Abstract
The C2 domain of factor VIII (FVIII) mediates FVIII binding to von Willebrand factor (VWF) and phospholipids (PLs), thereby determining the stability and the activity of FVIII. A deletion of Ala2201 (Del2201) was identified in the FVIII C2 domain of 2 unrelated patients with mild hemophilia A (FVIII:C 11%-33%). This mutation prevents FVIII binding to a human monoclonal antibody recognizing the C2 domain and inhibiting FVIII binding to VWF and phospholipids. By comparison to healthy FVIII, Del2201 FVIII had a significantly reduced binding to VWF, which likely contributes to reduced FVIII levels in plasma. Del2201 FVIII interaction with phospholipids was evaluated in an FXa generation assay, using various concentrations of synthetic phospholipid vesicles mimicking an activated platelet surface. At the lowest phospholipid concentration allowing FXa generation, Del2201 FVIII activity was reduced 3-fold. This is the first report of a mutation altering FVIII binding to phospholipids and occurring in patients with hemophilia A.
Introduction
The factor VIII (FVIII) C2 domain consists of the 170 carboxy-terminal residues of the molecule. It contributes to FVIII binding to von Willebrand factor (VWF)1-3 and to phospholipids (PLs),4,5 and thereby determines the stability in plasma and the activity of FVIII. More than 30 mutations associated with mild/moderate hemophilia A have been identified in this domain.6 However, in most cases, it remains unclear by which mechanism these mutations reduce plasma FVIII levels.
The crystal structure of the C2 domain was recently determined.7 At one side of the domain, hydrophobic residues were predicted to insert into the PL membrane, whereas basic residues above the hydrophobic surface interact with negatively charged PL by electrostatic interactions7 (Figure 1). The 2-dimensional crystallography of FVIII bound to PL,8 and site-directed mutagenesis9 has provided data in agreement with these predictions.
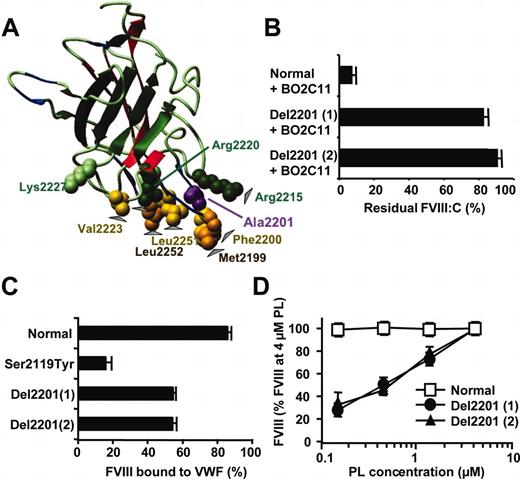
Interaction of healthy and mutated plasma FVIII with BO2C11. (A) Location of residues involved in FVIII binding to BO2C11 in the 3-dimensional structure of the C2 domain. The C2 domain is represented as ribbon diagram. The hydrophobic and basic residues mediating binding to PLs are highlighted as Corey-Pauling-Kultun (CPK) spheres. Arrowheads indicate residues mediating FVIII binding to BO2C11 as determined by crystallization of an Fab fragment of BO2C11 bound to a recombinant C2 domain.20 The human monoclonal antibody BO2C11 was obtained by immortalization of B lymphocytes of a patients with hemophilia A with a high-titer inhibitor. BO2C11 is representative of C2 inhibitor insofar as it belongs to the immunoglobulin G4 (IgG4) isotype and inhibits FVIII binding to both VWF and PLs.14 The figure was drawn using MolMol,21 using the published coordinates of the C2 domain.7 (B) Inhibition of healthy and mutated FVIII by BO2C11. Plasma from Del2201 of patients 1 and 2 and from healthy pool plasma were incubated with BO2C11 at 10 μg/mL for 2 hours at 37°C, and the residual FVIII activity was measured in a chromogenic assay. Results are expressed as residual FVIII activity (mean ± SD). (C) FVIII binding to VWF in plasma. Plasma from patient 1 or 2, diluted 20-fold, was incubated with either Sepharose beads coated with anti-VWF antibody or control Sepharose. Following centrifugation, FVIII was measured in the supernatant of anti-VWF Sepharose (free FVIII) and of control Sepharose (total FVIII) by using a FVIII chromogenic assay. FVIII bound to VWF was calculated by subtracting free FVIII from total FVIII. The fraction of FVIII bound to VWF is expressed as the percentage of total FVIII (mean ± SD). (D) Cofactor activity of plasma FVIII was evaluated in a chromogenic assay by using various concentrations of synthetic phospholipid vesicle containing 9% phosphatidyl-serine, 25% phosphatidyl-choline, 31% phosphatidyl-ethanolamine, 15% sphingomyelin, and 20% cholesterol and representative of PLs expressed at the surface of activated platelets. At each PL concentration, FXa generation was compared with that observed in the presence of a known concentration of healthy plasma FVIII. For each FVIII variant, results are expressed as the percentage (mean ± SD) of the FVIII activity measured at the highest PL concentration tested (4 μM).
Interaction of healthy and mutated plasma FVIII with BO2C11. (A) Location of residues involved in FVIII binding to BO2C11 in the 3-dimensional structure of the C2 domain. The C2 domain is represented as ribbon diagram. The hydrophobic and basic residues mediating binding to PLs are highlighted as Corey-Pauling-Kultun (CPK) spheres. Arrowheads indicate residues mediating FVIII binding to BO2C11 as determined by crystallization of an Fab fragment of BO2C11 bound to a recombinant C2 domain.20 The human monoclonal antibody BO2C11 was obtained by immortalization of B lymphocytes of a patients with hemophilia A with a high-titer inhibitor. BO2C11 is representative of C2 inhibitor insofar as it belongs to the immunoglobulin G4 (IgG4) isotype and inhibits FVIII binding to both VWF and PLs.14 The figure was drawn using MolMol,21 using the published coordinates of the C2 domain.7 (B) Inhibition of healthy and mutated FVIII by BO2C11. Plasma from Del2201 of patients 1 and 2 and from healthy pool plasma were incubated with BO2C11 at 10 μg/mL for 2 hours at 37°C, and the residual FVIII activity was measured in a chromogenic assay. Results are expressed as residual FVIII activity (mean ± SD). (C) FVIII binding to VWF in plasma. Plasma from patient 1 or 2, diluted 20-fold, was incubated with either Sepharose beads coated with anti-VWF antibody or control Sepharose. Following centrifugation, FVIII was measured in the supernatant of anti-VWF Sepharose (free FVIII) and of control Sepharose (total FVIII) by using a FVIII chromogenic assay. FVIII bound to VWF was calculated by subtracting free FVIII from total FVIII. The fraction of FVIII bound to VWF is expressed as the percentage of total FVIII (mean ± SD). (D) Cofactor activity of plasma FVIII was evaluated in a chromogenic assay by using various concentrations of synthetic phospholipid vesicle containing 9% phosphatidyl-serine, 25% phosphatidyl-choline, 31% phosphatidyl-ethanolamine, 15% sphingomyelin, and 20% cholesterol and representative of PLs expressed at the surface of activated platelets. At each PL concentration, FXa generation was compared with that observed in the presence of a known concentration of healthy plasma FVIII. For each FVIII variant, results are expressed as the percentage (mean ± SD) of the FVIII activity measured at the highest PL concentration tested (4 μM).
The C2 domain is frequently targeted by FVIII inhibitor antibodies occurring in patients with hemophilia A treated with FVIII concentrates.10-12 In addition, some point mutations, located in the amino-terminal part of the C2 domain of patients with mild/moderate hemophilia A, are associated with an increased incidence of inhibitor development.13
Despite the importance of C2 for FVIII function and antigenicity, only limited information is available regarding alterations of structure and function of the C2 domain in hemophilia A. We, therefore, screened plasma from patients with mild/moderate hemophilia A for alterations in the antigenic determinant recognized by a human monoclonal antibody to the C2 domain.14 We also investigated the interaction of such mutated FVIII molecules with PL and VWF.
Study design
Plasma was obtained, under institutionally approved informed consent, from healthy donors and 111 patients with mild hemophilia A.
B-domain–deleted recombinant (BDD-r) healthy Ser2119Tyr and Del2201 FVIII were produced as described.15 For Del2201 FVIII, mutagenesis was performed by using the primers 5′-ACC-AAT-ATg-TTT-ACC-Tgg-TCT-CCT-TCA-AAA-gCT-CgA-3′ and 5′-TgA-Agg-AgA-CCA-ggT-AAA-CAT-ATT-ggT-AAA-gTA-ggA-3′. BDD-rFVIII production was evaluated by using transiently transfected Chinese hamster ovary (CHO) cells, and BDD-rFVIII preparations were partially purified from VWF-free supernatant of stably transfected CHO cells.15
FVIII activity (FVIII:C) was measured in a 1-stage clotting assay using partial thromboplastin time (PTT) automate (Stago, Parsippany, NJ) or by using a chromogenic assay (Coatest FXa generation assay; Chromogenix-Instrumentation Laboratory SpA, Milano, Italy) as recommended for BDD-rFVIII.16 FVIII:Ag was measured by enzyme-linked immunosorbent assay (ELISA).15 The inhibition of plasma FVIII by purified BO2C11 was evaluated as described.15
FVIII binding to VWF in plasma was studied by using a centrifugation assay.15 The binding of recombinant FVIII (0.5 IU/mL) to VWF was evaluated in the presence of various concentrations of purified VWF, which was bound to anti-VWF antibodies coated on Sepharose.15 FVIII interactions with PLs were evaluated by using a modification of the Coatest FXa. Aliquots of plasma in 30-μL samples diluted 200-fold in Tris (tris(hydroxymethyl)aminomethane) 50 mM, pH 7.3, 0.2% bovine albumin, or BDD-rFVIII were mixed with 30 μL PL at various concentrations for a 10-minute incubation at 37°C. At each PL concentration, FVIII activity in the test sample was determined by comparing FXa generation with that obtained by using known concentrations of healthy plasma FVIII.15 For each FVIII variant, results were expressed as the percentage of the FVIII activity measured at the highest PL concentration. Small unilamellar PL microvesicles representative of an activated platelet surface were used.17-19
Results and discussion
To investigate the effect of mutations responsible for mild/moderate hemophilia A on antigenic determinant(s) in the C2 domain, we checked whether such mutations could prevent FVIII inactivation by BO2C11. The antibody inhibited more than 95% of plasma FVIII from all but 2 patients (Figure 1B). Both patients carried a deletion of Ala2201 (Del2201) in the C2 domain. The effect of this novel mutation is likely explained by the location of Ala2201 close to residues Met2199 and Phe2200, both shown by crystallography to mediate FVIII contact with BO2C1120 (Figure 1A).
The 2 patients carrying Del2201 are unrelated at least up to the second generation. FVIII:C ranged between 11% and 33%, with FVIII:Ag between 35% and 58%. No difference in FVIII:C activity was recorded by using either the 1-stage or the chromogenic FVIII assay. One patient (43 years old) experienced several bleeding episodes, including spontaneous and postoperative ocular angioma bleeding and gingivorrhagia, whereas the other patient (18 years old) suffered from frequent bruising and epistaxis. In case of minor surgery, both patients could always be efficiently treated with DDAVP (1-deamino-8-D-arginine vasopressin) and, therefore, never received FVIII concentrates.
Because BO2C11 inhibits FVIII binding to VWF and PLs, we investigated Del2201 FVIII interactions with VWF and PLs. In control assays, the fraction of healthy FVIII bound to VWF was 85% and that of a FVIII with mutation Ser2119Tyr in the C1 domain was 15%, in agreement with published values.15 For both patients with Del2201, plasma FVIII bound to VWF was less than 55% (Figure 1C). A reduced binding of FVIII to VWF was also observed when FVIII was measured by using a FVIII:Ag ELISA (data not shown).
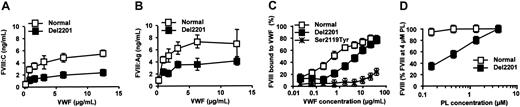
The functional alteration of Del2201 FVIII was further studied by using BDD-rFVIII. In the presence of VWF, the rate of production of Del2201 BDD-rFVIII was lower than that of healthy BDD-rFVIII. By contrast, in VWF-free medium, the rates of production and the FVIII:C/FVIII:Ag ratio of both FVIII proteins were similar (Figure 2A-B). In a FVIII 1-stage assay, the activity of both BDD-rFVIII proteins was 50% lower than in the chromogenic assay, in agreement with observations made with another BDD-rFVIII.16 To evaluate the binding of BDD-rFVIII to VWF, we measured the capture of rFVIII by various concentrations of VWF bound to Sepharose. Between 10- and 500-fold more VWF was required to capture 25% of Del2201 and Ser2119Tyr FVIII, respectively, than of healthy FVIII (Figure 2C). Similar results were obtained when FVIII bound to VWF was evaluated by measuring FVIII:Ag (data not shown). These observations confirm the role of the C2 domain in FVIII binding to VWF.1-3 Given the importance of VWF for FVIII stability, the mild reduction of Del2201 FVIII binding to VWF could contribute to low plasma FVIII levels.
Healthy and Del2201 BDD-rFVIII interactions with VWF and phospholipids. (A) CHO cells transfected with expression vectors for healthy and Del2201 BDD-rFVIII were incubated for 16 hours in the presence of various concentrations of VWF. FVIII activity in the cell culture supernatant was determined using a chromogenic assay (mean ± SD). (B) CHO cells transfected with expression vectors for healthy and Del2201 BDD-rFVIII were incubated for 16 hours in the presence of various concentrations of VWF as in panel A. FVIII:Ag in the cell culture supernatant was determined by ELISA (mean ± SD). (C) BDD-rFVIII binding to VWF. Various concentrations of VWF bound to Sepharose beads were incubated with healthy, Del2201, and Ser2119Tyr BDD-rFVIII (0.5 IU/mL). Following centrifugation, FVIII was measured in the supernatant of VWF Sepharose (free FVIII) and of control Sepharose (total FVIII) by using a FVIII chromogenic assay. FVIII bound to VWF was calculated by subtracting free FVIII from total FVIII. The fraction of FVIII bound to VWF is expressed as the percentage of total FVIII (mean ± SD). (D) Activity of BDDr-FVIII was evaluated in a chromogenic assay by using various concentrations of synthetic phospholipid vesicle, representative of PLs expressed at the surface of activated platelets. At each PL concentration, FXa generation was compared with that observed in the presence of a known concentration of healthy plasma FVIII. For each FVIII variant, results are expressed as a percentage (mean ± SD) of the FVIII activity measured at the highest PL concentration tested (4 μM).
Healthy and Del2201 BDD-rFVIII interactions with VWF and phospholipids. (A) CHO cells transfected with expression vectors for healthy and Del2201 BDD-rFVIII were incubated for 16 hours in the presence of various concentrations of VWF. FVIII activity in the cell culture supernatant was determined using a chromogenic assay (mean ± SD). (B) CHO cells transfected with expression vectors for healthy and Del2201 BDD-rFVIII were incubated for 16 hours in the presence of various concentrations of VWF as in panel A. FVIII:Ag in the cell culture supernatant was determined by ELISA (mean ± SD). (C) BDD-rFVIII binding to VWF. Various concentrations of VWF bound to Sepharose beads were incubated with healthy, Del2201, and Ser2119Tyr BDD-rFVIII (0.5 IU/mL). Following centrifugation, FVIII was measured in the supernatant of VWF Sepharose (free FVIII) and of control Sepharose (total FVIII) by using a FVIII chromogenic assay. FVIII bound to VWF was calculated by subtracting free FVIII from total FVIII. The fraction of FVIII bound to VWF is expressed as the percentage of total FVIII (mean ± SD). (D) Activity of BDDr-FVIII was evaluated in a chromogenic assay by using various concentrations of synthetic phospholipid vesicle, representative of PLs expressed at the surface of activated platelets. At each PL concentration, FXa generation was compared with that observed in the presence of a known concentration of healthy plasma FVIII. For each FVIII variant, results are expressed as a percentage (mean ± SD) of the FVIII activity measured at the highest PL concentration tested (4 μM).
Del2201 FVIII interaction with PLs was evaluated in a FXa generation assay, using synthetic PL vesicles representative of activated platelet surface. There was a good correlation between the FVIII:C obtained at 4 μM PL and that measured in standard FVIII:C assays. However, reducing PL concentrations resulted in a progressive decrease in FVIII activity in plasma of both patients carrying the deletion compared with the effect on healthy plasma samples. At the lowest PL concentration still allowing a significant FXa generation (1.5 μM PL), Del2201 FVIII:C was reduced 3-fold relative to healthy plasma (Figure 1D). Comparison of healthy and Del2201 BDD-rFVIII provided the same results as with healthy and patient plasma FVIII (Figure 2D).
In conclusion, Del2201 in the FVIII C2 domain eliminates a major antigenic determinant of the FVIII molecule and impairs FVIII interaction with both VWF and PLs. This first report of a mutation occurring in patients with mild/moderate hemophilia A and altering FVIII binding to PLs raises the question of whether mutation in the FVIII PL binding site can lead to mild hemophilia A, while escaping detection using conventional FVIII:C assays. However, as previously shown by site-directed mutagenesis,9 it is possible that any mutation in the FVIII PL binding site impairs FVIII binding to VWF, thereby resulting in low FVIII:C levels detectable with routine FVIII:C assays.
Prepublished online as Blood First Edition Paper, September 11, 2003; DOI 10.1182/blood-2003-04-1321.
Supported in part by grant G.0378.01 from the Flemish Research Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal