Abstract
The full potential of a graft-versusmyeloma effect after allogeneic hematopoietic cell transplantation (HCT) for patients with multiple myeloma (MM) has not been realized because of excessive early transplantation-related mortality (TRM) with conventional HCT. Autologous HCTs have been characterized by almost universal disease recurrences. The current trial combined autologous HCT with subsequent nonmyeloablative allogeneic HCT to maintain the benefits of both approaches with acceptable toxicity. Fifty-four patients, 52 years of age (median; range, 29-71 years), with previously treated stage II or III MM (52% refractory or relapsed disease) were given melphalan 200 mg/m2 and autologous HC transplants. Regimen-related toxicities after autologous HCT were moderate with a median of 6 days of neutropenia, 7 days of hospitalization, and 1 death from infection. Forty to 229 days later (median, 62 days), 52 patients received a single fraction dose of 2 Gy total body irradiation and HC transplants from HLA-identical siblings with postgrafting immunosuppression with mycophenolate mofetil (MMF) and cyclosporine (CSP). Patients experienced medians of 0 days of hospitalization, neutropenia, and thrombocytopenia. Sustained engraftment was uniform. With a median follow-up of 552 days after allografting, overall survival is 78%. One patient (2%) died before day 100 from disease progression. Thirty-eight percent of patients developed acute graft-versus-host disease (GVHD; grade II in all but 4 cases) and 46% chronic GVHD requiring therapy. Tumor responses occurred slowly. Thus far, 57% of patients have achieved complete remissions and 26% have achieved partial remissions for an overall response of 83%. Despite being evaluated in elderly patients with MM, this 2-step approach has reduced the acute toxicities of allogeneic HCT while achieving potent antitumor activities.
Introduction
High-dose therapy with autologous hematopoietic cell transplantation (HCT) for advanced-stage myeloma in patients younger than 65 years of age has survival advantages compared with conventional therapy.1,2 In the Intergroupe Francais du Myélome (IFM) 90 trial, the complete remission (CR) rate was higher and the 7-year event-free survival (EFS) 16% and overall survival (OS) 43% compared with 8% and 25% with conventional chemotherapy, respectively.1,3 The use of tandem autografts is also superior to standard chemotherapy4 and may be better than a single autograft. Recent data from the IFM94 trial, published in abstract form, comparing single dose of 140 mg/m2 melphalan (Mel140) plus total body irradiation (TBI; 8 Gy) versus tandem Mel140 followed by Mel140 + TBI (8 Gy) in 399 patients found a similar CR rate of 34% versus 35% but 7-year EFS of 10% versus 20% and 7-year OS of 21% versus 42% favoring the tandem transplant arm.3 Despite high response rates and relatively low transplantation-related mortality (TRM) of less than 10%, fewer than 30% of patients remain in remission 3 to 7 years later.5-13 The high rate of progression/relapse following autologous HCT is due to the inability to eliminate all tumor cells from the patients and, possibly, from the autografts. Purging of grafts to reduce tumor contamination has not been shown to be beneficial14-16 and may increase the risk of infections due to removal of host immune cells.17 While a number of conditioning regimens have been used, melphalan at 200 mg/m2 has been well tolerated, even in patients in their seventh decade of life,18-20 and has generally been accepted as the current standard.19,21
In contrast, the use of allogeneic HCT following high-dose conditioning provides a tumor-free stem cell source and graft-versusmyeloma activity through immune responses against minor antigen differences between donor and host.22-24 Allogeneic HCT has been associated with a higher frequency of sustained molecular remissions25,26 and a lower risk of relapse.22 The graft-versus-myeloma activity of donor lymphocyte infusions (DLIs) in patients who have relapsed after conventional allografting further demonstrates the allogeneic immune cells' potential27-32 ; however, these responses are often brief and associated with graft-versus-host disease (GVHD).33,34 Unfortunately, allogeneic HCT has been associated with high TRM (20%-50%+ in the first 180 days) even in adults younger than 55 years of age, which has tempered enthusiasm with this approach and led to the early closure of the allogeneic bone marrow transplantation (BMT) arm in the United States intergroup trial.35-38 Recent analysis of transplant registry data reported to the European Group for Blood and Marrow Transplant (EBMT) suggests that the TRM associated with conventional allografts has decreased in the more recent cohort of patients that received transplants between 1994-1998 with a TRM at 6 months of 21% compared with 38% in patients that received transplants between 1983-1993.39 This decrease was attributed to a lower risk of fatal infections and pulmonary toxicity and was likely due to better patient selection. The 3-year survival following HCT was 55% in the later cohort compared with 35% in the earlier experience. The use of peripheral blood stem cells (PBSCs) rather than BM was associated with earlier engraftment but no difference in survival or CR rates. However, it is important to note that the median age in the recent cohort was 44 years with a range of 18 to 57 years and patients received transplants a median of 10 months from diagnosis. Thus, the advantages of allografts have been outweighed by the higher TRM compared with autografts, resulting in similar or inferior survival at 3 to 6 years40 despite the lower risk of relapse.
Recently, a new approach to allografting has been developed using nonmyeloablative conditioning and novel posttransplantation immunosuppression to assure engraftment and graft-versus-tumor effects for eradication of various hematologic malignancies.41 In contrast to conventional high-dose allografting, a low overall TRM of approximately 15% was observed in patients either too old or medically infirm to undergo conventional HCT. The 100-day mortality was 4.5%. Taking advantage of this new approach, we postulated that by combining cytoreduction achieved with high-dose autologous HC transplants and graft-versus-myeloma effects of nonmyeloablative HC transplants from HLA-identical siblings, we could safely extend the benefits of allogeneic transplantation to older patients (aged to 65 years) and hopefully achieve cures of multiple myeloma.
Patients and methods
Patients
From August 14, 1998, to June 1, 2001, 54 patients with multiple myeloma (MM) were treated at the Fred Hutchinson Cancer Research Center, the University of Washington, and the Veterans Administration Hospital (Seattle, WA); the City of Hope Medical Center (Duarte, CA); Stanford University (Stanford, CA); the University of Leipzig (Leipzig, Germany); the University of Torino (Torino, Italy); and the University of Colorado (Denver, CO). Clinical characteristics of the patients are shown in Table 1. Their median age was 52 years (range, 29-71 years). Seven patients were older than 60 years of age, 2 of whom were older than 65 years of age. Eighty-seven percent of patients had stage II/III disease at diagnosis and others had progressed to require therapy. All patients had received prior therapy for their myeloma. At the time of autografting, patients had received a median of 4 cycles (range, 4-19 cycles) of prior chemotherapy with 93% having received the vincristine, adriamycin, and dexamethasone (VAD) regimen (median, 4; range, 2-10 cycles). Forty-eight percent of patients had received more than one treatment regimen; 8% had received prior radiation therapy and 8% had received prior thalidomide. At the time of autologous HCT, 48% had relapsed or refractory disease from their prior therapy. Fifty-two percent had disease that was responsive to their last treatment, including 41% who had achieved partial remissions (PRs) and 11% CRs. Thirty-two percent of patients had elevated beta-2 microglobulin higher than 2.5 μg/mL. Entry criteria included serum bilirubins less than twice normal; left ventricular ejection fractions greater than 40%; creatinine clearances greater than 40 mL/min; and Karnofsky performance status greater than 60. Patients and donors signed written informed consents on protocols approved by the institutional review boards of each participating institution.
Patient characteristics
N = 54 . | No. . | Percent . |
|---|---|---|
| Age, median, y (range) | 52 (29-71) | — |
| Male | 37 | 68.5 |
| Prior chemotherapy | 54 | 100 |
| VAD | 50 | 93 |
| No. of cycles, median (range) | 4 (2-10) | — |
| More than one regimen | 26 | 48 |
| XRT | 4 | 7.8 |
| Response from last therapy at time of auto HCT | ||
| CR | 6 | 11 |
| PR | 22 | 41 |
| Untreated relapse | 7 | 13 |
| Refractory | 19 | 35 |
| B2M, median, μg/mL (range) | 1.94 (0.6-5.8) | — |
| B2M, greater than 2.5 μg/mL | 16/50 | 32 |
| Days Dx to auto, median (range) | 282 (163-3629) | — |
N = 54 . | No. . | Percent . |
|---|---|---|
| Age, median, y (range) | 52 (29-71) | — |
| Male | 37 | 68.5 |
| Prior chemotherapy | 54 | 100 |
| VAD | 50 | 93 |
| No. of cycles, median (range) | 4 (2-10) | — |
| More than one regimen | 26 | 48 |
| XRT | 4 | 7.8 |
| Response from last therapy at time of auto HCT | ||
| CR | 6 | 11 |
| PR | 22 | 41 |
| Untreated relapse | 7 | 13 |
| Refractory | 19 | 35 |
| B2M, median, μg/mL (range) | 1.94 (0.6-5.8) | — |
| B2M, greater than 2.5 μg/mL | 16/50 | 32 |
| Days Dx to auto, median (range) | 282 (163-3629) | — |
VAD indicates vincristine, adriamycin, dexamethasone; XRT, radiation therapy; CR, complete remission; PR, partial remission; B2M, beta-2 microglobulin; Dx, diagnosis; and —, not applicable.
Treatment plan
PBSC mobilization / high-dose melphalan / autologous HC transplants. Unless previously cryopreserved, PBSCs (target of 5 × 106 CD34 cells/kg) were collected and cryopreserved following cyclophosphamide (3-4 g/m2) on day +1, with or without paclitaxel (250 mg/m2) on day +2; and granulocyte colony-stimulating factor (G-CSF; 10 μg/kg subcutaneously) from day 3 through collection.42-44 This regimen was based on experience demonstrating more effective stem cell mobilization compared with cyclophosphamide (CY)/G-CSF alone.45 The first 4 patients had CD34-selected PBSCs using the CellPro (Bothell, WA) CD34 cell selection device in attempts to decrease myeloma cell contamination. This was discontinued after higher risks of cytomegalovirus (CMV) and other infections were found following CD34 selection.17
Melphalan (200 mg/m2) was given at least 31 days after mobilization chemotherapy via central catheter (undiluted as bolus injection or diluted with sodium chloride) over 15 to 20 minutes. Autologous PBSCs were thawed and infused 48 hours after melphalan. The day of PBSC infusion was designated day 0. Patients received G-CSF 5 μg/kg from days 0 or 5 of autografting until neutrophil counts of more than 1 × 109/L (1000/μL) were achieved.
Donor HC transplant collection/nonmyeloablative allografting. Upon recovery from autologous HCT (planned range, 40-120 days), patients underwent allografting. Recovery was defined as follows: (1) resolved mucositis and no need for intravenous hydration, (2) renal and hepatic function returned to entry criteria, (3) no intravenous antibiotics, and (4) CMV antigen negative. Conditioning consisted of 2 Gy TBI at 7 cGy/minute by linear accelerator. Nine patients received in addition fludarabine 30 mg/m2 on days -4, -3, and -2. Donor PBSCs were infused on day 0 after TBI. Postgrafting immunosuppression included mycophenolate mofetil (MMF), 15 mg/kg orally twice a day from the evening of day 0 until day +27 and cyclosporine (CSP), 6.25 mg/kg orally twice a day from day -1 to day +35 or +56 and then tapered. CSP trough levels were evaluated on day 3, then twice weekly and targeted to approximately 500 ng/mL (Abbott TDx, Abbot Park, IL) until CSP taper. In case of toxicity, CSP was adjusted. MMF doses were rounded to the nearest 250 mg.
Donors were HLA-identical siblings, 31 to 73 years of age (median, 50 years; Table 2). Donor PBSCs were mobilized using G-CSF (16 μg/kg/d; day -4 to 0) with aphereses on days -1 and 0. PBSCs harvested on day -1 were stored (4°C overnight) and infused with the day 0 collection. The median numbers of CD34+ and CD3+ T cells infused were 8.5 × 106/kg (range, 2.0-28.0 × 106/kg) and 3.5 × 108/kg (range, 1.4-11.7 × 108/kg), respectively.
Characteristics and results following nonmyeloablative HCT (N = 52 patients receiving allografts)
. | No. . | % or range . |
|---|---|---|
| Days from auto to allograft, median | 62 | 40-299 |
| Donor age, median y | 50 | 31-73 |
| Number CD34 cells × 106 kg, median | 8.5 | 2.0-28.0 |
| Number CD3 cells × 108 kg, median | 3.5 | 1.41-11.73 |
| Granulocyte nadir, median cells/μL | 760 | 100-3 184 |
| Days granulocyte counts less than 500/μL, median | 0 | 0-1 |
| Platelet nadir, median cells/μL | 95 000 | 15 000-192 000 |
| Days platelet counts less than 20 000/μL, median | 0 | 0-1 |
| Number of platelet transfusions, median | 0 | 0-140 |
| Number RBC transfusions, median | 0 | 0-40 |
| Days hospitalized, median | 0 | 0-37 |
| GVHD | ||
| Acute GVHD II-IV | 20/52 | 38.5% |
| Grade I | 16/52 | 30.8% |
| Grade III | 1/52 | 1.9% |
| Grade IV | 3/52 | 5.8% |
| Chronic GVHD | 32/50 | 64% |
| Chronic extensive GVHD | 23/50 | 46% |
. | No. . | % or range . |
|---|---|---|
| Days from auto to allograft, median | 62 | 40-299 |
| Donor age, median y | 50 | 31-73 |
| Number CD34 cells × 106 kg, median | 8.5 | 2.0-28.0 |
| Number CD3 cells × 108 kg, median | 3.5 | 1.41-11.73 |
| Granulocyte nadir, median cells/μL | 760 | 100-3 184 |
| Days granulocyte counts less than 500/μL, median | 0 | 0-1 |
| Platelet nadir, median cells/μL | 95 000 | 15 000-192 000 |
| Days platelet counts less than 20 000/μL, median | 0 | 0-1 |
| Number of platelet transfusions, median | 0 | 0-140 |
| Number RBC transfusions, median | 0 | 0-40 |
| Days hospitalized, median | 0 | 0-37 |
| GVHD | ||
| Acute GVHD II-IV | 20/52 | 38.5% |
| Grade I | 16/52 | 30.8% |
| Grade III | 1/52 | 1.9% |
| Grade IV | 3/52 | 5.8% |
| Chronic GVHD | 32/50 | 64% |
| Chronic extensive GVHD | 23/50 | 46% |
GVHD indicates graft-versus-host disease.
Supportive care. Patients received standard prophylaxis against bacterial, Pneumocystis carinii, and fungal infections, and herpes simplex and varicella-zoster virus reactivation. CMV reactivation was monitored and treated with ganciclovir.46
Analyses of chimerism and residual disease. Degrees of donor chimerism of peripheral blood T cells and granulocytes and unfractionated marrow were assessed at days 28, 56, 180, and 360 after allogeneic HCT using fluorescence in situ hybridization (FISH) in sex-mismatched pairs and polymerase chain reaction (PCR) analyses of polymorphic microsatellite regions in sex-matched pairs.47
Disease responses were assessed using the American Bone Marrow Transplant Registry criteria with the following modifications.48 Complete remissions required absence of the original monoclonal proteins in serum and urine by protein electrophoreses and of clear bands on immunofixation, less than 5% plasma cells in marrow aspirates, without evidence of clonal disease by flow cytometry, and no increases in sizes or numbers of osteolytic bone lesions. Partial remissions were defined as more than 75% reduction in the levels of serum monoclonal protein, more than 90% reduction in 24-hour urinary light chain excretion, and no increases in sizes or numbers of lytic bone lesions. Patients with less than a PR, without disease progression, were considered to have stable disease (SD). Patients were evaluated for disease once prior to autologous conditioning and once prior to nonmyeloablative conditioning to estimate the baseline level of disease activity prior to each transplantation.
Endpoints. The primary purpose of the study was to reduce the day-100 mortality from the current 20% to 50% seen following myeloablative HCT to less than 20% following the tandem transplantations in this older population. Secondary endpoints included engraftment and the degree of donor chimerism, response, and disease-free survival and the incidence of acute and chronic GVHD.49,50 Data were analyzed as of June 1, 2002.
Donor lymphocyte infusions (DLIs). The original intent was to use nonmyeloablative allografting to establish mixed donor chimerism and serve as a platform for subsequent DLIs to convert partial to full donor chimerism and to eliminate residual or progressive disease. As all but one patient achieved full donor chimerism, DLI was administered to only one patient for mixed chimerism with subsequent conversion to full donor chimerism. No other patients have received DLIs.
Results
Autologous HCT
Table 3 summarizes the outcome following high-dose melphalan and autologous HCT. Patients received transplants a median of 9 months (range, 5-119 months) from their diagnosis of MM.
Disease status at study enrollment and disease responses during study
. | . | . | Outcome, months after allo . | . | |
|---|---|---|---|---|---|
| At time of auto, n = 54 . | After auto, n = 54 . | Best after allo, n = 52 . | Deceased, mo/cause n = 12 . | Alive, mo, n = 42 . | |
| 6 CR | 6 CR | 6 CR | 3/enceph, 7/cGVHD | 18, 20, 24R, 24 | |
| 22 PR | 5 CR | 5 CR | — | 13, 19, 20, 24, 27R | |
| 11 PR | 6 CR | — | 12, 12, 13, 20, 24, 37 | ||
| 5 PR | — | 7, 12, 13, 18, 24 | |||
| 6 SD | 3 CR | — | 12, 12, 13 | ||
| 1 PR | 14/pulmonary cGVHD | — | |||
| 2 SD | — | 9, 18 | |||
| 7 REL | 0 CR | — | — | — | |
| 1 PR | 1 PR | 6/GVHD | — | ||
| 6 SD | 1 CR | 37/GVHD | — | ||
| 3 PR | 14/lung Cancer | 19, 19 | |||
| 2 PD | 23/MM | 19 | |||
| 19 REF | 0 CR | — | — | — | |
| 8 PR | 6 CR | — | 12, 18, 18, 18R, 20, 31 | ||
| 2 PR | 13/GVHD | 13 | |||
| 6 SD | 3 CR | — | 18, 36, 36 | ||
| 1 PR | 10R/GVHD | — | |||
| 2 SD | 3/GVHD | 6 | |||
| 3 PD | 1 CR | — | 17 | ||
| 1 PR | — | 12 | |||
| 1 PD | 3/MM | — | |||
| 2 NE | 2 NE | CMV after auto | 1 alive no allo | ||
. | . | . | Outcome, months after allo . | . | |
|---|---|---|---|---|---|
| At time of auto, n = 54 . | After auto, n = 54 . | Best after allo, n = 52 . | Deceased, mo/cause n = 12 . | Alive, mo, n = 42 . | |
| 6 CR | 6 CR | 6 CR | 3/enceph, 7/cGVHD | 18, 20, 24R, 24 | |
| 22 PR | 5 CR | 5 CR | — | 13, 19, 20, 24, 27R | |
| 11 PR | 6 CR | — | 12, 12, 13, 20, 24, 37 | ||
| 5 PR | — | 7, 12, 13, 18, 24 | |||
| 6 SD | 3 CR | — | 12, 12, 13 | ||
| 1 PR | 14/pulmonary cGVHD | — | |||
| 2 SD | — | 9, 18 | |||
| 7 REL | 0 CR | — | — | — | |
| 1 PR | 1 PR | 6/GVHD | — | ||
| 6 SD | 1 CR | 37/GVHD | — | ||
| 3 PR | 14/lung Cancer | 19, 19 | |||
| 2 PD | 23/MM | 19 | |||
| 19 REF | 0 CR | — | — | — | |
| 8 PR | 6 CR | — | 12, 18, 18, 18R, 20, 31 | ||
| 2 PR | 13/GVHD | 13 | |||
| 6 SD | 3 CR | — | 18, 36, 36 | ||
| 1 PR | 10R/GVHD | — | |||
| 2 SD | 3/GVHD | 6 | |||
| 3 PD | 1 CR | — | 17 | ||
| 1 PR | — | 12 | |||
| 1 PD | 3/MM | — | |||
| 2 NE | 2 NE | CMV after auto | 1 alive no allo | ||
CR indicates complete remission; enceph, encephalopathy; cGVHD, chronic graft-versus-host disease; —, no data necessary; R, relapse; PR, partial remission; SD, stable disease; REL, untested relapsed disease; PD, progression of disease; MM, multiple myeloma; REF, refractory disease; and NE, not evaluable.
Regimen-related toxicities, peripheral blood cell changes, transfusions and infections. The majority of patients experienced mucositis, nausea and vomiting, and diarrhea and required a median of 7 days (range, 0-34 days) hospitalization following conditioning of high-dose melphalan. The median days of neutrophil counts less than 0.5 × 109/L (500/μL) and platelet counts less than 20 × 109/L (20 000/μL) were 6 days (range, 0-17 days) and 1 day (range, 0-36 days), respectively. Patients received transfusions a median of 1 unit (range, 0-21 units) of red blood cells (RBCs) and 1 unit (range, 0-130 units) of platelets, respectively.
Patients experienced 14 bacterial and 8 viral infections (3 CMV), and 5 patients had oral Candida albicans. One patient developed CMV pneumonitis and died 31 days after CD34-selected autologous HCT.
Estimation of disease response. Patients were evaluated following their autografts and prior to allogeneic HCT to estimate their response to the high-dose melphalan (Table 3). One patient who died from infection was not evaluable for response. Of 22 patients with responsive disease prior to the autograft, 5 achieved CR, 11 achieved PR, and 6 had stable disease. Of 25 patients with relapsed or refractory disease prior to the autograft, none had CR, 9 had PR, 13 had stable disease, and 3 had disease progression. The 6 patients in CR prior to the autograft remained in CR.
Allogeneic HCT
Fifty-two allografts were carried out a median of 62 days (range, 40-229 days) after autologous HCT. One patient died prior to allografting and one patient was felt to be too ill after autograft and was taken off the study. Characteristics and results following allografting for the 52 patients are shown in Table 2.
Regimen-related toxicities, peripheral blood cell changes, and allogeneic engraftment. Patients did not experience severe mucositis, diarrhea, or severe nausea and vomiting. During the first 2 months, 16 (31%) patients developed transient grade II renal toxicities (likely due to targeting high CSP levels). Transient grades II and III hepatic toxicities occurred in 7 (13%) and 5 (10%) patients, respectively. During the first 2 months, patients were hospitalized a median of 0 days (range, 0-37 days).
The median period of neutropenia was 0 days (range, 0-19 days). The median neutrophil nadir was 0.76 × 109/L (760 cells/μL) (range, 0.1-3.184 × 109/L [range, 100-3184 cells/μL]). The median period of thrombocytopenia was 0 days (range, 0-1 days). The median platelet nadir was 95 × 109/L (95 000 cells/μL) (range, 15-192 × 109/L [15 000-192 000 cells/μL]). The median numbers of platelet and RBC transfusions within the first 60 days were 0 (range, 0-40) and 0 (range, 0-140), respectively. No serious hemolysis occurred in 7 ABO-incompatible transplants.
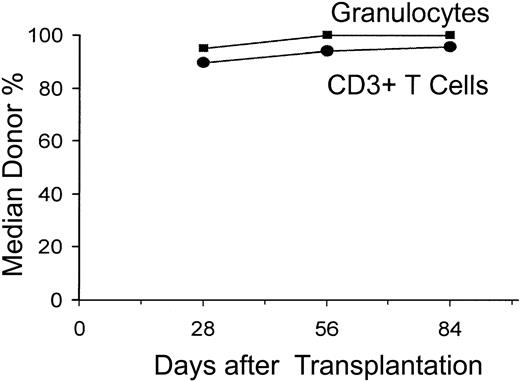
All 52 patients had sustained allografts (Figure 1). On day 28, 90%, 95%, and 95% of CD3+ T cells, granulocytes, and nucleated marrow cells, respectively, were of donor origin (median values). This increased to 96% to 100% through day 84. One patient with 83% donor T-cell chimerism on day +28 received a DLI of 106 CD3 cells/kg on day +85 and subsequently evolved to 97% donor T-cell chimerism.
Median percent donor chimerism after nonmyeloablative allogeneic HCT. The percentages of donor CD3+ T cells and granulocytes were measured on days 28, 56, and 84 after allografting.
Median percent donor chimerism after nonmyeloablative allogeneic HCT. The percentages of donor CD3+ T cells and granulocytes were measured on days 28, 56, and 84 after allografting.
Acute GVHD. Twenty (38.5%) patients developed grades II to IV acute GVHD at a median of 58 days (range, 10-107 days; Figure 2). This was grade II in 16, grade III in 1, and grade IV in 3 patients (Table 2). Fifteen patients had skin, 11 had gut, and 8 had liver involvement. GVHD responded in most patients either to resumption of CSP, MMF, or treatment with methylprednisolone (at 2 mg/kg/d with subsequent taper).
Cumulative incidence curves. Panel A shows the curve of acute GVHD, where the solid line indicates grade II and the dotted line indicates grade II/IV, and panel B shows the curve of chronic extensive GVHD.
Cumulative incidence curves. Panel A shows the curve of acute GVHD, where the solid line indicates grade II and the dotted line indicates grade II/IV, and panel B shows the curve of chronic extensive GVHD.
Chronic GVHD. Twenty-three patients (46%) developed chronic extensive GVHD requiring therapy with CSP, MMF, with or without prednisone (Table 2; Figure 2).
Infections. Pulmonary Aspergillus infections occurred in 2 patients who were successfully treated with amphotericin followed by itraconazole. Ten patients developed infections with blood cultures positive for coagulase-negative staphylococcus,6 and one each with Acinetobacter, Serratia marcescens, pseudomonas, and Listeria, and were successfully treated with appropriate antibiotics. Two patients developed urinary tract infections with Staphylococcus or Escherichia coli. Eleven of 38 patients (29%) who were CMV seropositive or had CMV-seropositive donors developed CMV antigenemia and were treated with prophylactic ganciclovir. One patient had CMV colitis. One patient developed BK virus in the urine and 1 patient each developed varicella-zoster virus (VZV) and herpes simplex virus (HSV) infections. Two patients were treated for Clostridium difficile following detection of toxin in the stool. Nine patients were treated for undiagnosed upper respiratory infections and 1 patient had parainfluenza isolated from nasopharyngeal washings.
Disease responses. Twenty-five of the 48 patients (52%) who were not in CR at study entry (including the 2 patients who did not proceed to allografting) achieved CR and 14 (29%) achieved PR with an overall response rate of 81% at a median follow-up of surviving patients of 550 days (range, 194-1114 days) after allografting. Twenty-five (46%) are surviving in CR (Tables 2, 3). One patient achieved CR following DLI and converted from partial to complete donor chimerism without GVHD. Nine additional patients have achieved at least PR to date with continued regressions of disease markers. Table 3 summarizes overall responses to each portion of the trial. Of 6 patients who were in CR at the start of the trial, 1 has relapsed and 2 have died from infection or GVHD. Overall, 3 of the 31 patients with CR have had disease reoccurrence. Of 22 patients in PR (responsive disease to last therapy) at study entry, 14 achieved CR, 6 achieved PR, and 2 had stable disease. Only one patient has progressed. Thus, of the 28 patients with responsive disease (CR + PR) at the time of study entry, only 2 patients have progressed and 16 of the 28 are in continuous CR, 5 are in PR, and 2 continue with stable disease. Of 26 patients with refractory or relapsed disease, 11 achieved CR, 8 achieved PR, 2 had stable disease, and 3 had disease progression. Two patients were not evaluable for response as they did not receive allografts.
Attainment of CR was gradual. Two patterns are illustrated in Figure 3. Most patients had continued regression of disease following each phase of treatment. Some patients had obvious regressions of disease following the allografts that were sometimes associated with GVHD. In most patients, monoclonal bands gradually disappeared over 6 to 12 months with a median time to CR of 6 months.
Examples of monoclonal paraprotein decline following autologous HCT (auto) and nonmyeloablative allogeneic HCT (allo). GVHD indicates graft-versus-host disease; mos, months after transplantation; Pre-Auto, disease workup immediately before autologous HCT; and Pre-Allo, disease workup after autologous and immediately before allogeneic HCT.
Examples of monoclonal paraprotein decline following autologous HCT (auto) and nonmyeloablative allogeneic HCT (allo). GVHD indicates graft-versus-host disease; mos, months after transplantation; Pre-Auto, disease workup immediately before autologous HCT; and Pre-Allo, disease workup after autologous and immediately before allogeneic HCT.
Overall and progression-free survivals
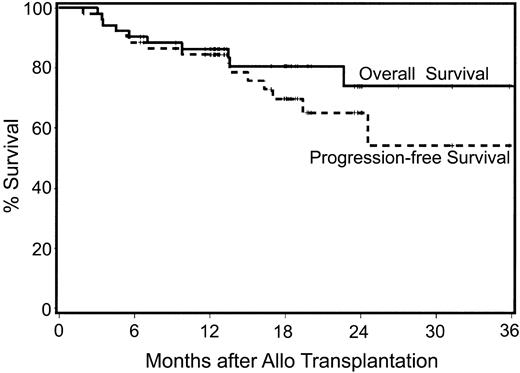
With median follow-ups for surviving patients of 611 days (range, 257-1210 days) after autologous and 550 days (range, 194-1114 days) after allogeneic HCT, 78% (42/54) of patients are surviving (Figure 4). Twelve patients have died; one patient died from CMV pneumonia following autologous HCT and 11 have died following allogeneic HCT. Of the 11, 3 died from disease progression at days +91, +296, and +689; 1 died from progressive encephalopathy at day +102; 3 died from complications associated with grade IV acute GVHD at days +104, +136, and +138; 1 died from pulmonary infection associated with chronic GVHD at day +212; and 2 died with pulmonary failure (BOOP) at days 413 and 1131 after transplantation, respectively. One additional patient died in CR from an unrelated malignancy, lung cancer, at day 410. The 100-day mortalities after auto and allo HCT were 2% (n = 1) and 2% (n = 1), respectively, with the one allogeneic recipient dying of disease progression. That patient had a 4-month delay between auto and allo HCT due to insurance issues. Estimated progression-free survival for all patients at 2 years is 55% (Figure 4).
Kaplan-Meier estimates of overall survival and progression-free survival following nonmyeloablative allografts for myeloma. Median follow-up is 18 months after allografting.
Kaplan-Meier estimates of overall survival and progression-free survival following nonmyeloablative allografts for myeloma. Median follow-up is 18 months after allografting.
Of the 28 patients with responsive disease entering the trial (CR + PR), only 3 have died and only 2 patients have had disease progression. In contrast, of the 26 patients with relapsed or refractory disease at study entry, 9 have died. The overall and progression-free survivals of patients with responsive compared with relapsed/refractory disease are shown in Figure 5.
Kaplan-Meier estimates of overall survival and progression-free survival following nonmyeloablative allografts. Panel A shows the Kaplan-Meier estimate for myeloma for patients with responsive disease at study entry (CR + PR) and panel B shows the estimate for relapsed/refractory disease.
Kaplan-Meier estimates of overall survival and progression-free survival following nonmyeloablative allografts. Panel A shows the Kaplan-Meier estimate for myeloma for patients with responsive disease at study entry (CR + PR) and panel B shows the estimate for relapsed/refractory disease.
Discussion
Conventional chemotherapy for MM is not curative.51 The use of high-dose myeloablative conditioning with autologous stem cell rescue has been associated with greater disease reduction and prolonged disease-free and overall survival compared with conventional therapy.1,3 In the IFM 90 trial, 38% of patients had a CR (22%) or very good PR with an EFS (median, 28 months) at 7 years of 16% and OS (median, 57 months) at 7 years of 43%, all superior to conventional chemotherapy.1,3 Additional randomized trials have generally favored autografting by demonstrating higher CR rates (22%-30%), although EFS and OS are confounded by the salvage use of autologous transplantation in the conventional chemotherapy arms.52-54 Autologous transplantation early, rather than as salvage of chemotherapy failure, was associated with longer EFS but similar OS.55 Among a multitude of conditioning regimens, single-agent melphalan (200 mg/m2) has emerged as the current standard by demonstrating the least toxicity with comparable results in patients in good medical condition as old as 70 years of age.56
The use of tandem high-dose regimens with autologous support has been explored by investigators at the University of Arkansas, Little Rock, and others.12,13 The use of the second autograft improved the CR rate from 24% to 43%.57 Tandem autografts were superior to conventional chemotherapy in a case-matched analysis with patients treated on Southwest Oncology Group protocols.4 Recent analysis of the IFM 94 trial has been presented in abstract form and suggests that tandem autografts are superior to a single autograft with median EFS 37 versus 31 months, 7-year EFS 20% versus 10%, and 7-year OS 42% versus 21%, respectively.3 Surprisingly, there was not an increase in CR rate (35% vs 34%) with the second transplantation, and the survival was superior only after 3 years. Despite this aggressive approach, nearly all patients eventually relapse or progress either from the reinfusion of tumor cells contaminating the PBSCs or from resistant cells remaining in the patient.
In contrast, the use of allogeneic stem cell support provides a tumor-free stem cell source and graft-versus-tumor immune effects. High-dose conditioning and allogeneic HCT has been associated with a high TRM in most trials and led to the premature closure of the allogeneic transplant arm of the North American Trial (TRM ∼40%). Overall 6-month TRM has ranged from 21% to 60%.36-40 Analysis of the EBMT database suggests that TRM has decreased in recent cohorts due to better patient selection resulting in decreased infectious and pulmonary toxicity.39 However, the 6-month TRM was still 21% with a 2-year TRM of 30% in patients a median age of 44 years (oldest patient 57 years of age). Overall, allogeneic HCT has been associated with a higher rate of molecular remission, but this advantage has been offset by the higher TRM when comparisons are made to single or tandem autologous HCT.
The current study employed a nonmyeloablative HC transplant regimen that reduces treatment-related toxicities and shifts the burden of tumor eradication toward the donor's T lymphocytes. In order to increase the likelihood of tumor eradication, the patients' disease was first reduced with high-dose melphalan and autologous HCT.
These results allowed the following observations. First, mortalities not related to relapse were 2% with autologous and 15% with allogeneic HCT. The 100-day transplant-related mortality after the allografts was 0%. Second, hematopoietic toxicities were greater following autologous compared with allogeneic HCT. Pancytopenias after nonmyeloablative allografts were minimal with both RBC and platelet transfusion requirements reduced compared with conventional grafts.58 Only 30% of patients had neutrophil nadirs of less than 0.5 × 109/L (500 cells/μL) and only 8% of patients had platelet nadirs less than 50 × 109/L (50 000/μL). Modest, reversible renal and hepatic toxicities were likely due to high-targeted CSP levels to facilitate engraftment.
Third, rapid, sustained allogeneic engraftment occurred. All but one patient had complete donor hematopoietic chimerism by day 84. That patient converted to complete donor chimerism with DLI. This rapid, uniform engraftment contrasts to a nonfatal rejection rate of 18% with the same regimen in patients without preceding autologous HCT.41 Although the protocol was originally conceived as establishing mixed donor chimerism that would serve as a platform for scheduled DLIs to convert from partial to full donor chimerism and treat persistent disease, we observed high donor chimerism, often in the setting of GVHD, precluding the need for DLI in all but one patient.
Fourth, grades II to IV acute GVHD was seen in 39% of patients. Severe disease was seen only in 4 patients (1 grade III, 3 grade IV). Three patients died from complications associated with acute GVHD or its treatment. In the initial phases of this study, CSP was discontinued by day 35. We observed that the median onset of GVHD was 72 days after HCT, and only one patient developed GVHD while being treated with both MMF and CSP. These observations have led us to extend CSP in subsequent patients through day 56 with a taper to day 80 or 180, depending on disease activity. Chronic GVHD requiring immunosuppressive therapy occurred in 46% of patients, and 3 patients have died from late complications resulting from pulmonary complications. Longer follow-up to assess the potential impact of chronic GVHD on outcome, either improved relapse-free survival or increased mortality, is still needed. Thus, while extending one or both of the immunosuppressive drugs may reduce GVHD, their effects on tumor responses will need to be determined. The incidence of GVHD in this study appears similar to standard allografts. Recent comparison of age-matched patients treated with conventional allografts versus nonmyeloablative allografts at our institution found lower grade III/IV acute GVHD (14% vs 38%) but similar chronic GVHD rates in the nonmyeloablative group.59
Finally, 81% of patients showed tumor responses with 52% achieving CR and 29% achieving PR. This is noteworthy as 48% of patients had relapsed or refractory disease before HCT. Separation of the antitumor effects due to the high-dose therapy before autologous HCT and those due to the allografts is difficult, and this issue can only be addressed in future prospective studies. We observed that the median time to CR after allogeneic HCT was 180 days (range, 100-730 days), consistent with gradual graft-versusmyeloma effects. Conceivably, patients currently in PR may ultimately achieve CR owing to these effects. Interestingly, 5 patients had CR within 9 months of allografting without any GVHD, suggesting subclinical graft-versus-host effects or other antitumor effects of this approach may control myeloma. Other patients achieved CR only after GVHD became symptomatic. The durability of the CRs observed is still unknown given that the longest follow-up is only approximately 3 years. To date, only 2 CR patients have had disease progression; however, longer follow-up is necessary.
Our approach compares favorably with other recent reports. Badros and colleagues32,60 treated 25 patients with intermediate-dose melphalan (100 mg/m2) following 1 or 2 prior autografts using matched-sibling HC transplants. Six additional patients received fludarabine/TBI and unrelated donor (URD) grafts. Overall, 58% developed acute GVHD and 61% a CR or near CR. Median OS was 15 months and better in patients who received the allograft as planned consolidation of a single autograft. Similar observations have been made by Peggs et al.61 Kröger et al62,63 have also used planned tandem auto/allografts using unrelated or mismatched related donors and conditioning with fludarabine, melphalan, and antithymocyte globulin in 17 patients. Day-100 TRM was 11%, and at a median follow-up after allografting of 13 months, the estimated 24-month OS was 74% and disease-free survival was 56%. Giralt et al64 reported on the use of reduced-intensity conditioning with fludarabine and melphalan (140-180 mg/m2) in 22 myeloma patients with advanced disease. Nine had failed prior autologous transplantation. Nonrelapse mortality was 19% at 100 days and 40% at 1 year. Survival at 2 years was estimated to be 30% with progression-free survival of 19%.
In conclusion, we have demonstrated in patients with myeloma the feasibility of combining autologous HCT after high-dose melphalan with nonmyeloablative allogeneic HCT. With no treatment-related deaths in the first 100 days after allografting, near-complete donor engraftment in all patients, and 81% responses, 52% of which were complete, this procedure appeared to have improved upon the current 20% early TRM seen in standard allogeneic HCT for younger patients with multiple myeloma, while maintaining potent antitumor effects and allowing allografting in patients up to 65 to 70 years of age. We plan to compare this approach with tandem autografting for patients without HLA-matched sibling donors in a clinical trial in the Bone Marrow Transplant-Clinical Trials Network (BMT-CTN). Patient selection may be critical as we and others have noted increased toxicity in relapsed and refractory patients. The BMT-CTN trial will enroll patients within 3 to 9 months from initiation of their first chemotherapy. Finally, based on these encouraging results, the use of autologous transplantation as cytoreduction for nonmyeloablative allografting may be an attractive approach to other malignancies such as advanced lymphoma.
Prepublished online as Blood First Edition Paper, July 10, 2003; DOI 10.1182/blood-2002-09-2955.
Supported in part by National Institutes of Health grants CA78902, CA18221, CA15704, and CA49605.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked ”advertisement” in accordance with 18 U.S.C. section 1734.
Our thanks to the nurses and staff for caring for the patients and for the research nurses, laboratory personnel, secretarial staff, and data coordinators, in particular, Debbie Bassuk, who helped capture the study information and follow-up, and Marie-Térèse Little, PhD, for chimerism data.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal