Abstract
Idiopathic pneumonia syndrome (IPS) is a significant noninfectious complication of hematopoietic stem cell transplantation (HSCT). We compared the incidences and outcomes of IPS among patients who underwent allogeneic HSCT after nonmyeloablative (n = 183) compared with conventional (n = 917) conditioning between December 1997 and December 2001. Patients given nonmyeloablative conditioning were older than those given conventional conditioning (median ages, 53 vs 41 years; P = .001). The cumulative incidence of IPS was significantly lower at 120 days after nonmyeloablative conditioning than conventional conditioning (2.2% vs 8.4%; P = .003). In addition, greater patient age (older than 40 years), diagnosis of acute leukemia or myelodys-plastic syndrome, and severe acute graft-versus-host disease were associated with significantly increased risks for IPS. Among older patients (older than 40 years) given conventional conditioning, high-dose total body irradiation (TBI) was associated with an increased risk for IPS than were non-TBI–based regimens (16% vs 5.8%; P = .001). IPS occurred early after transplantation, progressed rapidly, and was associated with a high mortality rate (75%) despite aggressive support. Initiation of mechanical ventilation and the presence of renal insufficiency at IPS onset were associated with increased risks for death after IPS. These findings support the concept that lung damage from the conditioning regimen plays a crucial role in the development of IPS after HSCT.
Introduction
Interstitial pneumonitis is a significant complication after conventional myeloablative hematopoietic stem cell transplantation (HSCT). In approximately half the patients, interstitial pneumonitis is the consequence of noninfectious diffuse lung injury.1,2 Several studies have reported the incidence of idiopathic interstitial pneumonitis, mainly diagnosed by lung biopsy or autopsy, to be 3% to 15% after allogeneic bone marrow transplantation with conventional conditioning.2-8 Previously reported risk factors for the development of idiopathic pneumonia include transplantation for hematologic malignancy, acute graft-versus-host disease (GVHD), and older patient age.3-5
In 1993, a National Institutes of Health workshop proposed a definition of idiopathic pneumonia syndrome (IPS) to include widespread alveolar injury in the absence of active lower respiratory tract infection after bone marrow transplantation.1 Using this definition, we previously reported the incidence, clinical course, and risk factors for IPS among 1165 patients who underwent bone marrow transplantation between 1988 and 1991.9 In that study, the cumulative incidence of IPS was 7.6% in the first 120 days after allogeneic HSCT, and the primary risk factors were grade IV acute GVHD and a diagnosis of malignancy other than leukemia. In 12 studies of 4496 HSCT patients summarized by Afessa et al,10 the overall incidence of IPS was 10% (range, 2%-17%), varying by the patient populations studied, by differences in the diagnostic methods used, and by the lack of a uniform definition of IPS. Outcome was poor among patients in whom IPS developed after conventional HSCT; the mortality rate estimate was 74% (range, 60%-86%) regardless of therapy.10
IPS encompasses a spectrum of clinical presentations and is thought to result from a diversity of lung insults, including toxic effects of myeloablative conditioning, immunologic cell-mediated injury, inflammatory cytokines, and occult pulmonary infections.1,11-17 Advances in transplantation medicine, such as modifications in cytoreductive regimens, changes in donor selection, and improvements in immune suppression and in the diagnosis and prevention of infection could alter the spectrum of lung injury in patients who have undergone allogeneic HSCT. In recent years, allogeneic HSCT with nonmyeloablative or reduced-intensity conditioning regimens has been developed and explored for the treatment of patients ineligible for conventional HSCT because of age or medical contraindications.18-23 Toxicity profiles of nonmyeloablative conditioning regimens are generally considered to be lower and to have fewer myelosuppression and gastrointestinal toxicities than those caused by conventional HSCT.
However, the incidence and clinical course of IPS after nonmyeloablative conditioning have not been investigated. Here, we describe the incidences, risk factors, and outcomes of IPS among patients who underwent allogeneic HSCT after nonmyeloablative conditioning compared with those who underwent it after conventional conditioning at the Fred Hutchinson Cancer Research Center (FHCRC).
Patients, materials, and methods
Study patients
We reviewed the medical records of 1100 patients who underwent allogeneic HSCT after nonmyeloablative (n = 183) or conventional (n = 917) conditioning at the FHCRC between December 1997 and December 2001. Patients who received cord blood, syngeneic grafts, or autologous grafts were not included. Patients who underwent allogeneic HSCT within the previous year were excluded from the analysis. The FHCRC Institutional Review Board approved this retrospective analysis.
The conventional transplantation study population included patients with malignant diseases treated with high-dose total body irradiation (TBI)–based (n = 528) or non–TBI–based (n = 361) myeloablative conditioning regimens and patients with nonmalignant diseases who were administered high-dose cyclophosphamide (n = 15), cyclophosphamide and busulfan (n = 12), or high-dose TBI-based regimens (n = 1) (Table 1). Two hundred ninety-two patients (75% of the patients given non-TBI–based regimens) received cyclophosphamide and targeted-dose busulfan, which was adjusted to achieve a steady state concentration of 800 to 900 ng/mL.24,25 Nonmyeloablative conditioning was given for patients ineligible for conventional HSCT because of age or comorbid conditions. All patients in the nonmyeloablative group received 2 Gy TBI on day 0 with (n = 127) or without (n = 56) preceding fludarabine therapy (30 mg/m2 body surface area per day) on days –4 to –2.18,23 Fractionated TBI was delivered at 6 cGy/min to 15 cGy/min for high-dose TBI regimens and at 7 cGy/min for nonmyeloablative conditioning from dual-opposing cobalt-60 (60Co) sources or a linear accelerator. Lung blocks at 2 half-value layers were used for the first half-fractions by a linear accelerator. Most patients who underwent conventional transplantation were given methotrexate (MTX) and cyclosporine (CSP),26 and all patients who underwent nonmyeloablative transplantation were given mycophenolate mofetil (MMF) and CSP for GVHD prophylaxis.18,23 Granulocyte colony-stimulating factor (G-CSF) was administered in patients with delayed engraftment, graft rejection, or secondary neutropenia. GVHD grading was performed with adjustment for comorbidity.27 Grade II indicates stage 3 skin disease or adjusted stage 1 liver or gut disease; grade III indicates nonfatal stage 4 skin disease or adjusted stage 2-4 liver or gut disease; and grade IV indicates death with uncontrolled GVHD as a major contributing cause. GVHD was treated with 1 to 2 mg/kg/d prednisolone equivalents, resumption of full-dose CSP administration if applicable, or both. Initial doses of corticosteroids and tapering schedules of immunosuppressive medications were modified at the discretion of the attending physicians according to the presence or absence of malignant cells and the severity of GVHD.
Patient and transplantation characteristics after allogeneic HSCT and conventional or nonmyeloablative conditioning
Factors . | Conventional; n = 917 . | Nonmyeloablative; n = 183 . | P . |
|---|---|---|---|
| Median patient age, y (range) | 41 (1-66) | 53 (1-73) | .001 |
| Male/female | 504/413 | 115/68 | .051 |
| Prior transplantation (%) | .001 | ||
| Yes | 20 (2) | 56 (31) | |
| No | 897 (98) | 127 (69) | |
| Underlying diagnosis (%) | .001 | ||
| CML | 316 (34) | 15 (8) | |
| Acute leukemia/MDS | 513 (56) | 52 (28) | |
| Other malignancy | 60 (7) | 108 (59) | |
| Lymphoma/CLL | 45 | 59 | |
| Multiple myeloma | 8 | 34 | |
| Other tumors* | 7 | 15 | |
| Nonmalignant disease | 28 (3) | 8 (4) | |
| Aplastic anemia | 14 | — | |
| Immunodeficiencies | 2 | 7 | |
| Other diseases* | 12 | 1 | |
| Disease risk (%)† | .001 | ||
| Low | 623 (68) | 89 (49) | |
| High | 294 (32) | 94 (51) | |
| Conditioning (%) | NE | ||
| TBI-based, more than 12 Gy | 273 (30) | — | |
| TBI-based, 12 Gy | 256 (28) | — | |
| Non-TBI-based, 0 Gy | 388 (42) | — | |
| 2 Gy TBI | — | 56 (31) | |
| 2 Gy TBI + fludarabine | — | 127 (69) | |
| Donor (%) | .001 | ||
| HLA-matched related | 429 (47) | 120 (66) | |
| HLA-mismatched related | 55 (6) | — | |
| Unrelated | 433 (47) | 63 (34) | |
| Stem cell source (%)‡ | .001 | ||
| PBSC | 317 (35) | 166 (91) | |
| Bone marrow | 600 (65) | 17 (9) | |
| Recipient CMV serostatus (%) | .035 | ||
| Positive | 446 (49) | 105 (57) | |
| Negative | 471 (51) | 78 (43) | |
| Donor CMV status (%) | NS | ||
| Positive | 371 (40) | 86 (47) | |
| Negative | 546 (60) | 97 (53) | |
| GVHD prophylaxis (%)§ | NE | ||
| MTX-containing regimens | 877 (96) | — | |
| Others | 40 (4) | 183 (100) |
Factors . | Conventional; n = 917 . | Nonmyeloablative; n = 183 . | P . |
|---|---|---|---|
| Median patient age, y (range) | 41 (1-66) | 53 (1-73) | .001 |
| Male/female | 504/413 | 115/68 | .051 |
| Prior transplantation (%) | .001 | ||
| Yes | 20 (2) | 56 (31) | |
| No | 897 (98) | 127 (69) | |
| Underlying diagnosis (%) | .001 | ||
| CML | 316 (34) | 15 (8) | |
| Acute leukemia/MDS | 513 (56) | 52 (28) | |
| Other malignancy | 60 (7) | 108 (59) | |
| Lymphoma/CLL | 45 | 59 | |
| Multiple myeloma | 8 | 34 | |
| Other tumors* | 7 | 15 | |
| Nonmalignant disease | 28 (3) | 8 (4) | |
| Aplastic anemia | 14 | — | |
| Immunodeficiencies | 2 | 7 | |
| Other diseases* | 12 | 1 | |
| Disease risk (%)† | .001 | ||
| Low | 623 (68) | 89 (49) | |
| High | 294 (32) | 94 (51) | |
| Conditioning (%) | NE | ||
| TBI-based, more than 12 Gy | 273 (30) | — | |
| TBI-based, 12 Gy | 256 (28) | — | |
| Non-TBI-based, 0 Gy | 388 (42) | — | |
| 2 Gy TBI | — | 56 (31) | |
| 2 Gy TBI + fludarabine | — | 127 (69) | |
| Donor (%) | .001 | ||
| HLA-matched related | 429 (47) | 120 (66) | |
| HLA-mismatched related | 55 (6) | — | |
| Unrelated | 433 (47) | 63 (34) | |
| Stem cell source (%)‡ | .001 | ||
| PBSC | 317 (35) | 166 (91) | |
| Bone marrow | 600 (65) | 17 (9) | |
| Recipient CMV serostatus (%) | .035 | ||
| Positive | 446 (49) | 105 (57) | |
| Negative | 471 (51) | 78 (43) | |
| Donor CMV status (%) | NS | ||
| Positive | 371 (40) | 86 (47) | |
| Negative | 546 (60) | 97 (53) | |
| GVHD prophylaxis (%)§ | NE | ||
| MTX-containing regimens | 877 (96) | — | |
| Others | 40 (4) | 183 (100) |
Numbers of patients (% of the total) are shown for all categories except patient age and patient sex. AML indicates acute myeloid leukemia; ALL, acute lymphocytic leukemia; HD, Hodgkin disease; NHL, non-Hodgkin lymphoma; CLL, chronic lymphocytic leukemia; MM, multiple myeloma; —, not applicable; NE, not evaluable; and NS, not statistically significant.
Other tumors include renal cell carcinoma. Other diseases include paroxysmal nocturnal hemoglobinuria.
Patients were stratified based on underlying disease, as described previously.31 High risk was defined as active, de novo, or relapsed AML, MDS (refractory anemia with excess blasts or excess blasts in transformation), myeloproliferative disorder, ALL, CLL, NHL, HD, MM regardless of status, accelerated phase or blastic crisis of CML, or other tumors such as renal cell carcinoma. Low risk was defined as nonmalignant disease, including aplastic anemia, immunodeficiency syndrome, any of the diseases mentioned with unknown disease status or in remission except for MM, CML chronic phase, and MDS (refractory anemia with or without ringed sideroblasts).
The PBSC group included 6 patients who received PBSCs and bone marrow.
After nonmyeloablative transplantation, all patients were given MMF and CSP. After conventional transplantation, most patients were given MTX and CSP, and others received MMF and CSP (n = 31) or CSP and anti-T-cell antibodies (n = 9).
Infection prophylaxis and diagnostic approach in patients with respiratory problems
Fluconazole (400 mg/d) or itraconazole (200 mg/d intravenously or 2.5 mg/kg 3 times daily by mouth) was administered to all patients for fungal prophylaxis.28,29 Patients were monitored with weekly cytomegalovirus (CMV) pp65 antigenemia testing, and antigenemia was treated with ganciclovir as described previously.30,31 Patients who had positive serologic test results for herpes simplex virus (HSV) received prophylactic low-dose acyclovir from day –5 to day 30 or until the resolution of mucositis, whichever occurred earlier.32 Patients who had positive serologic test results for varicella zoster virus (VZV) received prophylactic low-dose acyclovir until one year after transplantation.33 Prophylaxis against Pneumocystis carinii infection was provided primarily with trimethoprimsulfamethoxazole or, alternatively, with dapsone (50 mg twice daily).33 All patients received prophylactic antibacterial antibiotics (ceftazidime or ciprofloxacin) when absolute neutrophil counts (ANCs) were lower than 500/mm3.33 Patients with persistent fever during the administration of prophylactic antibiotics were treated with additional agents (vancomycin, aminoglycosides, amphotericin formulation) as clinically indicated. Physical examination and chest x-ray or computed tomography (CT) was performed and blood cultures were taken to identify the sources of infections.
All specimens of bronchoalveolar lavage (BAL), lung biopsy, and autopsy were submitted for cytologic and pathologic analyses with conventional staining or culture for bacteria, acid-fast bacilli, Legionella species, fungus, and Pneumocystis carinii; shell vial culture and routine culture for CMV and respiratory syncytial virus (RSV); and direct fluorescence staining with antibodies against CMV, HSV, VZV, RSV, parainfluenza virus, influenza virus, adenovirus, and Legionella as previously described.34 After July 2000, cultures for Mycoplasma and Chlamydia species were routinely performed on all specimens.
Definition of IPS
In this study, we retrospectively identified patients in whom IPS developed within the first 120 days of transplantation by examining a computerized microbiology database, histopathology reports, radiology reports, and chart review. IPS was defined as the presence of multilobar infiltrates by chest x-ray or CT scan, symptoms and signs of pneumonia with abnormal pulmonary physiology, and the absence of active lower respiratory tract infection as determined by BAL, lung biopsy, or autopsy.1 Invasive fungal infections were defined according to the European Organization for Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) criteria.35 Bacterial pneumonia was diagnosed if the BAL culture grew 104 or more colony-forming units (cfu) per milliliter or if pathogenic species were isolated in the setting of compatible radiographic changes.36 Patients with signs and symptoms of fluid overload were not categorized as having IPS if they responded to diuretic therapy. Patients with nondiagnostic BAL results who quickly responded to antimicrobial agents were not categorized as having IPS. The onset day of IPS was defined as the day on which multilobar infiltrates with abnormal pulmonary physiology were first recognized.
Statistical analyses
Groups categorized by conditioning regimen (conventional vs nonmyeloablative) were compared using the t test or the Wilcoxon rank sum test for continuous variables and the Fisher exact test for categorical factors. Probabilities of grades III-IV acute GVHD and IPS were estimated by cumulative incidence. Hazard rates were compared using log-rank tests. In addition to comparing conventional and nonmyeloablative conditioning groups, we analyzed several other factors for their association with IPS. Host factors of interest included patient age, sex, underlying diagnosis, and pretransplantation CMV serostatus. Transplantation characteristics included level of TBI conditioning, hematopoietic stem cell source (bone marrow or peripheral blood), donor type (human leukocyte antigen [HLA]-matched related or unrelated or HLA-mismatched related), donor CMV serostatus, and use of MTX for GVHD prophylaxis. Acute GVHD and engraftment (ANC greater than 500/mm3) were treated as time-dependent covariates in Cox regression models, such that the analysis considered only acute GVHD that preceded the onset of IPS. Multiple Cox regression models were used to assess the conditioning regimen comparison while controlling for other important prognostic factors.
Overall survival after IPS was estimated using Kaplan-Meier curves. We defined post-IPS mortality as death before or within 30 days of hospital discharge. Factors assessed for their association with post-IPS mortality included patient age, donor type, conditioning regimen, acute GVHD, mechanical ventilation, and corticosteroids for IPS treatment. Doses of corticosteroids were categorized as daily prednisolone equivalents administered for the treatment of IPS (none vs 2 mg/kg/d or less vs 4 mg/kg/d or more). Serum creatinine concentration, total bilirubin concentration, and vasopressor use measured within 3 days of IPS onset were also treated as IPS characteristics. Vasopressor use, which we used as a proxy for hemodynamic instability, was defined as a dopamine equivalent (more than 5 μg/kg/min for more than 4 hours).37 Logistic regression models were used to analyze the risk for post-IPS mortality for each factor in a univariable fashion. An exact multiple logistic regression model was used to assess the conditioning regimen and factors with univariable values (P < .10).
Two-sided P values from fitted regression models were derived from the Wald test. No adjustments were made for multiple comparisons; we considered P values less than .05 to be statistically significant.
Results
Patient and transplantation characteristics
Patient and transplantation characteristics of the 1100 patients are summarized in Table 1. Patients who underwent nonmyeloablative transplantation were older than those who underwent conventional transplantation (median ages, 53 vs 41 years; P = .001). The conventional transplantation group contained more patients with chronic myeloid leukemia (CML), acute myeloid and lymphocytic leukemia, and myelodysplastic syndrome (MDS), whereas patients in the nonmyeloablative group contained more patients with other malignancies such as lymphoma and multiple myeloma (P = .001). The nonmyeloablative group contained more patients with high-risk disease than the conventional group (51% vs 32%; P = .001). The conventional group contained more unrelated or HLA-mismatched donors (53% vs 34%; P = .001), whereas the nonmyeloablative group contained more patients who received G-CSF–mobilized peripheral blood stem cells (PBSCs) (91% vs 35%; P = .001). Twenty (2%) of the conventional transplantation patients and 56 (31%) of the nonmyeloablative transplantation patients previously underwent autologous, syngeneic, or allogeneic HSCT with myeloablative conditioning (P = .001).
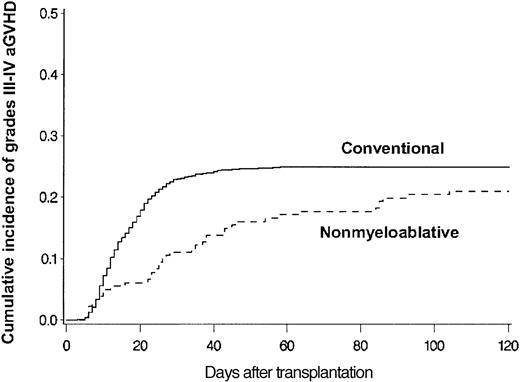
Median follow-up time was 365 days (range, 2-1554 days); more than 75% of patients were followed up for at least 120 days after transplantation. Estimated probabilities of grades III-IV acute GVHD at 120 days among 914 conventional and 181 nonmyeloablative transplantation patients were 25% and 21%, respectively (P = .14) (Figure 1). The onset of GVHD among patients with peak grades III-IV acute GVHD was delayed after nonmyeloablative conditioning (median, 28 days; range, 5-104 days) compared with conventional conditioning (median, 14 days; range, 3-58 days) (P = .001).
Grades III-IV acute GVHD. Cumulative incidence of grades III-IV acute GVHD among allogeneic HSCT patients after conventional (n = 914) or nonmyeloablative (n = 181) conditioning. Cumulative incidence rates of grades III-IV acute GVHD at 120 days after conventional (solid line) or nonmyeloablative (broken line) conditioning were 25% and 21%, respectively (P = .14). GVHD grades could not be assigned because of confounding from severe skin toxicity in 2 patients or from recurrent malignancy and premature termination of GVHD prophylaxis in 3 patients.
Grades III-IV acute GVHD. Cumulative incidence of grades III-IV acute GVHD among allogeneic HSCT patients after conventional (n = 914) or nonmyeloablative (n = 181) conditioning. Cumulative incidence rates of grades III-IV acute GVHD at 120 days after conventional (solid line) or nonmyeloablative (broken line) conditioning were 25% and 21%, respectively (P = .14). GVHD grades could not be assigned because of confounding from severe skin toxicity in 2 patients or from recurrent malignancy and premature termination of GVHD prophylaxis in 3 patients.
Incidence of IPS
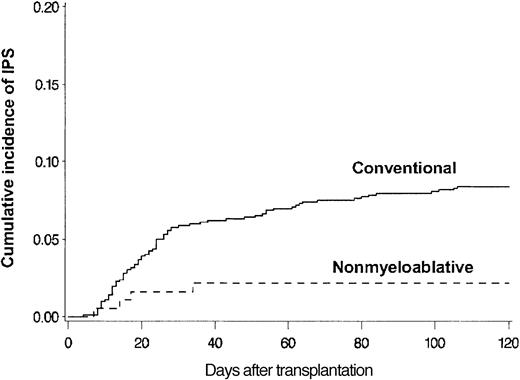
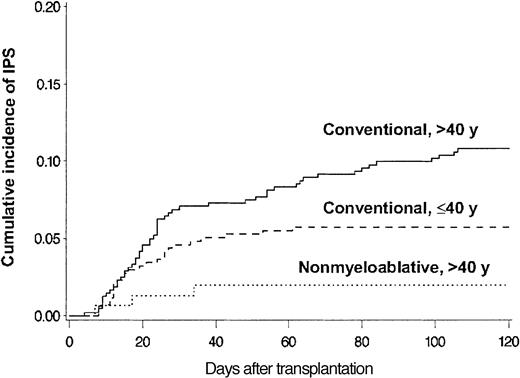
BAL, lung biopsy, or autopsy was performed in 224 patients in whom multilobar infiltrates developed after allogeneic HSCT. Among these patients, infectious organisms were identified in the specimens collected from 115 patients, which excluded these patients from the IPS cohort. Twenty-eight more patients with multilobar infiltrates were excluded from the IPS group because of pulmonary edema (13 patients), rapid response to empiric antibiotics (6 patients), malignant cell infiltrates (4 patients), absence of respiratory symptoms and signs (3 patients), aspiration pneumonia (1 patient), and alveolar proteinosis (1 patient). Eighty-one patients fulfilled the criteria for IPS. The diagnosis of IPS was supported by BAL in 62 patients, lung biopsy in 8, and autopsy in 11. Cumulative incidence rates of IPS at 120 days after allogeneic HSCT with conventional and nonmyeloablative conditioning were 8.4% (77 patients) and 2.2% (4 patients), respectively (P = .003) (Figure 2). Patients older than 40 years had IPS more frequently than younger patients at 120 days after conventional transplantation (11% vs 5.7%; P = .005) (Figure 3).
Differences attributed to conditioning. Cumulative incidence of IPS among allogeneic HSCT patients after conventional (n = 917) or nonmyeloablative (n = 183) conditioning. Cumulative incidence rates of IPS at 120 days after conventional (solid line) or nonmyeloablative (broken line) conditioning were 8.4% and 2.2%, respectively (P = .003).
Differences attributed to conditioning. Cumulative incidence of IPS among allogeneic HSCT patients after conventional (n = 917) or nonmyeloablative (n = 183) conditioning. Cumulative incidence rates of IPS at 120 days after conventional (solid line) or nonmyeloablative (broken line) conditioning were 8.4% and 2.2%, respectively (P = .003).
Differences attributed to patient age and conditioning. Cumulative incidence of IPS among allogeneic HSCT recipients stratified by patient age and conditioning regimen. Cumulative incidence rates of IPS at 120 days after conventional conditioning were 11% and 5.7% for patients older than 40 years (solid line; n = 481) or aged 40 years or younger (broken line; n = 436), respectively (P = .005). Cumulative incidence rate of IPS after nonmyeloablative conditioning was 2.0% for patients older than 40 years (dotted line; n = 152).
Differences attributed to patient age and conditioning. Cumulative incidence of IPS among allogeneic HSCT recipients stratified by patient age and conditioning regimen. Cumulative incidence rates of IPS at 120 days after conventional conditioning were 11% and 5.7% for patients older than 40 years (solid line; n = 481) or aged 40 years or younger (broken line; n = 436), respectively (P = .005). Cumulative incidence rate of IPS after nonmyeloablative conditioning was 2.0% for patients older than 40 years (dotted line; n = 152).
Patients older than 40 years who underwent nonmyeloablative transplantation had a lower cumulative incidence of IPS (2.0%) than older and younger patients who underwent conventional transplantation. Among 81 patients with IPS, 42 (52%) had acute GVHD before the onset of IPS, and 9 more were diagnosed with it after IPS. In 33% of patients, IPS occurred within 5 days of neutrophil engraftment. Importantly, among 30 patients who had IPS without acute GVHD, 12 died before engraftment.
Risk factors for IPS
Univariate analysis revealed that a conventional conditioning regimen (compared with a nonmyeloablative conditioning regimen), older patient age (older than 40 years), grades III-IV acute GVHD, high-dose TBI, underlying diagnosis of acute leukemia or MDS, and use of MTX for GVHD prophylaxis were associated with increased risk for IPS. Conventional conditioning was associated with a significantly increased risk for IPS compared with nonmyeloablative conditioning (hazard ratio [HR] = 4.7; 95% confidence interval [CI], 1.5-14; P = .006) after adjusting for other risk factors. Patient age older than 40 years (HR = 1.8; 95% CI, 1.1-2.9; P = .012) and grades III-IV acute GVHD (HR = 2.8; 95% CI, 1.7-4.5; P = .001) were also associated with significantly increased risks for IPS in a multiple Cox regression model (Table 2). A diagnosis of acute leukemia or MDS was associated with an increased risk for IPS compared with nonmalignant disease or CML (HR = 1.8; 95% CI, 1.1-3.0; P = .030). The risk for IPS did not differ significantly between patients with other malignancies and those with CML or nonmalignant diseases. Use of MTX was omitted from the multivariable model because IPS did not develop among conventional transplantation patients not given MTX prophylaxis (n = 40).
Multiple Cox regression analysis of idiopathic pneumonia syndrome among allogeneic HSCT recipients after conventional or nonmyeloablative conditioning
Variables . | Hazard ratio (95% CI) . | P . |
|---|---|---|
| Conditioning regimen | .006 | |
| Nonmyeloablative | 1.0 | |
| Conventional | 4.7 (1.5-14) | |
| Patient age | .012 | |
| 40 y and younger | 1.0 | |
| Older than 40 y | 1.8 (1.1-2.9) | |
| Acute GVHD* | .001 | |
| Grades 0-II | 1.0 | |
| Grades III-IV | 2.8 (1.7-4.5) | |
| Underlying diagnosis† | ||
| CML/nonmalignant disease | 1.0 | |
| Acute leukemia/MDS | 1.8 (1.1-3.0) | .030 |
| Other malignancy | 1.8 (0.74-4.4) | .20 |
Variables . | Hazard ratio (95% CI) . | P . |
|---|---|---|
| Conditioning regimen | .006 | |
| Nonmyeloablative | 1.0 | |
| Conventional | 4.7 (1.5-14) | |
| Patient age | .012 | |
| 40 y and younger | 1.0 | |
| Older than 40 y | 1.8 (1.1-2.9) | |
| Acute GVHD* | .001 | |
| Grades 0-II | 1.0 | |
| Grades III-IV | 2.8 (1.7-4.5) | |
| Underlying diagnosis† | ||
| CML/nonmalignant disease | 1.0 | |
| Acute leukemia/MDS | 1.8 (1.1-3.0) | .030 |
| Other malignancy | 1.8 (0.74-4.4) | .20 |
Conventional conditioning (n = 917 patients); nonmyeloablative conditioning (n = 183 patients).
Analysis considered only acute GVHD that preceded the onset of IPS.
Other malignancies included HD, NHL, CLL, and MM and other tumors such as renal cell carcinoma. Nonmalignant diseases included aplastic anemia, immunodeficiencies, and other diseases such as paroxysmal nocturnal hemoglobinuria. See Table 1 footnote for abbreviations.
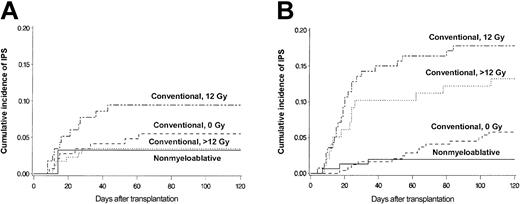
Figure 4 shows more detailed analyses of the contributions of patient age and conditioning regimen to the risk for IPS. Cumulative incidence curves stratified by conditioning regimen are shown separately for patients 40 and younger (Figure 4A) or older than 40 years (Figure 4B). Greater patient age (older than 40 years vs 40 years or younger) was associated with a significantly increased incidence of IPS among patients who received high-dose TBI (12 Gy: 18% vs 9.5%; P = .052; more than 12 Gy: 13% vs 3.4%; P = .001). In contrast, there were no differences in the incidence of IPS by age group after non–TBI–based conventional or nonmyeloablative regimens (5.8% vs 5.5% and 2.0% vs 3.2%, respectively). Conventional transplantation patients older than 40 years acquired IPS more frequently after high-dose TBI-based regimens (12 Gy or more) than after non–TBI–based regimens (16% vs 5.8%; P = .001).
Differences attributed to older and younger age. Cumulative incidence of IPS stratified by conditioning regimen among allogeneic HSCT patients aged 40 years or younger (panel A; n = 467) or older than 40 years (panel B; n = 633). Cumulative incidence rates of IPS among younger patients (panel A; aged 40 years or younger) were 3.4%, 9.5%, 5.5%, and 3.2% after more than 12 Gy TBI-based (dotted line; n = 175), 12 Gy TBI-based (semibroken line; n = 116), or non-TBI–based (broken line; n = 145) conventional conditioning, or nonmyeloablative (solid line; n = 31) conditioning, respectively. Cumulative incidence rates of IPS among older patients (panel B; older than 40 years) were 13%, 18%, 5.8%, and 2.0% after more than 12 Gy TBI-based (dotted line; n = 98), 12 Gy TBI-based (semibroken line; n = 140), or non-TBI–based (broken line; n = 243) conventional conditioning, or nonmyeloablative (solid line; n = 152) conditioning, respectively.
Differences attributed to older and younger age. Cumulative incidence of IPS stratified by conditioning regimen among allogeneic HSCT patients aged 40 years or younger (panel A; n = 467) or older than 40 years (panel B; n = 633). Cumulative incidence rates of IPS among younger patients (panel A; aged 40 years or younger) were 3.4%, 9.5%, 5.5%, and 3.2% after more than 12 Gy TBI-based (dotted line; n = 175), 12 Gy TBI-based (semibroken line; n = 116), or non-TBI–based (broken line; n = 145) conventional conditioning, or nonmyeloablative (solid line; n = 31) conditioning, respectively. Cumulative incidence rates of IPS among older patients (panel B; older than 40 years) were 13%, 18%, 5.8%, and 2.0% after more than 12 Gy TBI-based (dotted line; n = 98), 12 Gy TBI-based (semibroken line; n = 140), or non-TBI–based (broken line; n = 243) conventional conditioning, or nonmyeloablative (solid line; n = 152) conditioning, respectively.
The multiple Cox regression model shown in Tables 3 and 4 confirms these findings. Older and younger patients who received 12 Gy TBI and older patients who received more than 12 Gy TBI had a significantly increased risk for IPS than patients who received nonmyeloablative conditioning. Patients who received non-TBI–based conventional conditioning also showed a trend toward increased risk for IPS than those who received nonmyeloablative conditioning (P = .075). Among recipients of conventional conditioning regimens, older patients who received 12 Gy or more TBI had significantly increased risk for IPS than those who did not receive TBI (Table 4). The incidence of IPS did not differ significantly between patients who received high-dose TBI by 60Co or linear accelerator (data not shown).
Hazard ratios for IPS among patients undergoing allogeneic HSCT
Conditioning regimen . | Hazard ratio (95% CI) . | P . |
|---|---|---|
| Nonmyeloablative, all ages | 1.0 | — |
| Conventional | ||
| TBI 0 Gy, all ages | 2.9 (0.90-9.3) | .075 |
| TBI 12 Gy | ||
| 40 y or younger | 5.1 (1.5-17) | .010 |
| Older than 40 y | 9.3 (2.9-30) | .001 |
| TBI more than 12 Gy | ||
| 40 y or younger | 1.5 (0.39-5.6) | .57 |
| Older than 40 y | 6.2 (1.9-20) | .003 |
Conditioning regimen . | Hazard ratio (95% CI) . | P . |
|---|---|---|
| Nonmyeloablative, all ages | 1.0 | — |
| Conventional | ||
| TBI 0 Gy, all ages | 2.9 (0.90-9.3) | .075 |
| TBI 12 Gy | ||
| 40 y or younger | 5.1 (1.5-17) | .010 |
| Older than 40 y | 9.3 (2.9-30) | .001 |
| TBI more than 12 Gy | ||
| 40 y or younger | 1.5 (0.39-5.6) | .57 |
| Older than 40 y | 6.2 (1.9-20) | .003 |
Patients undergoing a nonmyeloablative conditioning regimen received a TBI dose of 2 Gy. Hazard ratios were determined by age and conditioning regimen, adjusted for acute GVHD and underlying diagnosis. — indicates the reference group.
Hazard ratios for IPS among patients undergoing conventional allogeneic HSCT
TBI dose . | Hazard ratio (95% CI) . | P . |
|---|---|---|
| 0 Gy, All ages | 1.0 | — |
| 12 Gy | ||
| 40 y or younger | 1.7 (0.83-3.6) | .14 |
| Older than 40 y | 3.2 (1.8-5.8) | .001 |
| Greater than 12 Gy | ||
| 40 y or younger | 0.50 (0.20-1.2) | .14 |
| Older than 40 y | 2.1 (1.0-4.3) | .044 |
TBI dose . | Hazard ratio (95% CI) . | P . |
|---|---|---|
| 0 Gy, All ages | 1.0 | — |
| 12 Gy | ||
| 40 y or younger | 1.7 (0.83-3.6) | .14 |
| Older than 40 y | 3.2 (1.8-5.8) | .001 |
| Greater than 12 Gy | ||
| 40 y or younger | 0.50 (0.20-1.2) | .14 |
| Older than 40 y | 2.1 (1.0-4.3) | .044 |
Hazard ratios were determined by patient age and conditioning regimen, adjusted for acute GVHD and underlying diagnosis. — indicates the reference group.
Clinical course and outcome of patients with IPS
We analyzed clinical courses and outcomes of 81 patients in whom IPS developed after allogeneic HSCT with conventional and nonmyeloablative conditioning. Clinical courses and outcomes of IPS were similar after conventional and nonmyeloablative conditioning regimens (Table 5). Median times to IPS onset were day 22 (range, days 4-106) and day 16 (range, days 7-34) after HSCT with conventional or nonmyeloablative regimens, respectively. Median times to IPS onset were day 23 and day 17 among patients with or without acute GVHD, respectively. Sixty of 81 (75%) patients died before or within 30 days of hospital discharge (post-IPS mortality). Most died with progressive respiratory failure, though some also had other critical medical conditions such as refractory GVHD and severe veno-occlusive disease (VOD) of the liver. The median time to death was 14 days (range, 1-979 days) from the onset of IPS. Overall survival was 23% at day 100 and 17% at 1 year after the onset of IPS. Among the 1019 patients who did not acquire IPS in the first 120 days, 165 (16%) died within 120 days of HSCT. In contrast, 62 (77%) of the 81 patients who acquired IPS died within the first 120 days of HSCT.
Clinical courses and outcomes of patients with IPS after allogeneic HSCT
. | Conventional; n = 77 . | Nonmyeloablative; n = 4 . |
|---|---|---|
| Time to IPS onset after HSCT, d, median (range) | 22 (4-106) | 16 (7-34) |
| Survival to 30 days after discharge, no. patients (%) | 20 (26) | 1 (25) |
| Time to death after IPS, d, median (range) | 14 (1-979) | 15 (4-279) |
| MV initiated, no. patients (%) | 48 (62) | 2 (50) |
| Time to MV after IPS, d, median (range) | 1 (0-57) | 2 (0-3) |
| Mortality after MV, no. patients (%) | 46 (96) | 2 (100) |
| Time to death after MV, d, median (range) | 7 (1-32) | 11 (4-17) |
. | Conventional; n = 77 . | Nonmyeloablative; n = 4 . |
|---|---|---|
| Time to IPS onset after HSCT, d, median (range) | 22 (4-106) | 16 (7-34) |
| Survival to 30 days after discharge, no. patients (%) | 20 (26) | 1 (25) |
| Time to death after IPS, d, median (range) | 14 (1-979) | 15 (4-279) |
| MV initiated, no. patients (%) | 48 (62) | 2 (50) |
| Time to MV after IPS, d, median (range) | 1 (0-57) | 2 (0-3) |
| Mortality after MV, no. patients (%) | 46 (96) | 2 (100) |
| Time to death after MV, d, median (range) | 7 (1-32) | 11 (4-17) |
Time to death after IPS (n = 70); time to MV after IPS (n = 50); and time to death after MV (n = 48). MV indicates mechanical ventilation.
Although a second confirmatory procedure was not routinely performed, repeat BAL or lung biopsy was performed if radiographic changes suggested possible infection. Subsequent BALs were performed in 8 patients, lung biopsies were performed in 5 patients, and autopsies were performed in 16 patients within 14 days of the onset of IPS. Among these 29 patients, CMV pneumonia was diagnosed in one of them 14 days after the onset of IPS. Nine (11%) more patients subsequently acquired pulmonary infections more than 14 days after the onset of IPS. Invasive pulmonary aspergillosis developed in 5 patients within a median of 36 days (range, 22-81 days) after IPS onset. Of these patients, 4 died of invasive Aspergillus infection. Three patients had CMV pneumonia within a median of 21 days (range, 19-34 days) after IPS onset, and 2 of these died. One of the 2 had Aspergillus and CMV infections. Other infections included pneumonia caused by adenovirus (1 patient) and Mycobacterium avium complex (1 patient).
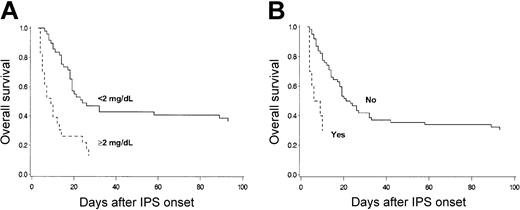
Mechanical ventilation was initiated for respiratory failure in 50 of 81 (62%) patients within a median of 1 day (range, 0-57 days) after the onset of IPS (Table 5). IPS patients in whom mechanical ventilation was initiated had a 96% mortality rate within a median of 7 days (range, 1-32 days) after intubation. The mortality of IPS patients not receiving mechanical ventilation was 39%. Six of 50 patients who underwent mechanical ventilation were successfully extubated after improvement of respiratory function, but 4 subsequently died of respiratory failure attributed to IPS or CMV pneumonia. Eleven patients died of respiratory failure without the initiation of mechanical ventilation. Mechanical ventilation was withheld in 5 patients in accordance with the patients' advance directives, in 1 patient with rapid progression of respiratory failure, in 1 patient with subsequent invasive aspergillosis, in 2 patients with refractory GVHD, and in 2 patients with severe VOD of the liver. Overall survival of the patients who had renal insufficiency, defined as a maximum serum creatinine concentration of 2 mg/dL or more within 3 days of IPS onset, was significantly lower than that of patients without renal insufficiency (Figure 5A). Survival was lower for patients who had hemodynamic instability requiring the use of vasopressor therapy within 3 days of IPS onset than for those without hemodynamic instability (Figure 5B).
OS after IPS. Overall survival after diagnosis of IPS stratified by maximum serum creatinine concentration (A) or vasopressor use (B) among 81 patients who acquired IPS after allogeneic HSCT. (A) Overall survival of the patients who had renal insufficiency, defined as maximum serum creatinine concentration of 2 mg/dL or more, within 3 days of IPS onset (broken line; n = 23) was lower than that of patients without renal insufficiency (solid line; n = 49) (P = .001). (B) Overall survival of patients who required vasopressor within 3 days of IPS onset (broken line; n = 10) was lower than that of patients who did not (solid line; n = 62) (P = .001). Nine patients who died within 3 days of IPS onset were excluded.
OS after IPS. Overall survival after diagnosis of IPS stratified by maximum serum creatinine concentration (A) or vasopressor use (B) among 81 patients who acquired IPS after allogeneic HSCT. (A) Overall survival of the patients who had renal insufficiency, defined as maximum serum creatinine concentration of 2 mg/dL or more, within 3 days of IPS onset (broken line; n = 23) was lower than that of patients without renal insufficiency (solid line; n = 49) (P = .001). (B) Overall survival of patients who required vasopressor within 3 days of IPS onset (broken line; n = 10) was lower than that of patients who did not (solid line; n = 62) (P = .001). Nine patients who died within 3 days of IPS onset were excluded.
Risk factors for post-IPS mortality
Risk factors for post-IPS mortality (death before or within 30 days of hospital discharge) were examined for the 81 patients with IPS (Table 6). Univariate analyses identified the initiation of mechanical ventilation for respiratory failure, the presence of renal or hepatic insufficiency or vasopressor use within 3 days of IPS onset, transplantation from an HLA-matched related donor, and absence of corticosteroid therapy as risk factors for death. Only initiation of mechanical ventilation and renal insufficiency within 3 days of IPS onset remained significant risk factors for death in a multiple logistic regression model. Older patient age, conventional conditioning, and acute GVHD, which were risk factors for the development of IPS, were not significant predictors for death after IPS in this model (data not shown).
Univariate analysis of risk factors for mortality after IPS
Variables . | Total no. patients . | Mortality after IPS, no. patients (%) . | P . |
|---|---|---|---|
| Mechanical ventilation | .001‡ | ||
| Not initiated | 31 | 12 (39) | |
| Initiated | 50 | 48 (96) | |
| Creatinine concentration at IPS* | .001‡ | ||
| Less than 2 mg/dL | 52 | 32 (62) | |
| 2 mg/dL or more | 29 | 28 (97) | |
| Total bilirubin concentration at IPS* | .037 | ||
| Less than 4 mg/dL | 49 | 32 (65) | |
| 4 mg/dL or more | 32 | 28 (87) | |
| Vasopressor use at IPS* | .017 | ||
| No | 68 | 47 (69) | |
| Yes | 13 | 13 (100) | |
| Donor | .023 | ||
| HLA-matched related | 37 | 32 (86) | |
| HLA-mismatched/unrelated | 44 | 28 (64) | |
| Corticosteroids for IPS† | .015 | ||
| None | 10 | 10 (100) | |
| 2 mg/kg/d or less | 28 | 16 (57) | |
| 4 mg/kg/d or more | 43 | 34 (79) |
Variables . | Total no. patients . | Mortality after IPS, no. patients (%) . | P . |
|---|---|---|---|
| Mechanical ventilation | .001‡ | ||
| Not initiated | 31 | 12 (39) | |
| Initiated | 50 | 48 (96) | |
| Creatinine concentration at IPS* | .001‡ | ||
| Less than 2 mg/dL | 52 | 32 (62) | |
| 2 mg/dL or more | 29 | 28 (97) | |
| Total bilirubin concentration at IPS* | .037 | ||
| Less than 4 mg/dL | 49 | 32 (65) | |
| 4 mg/dL or more | 32 | 28 (87) | |
| Vasopressor use at IPS* | .017 | ||
| No | 68 | 47 (69) | |
| Yes | 13 | 13 (100) | |
| Donor | .023 | ||
| HLA-matched related | 37 | 32 (86) | |
| HLA-mismatched/unrelated | 44 | 28 (64) | |
| Corticosteroids for IPS† | .015 | ||
| None | 10 | 10 (100) | |
| 2 mg/kg/d or less | 28 | 16 (57) | |
| 4 mg/kg/d or more | 43 | 34 (79) |
Death occurred before or within 30 days of hospital discharge in 81 patients in whom IPS developed after allogeneic HSCT.
Maximum serum creatinine level, total bilirubin level, and vasopressor use within 3 days of IPS onset.
Prednisolone equivalent.
Initiation of mechanical ventilation and renal insufficiency within 3 days of IPS onset remained significant in a multiple logistic regression model.
Most patients with IPS (88%) received corticosteroid therapy (2 mg/kg/d or less [n = 28] or 4 mg/kg/d or more [n = 43] prednisolone equivalent) within a median of 2 days (range, 0-14 days) after the onset of IPS. There was no significant difference in survival according to corticosteroid dosing strategy. Ten patients died within 10 days of IPS onset without receiving corticosteroid therapy because of the rapid progression of respiratory failure (n = 3), severe VOD of the liver (n = 4), graft failure plus VOD of the liver (n = 1), and possible invasive aspergillosis (n = 2).
Discussion
This study demonstrated a significantly lower incidence of IPS after nonmyeloablative conditioning than conventional conditioning among recipients of allogeneic HSCT. IPS typically occurred early after HSCT, with primary risk factors including older patient age, primary diagnosis of acute leukemia or MDS, use of high-dose TBI, and severe acute GVHD. Nonmyeloablative conditioning was associated with a significantly decreased risk for IPS than conventional conditioning despite older patient age and similar incidence of severe acute GVHD. The cumulative incidence of IPS at 120 days after conventional HSCT in the current study (8.4%) was equivalent to the incidence rates reported by others using a similar definition of the syndrome and comparable diagnostic methods.9,38 This observation confirms that IPS continues to cause transplantation-related morbidity and mortality despite advances in diagnostic methods for opportunistic infections and refinements in supportive care. Our study clearly demonstrated a lower incidence of IPS after nonmyeloablative conditioning than conventional conditioning during a similar time period.
It is postulated that IPS has a multifactorial etiology including toxic effects of myeloablative conditioning, immunologic cell-mediated injury, inflammatory cytokine-induced lung damage, and occult pulmonary infections.1,11-17 The contribution made by each of these potential pulmonary insults to the pathogenesis of IPS, however, remains obscure. We observed that grades III-IV acute GVHD remained prognostic for IPS after adjusting for other risk factors, confirming previous reports.3,5-9 It is important to emphasize that GVHD was analyzed as a time-dependent variable. Only acute GVHD that preceded the diagnosis of IPS was considered in our multiple Cox regression models. Although this observation does not demonstrate a causal relationship, it does support the hypothesis that acute GVHD is a risk factor for IPS. Although immune-mediated lung injury may contribute to the development of IPS, other risk factors must be involved because acute GVHD occurred with similar frequency in the 2 study populations, but the incidence of IPS was lower after nonmyeloablative conditioning than after conventional conditioning regimens.
Greater patient age, commonly defined as older than 20 years, has been reported to be a risk factor for IPS.4,6-8 In the current study, we divided patients based on age—older than 40 years and 40 years or younger—because the median ages of conventional and nonmyeloablative HSCT patients were 41 and 53 years, respectively. Greater patient age (older than 40 years) was associated with an increased risk for IPS, particularly among recipients of high-dose TBI-containing regimens. This observation may have important implications for the selection of conditioning regimens for older patients undergoing transplantation.
Several studies have reported that the underlying hematologic disorder is a risk factor for the development of IPS after HSCT. Patients with nonmalignant diseases such as aplastic anemia were at low risk for IPS, whereas those with malignant diseases were at increased risk.3-7 In our study IPS developed in few patients with nonmalignant disease. For this reason they were analyzed with another low-risk group, patients with CML. Consistent with previous reports, we found that acute leukemia or MDS was associated with increased risk for IPS compared with CML or nonmalignant disease after adjusting for the conditioning regimen and acute GVHD. In contrast to the prior report from our institution, the diagnosis of malignancy other than leukemia, such as lymphoma and myeloma, was not a significant risk for IPS in the current study. The difference observed between the 2 studies most likely reflects the shift from conventional to nonmyeloablative conditioning regimens for patients with lymphoma and myeloma to reduce unacceptably high transplantation-related mortality after conventional allogeneic HSCT.39,40
Regimen-related pulmonary toxicity is thought to be an important contributing factor to the development of IPS after HSCT. Inclusion of TBI in the preparative regimen and radiation dose delivered to the lung have been identified as risk factors for IPS.4,7,12,41 The current study showed that, among patients older than 40 years at transplantation, preparative regimens containing high-dose TBI were associated with a significantly higher risk for IPS than other conventional conditioning regimens, such as those containing busulfan plus cyclophosphamide, with which no TBI is administered. In contrast to our results, a meta-analysis of 5 randomized, prospective studies comparing TBI plus cyclophosphamide versus busulfan plus cyclophosphamide showed no statistically significant differences in the incidence of idiopathic pneumonia.42 The decreased incidence of IPS after busulfan plus cyclophosphamide observed in our study compared with previous reports may reflect the adjustment of busulfan doses to achieve a steady state concentration of 800 to 900 ng/mL, which has been shown to reduce regimen-related toxicity in CML and MDS patients.24,25
Earlier studies identified MTX as a risk factor for the development of interstitial pneumonitis; however, these studies did not distinguish idiopathic pneumonias from viral pneumonias.6,7,43,44 More recent studies have reported that MTX was not a risk factor for idiopathic pneumonia or for IPS.3,9 Moreover, 6 prospective, randomized studies comparing GVHD prophylaxis with or without MTX showed a similar incidence of idiopathic pneumonia for the 2 groups.26,45-49 In the current study, the small number of patients who received conventional conditioning but not MTX and the absence of IPS patients among them limited our ability to assess the risk for IPS attributable to MTX.
An unanticipated result of our study was the observation that the incidence of IPS was higher among patients treated with 12 Gy than with more than 12 Gy TBI. Aside from the total dose of TBI delivered, there were no differences in the methods of TBI administration or dose rate between the 2 groups. The observed difference in the incidence of IPS may be, in part, attributed to differences in the study patient population because patients who received more than 12 Gy TBI were significantly younger than those who received 12 Gy (median ages, 35 vs 41 years; P = .001). Interestingly, recipients of 2 Gy TBI as part of the nonmyeloablative regimen had a trend toward a lower risk for IPS than recipients of non-TBI–based conventional conditioning after adjusting for other covariables.
Our study showed that IPS was an early complication for allogeneic HSCT patients after conventional and nonmyeloablative conditioning regimens. The early onset of IPS observed in the current study is consistent with findings in previous studies.9,38 The observation that 33% of the IPS in the current study occurred within 5 days of neutrophil engraftment suggests that IPS often results from periengraftment respiratory distress syndrome.50
Historically, clinical outcomes among patients with IPS after conventional HSCT have been uniformly poor, with an estimated mortality rate of 74% (range, 60%-86%) regardless of therapy.10 Our study showed the overall mortality rate of patients with IPS was 75%, with a 2-week median time to death after IPS onset for patients who underwent conventional and nonmyeloablative HSCT. Pulmonary infections after the onset of IPS were less frequently identified (11%) in the current study than in previous reports from this institution,9,51 probably as the result of improved diagnostic methods and strategies for infection prophylaxis before and after the diagnosis of IPS. The typical clinical course with rapid progression to respiratory failure and death, however, remained unchanged.
Our study showed that renal insufficiency at IPS onset and the initiation of mechanical ventilation were important prognostic variables for outcome among HSCT recipients who acquired IPS. The poor outcome for patients requiring mechanical ventilation and patients with renal insufficiency at IPS onset was consistent with results reported in larger series for all HSCT recipients with respiratory failure of any cause.37,52 Greater patient age and acute GVHD, risk factors for the development of IPS, were not signifi-cant predictors of outcome after IPS. Although the number of patients with IPS after nonmyeloablative conditioning was small, once IPS was established, the outcomes were similar to those among patients who underwent conventional HSCT.
There is no established therapy for IPS after HSCT except for supportive care combined with the prevention and treatment of infections.10 High-dose corticosteroid therapy has generally been advocated for the treatment of IPS, but no study of sufficient sample size has shown unequivocal benefit from this intervention. In the current study, the absence of corticosteroid therapy for IPS was associated with a worse outcome in the univariate analysis, but this association was not significant in the multivariable model. More important, the use of corticosteroids at doses of at least 4 mg/kg/d prednisolone equivalent showed no better outcome than lower doses (2 mg/kg/d or less). Interpretation of these results is limited by the retrospective nature of this study.
This study has several limitations inherent in its outcome and patient populations. First, eligibility requirements for the conventional and nonmyeloablative conditioning regimens were different. Most nonmyeloablative HSCT patients were considered ineligible for conventional HSCT because of age or comorbid conditions. Second, there was a large difference between the number of patients undergoing a conventional conditioning regimen and those undergoing a nonmyeloablative regimen. This discrepancy reflects the fact that nonmyeloablative conditioning strategies represent a new approach reserved for patients deemed too old or too ill for conventional HSCT. Most patients are still conditioned with conventional regimens. Third, it was not possible to attribute the observed reduction in the incidence of IPS entirely to differences in the conditioning regimens because of differences between the groups with respect to patient age, underlying diagnosis, donor selection, hematopoietic stem cell source, and pretransplantation and posttransplantation immunosuppression. Fourth, it was diffi-cult to determine exactly the total number of patients with pulmonary signs or radiographic infiltrates because of the retrospective nature of the study. It is possible in some patients IPS might have been missed because of mild illness or because the patient was too ill to undergo an invasive diagnostic procedure. For these reasons, the incidence of IPS might have been underestimated. Nevertheless, the very large cohort of allogeneic HSCT recipients in the current study allowed us to make a number of clinically relevant observations.
The current study strongly suggests that if they can control the underlying disease and overcome the risk for graft rejection, nonmyeloablative or targeted dose chemotherapy-based myeloablative regimens may be preferred in the conditioning of patients older than 40 years who are undergoing allogeneic HSCT. The rapid progression to respiratory failure and the high mortality rate after IPS onset, despite advances in critical care, emphasize the need for new treatment modalities. Preclinical and clinical studies are needed to understand the pathogenesis of IPS after allogeneic HSCT. The reduced incidence of IPS among recipients of nonmyeloablative conditioning regimens may provide important clues for studies directed toward decreasing IPS-related nonrelapse mortality after allogeneic HSCT.
Prepublished online as Blood First Edition Paper, July 10, 2003; DOI 10.1182/blood-2003-05-1597.
Supported by National Institutes of Health grants CA78902, CA18029, CA15704, K23-CA92058, and HL36444. T.F. was supported by a fellowship from the Kirin Brewery Company, Ltd.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the medical, nursing, data processing, pathology, and laboratory staffs at the FHCRC for their important contributions to this study through dedicated care of the patients. We also thank Deborah M. Bassuk and Chris Davis for assistance with data collection; Joan G. Clark, Rachel A. Carter, Kieren A. Marr, and Richard A. Nash for their helpful discussions; and Helen S. Crawford and Bonnie S. Larson for manuscript preparation.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal