Abstract
The transcription factor Bach2, a member of the CNC family of proteins, binds to the Maf recognition element (MARE) by forming homodimers or dimerizing with small Maf transcription factors. Bach2-expressing cells show reduced proliferation and undergo spontaneous cell death. The inhibition of BCR/ABL tyrosine kinase activity by STI571 in chronic myeloid leukemia (CML) cell lines and CD34+ cells from patients with CML in lymphoid crisis results in induction of BACH2 expression. We show here that BACH2 modifies the in vitro cytotoxicity of anticancer drugs. The cytotoxic effects of commonly used anticancer agents were studied by overexpression of BACH2 in RAJI lymphoid cells, a cell line that does not express endogenous BACH2. Cell growth inhibition was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay. Clones overexpressing BACH2 were more sensitive to etoposide, doxorubicin, and cytarabine than control RAJI cells, whereas there were no significant differences in the sensitivity of either cells to methotrexate or vincristine. Interestingly, we found that the former drugs were oxidative stressors that induced the nuclear accumulation of BACH2. In contrast, methotrexate or vincristine did not induce production of intracellular reactive oxygen species (ROS) and nuclear accumulation of BACH2. These results, coupled with our previous data showing that BACH2 promotes oxidative stress-induced cell death, suggest that combination chemotherapy involving STI571 and anticancer drugs that produce ROS may be of benefit in the treatment of Philadelphia chromosome 1 (Ph1)–positive leukemia.
Introduction
About 20% to 30% of adult acute lymphoblastic leukemia (ALL) and 2% to 5% of childhood ALL show a reciprocal translocation between chromosomes 9 and 22, the so-called Philadelphia (Ph) chromosome.1 This translocation results in the head-to-tail fusion of the breakpoint cluster region (BCR) gene on chromosome 22 at band q11 with the ABL protooncogene located on chromosome 9 at band q34. In ALL cases, BCR/ABL chimeric transcripts are expressed, although different forms of the BCR/ABL protein can be generated by this translocation, depending on the breakpoint location within the BCR gene. In one third of Ph1-positive ALL cases, the P210 BCR/ABL transcript is expressed; the P190 BCR/ABL transcript is observed in the remaining Ph1-positive ALL cases.2 Both BCR/ABL fusion proteins exhibit deregulated tyrosine kinase activity compared with the native ABL protein.3 The leukemia-promoting function of this protein requires this deregulated tyrosine kinase activity.4
Despite steady improvements in the management of ALL in children, the prognosis of Ph1-positive ALL remains poor. Adult Ph1-positive ALL is also chemoresistant, and fewer than 5% of patients are cured by chemotherapy.5 Molecularly designed therapeutic approaches resulted in the development of STI571, a potent and selective inhibitor of the tyrosine kinase activity of BCR-ABL.5 STI571 is well tolerated and has significant antileukemic activity in patients with CML and Ph1-positive ALL.6,7 The overall response rate in patients with CML with lymphoid blast crisis or Ph1-positive ALL is 70%, and 20% have complete remissions. However, most patients with lymphoid blast crisis or Ph1-positive ALL relapse after a short remission period.8 The combination of STI571 with standard antileukemic agents may improve the outcome of patients with blast crisis or Ph1-positive ALL. STI571 has additive or synergetic effects when used in combination with cytarabine, doxorubicin, or etoposide,9-11 whereas it has an antagonistic effect when used with methotrexate.9 The mechanisms responsible for these additive or antagonistic effects are unknown.
A recent study showed that use of STI571 to inhibit BCR-ABL kinase activity in CML cell lines and CD34+ cells from patients with CML in lymphoid crisis induces the transcription factor BACH2.12 Bach2 is a basic-leucine zipper (bZip) protein that forms heterodimers with members of the Maf-related oncoprotein family.13 Like the erythroid transcription factor p45 erythroid nuclear factor 2 (NF-E2) and the related factors Nrf1,-2, and -3,14-18 their heterodimers with small Maf proteins (MafF, MafG, and MafK) bind to Maf recognition elements (MAREs).18 As MARE includes a 12-O-tetradecanoylphorbol-13-acetate (TPA)–responsive element (TRE), MARE can also be bound by Jun and Fos family members as well.19 The presence of numerous combinations of MARE-binding factors suggests that MARE is involved in many biologic processes and that deregulation of MARE-mediated gene expression underlies cell transformation by Maf and activated protein 1 (AP-1)–related factors. Among the transcription factors that bind to MARE, homodimers of the small Maf proteins and small Maf/Bach heterodimers are known to repress transcription (Figure 1).13,15 Thus, there appears to be a complex transcriptional antagonism that regulates MARE-dependent gene expression.
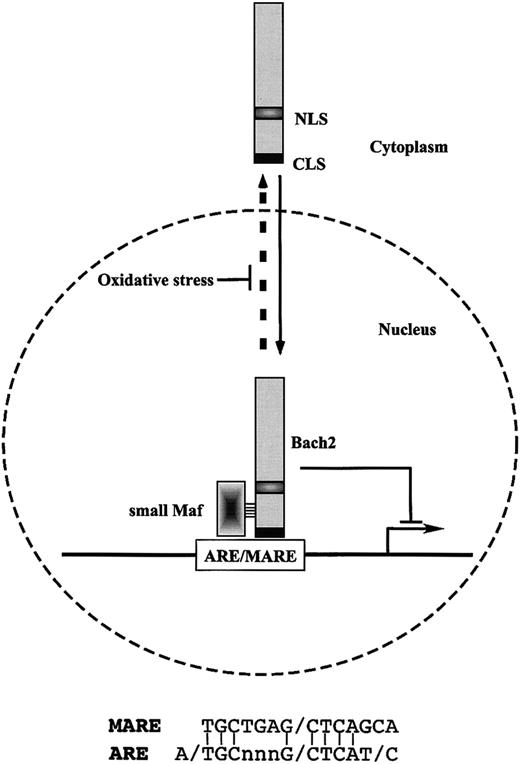
Schematic model of the regulation of inducible expression of the cellular antioxidative stress genes through Bach2 transcription factor. Bach2 possesses one nuclear localization signal (NLS) and one cytoplasmic localization signal (CLS). Bach2 is mainly localized in cytoplasm through its C-terminal evolutionarily conserved CLS. Oxidative stress induces the nuclear accumulation of Bach2 by inhibiting the CLS activity. Bach2 is a bZip protein that forms heterodimers with small Maf proteins. The heterodimers bind to MARE, which are most closely related to the antioxidant response elements (ARE) and repress transcription through ARE/MARE.
Schematic model of the regulation of inducible expression of the cellular antioxidative stress genes through Bach2 transcription factor. Bach2 possesses one nuclear localization signal (NLS) and one cytoplasmic localization signal (CLS). Bach2 is mainly localized in cytoplasm through its C-terminal evolutionarily conserved CLS. Oxidative stress induces the nuclear accumulation of Bach2 by inhibiting the CLS activity. Bach2 is a bZip protein that forms heterodimers with small Maf proteins. The heterodimers bind to MARE, which are most closely related to the antioxidant response elements (ARE) and repress transcription through ARE/MARE.
Cells possess various defense mechanisms to protect themselves against reactive oxygen species (ROS). These mechanisms are mediated by thiol-rich molecules such as glutathione and metallothioneins, detoxifying enzymes such as glutathione-S-transferase, and heme oxygenase 1, which protects cells from oxidative stress by generating a potent radical scavenger bilirubin. Inducible expression of these genes is mediated at least in part by a cis-regulatory element called the antioxidant responsive element (ARE), which is also referred to as an electrophile responsive element (EpRE). ARE is bound by various AP-1–related transcription factors in vitro. Compared with other AP-1–binding DNA sequences, the ARE is most closely related to MARE (Figure 1).20-22 Conversely, transfection of cells with reporter genes linked to MARE results in reporter gene expression on exposure to oxidative stress.23,24 Oxidative stress regulates the subcellular localization of Bach2 through oxidative stress–sensitive conditional nuclear export.24 This suggests a role for Bach2 as a biologic switch that processes and transduces an oxidative stress signal in the nucleus.
In hematopoiesis, Bach2 is expressed specifically by B cells.25,26 Forced expression of BACH2 in a human B-cell line, RAJI that does not express endogenous BACH2, results in a marked reduction in clonogenic activity.26 Furthermore, these Bach2-expressing cells underwent spontaneous cell death when a Bach2-enhanced green fluorescent protein (EGFP) bi-cistronic retrovirus was used to monitor the fate of cells at the single-cell level.27 They undergo massive apoptosis on addition of mild oxidative stress. Whereas Bach2 is predominately cytoplasmic in Bach2-overexpressing cells, oxidative stress efficiently induces its nuclear accumulation and forms nuclear foci that are associated with promyelocytic leukemia nuclear (PML) bodies.27
Here, we investigated the in vitro effects of commonly used anticancer drugs in BACH2-overexpressing RAJI lymphoid cells.26 BACH2-overexpressing clones were more sensitive to etoposide, doxorubicin, and cytarabine than control RAJI cells, whereas there were no significant differences in the sensitivity of either cells to methotrexate or vincristine. A large body of evidence indicates that some anticancer drugs may exhibit cellular toxicity in part through the generation of ROS.28 Of interest, we found that the former drugs were oxidative stressors that induced the nuclear accumulation of BACH2. In contrast, methotrexate or vincristine did not induce production of ROS and did not disturb the oxidative stress-sensitive conditional nuclear export of BACH2. These results suggest that effects of chemotherapeutic agents generating ROS and affecting localization of BACH2 may be ideally suited to synergize with STI571 through its induction of BACH2 in Ph1-positive lymphoid leukemia. These findings underline the importance of ROS productivity by anticancer drugs used in combination chemotherapy with STI571 in the treatment of BCR-ABL–positive leukemia.
Materials and methods
Reagents
STI571 was kindly provided by Novartis (Basel, Switzerland). Methotrexate (MTX), vincristine (VCR), etoposide (VP16), daunorubicin (DNR), and cytosine β-d-arabinofuranoside (Ara-C) were purchased from Sigma Chemical (St Louis, MO). All drugs were dissolved in dimethyl sulfoxide. The stock solutions of all drugs were prepared at 2 to 100 mg/mL and stored at –20°C. Appropriate drug concentrations were made by dilution with fresh medium immediately before each experiment. The final concentration of dimethyl sulfoxide in the media was less than 0.1%, and it had no effect on cell growth. When antioxidant agents were tested, they were added for the time period indicated in the text. The antioxidant agent catalase was dissolved in RPMI 1640 medium, and cells were suspended in the resulting solution. Anticancer drugs were added, and the cells were incubated at 37°C in 5% CO2 for the time periods indicated in the text.
Cell culture and cell lines
The Burkitt lymphoma cell line RAJI was provided by the Japanese Cancer Resources Bank (Tokyo). Control RAJI cell clones and clones overexpressing BACH2 were described previously.26 Cells were grown in RPMI 1640 medium containing 10% heat-inactivated fetal calf serum (FCS) at 37°C in 5% CO2. Exponentially growing cells were harvested by centrifugation and resuspended in fresh medium to achieve a culture density of 1 × 105 cells/mL. Drugs (MTX, VCR, VP16, DNR, and Ara-C) at the indicated concentrations were added the following day.
MTT assay
Viable cell growth was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay as described previously.29 For the background control (RPMI 1640 + 10% FCS), control (no drug), and each drug, the 4 intermediate data values were used for the analysis. Briefly, 0.1 mL cell cultures (1 × 104 cells) per well were seeded in 96-well plates 1 day before the experiments. MTT (5 mg/mL, 10 μL/well; Chemicon, Temecula, CA) was added to each well and incubated at 37°C for 4 hours. Cells were then lysed by adding 0.1 mL lysis solution (0.04 N HCl in isopropanol) per well. Absorbance was measured at 570 nm by using a microplate reader and reported as optical density (OD). The viability is calculated as OD with drug/OD without drug.
FACS analysis
Cells (2 × 105/well) were plated into 12-well collagen-coated plates 1 day before the experiments. On the day of the experiments, the medium was removed, and the plated cells were washed with phosphate-buffered saline (PBS) (–) and then stained with 2.5 μg/mL propidium iodide (PI; Sigma) in PBS. Cells were sorted using a fluorescence cell sorter (FACS) (Calibur; Becton Dickinson, Franklin Lakes, NJ). The collected data were analyzed by CellQuest software (Becton Dickinson).
Determination of intracellular ROS production
The production of hydrogen peroxide and superoxide anion, respectively, was monitored by flow cytometry using 2′, 7′-dichlorodihydrofluorescein diacetate (DCFH-DA) dyes (Sigma Chemical). Cells (2 × 105/well) were plated into 12-well collagen-coated plates 1 day prior to the experiments. The following day the cells were incubated with 10 μM DCFH-DA in 5% CO2 at 37°C for 30 minutes. After separation from the medium by centrifugation, the cells were washed with PBS (–), and cell fluorescence at 4°C was assessed by determination of the fluorescent signal as detected by flow cytometry laser (488 nm wavelength).
Immunofluorescence staining
RAJI cells were fixed with 4% paraformaldehyde in 1 × PBS. Thereafter cells were permeabilized with 0.5% Triton X-100/PBS for 5 minutes. To detect BACH2, the fixed cells were incubated with rabbit anti-Bach2 antiserum (F69-1) diluted at 1:500 in 1% bovine serum albumin (BSA)/PBS. AlexaFluor488-conjugated goat antirabbit immunoglobulin G (IgG) antibody (Molecular Probes, Eugene, OR) diluted in 1% BSA/1 × PBS was used as secondary antibodies. All antibody incubations were performed at 37°C for 30 minutes. Nuclei were stained with 10 μM Hoechst 33342.
Results
BACH2 transcription factor modifies the cytotoxic effects of anticancer drugs
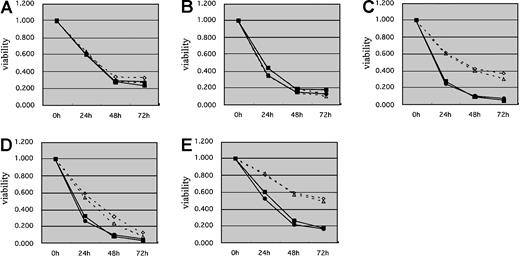
To investigate the effects of the transcription factor BACH2 on chemotherapy-induced cell death, we compared the sensitivities of 2 RAJI clones that overexpress BACH2 (no. 67 and no. 75) and 2 control RAJI clones (pDL2 and pDL3) to commonly used anticancer drugs with the use of the MTT assay. The results showed that BACH2-overexpressing cells had a significant decrease in viability compared with control cells after treatment with VP16 (Figure 2C), DNR (Figure 2D), or Ara-C (Figure 2E) for 24 or 48 hours. However, there were no significant differences in cell viability with MTX (Figure 2A) or VCR (Figure 2B) treatment. To confirm these results, we examined the sensitivities of these cell lines to anticancer drugs with the use of flow cytometric analysis. After treatment of cells with these drugs for the appropriate times, the cells were stained with PI, and the percentages of dead cells were measured by FACS. The percentages of PI-stained dead cells in BACH2-expressing and control cell lines were almost equal after treatment with MTX (Figure 3A) or VCR (Figure 3B). In contrast, the percentages of PI-stained dead cells in BACH2-expressing clones was significantly increased versus control clone cells after treatment with VP16 (Figure 3C), DNR (Figure 3D), or Ara-C (Figure 3E). These results were consistent with those obtained with the MTT assay.
Cells overexpressing BACH2 have increased sensitivity to antileukemic agents. BACH2-overexpressing (no. 67, no. 75) and control (pDL2, pDL3) RAJI clones were seeded (1 × 105/mL) in 96-well plates 1 day prior to experiments. Cells were treated with 0.02 μg/mL MTX (A), 0.01 μg/mL VCR (B), 2 μg/mL VP16 (C), 0.2 μg/mL DNR (D), or 0.1 μg/mL Ara-C (E). After incubation for 24, 48, and 72 hours, MTT (5 mg/mL, 10 μL/well) was added to each well, and the absorbance at 570 nm was measured with the use of a microplate reader. The results are representative of 3 separate experiments. ⋄ indicates PDL2; ▵, PDL3; ▪, no. 67; and •, no. 75.
Cells overexpressing BACH2 have increased sensitivity to antileukemic agents. BACH2-overexpressing (no. 67, no. 75) and control (pDL2, pDL3) RAJI clones were seeded (1 × 105/mL) in 96-well plates 1 day prior to experiments. Cells were treated with 0.02 μg/mL MTX (A), 0.01 μg/mL VCR (B), 2 μg/mL VP16 (C), 0.2 μg/mL DNR (D), or 0.1 μg/mL Ara-C (E). After incubation for 24, 48, and 72 hours, MTT (5 mg/mL, 10 μL/well) was added to each well, and the absorbance at 570 nm was measured with the use of a microplate reader. The results are representative of 3 separate experiments. ⋄ indicates PDL2; ▵, PDL3; ▪, no. 67; and •, no. 75.
Overexpression of BACH2 enhances cell sensitivity to antileukemic agents. BACH2-overexpressing (no. 67, gray) and control (pDL3, black) RAJI clones were seeded (2 × 105/mL) into 12-well plates and treated with 0.02 μg/mL MTX (A), 0.01 μg/mL VCR (B), 2 μg/mL VP16 (C), 0.2 μg/mL DNR (D), or 0.1 μg/mL Ara-C (E) for 24 hours. After the treatment, cells were stained with PI and analyzed by flow cytometry. The percentage of control (upper bars) and BACH2-overexpressing RAJI cells (lower bars) staining positive with PI (dead cells) after treated with drug was determined. Control cells are indicated by open area, BACH2-overexpressing RAJI cells by filled area. The results are representative of 3 separate experiments.
Overexpression of BACH2 enhances cell sensitivity to antileukemic agents. BACH2-overexpressing (no. 67, gray) and control (pDL3, black) RAJI clones were seeded (2 × 105/mL) into 12-well plates and treated with 0.02 μg/mL MTX (A), 0.01 μg/mL VCR (B), 2 μg/mL VP16 (C), 0.2 μg/mL DNR (D), or 0.1 μg/mL Ara-C (E) for 24 hours. After the treatment, cells were stained with PI and analyzed by flow cytometry. The percentage of control (upper bars) and BACH2-overexpressing RAJI cells (lower bars) staining positive with PI (dead cells) after treated with drug was determined. Control cells are indicated by open area, BACH2-overexpressing RAJI cells by filled area. The results are representative of 3 separate experiments.
Determination of intracellular ROS production by commonly used anticancer drugs
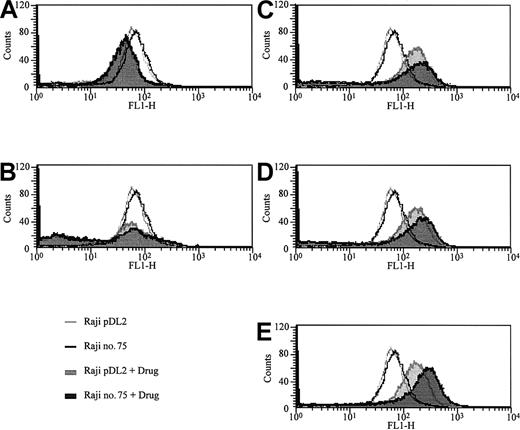
To understand the mechanisms responsible for the observed differences in sensitivity of these cells to some anticancer drugs, we examined the effects of anticancer drugs on generation of intracellular oxidants by measuring the extent of oxidation of a redox-sensitive dye DCFH-DA. DCFH-DA is a sensitive fluorometric probe of production of oxidative stress in living cells.30,31 DCFH-DA enters the cell and is deacetylated by esterases to DCFH, which, because of the negative charge, remains enclosed within the cell. When DCFH is oxidized by ROS, it is transformed into the highly fluorescent dye 2′,7′-dichlorofluorescein (DCF). Figure 4 is a representative picture of DCF fluorescence in RAJI cells following treatment by various antileukemic agents. It is obvious that VP16 (Figure 4C), DNR (Figure 4D), and Ara-C (Figure 4E) induced ROS production in the cells, whereas VCR did not (Figure 4B). Of interest, MTX decreased intracellular ROS production (Figure 4A).
Measurement of intracellular ROS production using a redox-sensitive dye and FACS. BACH2-overexpressing (no. 75) and control (pDL2) RAJI clones were seeded (2 × 105/mL) into 12-well plates and treated with 0.02 μg/mL MTX (A), 0.01 μg/mL VCR (B), 2 μg/mL VP16 (C), 0.2 μg/mL DNR (D), or 0.1 μg/mL Ara-C (E) for 24 hours. After treatment, the cells were stained with DCFH-DA and analyzed by flow cytometry as described in “Materials and methods.” Control cells are indicated by open area, and treated cells by filled area. The results are representative of 3 separate experiments.
Measurement of intracellular ROS production using a redox-sensitive dye and FACS. BACH2-overexpressing (no. 75) and control (pDL2) RAJI clones were seeded (2 × 105/mL) into 12-well plates and treated with 0.02 μg/mL MTX (A), 0.01 μg/mL VCR (B), 2 μg/mL VP16 (C), 0.2 μg/mL DNR (D), or 0.1 μg/mL Ara-C (E) for 24 hours. After treatment, the cells were stained with DCFH-DA and analyzed by flow cytometry as described in “Materials and methods.” Control cells are indicated by open area, and treated cells by filled area. The results are representative of 3 separate experiments.
Antioxidant treatment inhibits the effects of BACH2 on chemotherapy-induced cell death
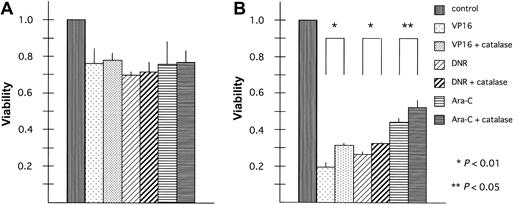
To evaluate whether ROS are involved in the increased cytotoxicity of anticancer drugs in BACH2-overexpressing cells, BACH2-overexpressing (no. 67) and control (pDL2) cell lines were treated with anticancer drugs alone or in combination with the antioxidant compound, catalase, which stimulates dismutation of H2O2.32 The results of experiments carried out using 50 U/mL catalase with VP16, DNR, or Ara-C are shown in Figure 5. Cells were pretreated for 1 hour with catalase to provide time for uptake of the compound into the cells. Catalase treatment did not affect chemotherapy-induced cytotoxicity in control cells (Figure 5A). However, it had significant effects when administered to the BACH2-overexpressing clone, (Figure 5B) (VP16, P < .01; DNR, P < .01; Ara-C, P < .05).
Antioxidant treatment inhibits BACH2 promotion of chemotherapy-induced cell death. Control (pDL2; panel A) and BACH2-overexpressing (no. 67; panel B) RAJI clones were seeded (1 × 105/mL) in 96-well plates 1 day before the experiments. Cells were treated with catalase 50 U/mL for 1 hour. Then 2 μg/mL VP16, 0.2 μg/mL DNR, or 0.1 μg/mL Ara-C was added to the suspension, and the cells were incubated at 37°C in 5% CO2 for 40 hours. After incubation, MTT (5 mg/mL, 10 μL/well) was added to each well, and the absorbance was measured. A statistically significant difference in cell viability was observed (*P < .01 or **P < .05). The results are representative of 3 separate experiments. Error bars represent standard deviation.
Antioxidant treatment inhibits BACH2 promotion of chemotherapy-induced cell death. Control (pDL2; panel A) and BACH2-overexpressing (no. 67; panel B) RAJI clones were seeded (1 × 105/mL) in 96-well plates 1 day before the experiments. Cells were treated with catalase 50 U/mL for 1 hour. Then 2 μg/mL VP16, 0.2 μg/mL DNR, or 0.1 μg/mL Ara-C was added to the suspension, and the cells were incubated at 37°C in 5% CO2 for 40 hours. After incubation, MTT (5 mg/mL, 10 μL/well) was added to each well, and the absorbance was measured. A statistically significant difference in cell viability was observed (*P < .01 or **P < .05). The results are representative of 3 separate experiments. Error bars represent standard deviation.
Antileukemic agents that generate intracellular ROS induce the nuclear accumulation of BACH2
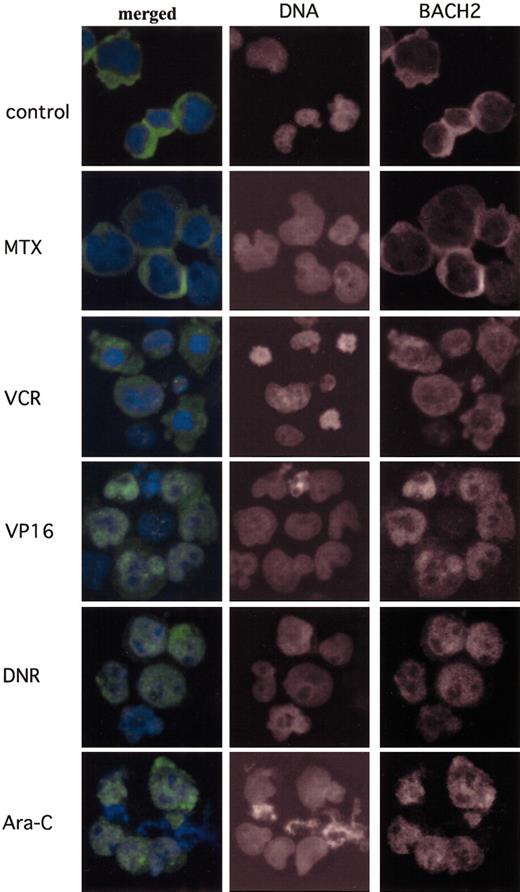
In further investigations of the mechanisms responsible for the increased cytotoxicity of antileukemic agents in BACH2-overexpressing cells we examined the subcellular localization of BACH2 on exposure to these drugs. As shown in Figure 6A, BACH2 is mainly located in the cytoplasm in the absence of these drugs. Within 24 hours of treatment of the cells with VP16 (Figure 6D), DNR (Figure 6E), or Ara-C (Figure 6F), BACH2 was translocated from the cytoplasm into nuclei. However, neither MTX (Figure 6B) nor VCR (Figure 6C) induced nuclear accumulation of BACH2 despite treatment for up to 36 hours.
Antileukemic agents that generate intracellular ROS induce the nuclear accumulation of BACH2. BACH2-overexpressing RAJI cells (no. 67) were treated with drugs at the indicated concentrations—control, not treated; MTX, 0.02 μg/mL; VCR, 0.01 μg/mL; VP16, 2 μg/mL; DNR, 0.2 μg/mL; and Ara-C, 0.1 μg/mL—a day after culture. Cells were fixed and then stained for Bach2 proteins and for DNA. The merged images show BACH2 (green) and DNA (blue). Original magnification, × 400.
Antileukemic agents that generate intracellular ROS induce the nuclear accumulation of BACH2. BACH2-overexpressing RAJI cells (no. 67) were treated with drugs at the indicated concentrations—control, not treated; MTX, 0.02 μg/mL; VCR, 0.01 μg/mL; VP16, 2 μg/mL; DNR, 0.2 μg/mL; and Ara-C, 0.1 μg/mL—a day after culture. Cells were fixed and then stained for Bach2 proteins and for DNA. The merged images show BACH2 (green) and DNA (blue). Original magnification, × 400.
Discussion
Here, we have shown that expression of BACH2 modifies the in vitro cytotoxicity of various anticancer drugs. BACH2-overexpressing cells were more sensitive to VP16, DNR, or Ara-C than cells with no endogenous expression of BACH2, whereas there were no significant differences in the sensitivity of these cells to MTX or VCR. We found that VP16, DNR, or Ara-C generated ROS, whereas VCR had no effect on ROS production, and MTX reduced ROS production. The former drugs induced nuclear accumulation of BACH2. These results, coupled with our previous data that BACH2 promotes oxidative stress-induced cell death,27 suggest that ROS induction by anticancer drugs may play an important role in the effects of combination chemotherapy with STI571 for Ph1-positive leukemia.
Bach2 is localized in the cytoplasm through its C-terminal CLS. The CLS directs leptomycin B-sensitive and Crm1/Exportin1-dependent nuclear export. However, CLS is distinct from the conventional leucine-rich class of nuclear export signal in that oxidative stress aborts CLS activity and induces the nuclear accumulation of Bach2.21 Our present data demonstrate that there is a close correlation between the nuclear accumulation of BACH2 and production of intracellular oxidants as measured by flow cytometry by using the oxidant sensitive dye DCFH-DA. Therefore, the examination of the effect of anticancer drugs on subcellular localization of BACH2 is a very sensitive and novel method for detection of the ROS productivity.
In the studies presented here, we found that BACH2-overexpressing cells showed increased sensitivity to 3 different drugs. Each drug represents the different class of chemotherapeutic agents: a topoisomerase II inhibitor (VP16), an anthracycline (DNR), and a metabolic inhibitor (Ara-C). Thus, the drug sensitivity was modified by expression of BACH2 independently of the mechanism of action of the drug. Ara-C is an arabinose nucleoside analog of deoxycytidine and remains one of the most effective drugs used in the treatment of acute leukemia and other hematologic malignancies.33 After intracellular activation, Ara-C interferes with DNA replication and repair through inhibition of DNA polymerase and incorporation into DNA. Although a report indicated that ROS generation by Ara-C was partially responsible for Ara-C–induced toxicity and cancer cell apoptosis,34 the mechanism of ROS generation still remains unknown. However, it is well established that the anthracyclines, DNR, doxorubicin, and idarubicin, induce intracellular oxidative stress.35 These agents are intercalators and induced topoisomerase-mediated single- and double-stranded breaks in DNA. They can undergo chemical reduction through several enzymatically catalyzed or ion-catalyzed pathways, yielding highly reactive free radical intermediates. Transfer of an electron from these unstable radicals to molecular oxygen yields hydroxyl radicals, compounds that can cause oxidative damage to cellular macromolecules. Here, we observed that Ara-C–treated cells had similar levels of ROS production as DNR-treated cells, indicating that Ara-C is also a strong oxidative stressor. An equivalent level of ROS was also detected in VP-16–treated cells. Our data suggest that ROS generated by each drug may be responsible for the increased drug sensitivity in BACH2-overexpressing cells despite the different mechanism of action.
MTX is the most widely used antimetabolite in pediatric oncology and a tight-binding inhibitor of dihydrofolate reductase (DHFR), enzyme responsible for converting folates to their active, chemically reduced (tetrahydrofolate) form. MTX is also widely used as antirheumatic drug. Of interest, MTX suppresses the interleukin-6–induced generation of ROS in synovial cells from patients with rheumatoid arthritis36 and inhibits the production of H2O2 and NO by articular chondrocytes in vitro.37 Consistent with these results, we found that treatment of RAJI cells with MTX decreased ROS production. Because antioxidant treatment partially prevented BACH2 promotion of chemotherapy-induced cell death (Figure 5), suppression of ROS production by MTX may contribute to the antagonistic effects of STI571 in Ph1-positive leukemia cells.
Overexpression of BACH2, coupled with its nuclear localization by ROS, leads to increased chemosensitivity measured by viability of the lymphoid cell line. Increase in ROS seems part of cytotoxic effect of a broad group, but not all, of standard chemotherapeutic agents used for lymphoid leukemias. Effects of chemotherapeutic agents generating ROS and affecting localization of BACH2 may be ideally suited to synergize with STI571 through its induction of BACH2 in Ph1-positive lymphoid leukemia.
Prepublished online as Blood First Edition Paper, June 26, 2003; DOI 10.1182/blood-2002-12-3656.
Supported by Grants-in-Aid for Scientific Research, Grants-in-Aid for Scientific Research on Priority Areas, and a Grant-in-Aid for Encouragement of Young Scientists from the Ministry of Education, Science, Sports and Culture, and grants from Uehara Memorial Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal