Abstract
To assess the clinical benefit of rituximab for HIV-associated Castleman disease, 5 patients infected with HIV with histologic-proven Castleman disease were prospectively enrolled to receive 4 infusions of rituximab. Clinical and biologic parameters (C-reactive protein, CD19 cell count, Kaposi sarcoma–associated herpesvirus [KSHV] viral load in peripheral blood mononuclear cells) were assessed before and at different time points following rituximab infusions. Two patients died very quickly after the beginning of rituximab therapy with no effect on both KSHV viral load and CD19 cell count. Three of 5 patients were considered in complete remission with no more clinical symptoms related to Castleman disease with a follow-up of 4 to 14 months. In 2 cases, clinical remission correlated with a dramatic decrease of KSHV viral load and C-reactive protein levels and a transitory but sharp decrease of CD19 cell count. In 2 responders, we observed an aggravation of Kaposi sarcoma. Our preliminary results suggest that rituximab may be effective in controlling Castleman disease in a subset of patients, although it may exacerbate concomitant Kaposi sarcoma.
Introduction
Kaposi sarcoma–associated herpesvirus (KSHV) is implicated in a limited subset of lymphoproliferative disorders such as primary effusion lymphoma (PEL) and multicentric Castleman disease (MCD).1 MCD is characterized by lymphadenopathy with angiofollicular hyperplasia and plasma cell infiltration. In the context of HIV infection, MCD is always associated with KSHV infection, and elevated KSHV viral load in peripheral blood mononuclear cells (PBMCs) is associated with exacerbation of MCD-related clinical symptoms.2,3 Although single-agent chemotherapy with vinblastine may be effective in MCD, clinical remission is typically short lived, there is no current treatment permitting a sustained response, and the median survival of patients after the diagnosis of the disease is 14 months, before highly active antiretroviral therapy (HAART).4 Although the benefit of HAART for Kaposi sarcoma has been reported with both clinical improvement and dramatic decrease of its incidence since 1996, initiation of HAART has little effect on the course of MCD, and in some instances HAART could even precipitate the onset of MCD.3,5-9
It has been shown that lymph nodes of patients with KSHV-related MCD harbored the virus in immunoglobulin M (IgM)/λ-restricted plasmablasts localized in the mantle zone with a variable expression of the CD20 surface antigen.10 A recent case study reported a long-term remission of MCD with a single dose of anti-CD20 monoclonal antibody.11 We, therefore, evaluated the virologic, biologic, and clinical outcome of 5 patients infected with HIV with MCD treated by 4 weekly infusions of rituximab (Mabthera; Roche, Basel, Switzerland), a monoclonal antibody to B-cell antigen CD20.
Study design
Five patients infected with HIV (mean age, 40 years; 4 men, 1 woman) with histologically proven Castleman disease were included prospectively in a nonrandomized study evaluating the effect of 4 weekly infusions of rituximab at the standard dose of 375 mg/m2 (Mabthera; Roche, Basel, Switzerland). The mean characteristics of patients are presented in Table 1. Two patients (patients 1 and 2) were previously treated with periodic infusions of vinblastine that led to a substantial response, but the discontinuation of treatment resulted in the resumption of clinical symptoms and inflammatory syndrome related to MCD within 2 to 3 weeks. In patient 5, onset of MCD was precipitated by HAART, and patient 4 received 2 infusions of vinblastine that had a transitory effect. Two patients (patients 2 and 3) received intravenous immunoglobulins for severe, life-threatening hematologic disorders (ie, hemolytic anemia in one case and cyclic neutropenia in the other case). During the follow-up, clinical symptoms were recorded (fever, lymph node enlargement, hepatosplenomegaly), and biologic parameters were determined (HIV-1 plasma viral load, CD4/CD8 and CD19 cell counts, and C-reactive protein [CRP] levels) at different time points using standard procedures. Before and sequentially after rituximab infusions, PBMCs were isolated by Ficoll-Hypaque density gradient centrifugation (Pharmacia, Uppsala, Sweden). DNA was extracted from PBMCs by using QIAamp system (QIAgen, Chatsworth, CA), according to the manufacturers' instructions, and was then subjected to a real-time polymerase chain reaction (PCR) assay combining the quantification of KSHV and albumin gene DNA.12 Each sample was evaluated in duplicate reactions. This evaluation was performed with the fluorescent TaqMan methodology on ABI Prism 7700 Sequence Detection System (PE Applied Biosystems, Foster City, CA). Negative controls for all assays included 2 reactions that contained no DNA. KSHV viral load in PBMCs was expressed as the absolute KSHV genome copy number in 150 000 human diploid cells, and the lower limit of quantification was 10 copies per 150 000 cells.
Clinical features of patients
Patients . | Date of HIV diagnosis . | CDC stage . | ARV treatment . | CD4 count, cells/mm3 . | HIV-RNA log, copies/mL . | Previous MCD treatment . | Associated treatment . | Associated pathology . | Clinical outcome . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1997 | A2 | d4T/ddl/EFV | 635 | < 2.3 | Vinblastine (2 y) | None | None | CR |
| 2 | 1989 | C3 | EFV/APV/LPV | 669 | < 2.3 | Vinblastine (6 y) | IVIG | KS, MAS, neutropenia | Death |
| 3 | 2000 | C3 | d4T/3TC/NVP | 170 | < 2.3 | None | IVIG, vinblastine, corticoids | KS, hemolytic anemia | Death |
| 4 | 1998 | C3 | ZDV/3TC/ABC | 76 | 3.9 | Vinblastine (2 inf) | None | KS | CR |
| 5 | 1997 | C3 | ddl/3TC/TDF/EFV/LPV | 192 | < 2.3 | None | None | KS | CR |
Patients . | Date of HIV diagnosis . | CDC stage . | ARV treatment . | CD4 count, cells/mm3 . | HIV-RNA log, copies/mL . | Previous MCD treatment . | Associated treatment . | Associated pathology . | Clinical outcome . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1997 | A2 | d4T/ddl/EFV | 635 | < 2.3 | Vinblastine (2 y) | None | None | CR |
| 2 | 1989 | C3 | EFV/APV/LPV | 669 | < 2.3 | Vinblastine (6 y) | IVIG | KS, MAS, neutropenia | Death |
| 3 | 2000 | C3 | d4T/3TC/NVP | 170 | < 2.3 | None | IVIG, vinblastine, corticoids | KS, hemolytic anemia | Death |
| 4 | 1998 | C3 | ZDV/3TC/ABC | 76 | 3.9 | Vinblastine (2 inf) | None | KS | CR |
| 5 | 1997 | C3 | ddl/3TC/TDF/EFV/LPV | 192 | < 2.3 | None | None | KS | CR |
CDC indicates Centers for Disease Control and Prevention Disease Status; ARV, antiretroviral; d4T, stavudine; 3TC, lamivudine; ZDV, zidovudine; ddl, didanosine; ABC, abacavir; TDF, tenofovir; APV, amprenavir; LPV, lopinavir; EFV, efavirenz; NVP, nevirapine; MCD, multicentric Castleman disease; IVIG, intravenous immunoglobulin; inf, infusions; KS, Kaposi sarcoma; MAS, macrophagic-activating syndrome; and CR, complete remission.
Results and discussion
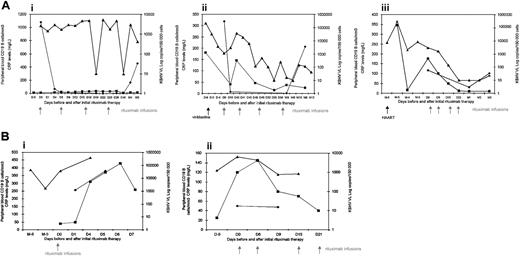
Three of 5 patients (patients 1, 4, and 5) were in complete remission with no clinical symptoms related to MCD (ie, fever, lymph node enlargement, and splenomegaly) with a follow-up of 4 to 14 months after infusions of rituximab. Longitudinal evaluation of KSHV DNA in PBMCs, CRP, and CD19 cell counts for these patients is presented in Figure 1. For patient 1, treatment with rituximab allowed the discontinuation of vinblastine infusions with a follow-up of 6 months, and KSHV viral load dropped substantially but temporarily after the third and the fourth infusion of rituximab. In patient 4 and patient 5, clinical remission correlated with biologic response (ie, the regression of inflammatory syndrome) and a significant decrease of KSHV viremia. KSHV DNA in PBMCs showed a consistent decrease after each rituximab infusion and finally was below the limit of detection or was present episodically and always at low titers. Interestingly, those 3 patients who responded to rituximab experienced a transitory but sharp decrease of the CD19 cell count following rituximab infusions, with a return to baseline level after a few months, without recurrence of clinical symptoms (Figure 1A). Patients remained on antiretroviral therapy during rituximab therapy, and no effects on virologic and immunologic markers of HIV-1 infection were observed (data not shown). No adverse reactions were encountered during infusions of rituximab, although in 2 cases (patients 4 and 5) we observed an aggravation of KS predominantly of the lower limbs requiring local electron therapy.
Longitudinal evolution of KSHV DNA copies in PBMCs, serum CRP, and peripheral blood CD19 B-cell counts in patients infected with HIV-1 with multicentric Castleman disease before and after treatment with 4 weekly infusions of rituximab. (A) Responders include patients 1 (i), 4 (ii), and 5 (iii) with complete remissions with no clinical symptoms with a follow-up of 3 to 12 months. (B) Nonresponders include patients 2 (i) and 3 (ii) without any response to rituximab. VL indicates viral load; D, day; W, week; and M, month. ▪ indicates CRP; ♦, CD19; and ▴, KSHV VL.
Longitudinal evolution of KSHV DNA copies in PBMCs, serum CRP, and peripheral blood CD19 B-cell counts in patients infected with HIV-1 with multicentric Castleman disease before and after treatment with 4 weekly infusions of rituximab. (A) Responders include patients 1 (i), 4 (ii), and 5 (iii) with complete remissions with no clinical symptoms with a follow-up of 3 to 12 months. (B) Nonresponders include patients 2 (i) and 3 (ii) without any response to rituximab. VL indicates viral load; D, day; W, week; and M, month. ▪ indicates CRP; ♦, CD19; and ▴, KSHV VL.
Two of 5 patients (patients 2 and 3) died very quickly after the beginning of treatment with rituximab. Both patients were sick prior to rituximab infusions and had severe hematologic autoimmune disorders associated with MCD, requiring iterative administration of corticosteroids and intravenous immunoglobulin. Patient 2 presented with macrophage activation syndrome (MAS) simultaneously with relapses of Castleman-related symptoms and pulmonary signs such as severe hypoxemia, associated with cyclic autoimmune neutropenia. Four days after one infusion of rituximab, he presented with acute respiratory distress syndrome and increased white blood cell count with 44 × 109/L neutrophils, plasmacytosis, and myelemia. He died at day 8 with multivisceral collapse and refractory lactic acidosis. Patient 3 presented with severe chronic hemolytic anemia resistant to iterative administration of intravenous immunoglobulin. Despite 4 infusions of rituximab, Castleman symptoms persisted and anemia worsened. This patient died 4 days after splenectomy with severe acidosis and multivisceral collapse. These patients had no response either on KSHV viral load or CD19 cell counts, whereas the 3 responders experienced a sharp decrease of circulating CD19+ B cells (Figure 1B).
The presence of clinical symptoms of MCD is strongly associated with high KSHV viral load in PBMCs, and blood viral monitoring may represent the most accurate marker of disease activity and response to therapy.2,3 Although HAART has been shown to improve KS, it has little effect on the evolution of MCD and the subsequent risk of development of non-Hodgkin lymphomas.2-9 Pathophysiology of MCD is still unclear, but KSHV seems to have a trigger role in the setting of HIV infection. KSHV is always found in HIV-associated MCD and exacerbations of clinical symptoms related to MCD are associated with a high increase of KSHV viral load in PBMCs as well as with elevation of human interleukin-6 (IL-6) and human IL-10 serum levels.2,3 In lymph nodes of patients with MCD, KSHV is present in IgM/λ-restricted plasmablasts localized in the mantle zone of the follicles, and 10% to 20% of KSHV-infected cells express viral IL-6 that purportedly plays a pivotal role in MCD pathogenesis.5 Nonrandomized studies have shown that monoclonal antibodies against human IL-6 may have a potential interest in the treatment of MCD in patients without HIV infection.13
Because CD20 is variably expressed by KSHV-infected cells, anti-CD20 monoclonal antibody (rituximab) may be of interest for the management of MCD. Rituximab is a chimeric human immunoglobulin G (IgG) anti-CD20 monoclonal antibody that mediates cytotoxicity against CD20+ malignant B cells in activating complement-dependent cytotoxicity (CDC) and antibody-dependent cell-mediated cytotoxicity (ADCC). One previous case study had reported long-term remission of MCD with a single dose of rituximab in a patient infected with HIV.11 Herein, we report the success of rituximab in controlling MCD symptoms in 3 of 5 patients with MCD. Clinical symptoms related to MCD did not recur with a follow-up of 4 to 14 months, and clinical response was associated with a large decrease of KSHV viral load and of the CRP levels. These patients experienced a sharp decrease of CD19+ cells in peripheral blood, which has been previously observed in patients treated with rituximab. These results argue for the role of rituximab in targeting and purging KSHV-infected neoplastic B cells. However, the fact that blood B cells dropped to very low levels while the viral load in PBMCs decreased gradually may indicate that other types of blood cells may contain the virus or that KSHV-infected B cells may express less CD20 or that KSHV may confer to cells a relative survival advantage.
The other 2 patients died quickly after the start of rituximab infusions, and these patients had no response either on KSHV viral load or CD19 cell count. The nonresponders had MCD associated with autoimmune disorders and were treated in parallel with intravenous immunoglobulin, suggesting that rituximab may worsen autoimmune disorders via the knock-out of a humoral immune control or that intravenous immunoglobulin may interact with rituximab and abrogate the potential interest of the drug, which is supported by the lack of CD19 cell decrease after infusions.
Two responders experienced an aggravation of their KS, although HIV-1 load and CD4 cell count remained as baseline, suggesting that rituximab may have exacerbated KS. Although KS and MCD are both associated with KSHV, these diseases are clearly different in terms of response to HAART and to rituximab. Studies evaluating the benefit of rituximab, combined or not with antiherpesviral agent are warranted, to determine potential factors associated with clinical response in patients with MCD and the long-term effect on KS in this instance.
Prepublished online as Blood First Edition Paper, July 3, 2003; DOI 10.1182/blood-2003-03-0951.
Supported in part by grants from Association pour la Recherche sur le Cancer (ARC), Sidaction, and Association de Recherche en Virologie et Dermatologie (ARVD).
Presented at the Seventh International Conference on Malignancies in AIDS and Other Immunodeficiencies, Bethesda, MD, April 28-29, 2003.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Françoise Picard for performing some of the CD19 phenotype and Didier Bouscary for clinical contributions.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal