Abstract
The transcription factor C/EBPα (CCAAT/enhancer binding protein α) is critical for granulopoiesis. Gene disruption in mice blocks early granulocyte differentiation and disruption of C/EBPα function has been implicated in human acute myeloid leukemia (AML), but no systematic structure-function analysis has been undertaken to identify the mechanisms involved in C/EBPα-mediated granulocyte differentiation. Here we demonstrate that loss of either of 2 key regions results in disruption of C/EBPα granulocytic development: the amino terminus and specific residues residing on the non-DNA binding face of the basic region. Mutation of either results in loss of C/EBPα inhibition of E2F and down-regulation of c-Myc, but only mutation of the basic region results in loss of physical interaction with E2F. In contrast, while the amino terminal mutant retains the ability to interact with E2F, this mutant fails to bind a C/EBPα site efficiently, fails to activate C/EBPα target genes, and is also defective in inhibition of E2F activity. These results further emphasize the importance of inhibition of proliferative pathways in granulopoiesis and demonstrate that several regions of the C/EBPα protein are involved in this mechanism.
Introduction
Recent studies have emphasized the importance of transcription factors in cell differentiation. One such factor is CCAAT/enhancer binding protein α (C/EBPα). C/EBPα is expressed in a number of different tissues, but in the hematopoietic system it is expressed in stem and myeloid progenitor cells and up-regulated with granulocytic differentiation and is not expressed in other blood lineages.1-3 Consistent with this specific pattern of expression is its critical role in granulopoiesis supported by studies demonstrating that disruption of the C/EBPα gene in mice results in a specific loss of granulocyte development and the accumulation of early myeloid blasts in the fetal liver.4 In addition, expression of C/EBPα in bipotential myeloid cells promotes granulocytic and blocks monocytic differentiation.2,5 Finally, consistent with its role in promoting granulopoiesis, a number of studies have implicated mutation and/or loss of C/EBPα expression as contributing to the pathogenesis of acute myeloid leukemia (AML), a disease characterized by an early block in granulopoiesis.6-9
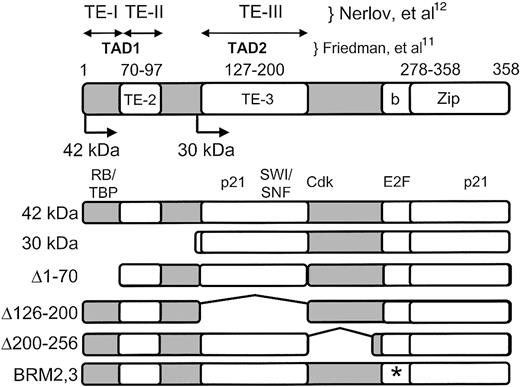
A number of studies have investigated the function of the domains of C/EBPα in an effort to understand its mechanism of action. C/EBPα was the first transcription factor described to have a basic-leucine zipper (bZip) domain, a characteristic shared by several families of transcription factors.10 The carboxyl-terminal leucine zipper forms a domain mediating homo- and heterodimerization with other members of the C/EBP family. Adjacent to the leucine zipper, in the direction of the amino terminus, is a basic region essential for DNA binding activity. Early studies also defined a number of transactivation domains, largely based on transactivation studies using nonmyeloid cells (Figure 1).11,12 In addition, a domain that interacts with the SWI/SNF chromatin remodeling complex that is critical for adipogenesis was described.13
C/EBPα functional and protein interaction domains. Above the diagram, the amino terminal transactivation domains are marked, defined by 2 previous studies: TE-I, TE-II, and TE-III12 ; and TAD1 and TAD2.11 Numbers directly above the diagram indicate the amino acids corresponding to TE-II (70-97), TE-III (127-200), and the basic-zipper domain (bZip; amino acids 278-358 of the rat C/EBPα protein10 ). Also shown are the positions of the ATG start translation site for the 42-kDa wild-type C/EBPα peptide as well as the start ATG encoding the 30-kDa peptide (at amino acid 120). Below the diagram are the locations of interaction of the retinoblastoma protein (“RB,” amino acids 68-8350 ); the cell cycle inhibitor p21 (“p21,” amino acids 119-226 and the leucine zipper, amino acids 313-36051 ); chromatin remodeling proteins (“SWI/SNF,” amino acids 126-200, corresponding to TE-III13 ); cyclin dependent kinases (“Cdk,” described in one study as amino acids 175-188,35 and in another study as amino acids 119-160 and 280-31351 ); and E2F proteins (“E2F,” amino acids 294 and 297, this study). Shown below are the 42-kDa wild-type and mutant C/EBPα proteins used in this study. * Indicates the location of the 2 point mutations in each BRM mutant (BRM-2: Ile294Ala, Arg297Ala; BRM-3: Asp301Ala, Lys304Ala21 ).
C/EBPα functional and protein interaction domains. Above the diagram, the amino terminal transactivation domains are marked, defined by 2 previous studies: TE-I, TE-II, and TE-III12 ; and TAD1 and TAD2.11 Numbers directly above the diagram indicate the amino acids corresponding to TE-II (70-97), TE-III (127-200), and the basic-zipper domain (bZip; amino acids 278-358 of the rat C/EBPα protein10 ). Also shown are the positions of the ATG start translation site for the 42-kDa wild-type C/EBPα peptide as well as the start ATG encoding the 30-kDa peptide (at amino acid 120). Below the diagram are the locations of interaction of the retinoblastoma protein (“RB,” amino acids 68-8350 ); the cell cycle inhibitor p21 (“p21,” amino acids 119-226 and the leucine zipper, amino acids 313-36051 ); chromatin remodeling proteins (“SWI/SNF,” amino acids 126-200, corresponding to TE-III13 ); cyclin dependent kinases (“Cdk,” described in one study as amino acids 175-188,35 and in another study as amino acids 119-160 and 280-31351 ); and E2F proteins (“E2F,” amino acids 294 and 297, this study). Shown below are the 42-kDa wild-type and mutant C/EBPα proteins used in this study. * Indicates the location of the 2 point mutations in each BRM mutant (BRM-2: Ile294Ala, Arg297Ala; BRM-3: Asp301Ala, Lys304Ala21 ).
In the hematopoietic system, a number of C/EBPα target genes have been described, including a number of primary granule protein genes.14,15 C/EBPα was also shown to regulate the genes encoding the receptors for the granulocytic growth factors granulocyte colony-stimulating factor (G-CSF) and interleukin 6 (IL-6).4,16,17 However, knockout studies demonstrated that these growth factor receptors were not the critical granulocytic target genes, since disruption of one or both growth factor pathways failed to recapitulate the absolute block in granulocyte development observed in C/EBPα knockout mice.18,19 Instead, several recent studies pointed to the importance of inhibition of E2F activity, as well as down-regulation of the E2F target gene c-Myc.20,21 Both representational difference analysis (RDA) and microarray analysis demonstrated that c-Myc RNA and protein were rapidly down-regulated following induction of C/EBPα and that enforced expression of c-Myc under an inducible promoter that could not be down-regulated by C/EBPα resulted in loss of C/EBPα-mediated granulocytic differentiation in cell lines, as well as induction of AML in vivo in mice.20,22 Additional studies indicated that the down-regulation of c-Myc was mediated by inhibition of E2F activity, thus preventing activation of the c-Myc promoter by E2F. While C/EBPα was shown to physically interact with E2F, it did not block the ability of E2F to bind to DNA but instead inhibited E2F activation function.20
These studies demonstrating the importance of inhibition of E2F and down-regulation of c-Myc were proven in a genetic model through the utilization of mutations in the basic region that altered amino acid residues on the non-DNA binding face of the protein.21 C/EBPα proteins with these mutations (basic region mutants [BRM]) failed to inhibit E2F activity in transfection assays. More significantly, when knocked into the murine C/EBPα locus, BRM mutations failed to support granulocytic differentiation in vivo, as was the case in C/EBPα knockout mice.21
Consistent with the role of C/EBPα in granulopoiesis are a number of recent studies that indicated that it is down-regulated and mutated in a number of different types of human AML and the myeloid blast crisis phase of chronic myeloid leukemia (CML).6-9,23-26 In addition to mutations of the carboxyl terminal DNA binding domain, approximately 50% of patients harbor small deletions of the amino terminus that result in loss of the full-length 42-kDa form of C/EBPα and enhancement of the shorter 30-kDa form whose translation is initiated from a downstream in-frame translational initiation site.6,9 This 30-kDa peptide has lost the amino terminal transactivation domains TE-I and TE-II but retains the basic-zipper DNA binding domain, binds DNA poorly relative to the 42-kDa form, and acts as a dominant-negative to block the function of the wild-type allele.6
The results of the studies cited above6,7,26 demonstrate the importance of understanding the mechanisms involved in C/EBPα-mediated granulopoiesis. Therefore, we undertook a study to define the regions of C/EBPα critical for granulopoiesis. Our results point to 2 critical regions: one in the amino terminus and a second in the BRM domain. Mutation of either one has a major effect on C/EBPα-mediated granulopoiesis through different mechanisms.
Materials and methods
Wild-type and mutant C/EBPα proteins
The 42-kDa (“wild-type”) pBabe-C/EBPα–estrogen receptor (pBabe-C/EBPα-ER) construct was provided by Alan Friedman.5 For 30-kDa–ER pBabe, a 30-kDa insert flanked by BamHI restriction sites was synthesized by polymerase chain reaction (PCR; 5′ primer: GGGGATCCGCCACCATGTCCGCGGGGGCGCAC and 3′ primer: ATGGATCCGGCGCGCAGTTG) and subsequently cloned into pBabe-ER digested with BamHI with the 30-kDa C/EBPα peptide in frame with the c-terminal ER ligand binding domain. C/EBPα deletions Δ1-70, Δ126-200, Δ200-256, and basic region mutant constructs BRM2 and BRM3 in the pBabe retroviral vector without the ER moiety were previously described.12,21 All constructs were cloned into the pBabe-ER construct in 2 steps. First, an NcoI fragment of the pBabe constructs was ligated into a pUC vector containing a wild-type C/EBPα cDNA missing an internal NcoI fragment. Next a BamHI fragment from the pUC clones was released and cloned into pBabe-ER restricted with BamHI so that resulting constructs contained C/EBPα mutant sequences in frame with the ER ligand binding domain on the c-terminus.
Generation of stable K562 cell lines, induction of granulocytic development, and assessment by Wright-Giemsa staining and NBT assay
K562 cells (1 × 107) were electroporated in a Gene Pulser apparatus (BioRad, Melville, NY) with 5 μg of ScaI-linearized plasmid at 220 V, 960 μF in 0.4-cm cuvettes (Molecular BioProducts, San Diego, CA) and plated on 96-well plates at 0.5 cells/well in phenol red–free RPMI/10% charcoal-stripped fetal bovine serum (FBS). Selection with 1 μg/mL puromycin began 48 hours after transfection. C/EBPα-ER nuclear translocation was induced by addition of 1 μM β-estradiol from a 1 mM stock in 100% ethanol (Sigma, St Louis, MO; catalog no. E-2257). To generate the 30-kDa and other mutant C/EBPα lines, K562 cells were stably transfected by electroporation with the pBabe–C/EBPα-ER fusion constructs after linearization with ScaI. Multiple individual clones for each mutant were isolated and analyzed by Western blot to confirm expression of the fusion protein and by immunofluorescence to ensure that the ER fusion protein was functional, localizing from the cytoplasm to the nucleus after β-estradiol treatment.7 The results from 2 representative clones are presented in the article. K562–C/EBPα-ER lines were grown in phenol red–free RPMI medium (Gibco-BRL, Grand Island, NY) supplemented with 10% FBS and 1 μg/mL puromycin. Nitroblue blue tetrazolium (NBT) analysis was performed using 5 × 105 cells incubated in a 1-mL solution containing phosphate-buffered saline (PBS), NBT (Sigma), and 0.33 μM phorbol myristate acetate (PMA) for 20 minutes at 37°C. The reaction was then stopped by incubation on ice. Cells were immediately fixed on slides by cytocentrifugation and counterstained with 0.5% safranin in 20% ethanol. The percentage of NBT-positive cells was quantitated by counting at least 100 cells for each mutant.
Northern blot analysis of c-Myc, C/EBPϵ, and G-CSF receptor RNA
Cell lines were treated with 1 μM β-estradiol and RNA isolated by Tri Reagent (Molecular Research Center, Cincinnati, OH). Ten micrograms of RNA was analyzed by Northern blotting as described previously15 and hybridized to a human c-Myc probe consisting of a 305-bp XbaI/EcoRI cDNA fragment,20 a C/EBPϵ probe (0.5-kb PstI fragment),2,27 a G-CSF receptor probe (0.72-kb SacII/NdeI fragment),4 and a glyceraldehyde-3-phosphate dehydrogenase (GAPDH) probe (a 1.5-kb PstI fragment)28 labeled with [α32P]-dCTP (deoxycytosine triphosphosphate).29 Northern blots were stripped between hybridizations by incubation in a solution containing 0.1 × SSC (0.15 M NaCl2 plus 0.015 M sodium citrate)/0.5% sodium dodecyl sulfate (SDS) starting at 100°C with shaking until the solution cooled to room temperature (20 minutes).
Electromobility mobility shift (EMSA)
Nuclear extracts were prepared as described.30 Briefly, 2-5 × 107 cells were washed once in PBS; resuspended in equal volume of ice-cold hypotonic buffer A (10 mM HEPES-KOH [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid–KOH], pH 7.9), 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol (DTT), and 0.5 mM phenylmethylsulfonyl fluoride (PMSF); and incubated on ice for 15 minutes. Cells were then lysed by 5 to 10 passages through a 23- to 26-gauge needle attached to a 1-mL syringe. Nuclei were harvested by 20-second centrifugation at 16 000g, and nuclear proteins were extracted by addition of 2:3 cell pellet volume of high-salt buffer C (20 mM HEPES-KOH, pH 7.9; 25% glycerol; 420 mM NaCl; 1.5 mM MgCl2; 0.2 mM EDTA (ethylenediaminetetraacetic acid); 0.5 mM DTT; and 0.5 mM PMSF). Nuclear extracts were recovered by centrifugation at 4°C, 16 000g, for 5 minutes. Total protein was assayed using the BioRad kit. Complementary oligonucleotides were annealed and labeled at the 5′ ends using [32P]ATP (adenosine triphosphate) (6000 Ci/mmol [2.22 × 1013 Bq]; Amersham, Arlington Heights, IL) and T4 polynucleotide kinase (NEB, Beverly, MA) and separated from unincorporated nucleotide by passage through a Sephadex G–25 column (Boehringer-Mannheim, Mannheim, Germany). EMSAs were performed by incubating 10-μg nuclear extracts with 50 000 counts per minute (cpm) double-stranded oligonucleotide in a 20-μL reaction mixture containing 10 mM HEPES-KOH buffer (pH 7.9), 50 mM KCl, 2.5 mM MgCl2, 1 mM DTT, 10% glycerol, 1 μg acetylated bovine serum albumin (NEB), and 0.5 μg poly(dI-dC) at 25°C for 20 minutes. For the supershift assay, 1 μL polyclonal anti-C/EBPα antibody (sc-61X; Santa Cruz Biotechnology, Santa Cruz, CA) was added to the binding reaction. Binding reactions were resolved on a 4% nondenaturing polyacrylamide gel containing 1 × TBE (0.089 M Tris borate, 0.089 M boric acid, and 0.002 M EDTA) and electrophoresed at 150 V at 4°C. Oligonucleotides used in EMSA were derived from the G-CSF receptor promoter (bp –57 to –38; with C/EBP binding site underlined): upper strand, 5′-AAGGTGTTGCAATCCCCAGC-3′; lower strand, 5′-GCTGGGGATTGCAACACCTT-3′.
Western blots of transfected K562 extracts used in EMSA were performed as described in “Coimmunoprecipitation of C/EBPα and E2F proteins,” using a rabbit anti-estrogen receptor antibody diluted 1:1000 (HC-20; Santa Cruz Biotechnology) and goat antirabbit immunoglobulin G–horseradish peroxidase (IgG-HRP) at 1:5000 dilution. Specific C/EBPα-ER fusion protein bands were quantitated with ImageQuant software (Molecular Dynamics, Sunnyvale, CA).
Cell growth and apoptosis assays
Cells were grown in 24-well plates in phenol red–free RPMI 1640 (Gibco-BRL, Gaithersburg, MD), 10% charcoal dextran–stripped FBS (Gemini Bio-Products, Woodland, CA), and 0.5 μg/mL puromycin, beginning at a density of 4 × 104 cells/mL. Two clones for each C/EBPα-ER construct were used. One-micromolar β-estradiol was added from a 2-mM stock solution in 100% ethanol to induce C/EBPα-ER nuclear translocation and a corresponding amount of ethanol (0.05%) to mock-treated cells as controls. Viable cells excluding trypan blue were enumerated every day for 4 days using a hemocytometer (Hausser Scientific, Horsham, PA).
The percentage of apoptotic cells was evaluated on day 4 by using an Annexin-V–FLUOS Staining Kit (Roche Diagnostic, Indianapolis, IN; catalog no. 1-988-549). Cells (3 × 105) were washed in PBS and resuspended in 100 uL Annexin-V–FLUOS labeling solution containing 0.2 uL Annexin-V–fluorescein and 2 uL propidium iodide (PI). After 15 minutes incubation at room temperature, cells were analyzed by flow cytometry (Cytomics FC500; Beckman Coulter, Hialeah, FL) counting 10 000 events per clone. Annexin-V binds cells that express phosphatidylserine on the outer layer of the cell membrane and PI stains cellular DNA of those cells with a compromised cell membrane. Thus, apoptotic cells (stained only with Annexin-V) were discriminated from live cells (unstained with either fluorochrome) and necrotic cells (stained with both Annexin and PI).
Coimmunoprecipitation of C/EBPα and E2F proteins
Coimmunoprecipitation binding assays were performed as described previously.20 Briefly, 2 × 107 cells were treated with β-estradiol to translocate ER fusion proteins. Cell lysates from equal numbers of cells were harvested at 24 hours following β-estradiol treatment by lysing cells in 200 uL lysis buffer (50 mM NACl2; 150 mM Tris [pH 7.6], 0.1% NP-40, 1 mM PMSF, and 10 μM aprotinin and leupeptin). One twentieth the amount of lysate was used in Western analyses without immunoprecipitation as a control for protein expression. Supernatants were precleared with 50 uL of a 1:1 slurry of protein A–agarose (Santa Cruz Biotechnology) in lysis buffer with 6 μg normal rabbit serum (NRS). The precleared supernatants were recovered and incubated with 12 μg of either C/EBPα antiserum (sc-61X; Santa Cruz Biotechnology) or NRS antiserum (as a control; Santa Cruz Biotechnology) and 50 uL slurry of protein A–agarose overnight at 4°C. The bound protein–protein A complexes were washed twice with lysis buffer and once with wash buffer. The resulting pellet was resuspended in 30 uL of 2 × protein-loading buffer. Bound complexes were released by heating to 95°C for 5 minutes resolved on a 10% SDS–polyacrylamide gel and analyzed by Western analysis. To detect E2F2 or E2F4 protein, membranes were hybridized with a 1:250 dilution of E2F2 or E2F4 antibody (sc-633x and sc-1082x, respectively; Santa Cruz Biotechnology) followed by a 1:5000 dilution of antigoat or antimouse IgG antibody conjugated with horseradish peroxidase (Santa Cruz Biotechnology). Detection of immune complexes was achieved by enhanced chemiluminescence (NEN). As a control to demonstrate that C/EBPα protein was immunoprecipitated, Western blots were stripped by incubating blots at 65°C for 5 minutes in buffer containing 62.5 mM Tris (pH 6.8), 0.02% SDS, and 10 mM β-mercaptoethanol, as previously described20 and incubated with a 1:200 dilution of C/EBPα antisera (sc-61; Santa Cruz Biotechnology) followed by a 1:5000 dilution of protein A conjugated with HRP (Santa Cruz Biotechnology).
Results
The C/EBPα 42-kDa wild-type protein, but not the 30-kDa dominant-negative or BRM2 mutant, induces granulocytic development of K562 cells
Previous results from several laboratories have indicated that induction of C/EBPα results in granulocytic differentiation of multipotential hematopoietic cell lines.2,5 In order to understand the mechanisms involved in this process, we used a series of stable cell lines in which wild-type or mutant C/EBPα proteins were introduced into the multipotential K562 line in an inducible manner (Figure 1). We elected to use K562 cells because they do not express endogenous C/EBPα.2 In addition, they are a human cell line derived from a patient with chronic myelogenous leukemia and represent a very early progenitor capable of multilineage differentiation.31,32 Furthermore, they undergo differentiation in 3 to 4 days, in contrast to the 17 days required for induction of differentiation of other human myeloid lines, such as U937.2 Given potential differences in function between human and murine cells,26 we felt that it was important to test these mutants in human cells.
Because expression of C/EBPα inhibits cellular proliferation,33-36 we introduced the wild-type and mutant C/EBPα proteins in the form of an estrogen receptor fusion protein. This type of fusion has been used to provide inducible expression of a number of different transcription factors37 and in particular has been used in multiple studies of C/EBPα function.5,7 The C/EBPα-estrogen receptor fusion proteins are expressed in a constitutive manner but remain cytoplasmic and therefore nonfunctional until nuclear translocation is induced by addition of β-estradiol, resulting in induction of C/EBPα DNA binding and activity.7
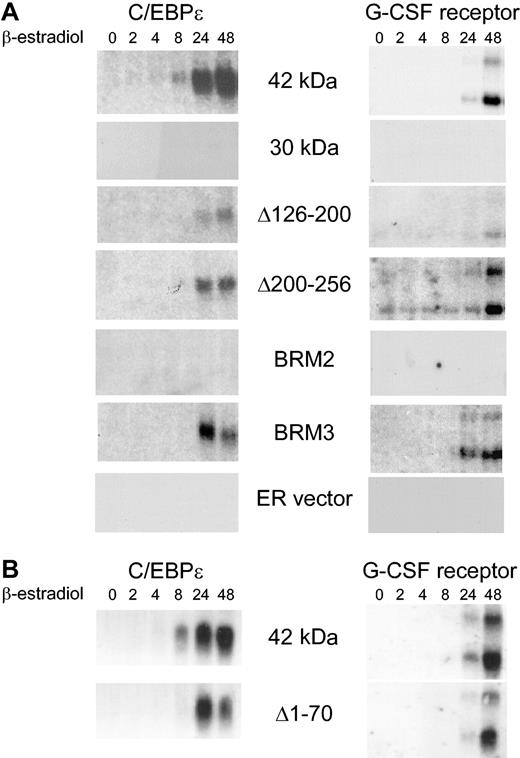
Using this system, we assessed the ability of 42 kDa (wild-type) and mutant C/EBPα to induce cellular differentiation. We analyzed multiple clones from each mutant that expressed similar levels of the C/EBPα-ER fusion proteins (see the coimmunoprecipitation experiments information in the last section of “Results” and in Figure 7). Induction of 42-kDa C/EBPα resulted in granulocytic development, as assessed by induction of NBT activity (Figure 2; Table 1) as well as induction of RNA for C/EBPϵ and the G-CSF receptor (Figure 3). Previous studies have indicated a tight correlation of induction of granulocytic differentiation and up-regulation of these 2 genes.2,27 We then tested several C/EBPα deletion mutants for their ability to induce differentiation. Deletion of amino acids 126 to 200, which removes a domain responsible for interaction with SWI/SNF,13 as well as a region mediating interactions with cyclin-dependent kinase (Cdk),35 resulted in a phenotype in which there was partial induction of NBT activity and RNA encoding C/EBPϵ and G-CSF receptor (Table 1; Figures 2,3). A similar partial differentiation phenotype was observed with a construct deleting amino acids 200 to 256, which includes glycogen synthase kinase-3 (GSK-3) phosphorylation sites.38 In contrast, deletion of the amino terminal 120 amino acids, characterized by the 30-kDa peptide, resulted in a nearly complete loss of NBT activity and no up-regulation of C/EBPϵ and G-CSF receptor RNA could be detected (Figures 2,3; Table 1), consistent with its role as a dominant-negative mutant.6 It has also been shown that the amino terminus is required for inhibition of E2F activity, a function tightly associated with ability to induce granulocytic differentiation.20,21 Previous studies have also indicated that a smaller amino terminal deletion, Δ1-70, was also deficient in inhibition of E2F activity in transient transfections of fibroblast cell lines.21 However, when we tested this mutant, its activity was more similar to that of the 42-kDa than that of the 30-kDa form in which the first 120 amino acids are deleted (Table 1; Figures 2,3). Therefore, we tested 2 additional mutants, BRM2 and BRM3. Both are 2 amino acid substitutions within the C/EBPα basic region, which is required for DNA binding, in residues that are predicted to face away from the bound DNA. Such mutations do not disrupt C/EBPα DNA binding. However, mutant BRM2 is unable to inhibit the activity of E2F and fails to restore granulopoiesis in vivo, whereas BRM3 resembles the wild-type 42-kDa form.21 Consistent with these findings, BRM2 was completely unable to induce K562 cell granulocytic development, as measured by NBT activity and induction of C/EBPϵ and G-CSF receptor RNA, whereas BRM3 was almost as active as the 42-kDa form (Table 1; Figures 2,3). In summary, mutations that disrupt the ability of C/EBPα to inhibit E2F activity, such as the 30-kDa and BRM2 mutants, are completely unable to induce granulopoietic development. Mutant Δ1-70, while incapable of inhibiting E2F activity in transient transfections of fibroblasts,21 was nevertheless able to induce granulocytic development in a manner almost as efficiently as the 42-kDa protein.
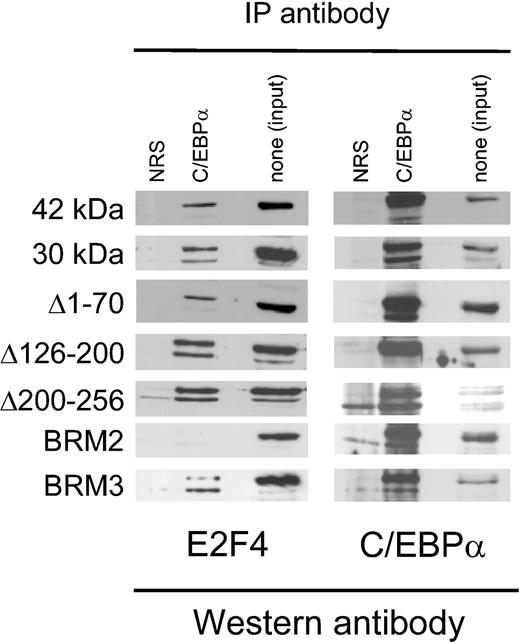
The C/EBPα BRM2 mutation abrogates physical interaction with E2F. Whole-cell protein lysates from K562 lines stably transfected with C/EBPα proteins shown on the left side were incubated with normal rabbit serum (NRS) or antibodies to C/EBPα. Immunoprecipitated proteins from equal number of cells were separated on SDS-PAGE and analyzed by Western analysis using an E2F4 antibody and then the same blot stripped and reprobed with an antibody recognizing C/EBPα. The third lane in each panel represents one twentieth of the amount of lysate in the absence of immunoprecipitation as a control.
The C/EBPα BRM2 mutation abrogates physical interaction with E2F. Whole-cell protein lysates from K562 lines stably transfected with C/EBPα proteins shown on the left side were incubated with normal rabbit serum (NRS) or antibodies to C/EBPα. Immunoprecipitated proteins from equal number of cells were separated on SDS-PAGE and analyzed by Western analysis using an E2F4 antibody and then the same blot stripped and reprobed with an antibody recognizing C/EBPα. The third lane in each panel represents one twentieth of the amount of lysate in the absence of immunoprecipitation as a control.
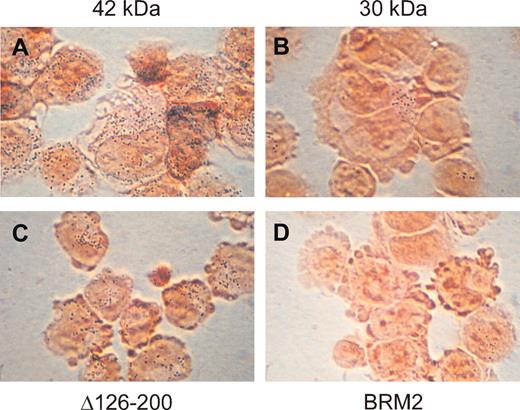
C/EBPα 30-kDa and BRM2 mutant proteins cannot induce granulocytic development of K562 cells with C/EBPα 42-kDa and mutant proteins. Shown are pictures of K562 cells stably transfected with the 42-kDa (A), 30-kDa (B), Δ126-200 (C), and BRM2 (D) C/EBPα-ER fusion proteins 3 days after induction of nuclear localization of the fusion protein with β-estradiol. NBT activity can be detected by the small blue dots observed after counterstaining the cells with safranin. As indicated in Table 1, less than 1% of BRM2 stably transfected cells stained positive; a single positively staining cells is shown in this field. Original magnification, × 1000.
C/EBPα 30-kDa and BRM2 mutant proteins cannot induce granulocytic development of K562 cells with C/EBPα 42-kDa and mutant proteins. Shown are pictures of K562 cells stably transfected with the 42-kDa (A), 30-kDa (B), Δ126-200 (C), and BRM2 (D) C/EBPα-ER fusion proteins 3 days after induction of nuclear localization of the fusion protein with β-estradiol. NBT activity can be detected by the small blue dots observed after counterstaining the cells with safranin. As indicated in Table 1, less than 1% of BRM2 stably transfected cells stained positive; a single positively staining cells is shown in this field. Original magnification, × 1000.
Percent of NBT-positive K562 cells following induction of C/EBPα
C/EBPα construct . | % NBT positive . |
|---|---|
| 42 kDa, wild-type | 87 ± 10 |
| 30 kDa | 8.1 ± 4.4 |
| Δ126-200 | 46.5 ± 19.4 |
| Δ200-256 | 86 ± 6.2 |
| BRM2 | 0 |
| BRM3 | 74 ± 10.5 |
| Vector | 0 |
| 42 kDa, wild-type* | 73 ± 8.1 |
| Δ1-70 | 34.5 ± 23.4 |
| Vector | 0 |
C/EBPα construct . | % NBT positive . |
|---|---|
| 42 kDa, wild-type | 87 ± 10 |
| 30 kDa | 8.1 ± 4.4 |
| Δ126-200 | 46.5 ± 19.4 |
| Δ200-256 | 86 ± 6.2 |
| BRM2 | 0 |
| BRM3 | 74 ± 10.5 |
| Vector | 0 |
| 42 kDa, wild-type* | 73 ± 8.1 |
| Δ1-70 | 34.5 ± 23.4 |
| Vector | 0 |
K562 C/EBPα-estrogen receptor fusion stable cell lines were treated with 1 uM β-estradiol for 3 to 4 days. At least 100 cells were counted from 2 or more clones of each line and results given ± standard deviation.
The data in the last 3 lines in the table were obtained in an independent experiment.
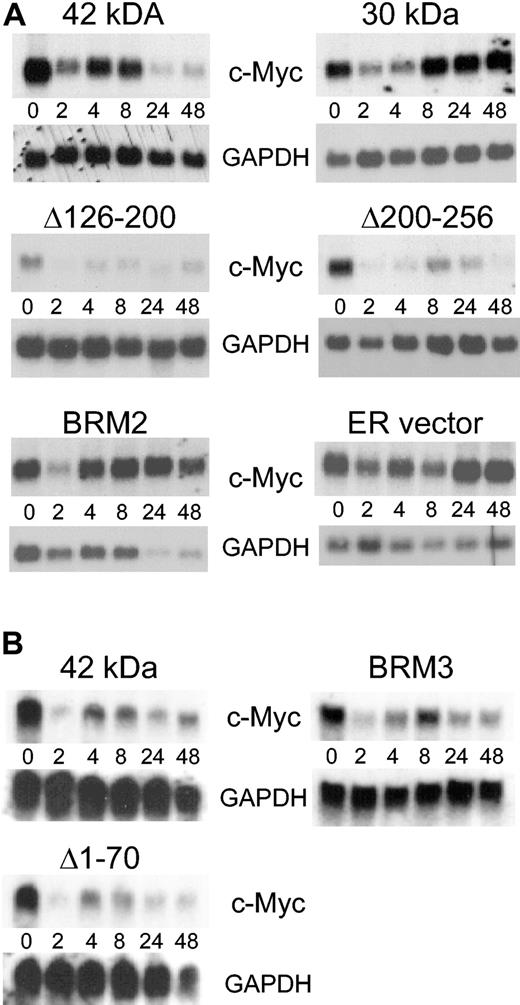
C/EBPα 30-kDa and BRM2 mutant proteins cannot induce the downstream granulocytic genes C/EBPϵ and the G-CSF receptor. (A) The figure demonstrates Northern blot analysis of C/EBPϵ and G-CSF receptor RNA in stably transfected K562 lines following induction with β-estradiol up to 48 hours. To control for RNA integrity and amount, the same blot was probed with c-Myc and GAPDH as shown in Figure 5A. (B) The procedure was the same as for panel A, except this was an independent experiment with different Northern blots. The same blots were probed with c-Myc and GAPDH as shown in Figure 5B. The blots for the 42-kDa C/EBPα-ER fusion in panels A and B are 2 completely independent experiments from 2 different inductions of transfected K562 cells.
C/EBPα 30-kDa and BRM2 mutant proteins cannot induce the downstream granulocytic genes C/EBPϵ and the G-CSF receptor. (A) The figure demonstrates Northern blot analysis of C/EBPϵ and G-CSF receptor RNA in stably transfected K562 lines following induction with β-estradiol up to 48 hours. To control for RNA integrity and amount, the same blot was probed with c-Myc and GAPDH as shown in Figure 5A. (B) The procedure was the same as for panel A, except this was an independent experiment with different Northern blots. The same blots were probed with c-Myc and GAPDH as shown in Figure 5B. The blots for the 42-kDa C/EBPα-ER fusion in panels A and B are 2 completely independent experiments from 2 different inductions of transfected K562 cells.
The C/EBPα 30-kDa form is defective in DNA binding
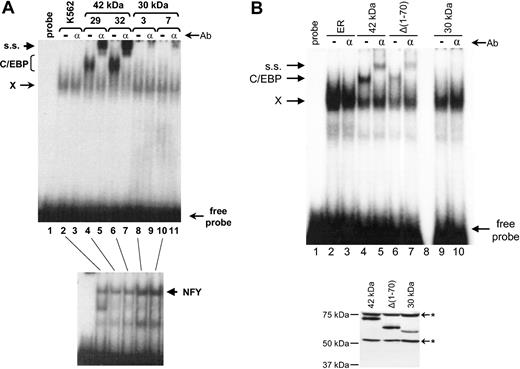
As noted in “Introduction,” mutations in AML that result in production of the 30-kDa form, which in turn binds DNA poorly and acts as a dominant-negative mutant, have been described.6 Since the 30-kDa form failed to induce granulocytic development of K562 cells (Table 1; Figures 2,3), we hypothesized that one mechanism might include a decrease in DNA binding activity. Therefore, we tested the C/EBPα DNA binding activity in K562 cells expressing the 42-kDa and 30-kDa proteins, respectively. As shown in Figure 4A, and consistent with our previous findings,2 untransfected K562 cells did not have any endogenous C/EBP DNA binding activity. Lines expressing the 42-kDa form contained strong C/EBP binding activity, which was completely shifted following addition of antibodies recognizing C/EBPα. In contrast, almost no detectable C/EBPα DNA binding activity could be detected by EMSA in cells expressing the 30-kDa form (Figure 4A), even though the cells express levels of C/EBPα 30-kDa protein that are comparable to that of the 42-kDa form (see the coimmunoprecipitation experiments information in the last section of “Results” and in Figure 7), and DNA binding activity of the ubiquitous transcription factor nuclear factor Y (NFY) was easily detected in these same extracts as a control (Figure 4A, bottom). Therefore, deletion of the amino terminus of C/EBPα results in loss of DNA binding activity in these cells.
The C/EBPα 30-kDa form is defective in DNA binding. (A, Top) EMSA was performed using a double-stranded C/EBP binding site from the human G-CSF receptor.16 Equal amounts of nuclear extracts from nontransfected K562 cells (lanes 2-3), 2 independent clones (nos. 29 and 32) expressing the 42-kDa C/EBPα protein (lanes 4-7), and 2 independent clones (nos. 3 and 7) expressing the 30-kDa C/EBPα protein (lanes 8-11) were used as a source of DNA binding proteins. Lane 1 contained probe only. In lanes 3, 5, 7, 9, and 11, 1 uL of a supershifting C/EBPα antibody was added. ss indicates supershifted complex; C/EBP, C/EBP complex; and X, nonspecific complex observed with this probe.6,16 Ab indicates antibody. (A, Bottom) The same extracts as used in lanes 2, 4, 6, 8, and 10 in the top panel were used in an EMSA assay with an NFY probe52 as a control for integrity and quantity of nuclear binding proteins. (B, Top) EMSA was performed as shown in panel A. Nuclear extracts from estrogen-receptor vector only (ER) transfected K562 cells (lanes 2-3) and clones expressing the 42-kDa C/EBPα protein (lanes 4-5), Δ1-70 (lanes 6-7), and the 30-kDa C/EBPα protein (lanes 9-10) were used as a source of DNA binding proteins. The amount of extract was adjusted according to the Western blot in the bottom panel so that the amount of C/EBPα-ER fusion protein was the same in each binding reaction. Lane 1 contained probe only and lane 8 is a blank lane. In lanes 3, 5, 7, and 10, 1 uL of a supershifting C/EBPα antibody was added. (B, Bottom) Western blot of transfected K562 extracts used in the top of panel B using a rabbit antiestrogen receptor antibody. * Indicates nonspecific bands. The specific C/EBPα-ER fusion protein bands were quantitated with a Phosphorimager (Amersham).
The C/EBPα 30-kDa form is defective in DNA binding. (A, Top) EMSA was performed using a double-stranded C/EBP binding site from the human G-CSF receptor.16 Equal amounts of nuclear extracts from nontransfected K562 cells (lanes 2-3), 2 independent clones (nos. 29 and 32) expressing the 42-kDa C/EBPα protein (lanes 4-7), and 2 independent clones (nos. 3 and 7) expressing the 30-kDa C/EBPα protein (lanes 8-11) were used as a source of DNA binding proteins. Lane 1 contained probe only. In lanes 3, 5, 7, 9, and 11, 1 uL of a supershifting C/EBPα antibody was added. ss indicates supershifted complex; C/EBP, C/EBP complex; and X, nonspecific complex observed with this probe.6,16 Ab indicates antibody. (A, Bottom) The same extracts as used in lanes 2, 4, 6, 8, and 10 in the top panel were used in an EMSA assay with an NFY probe52 as a control for integrity and quantity of nuclear binding proteins. (B, Top) EMSA was performed as shown in panel A. Nuclear extracts from estrogen-receptor vector only (ER) transfected K562 cells (lanes 2-3) and clones expressing the 42-kDa C/EBPα protein (lanes 4-5), Δ1-70 (lanes 6-7), and the 30-kDa C/EBPα protein (lanes 9-10) were used as a source of DNA binding proteins. The amount of extract was adjusted according to the Western blot in the bottom panel so that the amount of C/EBPα-ER fusion protein was the same in each binding reaction. Lane 1 contained probe only and lane 8 is a blank lane. In lanes 3, 5, 7, and 10, 1 uL of a supershifting C/EBPα antibody was added. (B, Bottom) Western blot of transfected K562 extracts used in the top of panel B using a rabbit antiestrogen receptor antibody. * Indicates nonspecific bands. The specific C/EBPα-ER fusion protein bands were quantitated with a Phosphorimager (Amersham).
Since the 30-kDa form (Δ1-120) fails to induce markers of differentiation, whereas the Δ1-70 does induce markers of differentiation, we asked whether the Δ1-70 protein was capable of binding C/EBPα target sites efficiently. As shown in Figure 4B, when the amount of C/EBPα-ER fusion protein was carefully matched, the Δ1-70 form bound DNA almost as efficiently as the 42-kDa form. The binding intensity of the band shift and supershift of the Δ1-70 mutant compared with the 42 kDa was 41% and 48%, respectively. Again, the 30-kDa form, even when present at the same protein levels as the 42 kDa and Δ1-70, failed to demonstrate any DNA binding to the C/EBPα site in the human G-CSF receptor. Therefore, one major difference between the Δ1-70 and Δ1-120 (30-kDa form) is the ability of the former, but not the latter, to bind to C/EBPα target sites in vitro.
The ability of C/EBPα to induce granulocytic development is associated with the ability to down-regulate c-Myc, inhibit growth, induce apoptosis, and interact with E2F
Previous studies have demonstrated that C/EBPα down-regulates c-Myc through inhibition of E2F function and that loss of this function is associated with loss of induction of granulocytic differentiation in cell lines and in vivo.20,21 The effect of the BRM2 mutant on c-Myc RNA has not been studied in vivo. However, we have demonstrated in cell lines that exogenous c-Myc prevented C/EBPα from inducing differentiation.20 Therefore, we investigated whether the ability of C/EBPα mutants to induce granulocytic development correlated with the ability to down-regulate endogenous c-Myc RNA. As shown in Figure 5 and Table 2, the 42-kDa form of C/EBPα was able to down-regulate endogenous c-Myc RNA, as was previously shown in several other myeloid cell lines.20 Although there was some fluctuation of c-Myc RNA levels at different time points, the BRM3 mutation, which was able to induce granulocytic development similar to that of the 42-kDa form (Table 1), down-regulated endogenous c-Myc almost to the same degree as the 42-kDa form (Figure 5; Table 2). The 30-kDa and BRM2 mutants, which were defective in granulocytic development induction, were unable to down-regulate c-Myc, as was the ER vector control. Mutants Δ1-70, Δ126-200, and Δ200-256 were intermediate in terms of induction of granulocytic development and down-regulation of c-Myc. Therefore, C/EBPα granulocytic development induction correlated with down-regulation of the C/EBPα target gene, c-Myc.
C/EBPα 30-kDa and BRM2 mutant proteins do not down-regulate c-Myc. (A) The figure shows the same set of Northern blots from Figure 3A hybridized to c-Myc and GAPDH probes. The hybridization signal was quantitated on a Phosphorimager and the data presented in Table 2. (B) The same set of Northern blots from Figure 3B hybridized to c-Myc and GAPDH probes. The blots for the 42-kDa C/EBPα-ER fusion in panel A and B are 2 completely independent experiments from 2 different inductions of transfected K562 cells.
C/EBPα 30-kDa and BRM2 mutant proteins do not down-regulate c-Myc. (A) The figure shows the same set of Northern blots from Figure 3A hybridized to c-Myc and GAPDH probes. The hybridization signal was quantitated on a Phosphorimager and the data presented in Table 2. (B) The same set of Northern blots from Figure 3B hybridized to c-Myc and GAPDH probes. The blots for the 42-kDa C/EBPα-ER fusion in panel A and B are 2 completely independent experiments from 2 different inductions of transfected K562 cells.
Percent c-Myc RNA decrease 24 hours after induction of C/EBPα
C/EBPα construct . | % c-Myc decrease . |
|---|---|
| 42 kDa, wild-type | 94 |
| 30 kDa | 4 |
| Δ126-200 | 42 |
| Δ200-256 | 83 |
| BRM2 | 0 |
| vector | 0 |
| 42 kDa, wild-type | 84 |
| Δ1-70 | 79 |
| BRM3 | 70 |
C/EBPα construct . | % c-Myc decrease . |
|---|---|
| 42 kDa, wild-type | 94 |
| 30 kDa | 4 |
| Δ126-200 | 42 |
| Δ200-256 | 83 |
| BRM2 | 0 |
| vector | 0 |
| 42 kDa, wild-type | 84 |
| Δ1-70 | 79 |
| BRM3 | 70 |
c-Myc RNA was quantitated using Phosphorimager software (Molecular Dynamics, Sunnyvale, CA). The results are presented as percent decrease in c-Myc RNA at 48 hours compared with 0 hours of 1 μM β-estradiol treatment for each stable K562 line as shown in Figure 5. The data in the first 6 lines were derived from Figure 5A and those in the last 3 lines from a completely independent experiment as shown in Figure 5B.
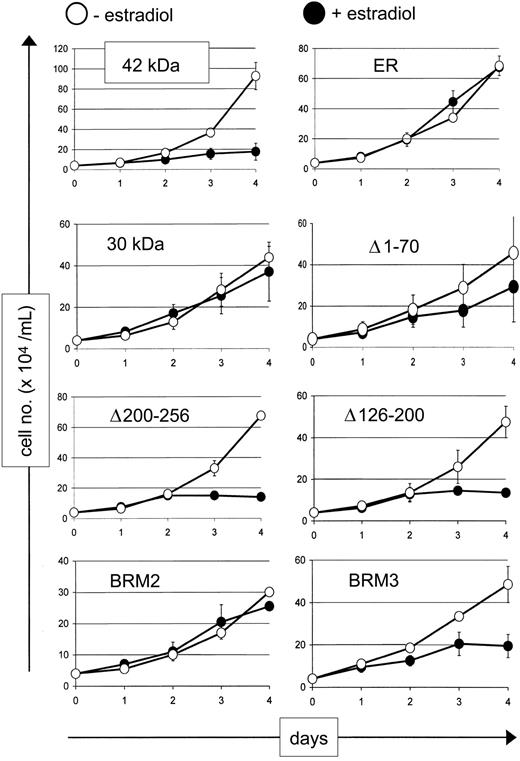
Because down-regulation of c-Myc is associated with granulocytic differentiation and inhibition of proliferation,20 we tested growth and apoptosis in the wild-type (42 kDa) and mutant cell lines. As shown in Figure 6, the 42-kDa, Δ126-200, Δ200-256, and BRM3 proteins were able to inhibit increase in cell numbers after 4 days of culture, whereas the 30-kDa and BRM2 mutants, as well as the vector control, did not show any inhibition of cell number as compared with vehicle control. Interestingly, the Δ1-70 mutant, which demonstrated intermediate properties in inducing differentiation markers and DNA binding, also demonstrated an intermediate inhibition of increase in cell number as well.
The C/EBPα 30-kDa and BRM2 mutations fail to inhibit cell growth. The vertical axis of each graph displays cell numbers of K562 cells stably transfected with the indicated C/EBPα-ER fusion proteins in the absence (○) or presence (•) of β-estradiol, which induces C/EBPα fusion protein expression. ER indicates estrogen receptor vector control. The error bars represent the standard deviation from 2 independent clones for each C/EBPα-ER fusion protein.
The C/EBPα 30-kDa and BRM2 mutations fail to inhibit cell growth. The vertical axis of each graph displays cell numbers of K562 cells stably transfected with the indicated C/EBPα-ER fusion proteins in the absence (○) or presence (•) of β-estradiol, which induces C/EBPα fusion protein expression. ER indicates estrogen receptor vector control. The error bars represent the standard deviation from 2 independent clones for each C/EBPα-ER fusion protein.
We also assessed the ability of the 42-kDa and mutant forms to induce apoptosis of these hematopoietic cell lines by flow cytometry following staining of the cells with Annexin-V and PI. After 4 days in culture, induction of the 42-kDa protein following treatment of the cells with β-estradiol led to a 6-fold increase in apoptosis compared with treatment with vehicle alone (data not shown). The 30-kDa and BRM2 mutants, along with the estrogen receptor vector control, failed to induce any increase in apoptosis, whereas the other mutants (Δ1-70, Δ126-200, Δ200-256, and BRM3 proteins) led to an intermediate (2-3 fold) increase in apoptotic cells. In summary, the ability of the C/EBPα proteins to inhibit growth and induce apoptosis correlated with their ability to induce granulocytic development.
Previously, we and others had demonstrated that C/EBPα physically interacts with E2F complexes, and that this interaction is associated with inhibition of E2F function and down-regulation of c-Myc RNA.20,21,39,40 Therefore, we also asked whether the different C/EBPα mutants interacted with E2F. As shown in Figure 7, all forms of C/EBPα physically interacted with E2F4 in the stably transfected cells with the exception of the BRM2 mutant. Similar results were obtained with the related family member E2F2 (data not shown). In addition, the right panel in Figure 7 demonstrates that all of the mutants tested, including BRM2, were capable of expressing significant amounts of C/EBPα protein in the stable cell lines. The BRM2 mutant, previously shown to be defective in inhibition of E2F,21 is both completely defective in down-regulation of c-Myc and fails to demonstrate a physical interaction with endogenous E2F family members. The functional effects of the various C/EBPα mutants are summarized in Table 3.
Summary of C/EBPα transfected K562 cell lines
K562 cell line . | NBT staining . | Down-regulate c-Myc . | Up-regulate C/EBPϵ . | Up-regulate G-CSFr . | Interact with E2F . |
|---|---|---|---|---|---|
| 42 kDa | ++++ | ++++ | ++++ | ++++ | ++++ |
| 30 kDa | + | + | 0 | 0 | +++ |
| Δ1-70 | ++ | +++ | +++ | ++ | +++ |
| Δ126-200 | ++ | ++ | ++ | +++ | +++ |
| Δ200-256 | ++++ | +++ | +++ | +++ | ++++ |
| BRM2 | 0 | 0 | 0 | 0 | 0 |
| BRM3 | +++ | +++ | +++ | ++++ | ++ |
| ER vector | 0 | 0 | 0 | 0 | 0 |
K562 cell line . | NBT staining . | Down-regulate c-Myc . | Up-regulate C/EBPϵ . | Up-regulate G-CSFr . | Interact with E2F . |
|---|---|---|---|---|---|
| 42 kDa | ++++ | ++++ | ++++ | ++++ | ++++ |
| 30 kDa | + | + | 0 | 0 | +++ |
| Δ1-70 | ++ | +++ | +++ | ++ | +++ |
| Δ126-200 | ++ | ++ | ++ | +++ | +++ |
| Δ200-256 | ++++ | +++ | +++ | +++ | ++++ |
| BRM2 | 0 | 0 | 0 | 0 | 0 |
| BRM3 | +++ | +++ | +++ | ++++ | ++ |
| ER vector | 0 | 0 | 0 | 0 | 0 |
++++ indicates very high level, such as 42 kDa (wild-type); +++, high level, but lower than 42 kDa; ++, intermediate between 42 kDa and ER vector; +, dramatically decreased; and 0, none.
Discussion
While results from a number of different laboratories have pointed to the importance of C/EBPα function in granulopoiesis, the mechanisms involved in normal bone marrow granulocytic development and in disruption of C/EBPα function in human AML are still not well defined. The results presented in this article provide further support of the concept that C/EBPα-mediated inhibition of E2F and subsequent down-regulation of c-Myc are critical for granulocytic development. In this study, 2 mutants were shown to be almost completely defective in induction of granulopoietic development using a human cell line model. One mutant, BRM2, has been demonstrated in murine systems to be critical for granulopoiesis. The mechanism by which BRM2 fails to inhibit E2F is likely a result of failure to interact with E2F (Figure 7). However, at this point we have not proven that the BRM region interacts with E2F, only that mutation of the region abrogates such an interaction. Another possibility is that the BRM mutation affects C/EBPα structure and loss of E2F interaction is a secondary effect. This is unlikely as the BRM2 mutant can bind DNA as well as the 42-kDa form.21 Although the BRM2 mutant binds C/EBP sites as well as the 42-kDa form, it fails to form a complex with E2F, consistent with its selective failure to coimmunoprecipitate with E2F (Figure 7).
The second mutant that failed to induce granulocytic development, the C/EBPα 30-kDa form, was previously demonstrated to display defective DNA binding properties and act as a dominant-negative in human AML cells with mutations in C/EBPα.6 It was also shown to be defective in inhibition of E2F.21 In this article, we demonstrate that human cells transfected with the 30-kDa form of human C/EBPα fail to bind DNA efficiently (Figure 4) but that the 30-kDa form can physically interact with E2F. Therefore, it is likely that the 30-kDa and BRM2 mutants fail to inhibit E2F and fail to induce granulocytic development through different mechanisms. The hypothesis that at least 2 mechanisms account for the failure of C/EBPα mutants to induce markers of differentiation is supported by our results with the Δ1-70 deletion mutant. This mutant has been demonstrated to be defective in induction of differentiation of adipocyte lines and fails to inhibit E2F in transient transfections in fibroblast lines.21 However, in hematopoietic cells, it was nearly as effective as the wild-type 42-kDa protein in terms of induction of NBT activity, up-regulation of C/EBPϵ and G-CSF receptor RNA, down-regulation of c-Myc, and intermediate in terms of DNA binding, inhibition of cell growth, and apoptosis (Figures 3,4,5,6; Tables 1,2). Like the 30-kDa form, and unlike BRM2, it can coimmunoprecipitate efficiently with E2F proteins (Figure 7). One possible explanation is that there may be different requirements in terms of the abilities of C/EBP proteins to induce granulocytic versus adipocytic differentiation.41,42 Additional studies will be required to understand whether the differences between the 30-kDa form, which harbors a deletion of amino acids 1-120, and the Δ1-70 protein result from functions specific to amino acids 71-120, or whether loss of these residues results in an alteration of the structure of the entire C/EBPα protein so that it no longer binds DNA effectively.
In these studies, we used human K562 cells as a model of C/EBPα induction of granulopoietic differentiation primarily because of the desire to use a human cell line that responded within a few days of expression of C/EBPα. K562 cells represent an early multipotential line derived from a patient with chronic myelogenous leukemia and do express BCR/ABL. It should be noted that BCR/ABL can inhibit C/EBPα-induced differentiation of murine 32D cells, which represent an early granulocytic cell, although it does not inhibit cell proliferation.5 The differences between the human K562 and murine 32D cells in their ability to differentiate in response to expression of C/EBPα could be due to differences in murine (32D) versus human (K562) systems, idiosyncratic differences in the cell lines, or different relative levels of BCR/ABL and C/EBPα expression. Supporting the last possibility, there was far more BCR/ABL protein detected in the transfected 32D lines compared with endogenous BCR/ABL in K5625 and relatively high levels of C/EBPα protein in our K562 lines (Figure 7). Therefore, the ratio of C/EBPα to BCR/ABL is likely to be much higher in our K562 stable lines than the 32D model,5 and this possibly explains the differences in these 2 systems. Furthermore, our results in the transfected K562 cells are consistent with those using the human U937 myeloid cell line,20 lines derived from murine bone marrow20,43 as well as from murine cells in vivo21 , and from human patients with AML.6,7 Therefore, we believe that our findings are not an artifactual result of the model system used.
The E2F family of transcription factors is composed of 6 members with conserved DNA binding and dimerization domains, termed E2F1-E2F6, that form heterodimers with DNA binding partners DP-1 or DP-2. In addition, E2F1-E2F5 proteins contain a retinoblastoma protein (RB) binding domain that also mediates interactions with other pocket proteins, such as p107 and p130, and these interactions may well modulate their function during the cell cycle.44,45 Knockout studies have not revealed the precise role of E2Fs in granulopoiesis but they have demonstrated that E2F family members affect other distinct hematopoietic lineages.46,47 However, other studies, including the BRM2 knock-in model, demonstrate that E2F proteins perform a role in myeloid differentiation.20,21,48,49 The inability of BRM2 to interact with E2F was shown previously by failure to induce supershift complexes in EMSA assays in liver extracts.21 Here, we have extended this observation using a different assay (coimmunoprecipitation) in hematopoietic cells. Whether E2F works predominantly through regulation of c-Myc or whether there are other critical E2F target genes in granulopoiesis, remains to be determined. While our previously published work indicates an effect on the c-Myc promoter,20 the reduction of endogenous c-Myc may be an indirect effect of differentiation and not a direct consequence of E2F inhibition. Furthermore, one hypothesis explaining why the BRM2 mutants do not induce C/EBPϵ and G-CSF receptor expression is related to the fact that BRM2 does not interact with E2F (Figure 7). E2F itself could be a cofactor required for C/EBPα transactivation. If so, this mechanism is specific for hematopoietic cells, since prior studies have shown that BRM2 can induce expression of C/EBP target genes in adipocyte differentiation.21
Previous studies have indicated that transactivation domain TE-III, corresponding to amino acids 126 to 200, is critical for activation of target genes through interactions with the SWI/SNF chromatin remodeling complex, and these interactions are essential for adipocyte differentiation.13 In our K562 model, Δ126-200 was capable of inducing granulocytic development to a degree approximately halfway between the wild-type 42-kDa form and that of mutants defective in granulocytic development, such as BRM2 (Tables 1,2; Figures 2,3,4). At this point in time, it is not clear that this same domain mediates interactions with SWI/SNF in granulocytic cells, but it is likely that loss of SWI/SNF interactions contributes to the decrease in target gene activation (Figure 3) and granulocytic development in this system. The region deleted in Δ200-256 includes amino acid residues mediating phosphorylation of C/EBPα by GSK-3,38 but to date no role for this modification in granulopoiesis has been established. In summary, our results suggest that both deletion of the C/EBPα amino terminus as well as selected small point mutations of the C/EBPα basic region lead to loss of inhibition of E2F activity and fail to induce granulocytic development. The similar phenotype of these 2 mutants is likely mediated by different mechanisms and further studies will be required to understand the precise nature of these differences.
Prepublished online as Blood First Edition Paper, July 17, 2003; DOI 10.1182/blood-2003-02-0479.
Supported by K01 DK62064 (H.S.R.) and National Institutes of Health (NIH) grant HL56745 (D.G.T.). E.A.N. is a Fellow of the Leukemia and Lymphoma Society of America.
F.D. and L.M.J. contributed equally to the work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Note added in proof. While this article was under review, Friedman et al published an assessment of the ability of C/EBPα amino terminal and BRM2 mutants to induce differentiation and inhibit cell cycle in the murine 320 cell line (Wang QF, Cleaves R, Kummalae T, Nerlov C, Friedman AD. Cell cycle inhibition mediated by the outer surface of the C/EBPα basic region is required but not sufficient for granulopoiesis. Oncogene. 2003;22: 2548-2557). Also, an additional manuscript assessing the function of amino terminal and BRM mutants was published (Ref. 43).
We thank members of the Tenen lab for their support; Katharina Wagner and Daniela Basseres for assistance with flow cytometry; Alan Friedman for the pBabe-C/EBPα-ER construct; Danilo Perrotti, Bruno Calabretta, and Nick Timchenko for discussion of their unpublished results; and Mary Singleton and Alison Lugay for expert assistance with preparation of the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal