Abstract
Thrombopoietin (TPO), the primary regulator of platelet production, also plays an important role in hematopoietic stem cell (HSC) biology. In previous studies we demonstrated that the self-renewal and expansion of HSCs is 10 to 20 times less robust in tpo–/– mice than in controls. To explore the molecular basis of this effect, we postulated that Hoxb4 might mediate at least part of the TPO effect on these cells. We first analyzed the effects of TPO on Hoxb4 expression in primitive hematopoietic cell lines; TPO increased expression of the gene 2- to 3-fold in EML and UT-7/TPO cells. We also compared Hoxb4 levels in a candidate HSC population derived from tpo–/– and control mice; Hoxb4 expression was 2- to 5-fold lower in null HSCs. Of the numerous signal transduction molecules induced by TPO, we found that p38 mitogen-activated protein kinase (MAPK) was responsible for the TPO-induced Hoxb4 elevation. We also demonstrated that upstream stimulating factor 1 (USF-1), a transcription factor previously shown to regulate Hoxb4 expression, is also induced by TPO in a p38-dependent manner. Together, these data provide a molecular pathway by which a growth factor can modulate a transcription factor and thereby help direct a critical developmental process.
Introduction
Thrombopoietin (TPO), initially cloned as a major regulator of platelet production,1 plays a pivotal role in hematopoietic stem cell (HSC) biology. Virtually all primitive HSCs that display repopulating activity express c-Mpl, the receptor of TPO.2 TPO alone or in combination with other early acting cytokines, such as stem cell factor (SCF), interleukin-3 (IL-3), or Flt-3 ligand, enhances proliferation of primitive hematopoietic cells in vitro.3,4 In vivo studies confirm these conclusions: all types of hematopoietic progenitors are reduced in the marrow of mpl–/– mice; the number of stem cell colony-forming units (CFU-Ss), a primitive hematopoietic progenitor, is reduced 8- to 10-fold5 ; and the frequency of repopulating HSCs is 7- to 8-fold lower in mpl–/– mice than in controls.2 The importance of TPO in stem cell self-renewal and expansion was also supported by the clinical observation that mutations of the c-Mpl gene cause congenital amegakaryocytic thrombocytopenia, a disease in which all hematopoietic lineages fail during childhood.6 More recently, we found that expansion of HSCs in adult bone marrow is 10 to 20 times less robust in tpo–/– mice following bone marrow transplantation. Exogenously added TPO substantially rescued this defect.7 These reports clearly indicate that TPO is a major nonredundant contributor to self-renewal and expansion of HSCs. However, the precise molecular mechanisms by which TPO regulates HSC expansion remain unexplored.
Homeobox proteins are transcription factors that play crucial roles in embryonic development. In addition to these developmental functions, recent studies revealed that homeobox proteins also contribute to adult physiologic and pathologic blood cell development.8 Although multiple homeobox proteins are expressed in primitive hematopoietic cells, Hoxb4 has been clearly demonstrated to function in HSCs; Hoxb4 is selectively expressed in primitive hematopoietic cells,9 retrovirus-mediated 2-fold overexpression of HOXB4 in bone marrow cells increases the recovery rate of HSCs in a transplantation model,10,11 HOXB4 increases the self-renewal activity of human cord blood–derived HSCs,12 and HSCs can be expanded ex vivo by introducing HOXB4 into bone marrow cells.13 During embryonic development, the expression of homeobox proteins is tightly regulated by other transcription factors, including homeobox proteins themselves. In contrast, our understanding of homeobox protein expression in adult hematopoietic cells is just emerging. Recently, Giannola and coworkers reported that combinations of early acting cytokines increased HOXB4 promoter activity in primitive hematopoietic cells.14 Based on these observations, we investigated whether Hoxb4 is a downstream mediator of TPO-induced HSC self-renewal and expansion.
The goal of our study was to investigate the molecular mechanisms by which TPO regulates self-renewal and expansion of HSCs; we hypothesized that Hoxb4 mediates these effects. We report here that TPO up-regulates Hoxb4 levels both in vitro and in vivo, and that p38 mitogen-activated protein kinase (MAPK) activation is required for its induction.
Materials and methods
Cell culture
EML cells15 were a gift of Dr Steve Collins (Fred Hutchinson Cancer Research Center, Seattle, WA) and were cultured with Iscove modified Dulbecco medium (IMDM; Gibco Laboratories, Grand Island, NY) containing 10% fetal calf serum (FCS) and 100 ng/mL murine (m) SCF (a gift of Akihiro Shimosaka, Kirin Pharmaceuticals, Tokyo, Japan). EML cells are immature murine hematopoietic progenitors immortalized by a dominantnegative retinoic acid receptor and display several characteristics of HSCs including expression of Sca-1 and CD34.15 UT-7/TPO cells16 were a gift of Dr Norio Komatsu (Jichi Medical School, Tochigi, Japan) and maintained in liquid culture with IMDM containing 10% FCS and 10 ng/mL human (h) TPO, a gift of Don Foster (Zymogenetics, Seattle, WA). UT-7/TPO is a human leukemic cell line and responds to multiple cytokines including granulocyte-macrophage colony-stimulating factor (GM-CSF), SCF, and TPO.16 GM-CSF was purchased from PeproTech (Rocky Hill, NJ).
Reagents
Neomycin was purchased from Gibco BRL Life Technologies (Gaithersburg, MD). SB203580 and PD98059 were purchased from Calbiochem (La Jolla, CA). Antisera for p38 MAPK and phsopho-p38-MAPK were purchased from Cell Signaling (Beverly, MA). Antisera for HoxB4 were purchased from Iowa Stock Center (Iowa City, IA). Antisera against upstream stimulating factor 1 and 2 (USF-1, -2) for supershift studies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Fluorescein isothiocyanate (FITC)–conjugated anti–Gr-1 and phycoerythrin (PE)–conjugated anti–c-kit antibodies were obtained from BD Biosciences Pharmingen (San Diego, CA).
RNA preparation and real-time RT-PCR
Total cellular RNA was extracted from cells using Rneasy Midi Kit (Qiagen, Valencia, CA). A quantitative real-time polymerase chain reaction (PCR) assay for HoxB4 and HoxA9 transcripts was developed using the ABI Prism 7700 Sequence Detection System (Applied Biosystems, Foster City, CA) or iCycler (Bio-Rad Laboratories, Hercules, CA). The following primer and probe sequences were used: human HOXB4 forward primer, 5′-CCTGGATGCGCAAAGTTCA-3′; human HOXB4 reverse primer, 5′-AATTCCTTCTCCAGCTCCAAGA-3′; murine Hoxb4 forward primer, 5′-CCTGGATGCGCAAAGTTCA-3′; murine Hoxb4 reverse primer, 5′-CGTCAGGTAGCGGTTGTAGTGA-3′; human HOXA9 forward primer; 5′-GCGCCTTCTCTGAAAACAAT-3′; human HOXA9 reverse primer; 5′-CAGTTCCAGGGTCTGGTGTT-3′; murine Hoxa9 forward primer; 5′-CCCACGCTTGACACTCACACTTTG-3′; murine Hoxa9 reverse primer; 5′-GAGTGGAGCGAGCATGTAG-3′ Hoxb4 probe, 5′-FAMTGAGCACGGTAAACCCCAATTACGCC-TAMRA-3′; human HOXA9 probe, 5′-FAM-CTCGGAAAAAGCGGTGCCCCTATAC-TAMRA3′; and murine Hoxa9 probe, 5′-FAM-CCCCATCGATCCCAATAACCCGGCTTAMRA-3′.
The primer pairs and probes are designed to span intron 1 to prevent reporting amplification of any contaminating gDNA. Primers for murine HoxA9 were previously reported.17 All primers were synthesized by Invitrogen Life Technologies (Carlsbad, CA). The probes were synthesized by Synthegen (Houston, TX). For analysis of HoxB4 expression, 50 ng total RNA was amplified and quantified in 40 μL reaction mixture containing 5 mM MgCl2, 300 μM dNTPs, 0.2 μL AmpliTaq Gold (Applied Biosystems), 0.05 μL M-MLV reverse transcriptase (Invitrogen Life Technologies), 0.4 μL Rnasin ribonuclease inhibitor (Promega, Madison, WI), 300 nM forward primer, 100 nM reverse primer, and 200 nM probe. For amplification of murine RNA, MgCl2 concentration was changed to 2.5 mM. In case of Hoxa9, we first synthesized cDNA using random hexamers and Superscript II reverse transcriptase (Invitrogen Life Technologies) and then performed real-time PCR with specific primers and probes. All reactions were performed in triplicate. For quantification of gene expression we used a relative standard curve method according to the technical manual of ABI prism 7700 sequence detection system. As an internal control we used glyceraldehyde-3-phosphate dehydrogenase (GAPDH) forward and reverse primers included in the GAPDH control reagents kit (Applied Biosystems).
Preparation of cell lysates and Western blotting
Cell lysates were prepared from EML or UT-7/TPO cells according to methods previously described.18 Cell lysate proteins were size-fractioned by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and then electroblotted onto polyvinylidene difluoride (PVDF) membranes. The blots were incubated with primary antibodies and visualized using a chemiluminescence detection kit (LumiGLO, Cell Signaling).
Preparation of nuclear extracts and electrophoretic mobility shift assay
Nuclear extracts were prepared as described previously.18 Nuclear extracts (10 μg) were incubated for 20 minutes at 20°C in 10 mM Tris (tris(hydroxymethyl)aminomethane)–HCL, pH 7.5, 50 mM NaCl, 0.5 mM dithiothreitol (DTT), 1.5 μg poly dIC/dAT, 0.5 mM ethylenediaminetetraacetic acid (EDTA), 4% glycerol with 2 ng of a 32P-labeled oligonucleotide. The probe was a double-stranded oligonucleotide corresponding to the USF-1–binding site of the human HOXB4 promoter (5′-CGGAGGATCACGTGGGCGCC-3′); the samples were then loaded on a 5% nondenaturating polyacrylamide gel, run for 1.5 hours at 150 V, vacuum dried, and exposed to x-ray film. For the supershift study, the nuclear extracts were incubated with antibodies at 20°C for 20 minutes before the above reaction.
Establishment of p38 dominant-negative mutant-expressing cells
An expression vector encoding a dominant-negative mutant form of p38 MAPK19 was a gift from Roger J. Davis (Howard Hughes Medical Institute, University of Massachusetts, Worcester). The vector was introduced into UT-7/TPO cells with Tfx-20 (Promega) according to the manufacturer's instructions and overexpressing cells were selected by culture in neomycin. Two highly expressing clones were selected for further study.
Isolation of immature hematopoietic cells
All mice used in this study were C57BL/6. TPO-null mice bred onto the C57BL/6 background were kindly provided by Fred de Sauvage (Genentech, South San Francisco, CA) and have been previously described.20 Whole bone marrow cells were harvested from 12-week-old mice by flushing the femoral contents with Dulbecco modified Eagle medium (DMEM) through 25-gauge needles. Then Sca-1+ cells were collected using MACS Sca-1 MultiSort kit (Miltenyi Biotec, Auburn, CA). The collected cells were stained with FITC-conjugated anti–Gr-1 and PE-conjugated anti–c-kit antibodies and c-kit+/Gr-1– cells were sorted using a FACstar flow cytometer (Becton Dickinson, Franklin Lakes, NJ).
Statistical analysis
Statistical analysis was performed using the Student t test. P < .05 was considered significant.
Results
TPO increases Hoxb4 expression in primitive hematopoietic cell lines
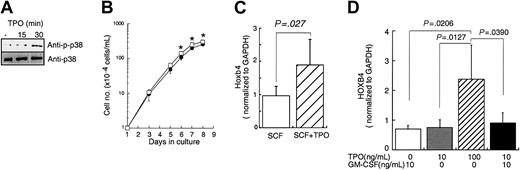
To begin to address our hypothesis we developed a quantitative assay for Hoxb4 mRNA using a real-time reverse transcription PCR (RT-PCR) strategy as described in “Materials and methods.” We first determined whether TPO exerts an effect on Hoxb4 expression in vitro. We used 2 primitive hematopoietic cell lines, EML cells15 and UT-7/TPO. First, we analyzed whether EML cells could respond to TPO. Treatment with TPO activated signal transduction molecules including p38 MAPK in EML cells (Figure 1A). Furthermore, the addition of TPO modestly enhanced the proliferation of EML cells (Figure 1B). To assess the effect of TPO on Hoxb4 expression, EML cells were cultured with SCF or SCF plus TPO for 24 hours and Hoxb4 levels were analyzed by real-time RT-PCR. Culture with TPO induced a 2-fold increase of Hoxb4 mRNA expression in EML cells compared with culture in SCF alone (Figure 1C). Similar results were obtained using another TPO-responsive primitive hematopoietic cell line, UT-7/TPO16 ; culture with an increased concentration of TPO increased HOXB4 levels 2- to 3-fold after 24 hours (Figure 1D). In contrast, GM-CSF failed to increase HOXB4 expression in UT-7/TPO cells.
Effects of TPO on primitive hematopoietic cell lines. (A) EML cells cultured with 100 ng/mL mSCF were treated with 100 ng/mL mTPO for the indicated time periods. Cell lysates were prepared, size fractionated, and probed for activation of p38 MAPK by Western blotting. (B) EML cells at a concentration of 1 × 104 cells/mL were cultured with SCF alone (•) or with SCF plus 100 ng/mL hTPO (□). Viable cell numbers were monitored for the duration of the culture by trypan blue dye exclusion. Each point represents the average ± SD of triplicate samples; *P < .05. (C) After a 24-hour culture of EML cells with or without TPO, total RNA was prepared and subjected to real time RT-PCR for Hoxb4 quantification. The data represent the average ± SD of several independent experiments (n = 6, control; n = 4, TPO treatment, respectively). (D) UT-7/TPO cells were cultured with the indicated combination of cytokines for 24 hours, total RNA was prepared, and real-time RT-PCR was performed to quantify HOXB4 level. The bars represent the average ± SD of several independent experiments (n = 4, GM-CSF 10 ng/mL; n = 5, TPO 10 ng/mL; n = 7, TPO 100 ng/mL; n = 4, TPO 10 ng/mL plus GM-CSF 10 ng/mL).
Effects of TPO on primitive hematopoietic cell lines. (A) EML cells cultured with 100 ng/mL mSCF were treated with 100 ng/mL mTPO for the indicated time periods. Cell lysates were prepared, size fractionated, and probed for activation of p38 MAPK by Western blotting. (B) EML cells at a concentration of 1 × 104 cells/mL were cultured with SCF alone (•) or with SCF plus 100 ng/mL hTPO (□). Viable cell numbers were monitored for the duration of the culture by trypan blue dye exclusion. Each point represents the average ± SD of triplicate samples; *P < .05. (C) After a 24-hour culture of EML cells with or without TPO, total RNA was prepared and subjected to real time RT-PCR for Hoxb4 quantification. The data represent the average ± SD of several independent experiments (n = 6, control; n = 4, TPO treatment, respectively). (D) UT-7/TPO cells were cultured with the indicated combination of cytokines for 24 hours, total RNA was prepared, and real-time RT-PCR was performed to quantify HOXB4 level. The bars represent the average ± SD of several independent experiments (n = 4, GM-CSF 10 ng/mL; n = 5, TPO 10 ng/mL; n = 7, TPO 100 ng/mL; n = 4, TPO 10 ng/mL plus GM-CSF 10 ng/mL).
Bone marrow cells from tpo–/– mice display reduced Hoxb4 expression
In an attempt to assess whether TPO has an effect on Hoxb4 expression in vivo, we compared Hoxb4 levels in primitive hematopoietic cells derived from tpo–/– and from control mice. Sca-1+/c-kit+/Gr-1– cells, a population highly enriched in hematopoietic stem and premature progenitor cells, were isolated from pools of whole bone marrow cells. As shown in Figure 2, we found 2- to 5-fold higher levels of Hoxb4 expression in wild-type than in tpo–/– mice in 2 sequence experiments. When combined these results were statistically different (P < .05).
Hoxb4level intpo[–/–]mice. Whole bone marrow cells were prepared from control C57BL6 and tpo[–/–] mice. The cells were pooled (experiment 1: n = 4, C57BL6, n = 4, tpo[–/–] mice; experiment 2: n = 4, C57BL6, n = 5, tpo[–/–] mice) and Sca-1+/c-kit+/Gr-1– cells were collected for real-time PCR analysis. Each column represents an average of HoxB4 level normalized to GAPDH internal control ± SD of 2 experiments.
Hoxb4level intpo[–/–]mice. Whole bone marrow cells were prepared from control C57BL6 and tpo[–/–] mice. The cells were pooled (experiment 1: n = 4, C57BL6, n = 4, tpo[–/–] mice; experiment 2: n = 4, C57BL6, n = 5, tpo[–/–] mice) and Sca-1+/c-kit+/Gr-1– cells were collected for real-time PCR analysis. Each column represents an average of HoxB4 level normalized to GAPDH internal control ± SD of 2 experiments.
TPO does not enhance the Hoxa9 level
To investigate whether TPO specially increases Hoxb4 mRNA levels in primitive hematopoietic cells, we analyzed the effect of TPO on Hoxa9 expression, another homeobox gene known to be involved in HSC self-renewal.21,22 In contrast to Hoxb4, TPO did not significantly enhance HOXA9 expression in UT-7/TPO cells (Figure 3A). We also compared the Hoxa9 level in Sca-1+/c-kit+/Gr-1– cells from control and tpo–/– mice. As shown in Figure 3B, the Hoxa9 level was slightly reduced in tpo–/– mice; however, these results did not reach statistical significance (P = .12). Interestingly, we could not find Hoxa9 expression in EML cells by real-time PCR methods.
TPO does not enhanceHoxa9expression. (A) UT-7/TPO cells were cultured with 10 ng/mL or 100 ng/mL TPO for 24 hours, total RNA was prepared, and real-time RT-PCR was performed to quantify HOXA9 level. The bars represent the average ± SD of several independent experiments (n = 3, TPO 10 ng/mL; n = 5, TPO 100 ng/mL). (B) Whole bone marrow cells were prepared from control C57BL6 and tpo[–/–] mice. The cells were pooled (experiment 1: n = 4, C57BL6, n = 4, tpo[–/–] mice; experiment 2: n = 4, C57BL6, n = 5, tpo[–/–] mice) and Sca-1+/c-kit+/Gr-1– cells were collected for real-time PCR analysis. Each column represents the average Hoxa9 level normalized to GAPDH internal control ± SD of 2 experiments (▪, C57/BL6; ▨, tpo[–/–] mice.)
TPO does not enhanceHoxa9expression. (A) UT-7/TPO cells were cultured with 10 ng/mL or 100 ng/mL TPO for 24 hours, total RNA was prepared, and real-time RT-PCR was performed to quantify HOXA9 level. The bars represent the average ± SD of several independent experiments (n = 3, TPO 10 ng/mL; n = 5, TPO 100 ng/mL). (B) Whole bone marrow cells were prepared from control C57BL6 and tpo[–/–] mice. The cells were pooled (experiment 1: n = 4, C57BL6, n = 4, tpo[–/–] mice; experiment 2: n = 4, C57BL6, n = 5, tpo[–/–] mice) and Sca-1+/c-kit+/Gr-1– cells were collected for real-time PCR analysis. Each column represents the average Hoxa9 level normalized to GAPDH internal control ± SD of 2 experiments (▪, C57/BL6; ▨, tpo[–/–] mice.)
Effects of chemical inhibitors on the HoxB4 level
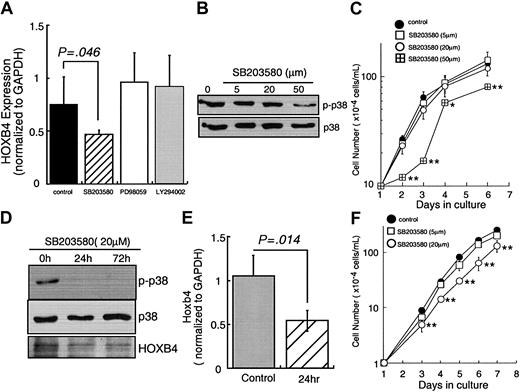
These results clearly indicate that TPO controls Hoxb4 expression at the level of RNA abundance. Next, we sought to identify the signal transduction molecules responsible for Hoxb4 induction. Because MAPKs and phosphoinositol 3-kinase (PI3K) have been shown to be critical in numerous studies of TPO-induced hematopoietic cell function, we initially used chemical inhibitors of these intracellular signal transducers and investigated the effects on HOXB4 levels in UT-7/TPO cells. As indicated in Figure 4A, neither PD98059 nor LY294002 inhibited TPO-induced HOXB4 expression despite blocking the phosphorylation of ERK1/2 and PI3K, respectively (data not shown). In contrast, treatment with 50 μM SB203580, a relatively specific inhibitor of p38 MAPK activation, reduced HOXB4 mRNA to 50% of that seen in TPO-induced control cells. At this concentration of inhibitor the activation of p38 MAPK was significantly inhibited (Figure 4B). SB203580 also inhibited TPO-dependent growth of UT-7/TPO cells at 50 μM (Figure 4C). We also confirmed the effects of SB203580 on Hoxb4 using EML cells. Use of 20 μM SB203580 reduced p38 MAPK activation (Figure 4D), Hoxb4 mRNA expression (Figure 4E), and Hoxb4 protein levels (Figure 4D). SB203580 also reduced the enhanced growth of SCF/TPO-treated EML cells in a dose-dependent manner (Figure 4F).
p38 MAPK inhibitor reducesHOXB4level. (A) UT-7/TPO cells were cultured in 10 ng/mL TPO and treated with 50 μM SB203580, 20 μM PD98059, or 20 μM LY294002 for 72 hours and total RNA was extracted. HOXB4 expression levels were analyzed by real-time RT-PCR; each column represents an average ± SD of several independent experiments (n = 5, control; n = 5, SB203580; n = 3, PD98059; n = 3, LY294002). (B) UT-7/TPO cells were cultured with the indicated concentrations of SB203580 for 72 hours. After each culture period, whole cell lysates were prepared and the activation of p38 MAPK was analyzed by Western blotting. (C) UT-7/TPO cells were cultured with the indicated concentrations of SB203580 and cell number was counted; *P < .05, **P < .01. (D) EML cells were cultured with SCF plus TPO and treated with SB203580 for 72 hours; total cell lysates were prepared and subjected to Western blotting with antiphospho-p38 MAPK, anti-p38 MAPK, and anti-HoxB4 antibodies. (E) EML cells were cultured with SCF plus TPO and treated with 20 μ M SB203580 for 24 hours. Then Hoxb4 levels were analyzed by real-time RT-PCR. Each column represents an average ± SD of several independent experiments (n = 5, control; n = 3, SB203580). (F) EML cells were cultured with SCF plus TPO and various concentrations of SB203580 and cell numbers were counted; *P < .05, **P < .01.
p38 MAPK inhibitor reducesHOXB4level. (A) UT-7/TPO cells were cultured in 10 ng/mL TPO and treated with 50 μM SB203580, 20 μM PD98059, or 20 μM LY294002 for 72 hours and total RNA was extracted. HOXB4 expression levels were analyzed by real-time RT-PCR; each column represents an average ± SD of several independent experiments (n = 5, control; n = 5, SB203580; n = 3, PD98059; n = 3, LY294002). (B) UT-7/TPO cells were cultured with the indicated concentrations of SB203580 for 72 hours. After each culture period, whole cell lysates were prepared and the activation of p38 MAPK was analyzed by Western blotting. (C) UT-7/TPO cells were cultured with the indicated concentrations of SB203580 and cell number was counted; *P < .05, **P < .01. (D) EML cells were cultured with SCF plus TPO and treated with SB203580 for 72 hours; total cell lysates were prepared and subjected to Western blotting with antiphospho-p38 MAPK, anti-p38 MAPK, and anti-HoxB4 antibodies. (E) EML cells were cultured with SCF plus TPO and treated with 20 μ M SB203580 for 24 hours. Then Hoxb4 levels were analyzed by real-time RT-PCR. Each column represents an average ± SD of several independent experiments (n = 5, control; n = 3, SB203580). (F) EML cells were cultured with SCF plus TPO and various concentrations of SB203580 and cell numbers were counted; *P < .05, **P < .01.
Dominant-negative mutant of p38MAPK inhibits TPO-induced HOXB4 elevation
Although we did not find any inhibitory effects of SB203580 on p42/p44 ERK or on c-Jun kinase (JNK) in either cell line (data not shown), it remained possible that SB203580 affected other signaling pathways. Thus, to confirm our results a dominant-negative mutant of p38 was used.19 We introduced this mutant cDNA into UT-7/TPO cells and established 2 independent clones. As shown in Figure 5A, phosphorylation of p38 MAPK is highly suppressed in these clones. In each of these clones, HOXB4 levels failed to increase after treatment with high concentrations of TPO (Figure 5B). Interestingly and consistent with an important HOXB4-induced growth effect on these cells, TPO-induced growth was significantly blunted in cells expressing the dominant-negative p38 (Figure 5C).
Effects of dominant-negative mutant p38 MAPK onHOXB4expression. (A) A cDNA expression vector containing a dominant-negative mutant of p38 MAPK was introduced by lipofection into UT-7/TPO cells and stable cell lines were selected. Overexpression of the cDNA and activity of p38 MAPK were monitored by Western blotting. (B) Parental UT-7/TPO cells and DN-p38 MAPK-expressing cells were treated with 10 ng/mL TPO (□) or 100 ng/mL TPO (▨) for 24 hours and total RNA was prepared. HOXB4 level was determined by real-time RT-PCR. The results represent the average ± SD of 3 independent experiments. ▪ represents the basal HOXB4 level in UT-7/TPO cells cultured with GM-CSF. (C) Parental UT-7/TPO cells and 2 clones of DN-p38 MAPK-expressing UT-7/TPO cells were cultured with 10 ng/mL TPO for the indicated time periods. Each point represents the average ± SD of triplicate samples.
Effects of dominant-negative mutant p38 MAPK onHOXB4expression. (A) A cDNA expression vector containing a dominant-negative mutant of p38 MAPK was introduced by lipofection into UT-7/TPO cells and stable cell lines were selected. Overexpression of the cDNA and activity of p38 MAPK were monitored by Western blotting. (B) Parental UT-7/TPO cells and DN-p38 MAPK-expressing cells were treated with 10 ng/mL TPO (□) or 100 ng/mL TPO (▨) for 24 hours and total RNA was prepared. HOXB4 level was determined by real-time RT-PCR. The results represent the average ± SD of 3 independent experiments. ▪ represents the basal HOXB4 level in UT-7/TPO cells cultured with GM-CSF. (C) Parental UT-7/TPO cells and 2 clones of DN-p38 MAPK-expressing UT-7/TPO cells were cultured with 10 ng/mL TPO for the indicated time periods. Each point represents the average ± SD of triplicate samples.
TPO induces USF-1 binding to the HOXB4 promoter
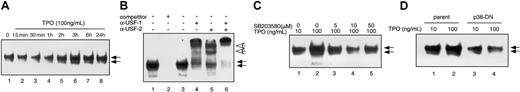
To further explore the mechanisms of TPO-induced expansion of primitive hematopoietic cells, we studied whether USF-1, a downstream mediator of p38 action, and a known HOXB4 transcriptional regulator, is affected by TPO treatment of cells. We performed gel-shift assays using a duplex oligonucleotide probe including the USF-1–binding site of the HOXB4 promoter. We found 2 specific DNA-binding complexes in nuclear extracts of UT-7/TPO cells, because an excess of competitor probe completely inhibited formation of either complex (Figure 6B, lanes 1 and 2). Addition of anti–USF-1 antibody completely shifted the lower complex and partially shifted the upper complex. In contrast, only the upper complex was shifted by an anti–USF-2 antibody (Figure 6B, lanes 3-6), consistent with previous reports concluding that the upper complex contains both USF-1 and USF-2 and the lower complex is composed of USF-1 homodimers. Treatment of UT-7/TPO cells with high concentrations of TPO significantly increased DNA binding in the lower complex (Figure 6A), and to a lesser extent, the upper complex. This result indicates that TPO mainly induces USF-1 binding on the HOXB4 promoter, also consistent with prior reports that USF-1 is phosphorylated by p38 MAPK.23
TPO enhances binding of USF-1 to theHOXB4promoter. (A) UT-7/TPO cells were stimulated with 100 ng/mL TPO for the indicated times and nuclear fractions were prepared for electrophoretic mobility shift assay (EMSA). EMSA was performed using oligonucleotides corresponding to the putative USF-1–binding site of the human HOXB4 gene promoter. Arrows indicate the DNA-protein complexes. (B) To confirm DNA-binding specificity, a 150 M excess of unlabeled probe was added to the reaction mixture (lane 2). Then, 10 μg of the nuclear extracts was incubated for 20 minutes at 20°C with antibody against USF-1 (lane 4), USF-2 (lane 5), or USF-1 and USF-2 (lane 6). White arrows indicate the shifted complexes. (C) UT-7/TPO cells were pretreated with the indicated concentration of SB203580 for 1 hour and then stimulated with 100 ng/mL TPO for 3 hours. Nuclear proteins were prepared and USF-1–binding activity was assessed by EMSA. (D) Parental UT-7/TPO cells and clone 1 p38-DN MAPK-expressing cells were stimulated with 100 ng/mL TPO for 3 hours and nuclear proteins were prepared for EMSA.
TPO enhances binding of USF-1 to theHOXB4promoter. (A) UT-7/TPO cells were stimulated with 100 ng/mL TPO for the indicated times and nuclear fractions were prepared for electrophoretic mobility shift assay (EMSA). EMSA was performed using oligonucleotides corresponding to the putative USF-1–binding site of the human HOXB4 gene promoter. Arrows indicate the DNA-protein complexes. (B) To confirm DNA-binding specificity, a 150 M excess of unlabeled probe was added to the reaction mixture (lane 2). Then, 10 μg of the nuclear extracts was incubated for 20 minutes at 20°C with antibody against USF-1 (lane 4), USF-2 (lane 5), or USF-1 and USF-2 (lane 6). White arrows indicate the shifted complexes. (C) UT-7/TPO cells were pretreated with the indicated concentration of SB203580 for 1 hour and then stimulated with 100 ng/mL TPO for 3 hours. Nuclear proteins were prepared and USF-1–binding activity was assessed by EMSA. (D) Parental UT-7/TPO cells and clone 1 p38-DN MAPK-expressing cells were stimulated with 100 ng/mL TPO for 3 hours and nuclear proteins were prepared for EMSA.
p38 MAPK is involved in TPO-induced USF-1 activation
Finally, we investigated whether TPO-induced USF-1 and USF-2 DNA binding is p38 dependent. UT-7/TPO cells were pretreated with several concentrations of the p38 MAPK inhibitor SB203580 for 1 hour, and then stimulated with 100 ng/mL TPO for 3 hours. SB203580 treatment decreased DNA binding of both complexes in a dose-dependent fashion (Figure 6C). In addition, the dominant-negative p38-expressing cells showed decreased USF-1 and USF-2 binding after treatment with high concentrations of TPO (Figure 6D). These results clearly demonstrate that TPO-induced USF-1 and USF-2 activation is at least partially dependent on p38 MAPK.
Discussion
Besides its important thrombopoietic functions, TPO has been repeatedly shown to support and enhance the expansion and self-renewal of HSCs.2-5,24 Recently, we tested the role of TPO in HSC engraftment. We found that 5-fold more bone marrow cells were required to radioprotect tpo–/– mice than wild-type mice in a transplantation setting.7 Furthermore, expansion of HSCs was reduced 10- to 20-fold in tpo–/– mice in a tandem transplantation protocol. Importantly, supplementation of TPO improved this loss of HSC expansion in tpo–/– mice. In this study we found that TPO increases Hoxb4 mRNA in vitro and in vivo, and that at least in part, TPO up-regulates Hoxb4 through p38 MAPK activation and subsequent DNA binding of transcription factor USF-1 on HOXB4 promoter. These results provide a molecular mechanism by which TPO enhances stem cell self-renewal and expansion.
TPO activates a wide variety of signal transduction molecules including p42/p44 ERK, the JAK-Stat pathway, PI3K, protein kinase A, and several isoforms of protein kinase C.25 Previous studies demonstrated that most of these molecules play important roles in the thrombopoietic function of TPO. However, little is known of the downstream molecules that mediate effects of TPO on HSCs.
To date, several molecules have been proposed to control the self-renewal of HSCs. Among these molecules, we focused our attention on one member of the homeobox family of proteins, Hoxb4, for several reasons; most primitive hematopoietic stem cells express Hoxb4,9 Hoxb4 induces the growth of hematopoietic progenitor cells in vitro,11 and in a bone marrow transplantation model, Hoxb4 overexpression accelerated stem cell regeneration and led to a marked competitive repopulating advantage.10,11 Hoxb4 overexpression also induces ex vivo expansion of HSCs.13 Recently, Giannola and colleagues found that HOXB4 promoter activity is increased by culturing primitive hematopoietic cells with several hematopoietic cytokines.14 This report suggested the possibility that TPO controlled Hoxb4 levels, and through this mechanism, influenced HSC self-renewal and expansion.
We first assessed the effects of TPO on Hoxb4 expression in vitro. TPO induced 2-fold higher levels of Hoxb4 mRNA in 2 primitive hematopoietic cell lines. More importantly, we demonstrated that a hematopoietic cell population highly enriched in HSCs derived from tpo-null mice displays 2 to 5 times lower Hoxb4 levels than control cells. Although our results were reproducible and statistically significant, it could be questioned whether this degree of enhancement is biologically relevant. However, using a retrovirus that doubled HOXB4 expression, Brun and colleagues demonstrated a remarkable degree of enhanced HSC repopulation and expansion in a transplantation study, whereas a 10-fold increased expression of HOXB4 increased myeloid differentiation at the expense of stem cell expansion.26 These results support our argument that the level of TPO induction of Hoxb4 is highly relevant.
Recently, Bjornsson and colleagues report that Hoxb4 and Hoxb3 knockout mice have a 2-fold reduction in repopulating ability in competitive transplantation experiments.27 This reduction level is modest compared with that present in tpo knockout mice, which demonstrated a 10- to 20-fold reduction in repopulating ability.7 This discrepancy would suggest that other molecules might be involved in TPO-induced HSC self-renewal and expansion. One possible target is Hoxa9. Hoxa9 also plays an important role in HSC biology. Hoxa9 is selectively expressed in primitive hematopoietic cells.9 Hoxa9-null mice showed 4- to 10-fold reduction of HSC numbers.21 Furthermore, overexpression of Hoxa9 enhanced the regenerative potential of HSCs.22 Thus it is possible that TPO also controls Hoxa9 expression. However, in contrast to Hoxb4, we could not find any effects of TPO on mRNA level of Hoxa9. This result, of course, does not eliminate other mechanisms of Hoxa9 expression or function by TPO.
Among numerous signal transduction molecules activated by TPO, we found that p38 MAPK is responsible for TPO-induced HOXB4 expression. Inhibitors of ERK or PI3K did not reduce HOXB4 levels. In contrast, the p38 inhibitor SB203580 decreased HOXB4 to 50% of control levels. p38 MAPK was initially reported as a stress-induced MAPK, which induces apoptosis of cells under such conditions.28 For example, UV treatment, irradiation, or deprivation of trophic factors induces activation of this kinase28 in several cell types. In contrast, it has been shown that a variety of growth factors, including hematopoietic cytokines, activate p38 MAPK. Moreover, some reports demonstrate that p38 is crucial for cytokine-induced cellular growth; SB203580 inhibits erythropoietin (EPO)–dependent growth of HCD57 cells.29 More recently, Kapur and coworkers reported that p38 MAPK is important for SCF-induced proliferation of erythroid progenitor cells.30 Consistent with this notion, we report here that inhibition of p38 MAPK results in decreased proliferation in TPO-responsive primitive hematopoietic cell lines. In contrast to these reports, some studies have indicated that p38 MAPK displays suppressive effects on hematopoiesis.31,32 Collectively, it is possible that p38 MAPK activates different downstream effectors dependent on cell type or cytokine. In a previous study, we found that p38 MAPK is activated by TPO in primary mature megakaryocytes,33 although inhibition of p38 MAPK in these cells did not affect TPO-induced proliferation or differentiation. These findings suggest that the function of p38 MAPK is also dependent on the differentiation stage of the target cells. Our present result that p38 MAPK but not ERK is involved in HOXB4 expression control differs from the observation of Giannola and colleagues, who reported that the RAS-ERK pathway is required for HOXB4 promoter activity in K562 cells.14 One possible explanation for the discrepancy is that SB203580 affects ERK activity in our system. However, we confirmed that SB203580 did not inhibit ERK function, rather, somewhat paradoxically, the p38 inhibitor activated ERK both in UT-7/TPO and in EML cells.33 In our experiments, we induced HOXB4 expression by treatment with TPO. In contrast, Giannola and coworkers used an active form of RAS to up-regulate HOXB4 promoter activity. Thus, these differences might explain the discrepancy of the 2 sets of results. Alternatively, differences of the cell types used in each study might affect the results.
To date numerous molecules have been known to be affected by p38 MAPK activation.28 A recent study by Galibert and colleagues indicated that UV-activated p38 MAPK activates USF-1,23 a transcription factor that belongs to the basic helix-loop-helix leucine zipper (b-HLH-LZ) family.34 Binding of USF-1 and USF-2 on the HOXB4 promoter was first reported by Giannola and coworkers. In the present study, we confirmed the binding of both USF-1 and USF-2 on this promoter.14 More importantly, we found that the binding of USF-1, and to a lesser extent USF-2, was enhanced by TPO stimulation. Furthermore, p38 MAPK acted as an upstream stimulator of this transcription factor. These results are consistent with results of Giannola et al, who showed that overexpression of USF-1 more effectively activates the HOXB4 promoter in primitive hematopoietic cells compared with USF-2.14 Gailbert and colleagues reported that USF-2 was also phosphorylated in response to p38.23 Thus, it is possible that other upstream factors or coactivator might be involved in activation of USF family transcription factors in hematopoietic cells.
Finally, as far as we can determine, our studies are the first to report that a hematopoietic cytokine influences HOX gene expression. One of the major controversies in the field of blood cell development is whether the cytokines that affect cellular proliferation and differentiation do so only by preventing programmed cell death (permissive) or actually direct or generate programs dictating cell fate. Proponents of the former theory posit that instead of cytokines, the displays of specific transcription factors are responsible for cell fate determination. Our results indicate that cytokine can actively direct these decisions through transcription factors.
In conclusion, we found that TPO regulates Hoxb4 level in hematopoietic cells through the activation of p38 MAPK and subsequent stimulation of Hoxb4 expression. We also show that TPO through p38 activates a known transcriptional activator of HOXB4, USF-1. In essence, these results strongly suggest that the favorable effects of TPO on HSC self-renewal and expansion are mediated, at least in part, by enhanced expression of Hoxb4.
Supported by National Institutes of Health grants R01 49855 and R01 31615.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Prepublished online as Blood First Edition Paper, July 10, 2003; DOI 10.1182/blood-2003-03-0944.
The authors wish to thank Roger J. Davis (Howard Hughes Medical Institute, University of Massachusetts) for kindly providing a dominant-negative p38 MAPK expression plasmid, Akihiro Shimosaka (Kirin Pharmaceuticals) for the gift of SCF, Donald Foster (Zymogenetics, Inc) for recombinant TPO, Steve Collins (Fred Hutchinson Cancer Research Center) for the EML cells and Norio Komatsu for providing UT-7/TPO cells.

![Figure 2. Hoxb4 level in tpo [–/–] mice. Whole bone marrow cells were prepared from control C57BL6 and tpo [–/–] mice. The cells were pooled (experiment 1: n = 4, C57BL6, n = 4, tpo [–/–] mice; experiment 2: n = 4, C57BL6, n = 5, tpo [–/–] mice) and Sca-1+/c-kit+/Gr-1– cells were collected for real-time PCR analysis. Each column represents an average of HoxB4 level normalized to GAPDH internal control ± SD of 2 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/9/10.1182_blood-2003-03-0944/6/m_h82135169002.jpeg?Expires=1769229296&Signature=WgZ9pZ5M6AJRpmzQopD55XvSPPtdANSohWxTnYuboGqhj3zY1cELAJWJPlYxz7lBXkvb26SzF5jltiD49g9Fg2923-ZSmIV20yMjixY6tLK7RTz5OsKHh~zhuyUZ565Lr7LZCv-ifnVl~4viPmwWBj~hm6yqbLm-uHggf6B~GH7KvlQO-pWxES9~mHHGgqXVNEFIrQlyVqI-yiwcUwjfo3slbiugSQsHwtpUd5R9bqrnNoY~lAoavokutripVLE6RUXuU7vHiu~VrKPujuqneBKBXT6v4U9Uad2Q8leiUeGEjajrZjTiCxR7e5Il-I01ayRJs9ggTyyhAnREf37tqQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. TPO does not enhance Hoxa9 expression. (A) UT-7/TPO cells were cultured with 10 ng/mL or 100 ng/mL TPO for 24 hours, total RNA was prepared, and real-time RT-PCR was performed to quantify HOXA9 level. The bars represent the average ± SD of several independent experiments (n = 3, TPO 10 ng/mL; n = 5, TPO 100 ng/mL). (B) Whole bone marrow cells were prepared from control C57BL6 and tpo[–/–] mice. The cells were pooled (experiment 1: n = 4, C57BL6, n = 4, tpo[–/–] mice; experiment 2: n = 4, C57BL6, n = 5, tpo[–/–] mice) and Sca-1+/c-kit+/Gr-1– cells were collected for real-time PCR analysis. Each column represents the average Hoxa9 level normalized to GAPDH internal control ± SD of 2 experiments (▪, C57/BL6; ▨, tpo[–/–] mice.)](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/9/10.1182_blood-2003-03-0944/6/m_h82135169003.jpeg?Expires=1769229296&Signature=aC7hBDJ78TDylQbieN-EbJTQwsiXDAFDTrz0d1ugQ6pT6zRSMaw9g~Q0xAzdvuNMGmqobXf9GpK2R2CFvXW8hjOJCndMO1sA1LSq6oc7Cq~iqsAHuICnkqyGgswfCzLFv0h15KQi6UXK2trDgYXhIfQwmq7l0ciC3F49I7~qmUIH0FkmGoUxdWeER3PI-sYsW201BRJ6r9kkJmD72YqEQxHEMb2RiUkcGCIM9vovkfGkehnShfpBDeu4HSMgFwq3CbA2H6o6V5s9FuZ4qzj7d138rHe0h4QVHmLqSBuvjYRH4hRqpGhwJ6GNqJF~LizcpuwZEGrS~JapX3up2B0aCw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal