Abstract
Crosslinking of the antigen receptors on the immature B-cell lymphoma, WEHI-231, leads to growth arrest and apoptosis. Commitment to such B-cell receptor (BCR)–mediated apoptosis correlates with mitochondrial phospholipase A2 activation, disruption of mitochondrial function, and cathepsin B activation. CD40 signaling has been reported to rescue WEHI-231 B cells from BCR-driven apoptosis primarily via up-regulation of the antiapoptotic protein Bcl-xL. Coupling of the BCR to the mitochondrial phospholipase A2–dependent apoptotic pathway can be prevented by rescue signals via CD40. We now show that overexpression of Bcl-xL can prevent mitochondrial phospholipase A2 activation, disruption of mitochondrial potential, and postmitochondrial execution of BCR-mediated apoptosis via cathepsin B activation. Moreover, overexpression of Bcl-xL protects WEHI-231 B cells from mitochondrial disruption and apoptosis resulting from culture with exogenous arachidonic acid, the product of phospholipase A2 action, suggesting that Bcl-xL may act to antagonize arachidonic acid–mediated disruption of mitochondrial integrity. However, although Bcl-xL expression can mimic CD40-mediated rescue of BCR-driven apoptosis, it cannot substitute for CD40 signaling in the reversal of BCR-mediated growth arrest of WEHI-231 B cells. Rather, CD40 signaling additionally induces conversion of arachidonic acid to prostaglandin E2 (PGE2), which promotes WEHI-231 B-cell proliferation by restoring the sustained, cycling extracellular signal–regulated/mitogen-activated protein kinase (ErkMAPkinase) signaling required for cell cycle progression.
Introduction
Apoptosis plays a key role in the selection processes that result in clonal deletion of autoreactive B lymphocytes during their development.1,2 The murine B-cell lymphoma cell line WEHI-231 has been widely used as a model for dissecting the molecular mechanisms underlying clonal deletion of immature B lymphocytes. This is because WEHI-231 cells have the cell surface phenotype of immature B cells (membrane immunoglobulin M–positive [mIgM+], mIgD–/low, FcRlow, Faslow, and major histocompatibility complex [MHC] class IIlow) and undergo growth arrest and apoptosis following ligation of the B-cell receptor (BCR). Moreover, WEHI-231 B cells can be rescued from BCR-mediated apoptosis by T-cell–dependent factors such as costimulation via CD40 ligation.3-5 The precise signaling mechanisms underlying such commitment to apoptosis or rescue of immature B cells remain to be defined. We have recently shown, however, that the BCR couples to up-regulation of cytosolic phospholipase A2 (cPLA2) expression, induction of mitochondrial phospholipase A2 activity, arachidonic acid–mediated collapse of mitochondrial potential (Δψm), and depletion of cellular adenosine triphosphate (ATP) under conditions of apoptotic, but not proliferative, signaling in WEHI-231 B cells.6,7 Importantly, such disruption of Δψm, ATP depletion, and apoptosis can be prevented by rescue signals via CD40.6,7
It is well established that CD40-mediated induction of Bcl-xL, and other antiapoptotic Bcl-2 family members, plays a key role in protecting WEHI-231 cells from BCR-driven apoptosis by stabilizing Δψm and mitochondrial homeostasis.5,8-14 Protection of mitochondrial integrity is central to cell survival as collapse of Δψm results in the release of factors that promote apoptosis. These factors (eg, cytochrome C, apoptosis-inducing factor, caspase-independent endonuclease) act to promote the activation of effector caspases, alternative executioner proteases, or directly induce apoptosis.15-21 The relative roles of various putative executioner proteases in BCR-driven apoptosis of WEHI-231 B cells are as yet unclear. For example, although caspase activation has been reported to be associated with such BCR-driven apoptosis,8,13,22,23 the release of cytochrome C from the mitochondria, which is critical for activation of effector caspases,15,24 does not appear to occur in these cells.6,23 Moreover, alternative executioner proteases such as calpains and cathepsins21,24,25 have recently been shown to play a role in BCR-driven apoptosis of WEHI-231 cells.6,23 Indeed, apoptosis of WEHI-231 cells resulting from BCR coupling to the mitochondrial PLA2 pathway is cathepsin B–dependent and occurs even in the presence of caspase inhibition. These apparently conflicting findings are likely to be reconciled, however, by the increasing evidence that caspases are not necessarily sufficient for apoptosis and that complex interactions of death signaling pathways are required for commitment to, and execution of, apoptosis.24
CD40 signaling can prevent BCR coupling to PLA2 activation, arachidonic acid–mediated disruption of mitochondrial function, and, ultimately, apoptosis of WEHI-231 B cells.6,7 Since induction of Bcl-xL expression resulting from CD40 signaling appears to be responsible for stabilizing the mitochondrial integrity of WEHI-231 cells, we investigated whether Bcl-xL expression is sufficient to antagonize BCR-driven mitochondrial PLA2 activation, mitochondrial disruption, and cathepsin B–mediated execution of apoptosis. We now show that expression of Bcl-xL can abrogate or overcome BCR-mediated recruitment of this death signaling cascade. Moreover, expression of Bcl-xL protects WEHI-231 B cells from mitochondrial disruption and apoptosis resulting from culture with exogenous arachidonic acid, the product of phospholipase A2 action suggesting that Bcl-xL acts, at least in part, to antagonize arachidonic acid–mediated disruption of mitochondrial integrity.
Materials and methods
Reagents and Abs
The panlipoxygenase inhibitor ethyl 3,4-dihydroxybenzylidenecyanoacetate (EDBC, median inhibitory concentration (IC50) values for 12-Lox, 0.1 μM; 15-Lox, 1μM, and 5-Lox, 10 μM) was from ALEXIS Biochemicals (Nottingham, United Kingdom), and the Cox2 inhibitor N-(2-Cyclohexyloxy-4-nitrophenyl)methanesulphonamide (NS-398) was from Calbiochem (Nottingham, United Kingdom). WEHI-231 cells transfected with the pSFFV-Neo plasmid containing human bcl-xL (WEHI-231 Bcl-xL) or no insert as control (WEHI-231 Neo) were selected for neomycin resistance by growth in the presence of G41826,27 (1 mg/mL) and were a gift from Dr C. B. Thompson (University of Pennsylvania). Purified monoclonal anti-IgM antibodies (Abs; antimouse μ-chain) and anti-CD40 Abs were produced from the B7.6 and FGK45 hybridomas, respectively, as described previously.28,29 Abs were as follows: goat anti-A1 (t-18) and rabbit anti–Bcl-xL/S (S-18) (Santa Cruz, Insight Biotechnology, Wembly, United Kingdom), mouse anti–Bcl-2,7 rabbit anti–Bcl-x (B22630), mouse anti–Mcl-1,11 and Cox2 Abs (all from Transduction Laboratories, Pharmingen, Oxford, United Kingdom), and active (phospho-) and total extracellular signal–regulated kinase (Erk) Abs and recombinant Erk2 and phosphoErk2 standards were from New England Biolabs (Hitchin, United Kingdom).7
DNA synthesis (thymidine uptake)
WEHI-231 cells (104/well) were cultured in RPMI medium containing 5% fetal calf serum, l-glutamine (2 mM), 2-mercaptoethanol (50 μM), penicillin (100 U/mL), streptomycin (100 μg/mL), sodium pyruvate (1 mM), and nonessential amino acids (1%), with the indicated stimuli for 44 hours at 37°C in 5% CO2. The cells were then pulsed with 0.5 μCi/well (0.0185 MBq) [3H]-thymidine (Amersham Life Sciences, Amersham, United Kingdom) before culturing for a further 4 hours. The cells were harvested and the level of [3H]-thymidine incorporated into DNA determined.28,29
Flow cytometry analysis of apoptosis (DNA content) and mitochondrial potential
Cells (5 × 105) were harvested at required time intervals. Following washing, the cells were resuspended in 100 μL PI stain (0.1% wt/vol sodium (tri) citrate, 0.1% vol/vol triton X-100, 50 μg/mL propidium iodide) before being incubated at 4°C for 10 minutes and then at room temperature for at least 30 minutes. Incorporation of the cationic lipophilic dye DiOC6 into mitochondria is proportional to the mitochondrial potential, Δψm.6,30 Cells were incubated for 30 minutes with 50 nM DiOC6 (Molecular Probes, Eugene, OR) and then washed once in phosphate-buffered saline (PBS).
At least 104 stained cells were analyzed on a FACScalibur (Becton Dickinson, Heidelberg, Germany) using CELLQuest software (Becton Dickinson).6
Western blotting
WEHI-231 cells (107 cells) were stimulated as indicated. Reactions were terminated by the addition of ice-cold 50 mM Tris (tris(hydroxymethyl) aminomethane) buffer, pH 7.4, containing 150 mM sodium chloride, 2% (vol/vol) nonidet P-40 (NP-40), 0.25% (wt/vol) sodium deoxycholate, 1 mM EGTA (ethylene glycol tetraacetic acid), 10 mM sodium orthovanadate, 0.5 mM phenylmethylsulfonylfluoride, chymostatin (10 μg/mL), leupeptin (10 μg/mL), antipain (10 μg/mL), and pepstatin A (10 μg/mL). The cells were solubilized for 30 minutes on ice before centrifugation of lysates at 12 000 rpm for 15 minutes. Equal protein loadings of lysates (bicichonic acid [BCA] protein assay; Pierce, Rockford, IL) were resolved by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), followed by transfer onto polyvinylidene fluoride (PVDF) filter membrane (Millipore, Billerica, Spain). Following blocking in PBS/0.1% Tween-20/10% nonfat milk for 1 hour at 20°C, membranes were incubated with the appropriate primary Ab overnight at 4°C followed by a 1-hour incubation at 20°C with the relevant horseradish peroxidase (HRP)–conjugated secondary Ab and developed by the enhanced chemiluminescence (ECL) system (Amersham, Aylesbury, United Kingdom).
cPLA2 assays
cPLA2 hydrolyzes phosphatidylcholine to generate arachidonic acid and lysophosphatidylcholine. Total cellular cPLA2 activity was assessed by measurement of [3H]arachidonic acid release from [3H]arachidonic acid–labeled phosphatidylcholine as described previously.6,28 Briefly, WEHI-231 B cells (106/mL) were incubated overnight with [3H]arachidonic acid (1 μCi/mL [0.037 MBq]) and following stimulation, lipids were extracted by the Bligh-Dyer method. [3H]arachidonate levels were determined by Silica Gel 60 thin-layer chromatography (Whatman, Clifton, NJ) in hexane–diethylether–formic acid (80:20:2, by volume).
Due to the loss of [3H]arachidonic acid during isolation of mitochondria, mitochondrial cPLA2 activity was measured by following the decrease in [3H]arachidonic acid–labeled phosphatidylcholine in mitochondrial extracts. Briefly, following stimulation of [3H]arachidonic acid–labeled cells (107) with anti-Ig for 3 hours at 37°C, mitochondria were isolated as described previously.6 Following extraction of the lipids by the Bligh-Dyer method, [3H]phosphatidylcholine levels were determined by Silica Gel 60 thin-layer chromatography in chloroform–acetone–methanol–glacial acetic acid–water (80:30:26:24:14 by volume).28,29
In some experiments, cPLA2 activity was determined using a cPLA2 assay kit (Cayman Chemical, Ann Arbor, MI) based on spectrophotometric detection (A414) of free thiol by Ellman reagent (5,5′-dithiobis(2-nitrobenzoic acid); DTNB) following hydrolysis of the arachidonyl thioester bond at the sn-2 position of the cPLA2 substrate, arachidonyl thio-phosphatidylcholine.6
Protease assays
WEHI-231 cells (5 × 106 cells per sample) were stimulated with anti-Ig (10 μg/mL) for 24 hours at 37°C. Cell lysates were then prepared in 50 mM Tris buffer, pH 7.4, containing 150 mM sodium chloride, 2% (vol/vol) NP-40, 0.25% (wt/vol) sodium deoxycholate, 1 mM EGTA, 10 mM sodium orthovanadate, and 0.5 mM phenylmethylsulfonylfluoride. Samples were then incubated for 30 minutes at room temperature with 100 μM cathepsin B substrate, zRR-pNA (z-Arg-Arg-pNA; Calbiochem), and the resultant generation of cleaved substrate was measured by reading absorbance (A) at 405 nm.6,31
Measurement of intracellular PGE2
Prostaglandin E2 (PGE2) activity in whole-cell lysates or cell supernatant was determined using a PGE2 competitive binding immunoassay assay kit (R & D Systems Europe, Abingdon, United Kingdom). Briefly, 50 μL whole-cell lysate (protein levels standardized to 1 μg/μL) or 100 μL cell supernatant was added to 100 μL assay buffer, followed by 50 μL PGE2 conjugate (alkaline phosphatase) and 50 μL PGE2 Ab solution. Samples and appropriate controls were incubated at room temperature for 2 hours. p-Nitrophenyl phosphate (pNPP) substrate (200 μL) was added and samples were incubated for one hour at room temperature. The optical density of each well was then determined using a microplate reader set to 405 nm with wavelength correction set at 570 and 590 nm.
Results
Overexpression of Bcl-xL prevents arachidonic acid–mediated apoptosis, but not growth arrest, of WEHI-231 B cells
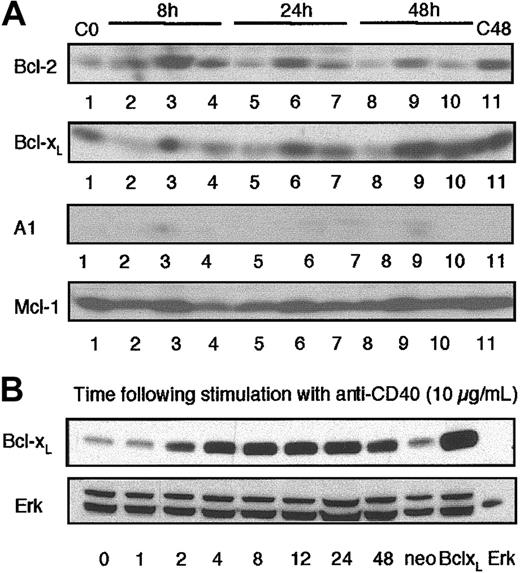
It has become widely accepted that the antiapoptotic protein Bcl-xL plays a pivotal role in the CD40-mediated rescue of BCR-driven apoptosis of WEHI-231 cells. However, recent reports have suggested that novel Bcl family members such as A1 or Mcl-1 may also play a role in such rescue.11-13,32-34 We therefore investigated the regulation of expression of these molecules in WEHI-231 B cells under conditions that induce apoptosis (ligation of the BCR by anti-Ig) or rescue from apoptosis (coligation of CD40 by anti-CD40 mAbs). Analysis by Western blotting showed that crosslinking of the antigen receptors with anti-Ig leads to a down-regulation of Bcl-2, Bcl-xL, and Mcl-1 expression within 24 hours. Little or no expression of A1 could be detected in either unstimulated or anti-Ig–treated cell lysates (Figure 1A). However, while stimulation with anti-CD40 treatment alone up-regulates expression of all of these Bcl-2 family members, only Bcl-xL expression is up-regulated during CD40-mediated rescue from BCR-driven apoptosis (Figure 1A). Since these data supported a key role for Bcl-xL in mediating CD40-driven rescue from BCR-induced apoptosis, we investigated whether overexpression of Bcl-xL in WEHI-231 B cells could prevent/overcome coupling to the mitochondrial PLA2 pathway and resultant growth arrest and apoptosis. To do this, we used Bcl-xL–overexpressing WEHI-231 B cells that exhibited levels of Bcl-xL comparable with those observed following stimulation of wild-type cells with anti-CD40 (Figure 1B).
Expression of Bcl-xLin WEHI-231 immature B cells. (A) WEHI-231 B cells (107 cells/lane) were cultured with medium, anti-IgM (5 μg/mL), or anti-CD40 (10 μg/mL), either alone or in combination, for up to 48 hours. Cell lysates were prepared and Western blot analysis of Bcl-2, Bcl-xL, A1, or Mcl-1 expression was performed as described in “Materials and methods.” Experimental conditions were as follows: lane 1, medium 0 hours (C0), lane 2, anti-Ig 8 hours; lane 3, anti-CD40 8 hours; lane 4, anti-Ig plus anti-CD40 8 hours; lane 5, anti-Ig 24 hours; lane 6, anti-CD40 24 hours; lane 7, anti-Ig plus anti-CD40 24 hours; lane 8, anti-Ig 48 hours; lane 9, anti-CD40 48 hours; lane 10, anti-Ig plus anti-CD40 48 hours; and lane 11, medium 48 hours (C48). (B) Wild-type WEHI-231 cells were stimulated with anti-CD40 (10 μg/mL) for up to 48 hours, cell lysates were prepared, and Western blot analysis of Bcl-xL expression was performed as described in “Materials and methods.” Expression of Bcl-xL in anti-CD40–treated wild-type WEHI-231 cells was compared with that of unstimulated WEHI-231 cells transfected with the empty vector (Neo) or overexpressing Bcl-xL (Bcl-xL). Recombinant p42 Erk2 was also loaded as an additional standard. Data are representative of at least 3 independent experiments.
Expression of Bcl-xLin WEHI-231 immature B cells. (A) WEHI-231 B cells (107 cells/lane) were cultured with medium, anti-IgM (5 μg/mL), or anti-CD40 (10 μg/mL), either alone or in combination, for up to 48 hours. Cell lysates were prepared and Western blot analysis of Bcl-2, Bcl-xL, A1, or Mcl-1 expression was performed as described in “Materials and methods.” Experimental conditions were as follows: lane 1, medium 0 hours (C0), lane 2, anti-Ig 8 hours; lane 3, anti-CD40 8 hours; lane 4, anti-Ig plus anti-CD40 8 hours; lane 5, anti-Ig 24 hours; lane 6, anti-CD40 24 hours; lane 7, anti-Ig plus anti-CD40 24 hours; lane 8, anti-Ig 48 hours; lane 9, anti-CD40 48 hours; lane 10, anti-Ig plus anti-CD40 48 hours; and lane 11, medium 48 hours (C48). (B) Wild-type WEHI-231 cells were stimulated with anti-CD40 (10 μg/mL) for up to 48 hours, cell lysates were prepared, and Western blot analysis of Bcl-xL expression was performed as described in “Materials and methods.” Expression of Bcl-xL in anti-CD40–treated wild-type WEHI-231 cells was compared with that of unstimulated WEHI-231 cells transfected with the empty vector (Neo) or overexpressing Bcl-xL (Bcl-xL). Recombinant p42 Erk2 was also loaded as an additional standard. Data are representative of at least 3 independent experiments.
Growth arrest was assessed by the anti-Ig–mediated suppression of DNA synthesis in wild-type (Neo) and Bcl-xL–overexpressing WEHI-231 B cells (Figure 2A). Analysis of the concentration dependence showed that maximal growth inhibition was achieved by concentrations of anti-Ig of 1 μg/mL in both cell lines confirming previous studies that Bcl-xL overexpression does not prevent BCR-driven growth arrest.9,10 Growth arrest was also assessed in response to exogenous arachidonic acid in order to address the role of the endogenous arachidonic acid generated during BCR coupling to PLA2 and apoptosis.6,7 Arachidonic acid–mediated growth arrest was similarly not prevented by Bcl-xL expression (Figure 2B). In contrast, apoptosis resulting from culture with either anti-Ig or exogenous arachidonic acid was substantially reduced in WEHI-231 B cells overexpressing Bcl-xL relative to wild-type controls (Figure 2C-D), although treatment with anti-CD40 could further suppress apoptosis resulting from either stimulus.
Overexpression of Bcl-xLprevents BCR- or arachidonic acid–mediated apoptosis, but not growth arrest, in WEHI-231 immature B cells. Growth arrest was assessed by measurement of anti-Ig- or arachidonic acid–mediated suppression of DNA synthesis by WEHI-231 B cells. (A) WEHI-231-Neo (□) or -Bcl-xL (▪) B cells were treated for 48 hours with 0 to 10 μg/mL anti-IgM or anti-IgM plus anti-CD40 (aIg + aCD40, both at 10 μg/mL), and levels of [3H]-thymidine incorporation into DNA were measured. (B) WEHI-231-Neo (□) or -Bcl-xL (▪) B cells were treated for 48 hours with 0 to 100 μM arachidonic acid or 10 μg/mL anti-IgM, and levels of [3H]-thymidine incorporation into DNA were measured. Data are expressed as means ± SD (n = 3) from single experiments representative of at least 2 other independent experiments. (C) WEHI-231-Neo (□) or -Bcl-xL (▪) B cells were treated for 48 hours with 100 μM arachidonic acid (AA), 10 μg/mL of anti-IgM (aIg), 10 μg/mL anti-CD40 (aCD40), or anti-IgM plus anti-CD40 (aIg/CD40, both at 10 μg/mL), and percentages of apoptotic cells were determined. Data are expressed as means ± SEM and are pooled from up to 13 individual experiments. (D) WEHI-231-Neo (open symbols) or WEHI-231Bcl-xL (filled symbols) B cells were treated for up to 72 hours with medium (squares), 100 μM arachidonic acid (circles), or 10 μg/mL anti-IgM (diamonds) before determining the resulting levels of apoptosis. Apoptosis was measured by propidium iodide (PI) staining of subdiploid DNA content as described in “Materials and methods.”
Overexpression of Bcl-xLprevents BCR- or arachidonic acid–mediated apoptosis, but not growth arrest, in WEHI-231 immature B cells. Growth arrest was assessed by measurement of anti-Ig- or arachidonic acid–mediated suppression of DNA synthesis by WEHI-231 B cells. (A) WEHI-231-Neo (□) or -Bcl-xL (▪) B cells were treated for 48 hours with 0 to 10 μg/mL anti-IgM or anti-IgM plus anti-CD40 (aIg + aCD40, both at 10 μg/mL), and levels of [3H]-thymidine incorporation into DNA were measured. (B) WEHI-231-Neo (□) or -Bcl-xL (▪) B cells were treated for 48 hours with 0 to 100 μM arachidonic acid or 10 μg/mL anti-IgM, and levels of [3H]-thymidine incorporation into DNA were measured. Data are expressed as means ± SD (n = 3) from single experiments representative of at least 2 other independent experiments. (C) WEHI-231-Neo (□) or -Bcl-xL (▪) B cells were treated for 48 hours with 100 μM arachidonic acid (AA), 10 μg/mL of anti-IgM (aIg), 10 μg/mL anti-CD40 (aCD40), or anti-IgM plus anti-CD40 (aIg/CD40, both at 10 μg/mL), and percentages of apoptotic cells were determined. Data are expressed as means ± SEM and are pooled from up to 13 individual experiments. (D) WEHI-231-Neo (open symbols) or WEHI-231Bcl-xL (filled symbols) B cells were treated for up to 72 hours with medium (squares), 100 μM arachidonic acid (circles), or 10 μg/mL anti-IgM (diamonds) before determining the resulting levels of apoptosis. Apoptosis was measured by propidium iodide (PI) staining of subdiploid DNA content as described in “Materials and methods.”
Overexpression of Bcl-xL suppresses coupling of the BCR to mitochondrial PLA2 activation
The finding that Bcl-xL expression can partially suppress arachidonic acid–induced apoptosis of WEHI-231 B cells suggested that this antiapoptotic protein was likely to act downstream of PLA2 activation, presumably by antagonizing the reported permeabilizing effects of arachidonic acid on mitochondrial membranes (reviewed in Chakraborti et al35 ). However, as reported in other systems,36,37 we have found that arachidonic acid can stimulate the activation of PLA2 resulting in the generation of enhanced arachidonic acid, either alone or in conjunction with anti-Ig (Figure 3A). This finding raised the possibility that Bcl-xL might also act to antagonize mitochondrial PLA2 activity. Investigation of anti-Ig–stimulated PLA2 activity in isolated mitochondria derived from wild-type and Bcl-xL–overexpressing WEHI-231 cells demonstrated that BCR coupling to this mitochondrial signaling pathway was indeed reduced by Bcl-xL expression (Figure 3B-C). To rule out the possibility that PLA2 was simply activated as a consequence of mitochondrial dysfunction, we determined the effect of mitochondrial stabilizers, such as oligomycin and antimycin, which we have previously shown to protect against anti-Ig–mediated disruption of Δψm, ATP depletion, and apoptosis,6 on BCR-coupled mitochondrial PLA2 activity. To complement these studies we determined whether modulation of cyclophilin activity (cyclophilin D is a component of the permeability transition pore in mitochondria) and hence apoptosis by cyclosporin A17,35,38,39 could also prevent coupling of the BCR to mitochondrial PLA2 in WEHI-231 cells. Neither class of mitochondrial stabilizer was able to prevent coupling of the BCR to mitochondrial PLA2 activity despite being able to protect against BCR-driven apoptosis6 (Figure 3D-E). These results therefore suggest that Bcl-xL induces inhibition of mitochondrial PLA2 activity independently of its regulation of mitochondrial membrane potential.
Bcl-xLexpression suppresses activation of mitochondrial phospholipase A2. Increasing concentrations (0.1-100 μM as indicated) of arachidonic acid induce phospholipase A2 activation and enhance anti-IgM–(1 μg/mL) stimulated PLA2 activation (A) as determined by the increased release (intracellular and extracellular) of [3H]arachidonic acid from cellular [3H]arachidonic acid–labeled phosphatidylcholine as described in “Materials and methods.” Due to the loss of [3H]arachidonic acid during the cellular fractionation process, anti-IgM–(1 μg/mL) stimulated mitochondrial phospholipase A2 activation (B-C) was therefore determined by the measurement of decreased levels of [3H]arachidonic acid–labeled phosphatidylcholine in isolated mitochondria derived from wild-type (B) and Bcl-xL (C) overexpressing WEHI-231 B cells. Data are representative of at least 3 independent experiments. (D) WEHI-231 B cells were cultured for 48 hours with medium or anti-IgM (10 μg/mL) in the presence and absence of oligomycin (OM, 8 ng/mL) or cyclosporin A (CSA, 1 nM), and percent of apoptotic cells was measured by PI staining of subdiploid DNA content as described in “Materials and methods.” (E) WEHI-231 B cells were cultured for 3 hours with medium or anti-IgM (10 μg/mL) in the presence and absence of oligomycin (OM, 8 ng/mL) or cyclosporin A (CSA, 1 nM), and isolated mitochondria were prepared. PLA2 activity was assayed using the PLA2 assay kit as described in “Materials and methods.” Data are presented as means ± SD in which n = 3 and from single experiments that are representative of at least 3 independent experiments.
Bcl-xLexpression suppresses activation of mitochondrial phospholipase A2. Increasing concentrations (0.1-100 μM as indicated) of arachidonic acid induce phospholipase A2 activation and enhance anti-IgM–(1 μg/mL) stimulated PLA2 activation (A) as determined by the increased release (intracellular and extracellular) of [3H]arachidonic acid from cellular [3H]arachidonic acid–labeled phosphatidylcholine as described in “Materials and methods.” Due to the loss of [3H]arachidonic acid during the cellular fractionation process, anti-IgM–(1 μg/mL) stimulated mitochondrial phospholipase A2 activation (B-C) was therefore determined by the measurement of decreased levels of [3H]arachidonic acid–labeled phosphatidylcholine in isolated mitochondria derived from wild-type (B) and Bcl-xL (C) overexpressing WEHI-231 B cells. Data are representative of at least 3 independent experiments. (D) WEHI-231 B cells were cultured for 48 hours with medium or anti-IgM (10 μg/mL) in the presence and absence of oligomycin (OM, 8 ng/mL) or cyclosporin A (CSA, 1 nM), and percent of apoptotic cells was measured by PI staining of subdiploid DNA content as described in “Materials and methods.” (E) WEHI-231 B cells were cultured for 3 hours with medium or anti-IgM (10 μg/mL) in the presence and absence of oligomycin (OM, 8 ng/mL) or cyclosporin A (CSA, 1 nM), and isolated mitochondria were prepared. PLA2 activity was assayed using the PLA2 assay kit as described in “Materials and methods.” Data are presented as means ± SD in which n = 3 and from single experiments that are representative of at least 3 independent experiments.
Overexpression of Bcl-xL antagonizes arachidonic acid- or BCR-mediated disruption of Δψm
We have previously shown that addition of exogenous arachidonic acid can mimic the BCR-driven disruption of mitochondrial function and integrity that plays a central role in the commitment to and execution of the later stages of apoptosis in WEHI-231 B cells.6 We therefore investigated whether overexpression of Bcl-xL prevents arachidonic acid and BCR-mediated disruption of the mitochondrial potential, Δψ. To do this quantitatively, we analyzed the incorporation of the cationic lipophilic dye DiOC6 into WEHI-231 B cells as the uptake of this dye into mitochondria is directly proportional to Δψm.30 Crosslinking of the antigen receptors, or culture with arachidonic acid, of wild-type WEHI-231 immature B cells induced a profound dissipation of Δψm, which was maximal by 20 to 24 hours.6 In contrast, the Δψm of Bcl-xL–overexpressing WEHI-231 B cells was more resistant to destabilization (Figure 4A) in keeping with their relative insensitivity to induction of apoptosis by these agents.
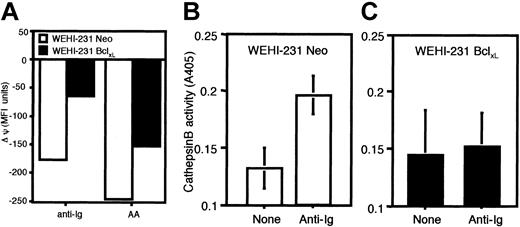
Bcl-xLstabilizes the Δψmand abrogates the BCR coupling to the postmitochondrial activation of cathepsin B in WEHI-231 immature B cells. (A) WEHI-231-Neo (□) and WEHI-231-Bcl-xL (▪) immature B cells were treated with anti-Ig (10 μg/mL) or arachidonic acid (100 μM) for 24 hours, and mitochondrial potential was assessed using the dye DiOC6. The data are represented as the change in mean fluorescence intensity (MFI) of DiOC6 staining following stimulation to demonstrate the extent of depolarization. WEHI-231-Neo (B) or -Bcl-xL (C) immature B cells were stimulated for 24 hours with anti-Ig (10 μg/mL), and cell lysates were prepared. Cathepsin B activity as evidenced by cleavage of the cathepsin B substrate, zRR-pNA (z-Arg-Arg-pNA; Calbiochem), was then assayed as described in “Materials and methods.” Data are representative of at least 3 independent experiments. Error bars indicate means ± SDs; n = 3.
Bcl-xLstabilizes the Δψmand abrogates the BCR coupling to the postmitochondrial activation of cathepsin B in WEHI-231 immature B cells. (A) WEHI-231-Neo (□) and WEHI-231-Bcl-xL (▪) immature B cells were treated with anti-Ig (10 μg/mL) or arachidonic acid (100 μM) for 24 hours, and mitochondrial potential was assessed using the dye DiOC6. The data are represented as the change in mean fluorescence intensity (MFI) of DiOC6 staining following stimulation to demonstrate the extent of depolarization. WEHI-231-Neo (B) or -Bcl-xL (C) immature B cells were stimulated for 24 hours with anti-Ig (10 μg/mL), and cell lysates were prepared. Cathepsin B activity as evidenced by cleavage of the cathepsin B substrate, zRR-pNA (z-Arg-Arg-pNA; Calbiochem), was then assayed as described in “Materials and methods.” Data are representative of at least 3 independent experiments. Error bars indicate means ± SDs; n = 3.
Bcl-xL antagonizes the postmitochondrial induction of the executioner protease, cathepsin B, in WEHI-231 immature B cells
We have previously shown that the BCR induces cathepsin B activation in a postmitochondrial manner and that such cathepsin B activity contributes to the executioner protease activity of apoptosis in WEHI-231 B cells.6 In keeping with the ability of Bcl-xL to prevent the anti-Ig–, or arachidonic acid–, driven collapse of Δψm and induction of apoptosis, we now find that BCR coupling to cathepsin B activation is abrogated in Bcl-xL–overexpressing WEHI-231 cells relative to their Neo wild-type controls (Figure 4B-C).
Overexpression of Bcl-xL does not restore the cycling Erk activation required for proliferation in WEHI-231 cells
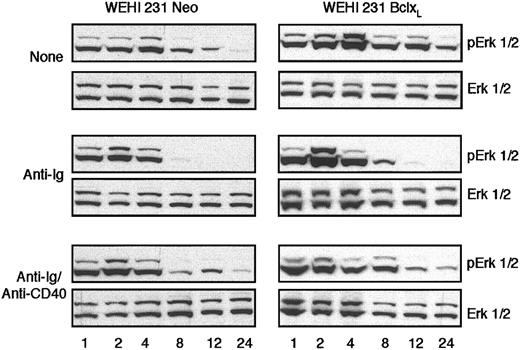
Although Bcl-xL expression can mimic CD40-mediated rescue of BCR-driven apoptosis of WEHI-231 B cells, we (Figure 2) and others9,10 have shown that expression of Bcl-xL cannot substitute for CD40 signaling in the reversal of anti-Ig–mediated growth arrest of these cells. The mechanisms underlying such CD40-mediated rescue of anti-Ig–induced growth arrest have not been delineated, but we have recently shown that a key event appears to be the anti-CD40–stimulated reversal of BCR-induced abrogation of sustained, cycling extracellular signal–regulated/mitogen-activated protein kinase (ErkMAPkinase) signaling inWEHI-231 B cells.7 We therefore investigated whether the inability of Bcl-xL expression to prevent BCR-driven growth arrest reflected a failure to reverse this anti-Ig–mediated desensitization of Erk activation. Indeed, overexpression of Bcl-xL did not restore sustained, cycling Erk activation in the presence of anti-Ig (Figure 5) suggesting that Bcl-xL up-regulation alone is not sufficient to overcome such BCR signaling and that additional CD40-derived signals are required to restore ErkMAPkinase activation and rescue from growth arrest.
Bcl-xLdoes not prevent BCR-mediated desensitization of sustained, cycling ErkMAPkinase activation in WEHI-231 B cells. WEHI-231-Neo or -Bcl-xL cells were stimulated with media, anti-IgM (1 μg/mL), or anti-IgM (1 μg/mL) plus anti-CD40 (10 μg/mL) for the time (h) indicated, up to 24 hours, and cell lysates were prepared. ErkMAPkinase activation was assessed by Western blot analysis of active, dually phosphorylated Erk and total Erk levels as described in “Materials and methods.” Data are representative of at least 10 experiments.
Bcl-xLdoes not prevent BCR-mediated desensitization of sustained, cycling ErkMAPkinase activation in WEHI-231 B cells. WEHI-231-Neo or -Bcl-xL cells were stimulated with media, anti-IgM (1 μg/mL), or anti-IgM (1 μg/mL) plus anti-CD40 (10 μg/mL) for the time (h) indicated, up to 24 hours, and cell lysates were prepared. ErkMAPkinase activation was assessed by Western blot analysis of active, dually phosphorylated Erk and total Erk levels as described in “Materials and methods.” Data are representative of at least 10 experiments.
Cox and Lox inhibitors implicate a key role for arachidonic acid and its metabolites in directing WEHI-231 cell fate
Exogenous arachidonic acid can mimic BCR-driven disruption of mitochondrial function and induction of apoptosis. Moreover, Bcl-xL expression can block BCR coupling to PLA2 and induction of apoptosis by arachidonate or via the BCR. Together, these findings support our proposal that BCR coupling to PLA2 plays a key role in mediating disruption of Δψm and inducing apoptosis. Unfortunately, it has not proved possible to demonstrate a causal role for PLA2 in this process using specific cPLA2 inhibitors as these reagents, which are nonmetabolizable analogs of arachidonate, also cause apoptosis. Nevertheless, we have found that inhibitors of cycloxygenase 2 (Cox2) and lipoxygenase (Lox), key enzymes in the conversion of arachidonate to prostaglandins and leukotrienes, respectively, promote BCR-mediated growth arrest and apoptosis (Figure 6). For example, inhibitors of Cox2 (NS-398) and Lox (EDBC, pan-Lox inhibitor) induce growth arrest and promote BCR-mediated growth arrest in a concentration-dependent manner (Figure 6A-B). Overexpression of Bcl-xL cannot prevent such growth arrest (Figure 6C-D). However, culture of WEHI-231 B cells with anti-Ig in the presence of these inhibitors leads to enhanced disruption of Δψ and superinduction of apoptosis that can be rescued by Bcl-xL expression (Figure 6E-F). Taken together, these data implicate a causal role for BCR-coupled arachidonic acid generation in mediating the mitochondrial death pathway.
Cox2 and Lox inhibitors enhance BCR-mediated growth arrest and apoptosis of WEHI-231 cells. (A) WEHI-231 cells were stimulated with 0 to 10 μg/mL anti-IgM in the absence (□) and presence of 4 μM (•) or 10 μM (▵) of NS-398 (Cox2 inhibitor) for 48 hours before levels of [3H]thymidine incorporated into DNA were determined. (B) Similarly, WEHI-231 cells were stimulated with 0 to 10 μg/mL anti-IgM in the absence (□) and presence of 1 μM(•)or10 μM(▵) EDBC (pan Lox inhibitor) for 48 hours before levels of [3H]thymidine incorporated into DNA were determined. WEHI-231-Neo (C) and -Bcl-xL (D) B cells were stimulated with 0 to 10 μg/mL anti-IgM in the absence (□) and presence of 10 μM NS-398 plus 10 μM EDBC (•) for 48 hours before levels of [3H]thymidine incorporated into DNA were determined. All data are presented as means ± SD, n = 3 from single experiments representative of at least 3 independent experiments. (E) Neo- or Bcl-xL–expressing WEHI-231 B cells were stimulated with 0 to 10 μg/mL anti-IgM in the absence and presence of 10 μM NS-398 for 24 hours before determining the percent of cells exhibiting low Δψm as described in “Materials and methods.” (F) WEHI-231-Neo (□) and -Bcl-xL (▪) B cells were stimulated with 10 μg/mL anti-IgM in the absence and presence of 10 μM NS-398 plus 10 μM EDBC for 48 hours. The percent of apoptotic cells, as indicated by PI staining of subdiploid DNA content, was then determined. WEHI-231-Neo (G) and -Bcl-xL (H) B cells were stimulated with media, 10 μg/mL anti-IgM, anti-CD40 (10 μg/mL), or anti-IgM plus anti-CD40 (anti-Ig/CD40, both at 10 μg/mL) in the absence (□) and presence of 10 μM NS-398 plus 10 μM EDBC (▪) for 48 hours before levels of [3H]thymidine incorporated into DNA were determined. Data are presented as means ± SD, n = 3 from single experiments representative of at least 3 independent experiments.
Cox2 and Lox inhibitors enhance BCR-mediated growth arrest and apoptosis of WEHI-231 cells. (A) WEHI-231 cells were stimulated with 0 to 10 μg/mL anti-IgM in the absence (□) and presence of 4 μM (•) or 10 μM (▵) of NS-398 (Cox2 inhibitor) for 48 hours before levels of [3H]thymidine incorporated into DNA were determined. (B) Similarly, WEHI-231 cells were stimulated with 0 to 10 μg/mL anti-IgM in the absence (□) and presence of 1 μM(•)or10 μM(▵) EDBC (pan Lox inhibitor) for 48 hours before levels of [3H]thymidine incorporated into DNA were determined. WEHI-231-Neo (C) and -Bcl-xL (D) B cells were stimulated with 0 to 10 μg/mL anti-IgM in the absence (□) and presence of 10 μM NS-398 plus 10 μM EDBC (•) for 48 hours before levels of [3H]thymidine incorporated into DNA were determined. All data are presented as means ± SD, n = 3 from single experiments representative of at least 3 independent experiments. (E) Neo- or Bcl-xL–expressing WEHI-231 B cells were stimulated with 0 to 10 μg/mL anti-IgM in the absence and presence of 10 μM NS-398 for 24 hours before determining the percent of cells exhibiting low Δψm as described in “Materials and methods.” (F) WEHI-231-Neo (□) and -Bcl-xL (▪) B cells were stimulated with 10 μg/mL anti-IgM in the absence and presence of 10 μM NS-398 plus 10 μM EDBC for 48 hours. The percent of apoptotic cells, as indicated by PI staining of subdiploid DNA content, was then determined. WEHI-231-Neo (G) and -Bcl-xL (H) B cells were stimulated with media, 10 μg/mL anti-IgM, anti-CD40 (10 μg/mL), or anti-IgM plus anti-CD40 (anti-Ig/CD40, both at 10 μg/mL) in the absence (□) and presence of 10 μM NS-398 plus 10 μM EDBC (▪) for 48 hours before levels of [3H]thymidine incorporated into DNA were determined. Data are presented as means ± SD, n = 3 from single experiments representative of at least 3 independent experiments.
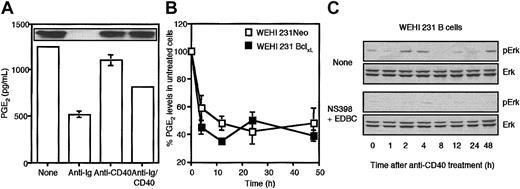
The finding that culture with the inhibitors alone resulted in growth arrest indicated that generation of arachidonate metabolites such as prostaglandins and/or leukotrienes may play a role in switching off the arachidonate death signal and/or promoting growth of WEHI-231 cells. Consistent with this, these inhibitors were also found to suppress CD40-mediated rescue of BCR-mediated growth arrest in both WEHI-231-Neo and -Bcl-xL cells (Figure 6G-H). To address this possibility, we measured the levels of PGE2 generated by WEHI-231 B cells stimulated with anti-Ig and/or anti-CD40. Consistent with PGE2 playing a role in promoting cell growth and proliferation, we found that stimulation with anti-Ig suppressed intracellular PGE2 levels, while costimulation with anti-CD40 restored them toward the levels observed in unstimulated, proliferating cells (Figure 7A). Such PGE2 generation appears to be solely intracellular as no release of PGE2 could be detected in cell supernatants (results not shown). Moreover, this CD40-mediated rescue of PGE2 levels was independent of Bcl-xL expression as BCR signaling suppressed PGE2 generation equally effectively in both WEHI-231-Neo and WEHI-231-Bcl-xL cells (Figure 7B). Although the precise details underlying BCR-mediated suppression of PGE2 and its rescue by CD40 signaling have not been defined, we have found that anti-Ig treatment suppresses Cox2 expression and that this is restored following stimulation via CD40 (Figure 7A, insert). Finally, a mechanism for PGE2 action in promoting cell proliferation was suggested by experiments examining the effects of Cox2/Lox inhibitors on the sustained, cycling pattern of Erk activation exhibited by anti-CD40–treated WEHI-231 cells. Culture with Cox2 and Lox inhibitors suppressed cycling Erk activation providing a mechanism for the observed growth arrest (Figure 7C).
CD40-mediated rescue of BCR-driven desensitization of ErkMAPkinase correlates with generation of arachidonate-derived metabolites such as PGE2. (A) WEHI-231 B cells were stimulated in the presence and absence of anti-IgM (1 μg/mL) and/or anti-CD40 (10 μg/mL) for 24 hours and then cell lysates were prepared and PGE2 levels were estimated by enzyme-linked immunosorbent assay (ELISA) and presented as means ± SD, n = 3 as described in “Materials and methods.” In the insert panel, such lysates were probed for Cox2 expression by Western blot analysis. (B) WEHI-231-Neo (□) and -Bcl-xL (▪) B cells were stimulated in the presence and absence of 1 μg/mL anti-IgM for up to 48 hours before estimating intracellular levels of PGE2. Each anti-Ig–treated time point sample is expressed as a percent of its unstimulated control and data are presented as means ± SD, n = 3. (C) WEHI-231 B cells were stimulated with anti-CD40 (10 μg/mL) for up to 48 hours in the presence and absence of NS-398 plus EDBC (both at 10 μM), and cell lysates were prepared. ErkMAPkinase activity was determined by Western blot analysis of phospho-Erk and total Erk reactivity as described in “Materials and methods.” The data presented are representative of at least 3 independent experiments.
CD40-mediated rescue of BCR-driven desensitization of ErkMAPkinase correlates with generation of arachidonate-derived metabolites such as PGE2. (A) WEHI-231 B cells were stimulated in the presence and absence of anti-IgM (1 μg/mL) and/or anti-CD40 (10 μg/mL) for 24 hours and then cell lysates were prepared and PGE2 levels were estimated by enzyme-linked immunosorbent assay (ELISA) and presented as means ± SD, n = 3 as described in “Materials and methods.” In the insert panel, such lysates were probed for Cox2 expression by Western blot analysis. (B) WEHI-231-Neo (□) and -Bcl-xL (▪) B cells were stimulated in the presence and absence of 1 μg/mL anti-IgM for up to 48 hours before estimating intracellular levels of PGE2. Each anti-Ig–treated time point sample is expressed as a percent of its unstimulated control and data are presented as means ± SD, n = 3. (C) WEHI-231 B cells were stimulated with anti-CD40 (10 μg/mL) for up to 48 hours in the presence and absence of NS-398 plus EDBC (both at 10 μM), and cell lysates were prepared. ErkMAPkinase activity was determined by Western blot analysis of phospho-Erk and total Erk reactivity as described in “Materials and methods.” The data presented are representative of at least 3 independent experiments.
Discussion
We have recently shown that commitment to BCR-mediated growth arrest and apoptosis of WEHI-231 B cells correlates with mitochondrial phospholipase A2 activation, arachidonic acid–mediated disruption of mitochondrial function, and cathepsin B activation.6 Coupling of the BCR to this apoptotic pathway can be abrogated by T-cell–dependent signals such as ligation of CD406,7 or addition of interleukin-4 (IL-4).28 Since CD40 signaling has been widely reported to rescue WEHI-231 B cells from BCR-driven apoptosis primarily via up-regulation of the antiapoptotic protein Bcl-xL, we investigated whether up-regulation of expression of Bcl-xL is responsible for abrogating or overcoming BCR-mediated recruitment of this apoptotic signaling cascade. We now show that simply expressing Bcl-xL can indeed prevent BCR-mediated mitochondrial phospholipase A2 activation, disruption of mitochondrial potential, and postmitochondrial execution of BCR-mediated apoptosis via cathepsin B activation resulting in survival of anti-Ig–treated WEHI-231 B cells. However, although Bcl-xL expression can therefore mimic CD40-mediated rescue of BCR-driven apoptosis of WEHI-231 B cells, we and others9,10 have shown that expression of Bcl-xL cannot substitute for CD40 signaling in the reversal of anti-Ig–mediated growth arrest of these cells. The mechanisms underlying such CD40-mediated rescue of anti-Ig–induced growth arrest have not been delineated, but we have recently shown that a key event appears to be the anti-CD40–stimulated reversal of BCR-induced abrogation of sustained, cycling ErkMAPkinase signaling in WEHI-231 B cells.7 Interestingly, we have observed that, unlike CD40 signaling, expression of Bcl-xL is unable to reverse this anti-Ig–mediated desensitization of Erk activation. These results suggest that Bcl-xL up-regulation alone is not sufficient to overcome such BCR signaling and that additional CD40-derived signals are required to restore ErkMAPkinase activation and rescue from growth arrest.
One candidate signal is the Cox2-mediated metabolism of arachidonic acid leading to the generation of PGE2. Indeed, elevated levels of PGE2 and Cox2 have previously been reported to lead to decreases in apoptosis by up-regulating Bcl-2 expression and stimulating mitogenic stimuli such as ErkMAPkinase in a number of cell types.40-42 Thus, our findings that (1) BCR signaling acts to suppress Cox2 and PGE2 levels while CD40 costimulation restores them and (2) culture with Cox2/Lox inhibitors blocks the sustained, cycling ErkMAPkinase signaling necessary for proliferation of WEHI-231 cells7 are consistent with the proposal that CD40, but not Bcl-xL expression, can promote cell growth and proliferation by generating arachidonic acid metabolites such as PGE2. We have previously shown that CD40 ligation can antagonize BCR coupling to PLA2 activation and apoptosis.6,7 However, the finding that CD40, but not Bcl-xL, signaling can also promote removal of the death signal arachidonate by conversion to Cox2/Lox metabolites possibly explains how CD40 stimulation can further enhance the protective effects of Bcl-xL overexpression against BCR-coupled apoptosis. We were initially rather surprised by the growth-promoting effects of PGE2, as exogenous PGE2 has been widely reported to have immunosuppressant effects. This is presumed to be due to recruitment of EP2 receptors that signal via elevation of cyclic adenosine monophosphate (cAMP),42 a second messenger widely established to induce growth arrest and apoptosis of lymphocytes.43 However, we have not been able to detect extracellular release of PGE2, suggesting that intracellular pools of such metabolites may transduce quite distinct signals in a manner analogous to that described for the differential signaling of intracellular sphingosine-1-phosphate versus extracellular signaling via Edg receptors.44 Alternatively, as it is not possible to completely rule out low-level secretion of PGE2, it has recently emerged that the EP4 receptor (which has a 7 times higher affinity for PGE2 than EP245 ) can signal to induce early growth response factor 1 (Egr-1) in a PI 3kinase and ErkMAPkinase-dependent manner.42 Similarly, the EP1 receptor has also recently been shown to couple to ErkMAPkinase activation.46 Thus the functional outcome of PGE2 generation may be dependent on PGE2 signal strength/EP receptor affinity and/or the maturation/activation status-dependent expression of EP subclasses in B cells. Such differential signaling would therefore reconcile the apparent discrepancy between the literature reporting immunosuppressive effects of prostaglandins/leukotrienes47-50 and our results and those of others51 in which PGE2 had been shown to act in synergy with a number of cell surface receptors such as CD40, IL-4R, and IL-10R to enhance B-cell proliferation.51
The proposal that Cox2/Lox metabolites such as PGE2 act as antiapoptotic/promitogenic lipid second messengers suggests that the receptor (BCR versus CD40)–driven interconversion of arachidonate and PGE2 provides a dynamic switch mechanism for regulating commitment to and rescue from apoptosis. As PGE2 is derived from arachidonate, and the actions of arachidonate and PGE2 are mutually antagonistic, the balance of signal dictates functional outcome. Indeed, this rheostat model provides a dynamic and amplifying system of regulation. Thus while arachidonate acts to down-regulate mitogenic signals such as ErkMAPkinase and induce apoptosis, conversion to PGE2 both relieves such negative signaling and actively promotes positive signaling by inducing mitogenic signals such as ErkMAPkinase.
In addition to antagonizing BCR-coupled PLA2 activation and associated apoptosis, overexpression of Bcl-xL can also protect WEHI-231 B cells from mitochondrial disruption and apoptosis resulting from culture with exogenous arachidonic acid, the product of phospholipase A2 action. Thus, this latter result may suggest that Bcl-xL acts, at least in part, to antagonize the increased permeability of the mitochondrial inner membrane reported to result from the accumulation of unsaturated fatty acids (arachidonic acid) consequent to the stimulation of mitochondrial phospholipase A2 activity (reviewed in Chakraborti et al35 ). However, as in other systems,36,37 we have shown that arachidonic acid can itself stimulate PLA2 activity presumably via a positive feedback loop. Moreover, Bcl-xL expression appears to be able to inhibit BCR coupling to mitochondrial PLA2 activation and this may reflect our previous findings that CD40 signaling prevents BCR-mediated cPLA2 translocation to the mitochondria.6 Taken together, these results suggest that, in addition to stabilizing mitochondrial permeability, up-regulation of Bcl-xL expression may also act to prevent mitochondrial localization and activation of cPLA2. This dual-pronged model of antagonism of arachidonic acid accumulation at the mitochondria may provide a mechanism to explain CD40-mediated protection of mitochondrial integrity. Indeed, the level of protection of Δψm afforded by expression of Bcl-xL was commensurate with that we have previously shown following costimulation of WEHI-231 B cells with anti-Ig plus anti-CD40.6
Although Bcl-xL has been widely reported to play a key role in regulating commitment to BCR-mediated apoptosis and CD40 rescue from such cell death, there is increasing evidence for putative key roles for other antiapoptotic proteins such as A1 and Mcl-1 in regulating B-cell survival.11-13,32-34 Indeed, recent studies by Kuss et al11 and Herold et al13 indicate that ectopic expression of A1 can partially rescue WEHI-231 B cells from anti-Ig–dependent apoptosis and that A1 can interfere with the caspase-9–mediated activation of caspase-7 in such B cells.13 However, although these authors showed that overexpression of A1 or dominant-negative caspase-9 almost completely inhibited BCR-mediated caspase-7 processing and effector caspase activity, these constructs inhibited BCR-mediated apoptosis by only some 40%.13 Consistent with this, we and others6,23 have been unable to detect the BCR-driven release of cytochrome C from the mitochondria, which is reported to be critical for execution of apoptosis by effector caspases.15,24 Moreover, although caspase activation has been widely reported to be associated with BCR-driven apoptosis of WEHI-231 B cells,8,13,22,23 we were unable to block BCR-driven apoptosis of WEHI-231 B cells with caspase inhibitors.6 Taken together, these results are consistent with the proposal that caspases are not necessarily sufficient for apoptosis and that complex interactions of death signaling pathways are required for commitment to, and execution of, apoptosis.21,24 Indeed, we have found BCR-driven apoptosis of WEHI-231 B cells to be cathepsin B–dependent6 suggesting that some interplay between BCR-mediated caspase and cathepsin B activities might be required for full induction of apoptosis in these B cells. Consistent with this, Madge et al have recently reported that, in vascular endothelial cells, cytokines such as tumor necrosis factor (TNF) or IL-1 can activate a cathepsin B–dependent, mitochondrial death pathway in which caspase activation is secondary to cathepsin regulation and not essential for cell death.52
Intriguingly, we have found that ceramide, another lipid that has been reported to play a role in transducing BCR-mediated apoptosis of WEHI-231 cells,53,54 induces the collapse of Δψm, mitochondrial release of cytochrome C, and apoptosis in a caspase-3–like (presumably caspase-7) manner.6 Moreover, we have found such ceramide-induced apoptosis of WEHI-231 B cells to be refractory to rescue by Bcl-xL overexpression (results not shown). Taken together with the report that ectopic expression of A1 can rescue caspase-7–mediated apoptosis,13 this latter result may suggest that while A1 expression can prevent effector caspase activation, Bcl-xL may act to counter the non–caspase-dependent execution of BCR-driven apoptosis.
In summary, we now show that overexpression of Bcl-xL can prevent mitochondrial phospholipase A2 activation, disruption of mitochondrial potential, and postmitochondrial execution of BCR-mediated apoptosis via cathepsin B activation. Moreover, overexpression of Bcl-xL protects WEHI-231 B cells from mitochondrial disruption and apoptosis resulting from culture with exogenous arachidonic acid, suggesting that CD40-mediated up-regulation of Bcl-xL acts, at least in part, to antagonize BCR-coupled arachidonic acid–mediated disruption of mitochondrial integrity. In contrast, Bcl-xL expression cannot substitute for CD40 signaling in restoring the sustained, cycling ErkMAPkinase activity required for CD40-mediated rescue from BCR-driven growth arrest in WEHI-231 B cells.
Prepublished online as Blood First Edition Paper, September 11, 2003; DOI 10.1182/blood-2003-07-2473.
Supported by Tenovus, Scottish Hospital Endowments Research Trust (SHERT), and the Biotechnology and Biological Sciences Research Council (BBSRC). E.K. was a recipient of a University of Glasgow Studentship from the Faculty of Medicine and a holder of an Overseas Research Students (ORS) award. S.B.G. held a Medical Research Council (MRC) PhD training studentship and C.A.F. and N.A.C. are Wellcome Trust PhD training students.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to thank Drs Craig Thomson and David Plas, University of Pennsylvania, for the gift of the Bcl-xL–overexpressing and Neo control WEHI-231 B-cell lines and for their help and advice with respect to these cell lines.

![Figure 2. Overexpression of Bcl-xL prevents BCR- or arachidonic acid–mediated apoptosis, but not growth arrest, in WEHI-231 immature B cells. Growth arrest was assessed by measurement of anti-Ig- or arachidonic acid–mediated suppression of DNA synthesis by WEHI-231 B cells. (A) WEHI-231-Neo (□) or -Bcl-xL (▪) B cells were treated for 48 hours with 0 to 10 μg/mL anti-IgM or anti-IgM plus anti-CD40 (aIg + aCD40, both at 10 μg/mL), and levels of [3H]-thymidine incorporation into DNA were measured. (B) WEHI-231-Neo (□) or -Bcl-xL (▪) B cells were treated for 48 hours with 0 to 100 μM arachidonic acid or 10 μg/mL anti-IgM, and levels of [3H]-thymidine incorporation into DNA were measured. Data are expressed as means ± SD (n = 3) from single experiments representative of at least 2 other independent experiments. (C) WEHI-231-Neo (□) or -Bcl-xL (▪) B cells were treated for 48 hours with 100 μM arachidonic acid (AA), 10 μg/mL of anti-IgM (aIg), 10 μg/mL anti-CD40 (aCD40), or anti-IgM plus anti-CD40 (aIg/CD40, both at 10 μg/mL), and percentages of apoptotic cells were determined. Data are expressed as means ± SEM and are pooled from up to 13 individual experiments. (D) WEHI-231-Neo (open symbols) or WEHI-231Bcl-xL (filled symbols) B cells were treated for up to 72 hours with medium (squares), 100 μM arachidonic acid (circles), or 10 μg/mL anti-IgM (diamonds) before determining the resulting levels of apoptosis. Apoptosis was measured by propidium iodide (PI) staining of subdiploid DNA content as described in “Materials and methods.”](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/1/10.1182_blood-2003-07-2473/6/m_h80145413002.jpeg?Expires=1769587528&Signature=CoeNGDQvzTNwKdZpyWhsza84oUt8wAFPdD7D2LJzLy8ojMtfC5w7mSDfo2NzSMJlqRlw63N3qmaYvH0Q4VqGAjP54Yi3znjLienpUagnlfTRHpv8mzITt1wa5vZygvYnX7Wa4Ojp-kIkTVsbAWoWe7t-iMEqCkZVq-55GKKLMms1Uppy68~5dXvaKOZ222U~dJk2ed8ltpksLUqnC6io9C0M8t7VMHFTcX5Gt6FmPk3a8sykEGmy8H8AOxBZldRMUjpfQGwqOlkSA22CDZ6OcL18uP4W77y0N0UmIXH3TxYeUtqh-9nhFlBDh3pVv12N~SBJ6PsuJb9wnrA8ZNJ3ZA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Bcl-xL expression suppresses activation of mitochondrial phospholipase A2. Increasing concentrations (0.1-100 μM as indicated) of arachidonic acid induce phospholipase A2 activation and enhance anti-IgM–(1 μg/mL) stimulated PLA2 activation (A) as determined by the increased release (intracellular and extracellular) of [3H]arachidonic acid from cellular [3H]arachidonic acid–labeled phosphatidylcholine as described in “Materials and methods.” Due to the loss of [3H]arachidonic acid during the cellular fractionation process, anti-IgM–(1 μg/mL) stimulated mitochondrial phospholipase A2 activation (B-C) was therefore determined by the measurement of decreased levels of [3H]arachidonic acid–labeled phosphatidylcholine in isolated mitochondria derived from wild-type (B) and Bcl-xL (C) overexpressing WEHI-231 B cells. Data are representative of at least 3 independent experiments. (D) WEHI-231 B cells were cultured for 48 hours with medium or anti-IgM (10 μg/mL) in the presence and absence of oligomycin (OM, 8 ng/mL) or cyclosporin A (CSA, 1 nM), and percent of apoptotic cells was measured by PI staining of subdiploid DNA content as described in “Materials and methods.” (E) WEHI-231 B cells were cultured for 3 hours with medium or anti-IgM (10 μg/mL) in the presence and absence of oligomycin (OM, 8 ng/mL) or cyclosporin A (CSA, 1 nM), and isolated mitochondria were prepared. PLA2 activity was assayed using the PLA2 assay kit as described in “Materials and methods.” Data are presented as means ± SD in which n = 3 and from single experiments that are representative of at least 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/1/10.1182_blood-2003-07-2473/6/m_h80145413003.jpeg?Expires=1769587528&Signature=Eg53up1YoyXfrTaOR1pScT8irDoPfbryi5UcTvFIRrMYP~AAgGCHCtCIOX4Gu30uC4ILzj2TvogkxsmLfOIE4QUuh~Xs9SXtnFyKmEmd6pEFhMZn-Z82xiPKMpktr-GVweZMvYh2IJLwRrHp6maOMg4-5jWRCGhBaS2yBQBTPpRTV9N3Ehye65oK1hhiQD1fFDjxzymGUGGjxFZV356pbQj65Z6RCI-xMTcB~9M7iP3L9uizbbVDkzwHBMq9kycxFFV9ValTu3V0jepDDRUpya0EI8H6Y1DYmuIgyGTl-Dgq5oBD3aaJfhFeB~U6I~Sm4ujdmLQzfheMybPGoBclMQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Cox2 and Lox inhibitors enhance BCR-mediated growth arrest and apoptosis of WEHI-231 cells. (A) WEHI-231 cells were stimulated with 0 to 10 μg/mL anti-IgM in the absence (□) and presence of 4 μM (•) or 10 μM (▵) of NS-398 (Cox2 inhibitor) for 48 hours before levels of [3H]thymidine incorporated into DNA were determined. (B) Similarly, WEHI-231 cells were stimulated with 0 to 10 μg/mL anti-IgM in the absence (□) and presence of 1 μM(•)or10 μM(▵) EDBC (pan Lox inhibitor) for 48 hours before levels of [3H]thymidine incorporated into DNA were determined. WEHI-231-Neo (C) and -Bcl-xL (D) B cells were stimulated with 0 to 10 μg/mL anti-IgM in the absence (□) and presence of 10 μM NS-398 plus 10 μM EDBC (•) for 48 hours before levels of [3H]thymidine incorporated into DNA were determined. All data are presented as means ± SD, n = 3 from single experiments representative of at least 3 independent experiments. (E) Neo- or Bcl-xL–expressing WEHI-231 B cells were stimulated with 0 to 10 μg/mL anti-IgM in the absence and presence of 10 μM NS-398 for 24 hours before determining the percent of cells exhibiting low Δψm as described in “Materials and methods.” (F) WEHI-231-Neo (□) and -Bcl-xL (▪) B cells were stimulated with 10 μg/mL anti-IgM in the absence and presence of 10 μM NS-398 plus 10 μM EDBC for 48 hours. The percent of apoptotic cells, as indicated by PI staining of subdiploid DNA content, was then determined. WEHI-231-Neo (G) and -Bcl-xL (H) B cells were stimulated with media, 10 μg/mL anti-IgM, anti-CD40 (10 μg/mL), or anti-IgM plus anti-CD40 (anti-Ig/CD40, both at 10 μg/mL) in the absence (□) and presence of 10 μM NS-398 plus 10 μM EDBC (▪) for 48 hours before levels of [3H]thymidine incorporated into DNA were determined. Data are presented as means ± SD, n = 3 from single experiments representative of at least 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/1/10.1182_blood-2003-07-2473/6/m_h80145413006.jpeg?Expires=1769587528&Signature=ICVVRpDvbWSht3~DMHW2mmCDCmkAPpI8mxFT1LYq0dscvUpquZ30vod68Wll6jYHt8GokR36BK8jHNZjZoOSM7BKD2AS6FE-CvuPX0Wzgg6LyfZOiARVmV~mWxFf0o-zne6w1iLjAxi5m94MxE8apl0Prsm7QBpl6H~YWWVZInqEzjkbyym90xjQf3bYHCnE~UP3O4iY5eyQ1AIroUhujaxXhCKnEwhVA3O7Ypyn8N7OpOa1NwjL1RFAGgxhCrXuWbTuFxk10VVRoWySh4alAjqGCp8ciScnGZ6wsCAi8neZUN2sVxRU2D1YBvhZh66D1nt4PPuwCOqsfmf9emkr0Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal