Abstract
Excessive proliferation of immune cells and vascular smooth myocytes (VSMCs) contributes to atherosclerosis. We have previously shown that whole-body inactivation of the growth suppressor p27 exacerbates atherosclerosis in apolipoprotein E-null mice (apoE–/–), and this correlated with increased proliferation of arterial macrophages and VSMCs. In the present study, we postulated that targeted disruption of bone marrow (BM) p27 is sufficient to enhance arterial macrophage proliferation and atherosclerosis. To test this hypothesis, sublethally irradiated apoE–/– mice with an intact p27 gene received a BM transplant from either apoE–/– or p27–/–apoE–/– doubly deficient donor mice and challenged with a high-cholesterol diet. Compared with mice that received an apoE–/– BM transplant, reconstitution with p27–/–apoE–/– doubly deficient marrow increased the expression of proliferating cell nuclear antigen in neointimal macrophages and accelerated aortic atherosclerosis, and this correlated with augmented aortic expression of the inflammatory cytokines CCL2/MCP-1 (monocyte chemoattractant protein 1) and CCL5/RANTES (regulated on activation, normal T-cell expressed and secreted). Overall, these findings provide evidence that p27 deficiency in hematopoietic progenitor cells enhances the inflammatory/proliferative response induced by dietary cholesterol and accelerates atherosclerosis.
Introduction
Atherosclerotic plaque development is due in part to the recruitment of circulating blood leukocytes into the artery wall, the migration of vascular smooth myocytes (VSMCs) from the media toward the growing neointimal lesion, and hyperplastic cell growth.1,2 The growth suppressor p27 coordinately inhibits cell proliferation and migration.3 Notably, p27 expression inversely correlates with macrophage and VSMC proliferation within human atherosclerotic tissue,4 and transforming growth factor β (TGF-β) present in human atherosclerotic tissue may mediate its growth-suppressive activity through p27.5 Furthermore, doubly deficient p27–/–apoE–/– mice display accelerated atherogenesis as compared with apoE–/– mice with an intact p27 gene.6 This effect of p27 inactivation was dose dependent and correlated with augmented proliferation of arterial macrophages and VSMCs. In the present study, we used a bone marrow (BM) transplantation model to examine whether targeted inactivation of p27 in hematopoietic precursors is sufficient to increase arterial macrophage proliferation and exacerbate atherosclerosis development.
Study design
BM transplantation
Female apoE–/– mice (C57BL/6J; Taconic M&B, Ry, Denmark) irradiated with 2 doses of 5.1 Gy spaced 4 hours apart received transplants of 2 × 106 BM cells obtained from the pooled femur of 2 male apoE–/– or doubly deficient p27–/–apoE–/– mice (C57BL/6J).6 After 4 weeks on a standard diet (2.8% fat; Panlab, Barcelona, Spain), the mice that received transplants were fed an atherogenic diet for 2 months (15.8% fat, 1.25% cholesterol, 0.5% sodium cholate, S4892-S010; Ssniff, Soest, Germany). Plasma cholesterol level was determined as described.6
Quantification of transplantation efficiency, atherosclerosis, and cytokine expression
Fat-fed mice were killed and genomic DNA was isolated from BM, spleen, and thymus to quantify transplantation efficiency by polymerase chain reaction (PCR) amplification of a 184–base pair (bp) fragment of the Y chromosome–specific Zfy-1 gene. DNA amount was normalized by quantitative amplification of a 190-bp fragment of the ras gene. Genomic DNA (20 ng) was amplified in a LightCycler (Roche, Mannheim, Germany) using the SYBR Green I reaction mix (Roche). Serially diluted standard samples of male and female DNA were simultaneously amplified using the same reaction mixture. To quantify cytokine mRNA expression by real-time PCR, 2 μg DNaseI-treated total RNA obtained from snap-frozen aortic arch tissue (Ultraspec RNA isolation system; Biotecx, Houston, TX) was reverse transcribed with MuLV reverse transcriptase (RT) (Roche). Primer sequences and amplification programs are available upon request. Detection of fluorescent product was carried out at the last step of each cycle.
Aortic atherosclerosis was quantified as previously described.6
Immunohistochemistry and Western blot
Aortic arch cross-sections were immunostained with antibodies against Mac-3 (1/100, sc-19991; Santa Cruz Biotechnology, Santa Cruz, CA), smooth muscle α-actin (SMα-actin) (using an alkaline phosphatase– conjugated antibody 1/100, clone 1A4; Sigma, St Louis, MO), and proliferating cell nuclear antigen (PCNA; 1/50, sc-7907; Santa Cruz Biotechnology). Immunocomplexes containing anti–Mac-3 and anti– PCNA antibodies were detected using the Ultra Streptavidin detection system (Level 2; Signet Laboratories, Cambridge, MA) and 3,3′-diaminobenzidine tetrahydrochloride dihydrate substrate (DAB; Sigma). SMα-actin expression was detected using Fast Red substrate (Sigma).
Whole-cell extracts were prepared from cultured NIH3T3 fibroblasts (American Type Culture Collection, Manassas, VA), BM-derived macrophages,7 and from the pooled aortic arch tissue from 4 mice that received transplants. Lysis buffer (50 mM Tris(tris(hydroxymethyl)aminomethane)– HCl [pH 7.5], 1% Nonidet P-40 (NP-40), 0.25% sodium deoxycholate, 150 mM NaCl, 150 mM EDTA [ethylenediaminetetraacetic acid], 1 mM PMSF [phenylmethylsulfonyl fluoride], 1 mM orthovanadate, 1 mM NaF, 0.1% sodium dodecyl sulfate [SDS]) was supplemented with protease inhibitor Complete Mini cocktail (Roche). Western blot analysis was carried out with antitubulin (1/200, sc-3035) and anti–Mac-3 (1/200, sc-19991) antibodies (Santa Cruz Biotechnologies) as previously described.6
Results and discussion
Because reconstitution of apoE–/– mice with wild-type BM markedly reduces atherogenesis,8 we performed our studies with apoE-null marrow. Female apoE–/– mice were sublethally irradiated and reconstituted with BM from male apoE–/– and p27–/–apoE–/– doubly deficient donors (apoE–/– → apoE–/– and p27–/–apoE–/– → apoE–/– mice, respectively). Animals were challenged for 8 weeks with a high-fat, cholesterol-rich diet beginning 1 month after transplantation. apoE–/– → apoE–/– and p27–/–apoE–/– → apoE–/– mice had comparable body weight and developed similar hypercholesterolemia (Table 1). Likewise, no statistically significant differences were observed in transplant efficiency in BM and thymus when comparing both groups. However, repopulation efficiency in the spleen was significantly higher in p27–/–apoE–/– → apoE–/– mice, consistent with previous transplantation studies with p27-null BM.9
Physiologic measurements in apoE-/- → apoE-/- and p27-/- apoE-/- → apoE-/- mice
. | . | Plasma cholesterol (mg/dL) . | . | Transplant donor engraftment (%) . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Bone marrow donor genotype . | Weight (g) . | Before diet . | After diet . | Bone marrow . | Spleen . | Thymus . | |||
| apoE-/- → apoE-/- | 19.6 ± 0.6 (n = 15) | 336 ± 35 (n = 15) | 2733 ± 266 (n = 15) | 43 ± 4 (n = 5) | 49 ± 5 (n = 5) | 48 ± 3 (n = 5) | |||
| p27-/- apoE-/- → apoE-/- | 20.4 ± 0.8 (n = 15) | 363 ± 21 (n = 15) | 2626 ± 109 (n = 15) | 44 ± 9 (n = 4) | 87 ± 5 (n = 4)* | 67 ± 6 (n = 4) | |||
. | . | Plasma cholesterol (mg/dL) . | . | Transplant donor engraftment (%) . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Bone marrow donor genotype . | Weight (g) . | Before diet . | After diet . | Bone marrow . | Spleen . | Thymus . | |||
| apoE-/- → apoE-/- | 19.6 ± 0.6 (n = 15) | 336 ± 35 (n = 15) | 2733 ± 266 (n = 15) | 43 ± 4 (n = 5) | 49 ± 5 (n = 5) | 48 ± 3 (n = 5) | |||
| p27-/- apoE-/- → apoE-/- | 20.4 ± 0.8 (n = 15) | 363 ± 21 (n = 15) | 2626 ± 109 (n = 15) | 44 ± 9 (n = 4) | 87 ± 5 (n = 4)* | 67 ± 6 (n = 4) | |||
With the exception of prediet plasma cholesterol, all parameters were measured after mice had been killed. Results were compared by 2-tailed, unpaired, Student t test. Only statistically significant differences are shown (P < .05). Results are shown as mean ± SE.
P < .002.
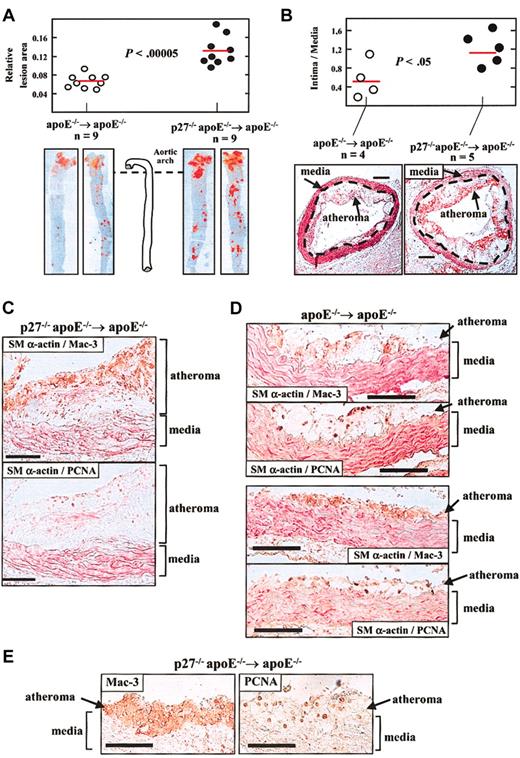
Aortic tissue was examined to quantify the extent of atherosclerosis by 2 independent approaches. First, we examined en face preparations of the aorta stained with Oil Red O (Sigma) to mark lipid-laden lesions. Computerized planimetric analysis revealed a 1.85-fold increase in mean lesion area in p27–/–apoE–/– → apoE–/– versus apoE–/– → apoE–/– mice (P < .00005; Figure 1A). Second, quantification of the area of atheroma (intima) and media in aortic arch cross-sections in another set of mice disclosed a 2.1-fold increase in the intima-to-media ratio in p27–/–apoE–/– → apoE–/– compared with apoE–/– → apoE–/– counterparts (P < .05; Figure 1B). Thus, reconstitution of apoE-null mice with p27-deficient BM enhances diet-induced atherosclerosis.
Reconstitution of apoE–/– mice with BM lacking p27 accelerates diet-induced atherosclerosis. One month after transplantation, mice were challenged with a high-cholesterol diet for 2 months. Animals were then killed and aortic tissue was retrieved to quantify atherosclerosis (A, B) and for immunohistochemical analysis (C-E). Results in A and B are given as mean ± SE, and differences between groups were evaluated using 2-tailed, unpaired Student t test. (A) The aorta was removed from the heart to the renal artery and was stained with Oil Red O. The area of atheroma (red staining) was quantified by computerized planimetry. Results are represented relative to total aortic area. The photomicrographs show representative examples. (B) Cross-sections of the aortic arch region were analyzed to measure the area of atheroma (intimal lesion) and media to determine the intima-to-media ratio. The red horizontal bars show the average value in each group. The representative photomicrographs in B show specimens doubly immunostained with antibodies against the macrophage-specific Mac-3 protein (brown signal) and the smooth muscle–specific α isoform of actin (SMα-actin; red signal). The discontinuous lines mark the boundary between the tunica media and the atheroma. The bars represent 0.2 mm. (C-E) Immunohistochemistry of aortic arch cross-sections. Each pair of photomicrographs correspond to immediately adjacent cross-sections of the same mouse (bars represent 100 μm). Mac-3 and PCNA are shown in brown, and SMα-actin in red. Analysis included double immunohistochemistry using the indicated pairs of antibodies (C, D) and single immunostaining using anti–Mac-3 and anti-PCNA antibodies (E). For double immunhistochemistry, specimens were first stained for either PCNA or Mac-3, and then with the alkaline phosphatase– conjugated anti–SMα-actin antibody. In both groups of mice, abundant immunoreactivity for Mac-3 and SMα-actin is detected within the atheroma and the media, respectively, and PCNA expression predominates in macrophage-rich regions of atheromas.
Reconstitution of apoE–/– mice with BM lacking p27 accelerates diet-induced atherosclerosis. One month after transplantation, mice were challenged with a high-cholesterol diet for 2 months. Animals were then killed and aortic tissue was retrieved to quantify atherosclerosis (A, B) and for immunohistochemical analysis (C-E). Results in A and B are given as mean ± SE, and differences between groups were evaluated using 2-tailed, unpaired Student t test. (A) The aorta was removed from the heart to the renal artery and was stained with Oil Red O. The area of atheroma (red staining) was quantified by computerized planimetry. Results are represented relative to total aortic area. The photomicrographs show representative examples. (B) Cross-sections of the aortic arch region were analyzed to measure the area of atheroma (intimal lesion) and media to determine the intima-to-media ratio. The red horizontal bars show the average value in each group. The representative photomicrographs in B show specimens doubly immunostained with antibodies against the macrophage-specific Mac-3 protein (brown signal) and the smooth muscle–specific α isoform of actin (SMα-actin; red signal). The discontinuous lines mark the boundary between the tunica media and the atheroma. The bars represent 0.2 mm. (C-E) Immunohistochemistry of aortic arch cross-sections. Each pair of photomicrographs correspond to immediately adjacent cross-sections of the same mouse (bars represent 100 μm). Mac-3 and PCNA are shown in brown, and SMα-actin in red. Analysis included double immunohistochemistry using the indicated pairs of antibodies (C, D) and single immunostaining using anti–Mac-3 and anti-PCNA antibodies (E). For double immunhistochemistry, specimens were first stained for either PCNA or Mac-3, and then with the alkaline phosphatase– conjugated anti–SMα-actin antibody. In both groups of mice, abundant immunoreactivity for Mac-3 and SMα-actin is detected within the atheroma and the media, respectively, and PCNA expression predominates in macrophage-rich regions of atheromas.
The cellular composition of atherosclerotic lesions was examined in aortic arch cross-sections by immunohistochemistry with anti–Mac-3 and anti–SMα-actin antibodies to visualize macrophages and VSMCs, respectively, which disclosed an overtly similar histopathology of the atheromas in both groups of mice. SMα-actin was predominantly expressed in the media (Figure 1C-D). In contrast, macrophages prevailed in the atheroma in both groups (Figure 1C-E). We estimated the relative abundance of macrophages in aortic tissue by Western blot analysis using the anti–Mac-3 rat monoclonal antibody. To verify the specificity of this antibody, we first examined cultures of fibroblasts and macrophages obtained from wild-type and p27-null mice, which revealed 2 macrophage-specific bands of similar intensity in wild-type and p27-null cells (Figure 2A, left). As shown in Figure 2A (right), Mac-3 expression was increased in the aorta of fat-fed p27–/–apoE–/– → apoE–/– mice versus apoE–/– → apoE–/– counterparts. Averaged over 3 independent experiments, densitometric analysis normalized by the internal tubulin loading control revealed a 2-fold increase in aortic Mac-3 expression in p27–/–apoE–/– → apoE–/– versus apoE–/– → apoE–/– mice. Because the main cellular components of the atherosclerotic lesions in our studies are macrophages (Figure 1C-E), increased Mac-3 expression in the aorta of fat-fed p27–/–apoE–/– → apoE–/– mice is consistent with larger plaques in these animals.
Expression studies in aortic tissue of fat-fed mice that received transplants. (A) Immunoblot analysis using the Mac-3 antibody and lysates of cultured cells (NIH 3T3 fibroblasts and macrophages—Mϕ—obtained from the BM of wild-type and p27–/– mice) and aortic tissue prepared from the pooled descending aortic arch of 3 fat-fed mice. The arrowheads point to macrophage-specific bands. (B) PCNA-immunostained specimens (Figure 1) were analyzed to quantify PCNA-immunoreactive cells per cross-section, both in the media and atheroma. PCNA-positive cells were counted in at least 3 sections from different regions of the aortic arch and all independent values were averaged. The dotted horizontal bars indicate the average value in each group. (C) Real-time quantitative RT-PCR analysis of total RNA isolated from the descending aortic arch to quantify cytokine expression within the atherosclerotic vessel wall. The number of animals in each group is indicated (n). Results are given as mean ± SE (ratio of cytokine/GAPDH [glyceraldehyde-3-phosphate dehydrogenase] mRNA). For each cytokine, average values in both groups were compared using the nonparametric Mann-Whitney U test (*, P < .01; **, P < .001). Error bars indicate SE.
Expression studies in aortic tissue of fat-fed mice that received transplants. (A) Immunoblot analysis using the Mac-3 antibody and lysates of cultured cells (NIH 3T3 fibroblasts and macrophages—Mϕ—obtained from the BM of wild-type and p27–/– mice) and aortic tissue prepared from the pooled descending aortic arch of 3 fat-fed mice. The arrowheads point to macrophage-specific bands. (B) PCNA-immunostained specimens (Figure 1) were analyzed to quantify PCNA-immunoreactive cells per cross-section, both in the media and atheroma. PCNA-positive cells were counted in at least 3 sections from different regions of the aortic arch and all independent values were averaged. The dotted horizontal bars indicate the average value in each group. (C) Real-time quantitative RT-PCR analysis of total RNA isolated from the descending aortic arch to quantify cytokine expression within the atherosclerotic vessel wall. The number of animals in each group is indicated (n). Results are given as mean ± SE (ratio of cytokine/GAPDH [glyceraldehyde-3-phosphate dehydrogenase] mRNA). For each cytokine, average values in both groups were compared using the nonparametric Mann-Whitney U test (*, P < .01; **, P < .001). Error bars indicate SE.
To examine whether p27 inactivation in the transplanted marrow increases arterial cell growth in recipient mice, we examined in aortic tissue the expression of the proliferation marker proliferating cell nuclear antigen (PCNA).10-12 To ascertain the identity of PCNA-immunoreactive cells, consecutive sections were examined by either double SMα-actin/Mac-3 and SMα-actin/PCNA immunohistochemistry (Figure 1C-D), or by single immunostaining with anti-PCNA and anti–Mac-3 antibodies (Figure 1E). SMα-actin/PCNA colocalization was scant, even in intimal SMα-actin–immunoreactive cells (Figure 1C, bottom photomicrograph). In contrast, Mac-3/PCNA coexpression was abundant in the atheromas of both apoE–/– → apoE–/– and p27–/–apoE–/– → apoE–/– mice (Figure 1C-E). PCNA immunoreactivity was higher in the atheroma versus the media in both groups, and a tendency toward more PCNA-expressing cells was noted in the atheromas of p27–/–apoE–/– → apoE–/– compared with apoE–/– → apoE–/– mice (Figure 2B). Moreover, the proliferative capacity of cultured p27-null macrophages was significantly higher than that of wild-type counterparts (data not shown). Overall, these findings are in agreement with previous studies showing that p27 inactivation enhances hematopoietic progenitor cell proliferation9 and implicating p27 as a critical macrophage growth suppressor.13,14 Because macrophages were the most abundant cells in our study, we suggest that macrophage p27 safeguards against the inflammatory/proliferative response induced by dietary cholesterol in apoE-null mice. These results are consistent with our studies demonstrating that whole-body inactivation of p27 enhances atherosclerosis.6 Similarly, both systemic15 and macrophage-specific16,17 p53 deficiency enhances diet-induced atherosclerosis. Thus, macrophage expression of growth suppressors seems to play a crucial role in atherosclerosis.
We next examined cytokine expression in aortic tissue by quantitative RT-PCR (Figure 2C). CCL2/MCP-1 (monocyte chemoattractant protein 1) and CCL5/RANTES (regulated on activation, normal T cell expressed and secreted) mRNA were significantly increased in p27–/–apoE–/– → apoE–/– versus apoE–/– → apoE–/– mice. In contrast, differences in interleukin-4 (IL-4) and interferon γ (IFN-γ) mRNA expression did not reach statistical significance. Because CCL2/MCP-1 and CCL5/RANTES promote macrophage infiltration and atheroma formation,18-20 increased macrophage infiltration in response to augmented aortic CCL2/MCP-1 and CCL5/RANTES expression in p27–/–apoE–/– → apoE–/– mice might contribute to accelerated atherosclerosis in these animals. It should be noted that lymphocyte recruitment and proliferation are also implicated in atherogenesis,1,2 and that p27 is a key regulator of lymphocyte proliferation in different physiopathologic conditions.21-27 Thus, enhanced lymphocyte activation may also contribute to the phenotype of p27–/–apoE–/– → apoE–/– mice.
In summary, we have shown that targeted p27 inactivation in hematopoietic progenitors enhances arterial macrophage proliferation and inflammatory response resulting in accelerated atherosclerosis. It is noteworthy that retrovirus-mediated constitutive p27 overexpression impaired the engraftment of transplanted cells in our murine model (V. A., A. D.-J., M. A., and A. B, unpublished results, October 2002). Thus, future studies using an inducible, macrophage-specific promoter are warranted to examine whether increasing p27 expression in macrophages may reduce atheroma development.
Prepublished online as Blood First Edition Paper, September 22, 2003; DOI 10.1182/blood-2003-07-2319.
Supported in part by the Ministerio de Ciencia y Tecnología of Spain and Fondo Europeo de Desarrollo Regional (grant SAF2001-2358), and from Instituto de Salud Carlos III (Red de Centros C03/01).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank M. J. Andrés-Manzano for preparing the figures, P. Carbonell for technical assistance, J. Cubells for animal care, and C. Caelles for L929 cells.

![Figure 2. Expression studies in aortic tissue of fat-fed mice that received transplants. (A) Immunoblot analysis using the Mac-3 antibody and lysates of cultured cells (NIH 3T3 fibroblasts and macrophages—Mϕ—obtained from the BM of wild-type and p27–/– mice) and aortic tissue prepared from the pooled descending aortic arch of 3 fat-fed mice. The arrowheads point to macrophage-specific bands. (B) PCNA-immunostained specimens (Figure 1) were analyzed to quantify PCNA-immunoreactive cells per cross-section, both in the media and atheroma. PCNA-positive cells were counted in at least 3 sections from different regions of the aortic arch and all independent values were averaged. The dotted horizontal bars indicate the average value in each group. (C) Real-time quantitative RT-PCR analysis of total RNA isolated from the descending aortic arch to quantify cytokine expression within the atherosclerotic vessel wall. The number of animals in each group is indicated (n). Results are given as mean ± SE (ratio of cytokine/GAPDH [glyceraldehyde-3-phosphate dehydrogenase] mRNA). For each cytokine, average values in both groups were compared using the nonparametric Mann-Whitney U test (*, P < .01; **, P < .001). Error bars indicate SE.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/1/10.1182_blood-2003-07-2319/6/m_h80145407002.jpeg?Expires=1767941234&Signature=p-R8QTnMd6A5ZSxEB4A5JKpDDP~14ZFVbUxbgED9keihVp1gbBiM6knPHTpFZOqRef5G861xpceJ-r4f4CR7hEwtbtJKIZcV46XBIBvg4khKLgNKr5-imWYsZMRbeDzNdrXNuRk4xY~koRCMOOQjayozLIdorF8Cxj9GrBfk0bztypT-LlcPF8SQzElrw0oABgOhkjfxwEadWt~IkpHDHgq4oUgyGJMV9jO6oD8FmTc--usOQBwX8Fdy5W5KpQEVz1AUh02nEtFeiggtGpEM3SJQLqNoLhu5io9YD7b7bW0Mxp8klfD0rVfDiZlyEHXjLVusdtXdh0Xa8fDjLY15dA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal