1A peptide induces T-cell tolerance and permits progressive growth of P815R tumors that are otherwise self-regressive.32 In this system, the administration of CD137 mAb at the time of peptide injection prevented the induction of tolerance and conferred protection against tumor challenge. While tumors failed to regress in the tolerized mice that received the control antibody, tumor regression was observed in CD137 mAb–treated mice that developed tumors. Therefore, CD137 mAb administration at the time of peptide administration transformed an otherwise tolerogenic vaccine into an immunogenic one. More importantly, CD137 mAb administration reversed tolerance in this model, resulting in tumor regression in 100% of treated mice. Our results thus support a new mechanism for the effect of CD137 receptor–mediated stimulation of CD8+ CTL responses in vivo.
Prepublished online as Blood First Edition Paper, September 11, 2003; DOI 10.1182/blood-2003-06-2184.
Supported by grants from the MD/PhD program of the Mayo Graduate and Medical Schools (R.A.W), National Institutes of Health (NIH) grants CA79915 and CA85721 (L.C.), the NIH (CA/AI 78399) (W.M.K), NIH postdoctoral fellowship (CA09127) (G.Z.), and the US Army Medical Research and Materiel Command postdoctoral fellowship (K.T.).
R.A.W. and K.T. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the NIH tetramer core facility (Emory University, Atlanta, GA) for providing the Kb/OVA tetramer, and Julie S. Lau and Kathy A. Jensen for editing the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal