Abstract
Acute graft-versus-host disease (GVHD) is a common complication of allogeneic hematopoietic stem cell transplantation (HSCT). It has been proposed that tumor necrosis factor α (TNF-α) blockade with infliximab may be an effective treatment for severe (grades III-IV) GVHD. We determined if infliximab use in this high-risk population was associated with an additional increased risk of non-Candida invasive fungal infections (IFIs). Records of the 2000-2001 HSCT cohort at our institution were reviewed. Fifty-three (20%) of 264 evaluable patients developed severe GVHD and 11 of these 53 (21%) received infliximab for treatment. Proven or probable IFI was documented in 10 (19%) of 53 patients with severe GVHD (incidence rate of 0.99 cases/1000 GVHD patient-days). When stratified by infliximab use, 5 of 11 infliximab recipients developed an IFI (6.78 cases/1000 GVHD patient-days), compared with 5 of 42 IFI cases among nonrecipients (0.53 cases/1000 GVHD patient-days). In a time-dependent Cox regression model among patients with severe GVHD, the adjusted IFI hazard ratio of infliximab exposure was 13.6 (P = .004; 95% CI, 2.29-80.2). We conclude that infliximab administration is associated with a significantly increased risk of non-Candida IFI in HSCT recipients with severe GVHD disease. Pre-emptive systemic antifungal therapy against molds should be considered in patients who develop severe GVHD after HSCT if infliximab is used.
Introduction
Acute graft-versus-host disease (GVHD) is a common complication of allogeneic hematopoietic stem cell transplantation (HSCT).1 Severe GVHD has been consistently associated with an increased relative risk (RR) of treatment-related mortality (RR of 5.33 when comparing patients with grades III-IV GVHD and those with grade II GVHD).1 Excess mortality is due to irreversible damage to skin, liver, and gastrointestinal tract and to infectious complications related to both loss of barrier integrity and to increased immunosuppression needed to control GVHD.2 Invasive fungal infections (IFIs) have become a leading cause of morbidity and mortality in this population.3,4
Patients with grades III to IV GVHD are usually treated with intensification of their immunosuppressive regimen, typically with high-dose corticosteroids; however, there is no standard therapy for patients with GVHD who fail an initial course of corticosteroids.2 Tumor necrosis factor α (TNF-α) is an important cytokine involved in the development of GVHD,5-8 and studies have shown possible benefit of anti–TNF-α antibody administration in treating GVHD.9,10
Infliximab was administered to patients in our institution for treatment of severe or refractory grades III to IV GVHD, and although responses were observed, many died of opportunistic infections. We evaluated a cohort of patients who underwent HSCT to assess the risk of non-Candida IFIs associated with infliximab use.
Patients and methods
Patients
Patients who received an allogeneic hematopoietic stem cell (HSC) transplant between January 1, 2000, and December 31, 2001, were identified through the clinical database at Dana-Farber Cancer Institute/Brigham and Women's Hospital Hematopoietic Stem Cell Transplantation Service. Information regarding age, sex, diagnosis, transplant type and treatment protocol, source of stem cells, risk group, conditioning regimen, donor relatedness and HLA matching, date of transplantation, cytomegalovirus (CMV) serology of recipients and donors, and date and causes of death were collected. Indications for HSCT considered low risk were acute myelogenous leukemia or acute lymphocytic leukemia in first complete remission, chronic myelogenous leukemia in first complete response, aplastic anemia, refractory anemia, and refractory anemia with ringed sideroblasts; all other indications were considered high risk.11
Acute GVHD was defined according to the modified Glucksberg scale12 and data were collected on GVHD prophylaxis regimen, date of onset, and maximum overall and organ-specific grade. Severe GVHD was defined as an overall grade of III or IV.12
Detailed information about all drugs used for treatment of patients who developed severe GVHD was obtained. These included oral and intravenous corticosteroids, cyclosporine, tacrolimus, sirolimus, mycophenolate mofetil (MMF), daclizumab, and infliximab.
IFIs were classified according to the 2002 European Organisation for Research and Treatment of Cancer (EORTC)/National Institute of Allergy and Infectious Deseases (NIAID) international consensus.13 Cases of IFI were identified by review of the medical records of all patients identified in the cohort and by review of all pathology, microbiology, infection control, and radiology databases. Physicians documented their findings without knowledge of infliximab exposure. Only proven or probable IFI not due to Candida species were considered for the analysis. IFI date was documented as the day when the diagnostic procedure was performed for proven IFI, or the day when both radiology and microbiology data were available to the clinician for probable IFI. If a diagnosis of IFI was only made after death, the IFI date was considered the date of death, but if a probable IFI diagnosis was confirmed at postmortem examination, the IFI date was documented as the day when the probable IFI diagnosis was made.
All doses of any corticosteroid received by patients with severe GVHD were transformed into prednisone equivalents using the corticosteroid equivalence table.14 The cumulative corticosteroid dose, adjusted to body weight, was calculated from the day of HSCT until death, the development of IFI, the end of cohort follow-up period, or when corticosteroids where tapered below 20 mg/d for more than 30 days. Empiric and prophylactic antifungal use was documented.
Follow-up ended on June 30, 2002, for analysis. Surviving patients were censored on that date or on the last visit before that date. The study was approved by the Human Research Committee at Dana-Farber/Partners Cancer Care.
Statistical analysis
The 2-sided Fisher exact test, Wilcoxon test, or t test was used as appropriate for comparison of baseline characteristics. IFI incidence rates and incidence rate ratios were calculated according to different exposure categories from day of transplantation in the HSCT cohort, and from day of onset of GVHD in those who developed severe GVHD; patients were censored at death or last visit before the end of follow-up. Confidence intervals for incidence rates and incidence rate ratios were calculated using the Haenszel15 and Byar method,16 respectively. Kaplan-Meier curves were calculated for survival and for time to IFI from date of transplantation. In those patients with severe GVHD, time to IFI from onset of acute GVHD was also calculated. Times to event were compared by using the log-rank test. Time-dependent Cox regression analysis of time to IFI from onset of GVHD was done to control for possible confounding or interactions among variables for patients with severe GVHD. Univariate Cox models were calculated for all possible risk factors among patients with severe GVHD. All covariates with a P value of less than .2 on univariate Cox analysis of IFI were considered in the multivariable Cox model. Infliximab was modeled as a time-dependent variable17 ; its exposure was assumed constant once weekly infusions were initiated. Only candidate variables that were statistically significantly associated (P < .05) with IFI in the final model were retained unless significant confounding was noted. The SAS System for Windows, version 8.01 (SAS Institute, Carey, NC), was used for the above analyses.
Results
A total of 270 HSCTs were performed from January 1, 2000, until December 31, 2001. Three patients received a myeloablative HSC transplant followed later by a nonmyeloablative HSC transplant for relapsed disease. These patients were withdrawn from the analysis; none of them developed severe GVHD or an IFI. A total of 197 evaluable patients received a myeloablative HSC transplant and 67 received a nonmyeloablative HSC transplant during this period. The baseline characteristics of the cohort according to transplant type are presented in Table 1.
Characteristics of the HSCT cohort according to transplant type
Variable . | Myeloablative . | Nonmyeloablative . |
|---|---|---|
| No. of patients | 197 | 67 |
| Median age, y (range) | 42 (18-62) | 52 (21-66) |
| Male, % | 58.4 | 62.7 |
| HLA matching, %* | ||
| Matched-related | 50.8 | 46.3 |
| Matched-unrelated | 30.9 | 49.2 |
| Mismatched-related | 5.1 | 3.0 |
| Mismatched-unrelated | 13.2 | 1.5 |
| Stem cell source, % | ||
| Bone marrow | 82.2 | 3.0 |
| Peripheral | 17.8 | 97.0 |
| Conditioning regimen, % | ||
| Cyclophosphamide | 100 | 0 |
| TBI | 94.9 | 0 |
| ATG | 1.5 | 0 |
| Busulfan | 3.6 | 100 |
| Fludarabine | 0 | 100 |
| Diagnosis, % | ||
| AML | 31.0 | 28.4 |
| CML | 18.3 | 11.9 |
| MDS | 15.2 | 13.4 |
| NHL | 9.6 | 19.4 |
| ALL | 14.7 | 3.0 |
| CLL | 2.0 | 11.9 |
| Other | 9.1 | 11.9 |
| Risk group, %† | ||
| Low | 36.6 | 11.9 |
| High | 63.4 | 88.1 |
| CMV serology of donor/recipient, %‡ | ||
| D-/R- | 48.2 | 39.7 |
| D-/R+ | 13.7 | 22.4 |
| D+/R- | 19.3 | 25.9 |
| D+/R+ | 18.8 | 12.1 |
| GVHD prophylaxis, %§ | ||
| Methotrexate | 72.1 | 0 |
| Cyclosporine | 27.4 | 100 |
| Tacrolimus | 54.3 | 0 |
| Sirolimus | 21.8 | 0 |
| MMF | 1.0 | 16.4 |
| T-cell manipulation∥ | 22.8 | 6.0 |
| Corticosteroids | 18.8 | 94.0 |
| Total patient-days | 64 759 | 18 485 |
| Median follow-up, d (IQR) | 255 (102-527) | 250 (181-341) |
| Median survival, d (95% CI) | 468 (257-871 +) | 341 (249-503) |
Variable . | Myeloablative . | Nonmyeloablative . |
|---|---|---|
| No. of patients | 197 | 67 |
| Median age, y (range) | 42 (18-62) | 52 (21-66) |
| Male, % | 58.4 | 62.7 |
| HLA matching, %* | ||
| Matched-related | 50.8 | 46.3 |
| Matched-unrelated | 30.9 | 49.2 |
| Mismatched-related | 5.1 | 3.0 |
| Mismatched-unrelated | 13.2 | 1.5 |
| Stem cell source, % | ||
| Bone marrow | 82.2 | 3.0 |
| Peripheral | 17.8 | 97.0 |
| Conditioning regimen, % | ||
| Cyclophosphamide | 100 | 0 |
| TBI | 94.9 | 0 |
| ATG | 1.5 | 0 |
| Busulfan | 3.6 | 100 |
| Fludarabine | 0 | 100 |
| Diagnosis, % | ||
| AML | 31.0 | 28.4 |
| CML | 18.3 | 11.9 |
| MDS | 15.2 | 13.4 |
| NHL | 9.6 | 19.4 |
| ALL | 14.7 | 3.0 |
| CLL | 2.0 | 11.9 |
| Other | 9.1 | 11.9 |
| Risk group, %† | ||
| Low | 36.6 | 11.9 |
| High | 63.4 | 88.1 |
| CMV serology of donor/recipient, %‡ | ||
| D-/R- | 48.2 | 39.7 |
| D-/R+ | 13.7 | 22.4 |
| D+/R- | 19.3 | 25.9 |
| D+/R+ | 18.8 | 12.1 |
| GVHD prophylaxis, %§ | ||
| Methotrexate | 72.1 | 0 |
| Cyclosporine | 27.4 | 100 |
| Tacrolimus | 54.3 | 0 |
| Sirolimus | 21.8 | 0 |
| MMF | 1.0 | 16.4 |
| T-cell manipulation∥ | 22.8 | 6.0 |
| Corticosteroids | 18.8 | 94.0 |
| Total patient-days | 64 759 | 18 485 |
| Median follow-up, d (IQR) | 255 (102-527) | 250 (181-341) |
| Median survival, d (95% CI) | 468 (257-871 +) | 341 (249-503) |
Follow-up ended on June 30, 2002, for analysis. The table includes the patients who received an HSC transplant from January 1, 2000, to December 31, 2001, except those excluded as explained in the text. TBI indicates total body irradiation; ATG, antithymocyte globulin; AML, acute myelogenous leukemia; CML, chronic myelogenous leukemia; MDS, myelodysplastic syndrome; NHL, non-Hodgkin lymphoma; ALL, acute lymphocytic leukemia; CLL, chronic lymphocytic leukemia; and other, other hematologic malignancies (myeloproliferative syndromes, Hodgkin disease, multiple myeloma, etc).
Patients were considered HLA matched if all 6 of 6 HLA-A, -B and -DR were identical.
Diseases considered low risk were AML or ALL in first complete remission, CML in first chronic phase, aplastic anemia, refractory anemia, and refractory anemia with ringed sideroblasts; all other diseases were considered high risk.
CMV serology data were incomplete in 9 nonmyeloablative recipients (n = 58).
Most patients received more than one intervention for GVHD prophylaxis except for the subgroup that received a CD6 T-cell—depleted transplant, which received no other prophylaxis.
T-cell manipulations included CD6 depletion, CD8 depletion, or CD34 selection, depending on the treatment protocol used.
When compared with patients who received a myeloablative HSC transplant, patients who received nonmyeloablative HSC transplants were older (median age, 52 versus 42 years; P < .0001), had diagnoses that were different (P = .0009), and were more likely to have diseases considered high risk (88.1% versus 62.9%; P < .0001). They were more likely to receive HLA-matched unrelated donor stem cell grafts (49.2% versus 30.9%; P = .004), and stem cells were more likely to be obtained from peripheral blood (97% versus 17.8%; P < .0001). All patients receiving nonmyeloablative HSC transplants received conditioning chemotherapy with busulfan and fludarabine, and overall different GVHD prophylaxis regimens as shown in Table 1. CMV serostatus of donor-recipient pairs and median follow-up times were similar.
There were 83 244 patient-days of observation at the time the cohort was closed for analysis. The median follow-up time was 252 days (interquartile range [IQR], 121-493 days). No patient was lost to follow-up.
Incidence of and treatments for acute GVHD
A preliminary analysis demonstrated similar survival and IFI rates among patients with no GVHD and those with grades I to II GVHD; consequently, these groups were pooled together into no or nonsevere GVHD. Severe GVHD was defined as an overall grade of III or IV.12 Fifty-three patients (20%) in the cohort (20%) developed acute severe GVHD. The proportion of unrelated donors was higher in patients with severe GVHD when compared with the rest of the cohort (66.0% versus 40.8%, P = .001); otherwise, the baseline characteristics were similar. Among myeloablative and nonmyeloablative HSC transplant recipients, the proportion of any degree of GVHD (57.4% versus 47.8%) or severe GVHD (18.3% versus 25.4%) was similar. The baseline characteristics of the whole cohort, those with severe GVHD, and those who received infliximab for treatment of severe GVHD are presented in Table 2.
Characteristics of the cohort stratified by GVHD severity and infliximab use
Variable . | Cohort . | No and nonsevere GVHD . | Severe GVHD . | Non-infliximab recipients . | Infliximab recipients . |
|---|---|---|---|---|---|
| No. of patients | 264 | 211 | 53 | 42 | 11 |
| Median age, y (range) | 44 (18-66) | 44 (18-66) | 46 (20-62) | 46 (23-60) | 47 (20-62) |
| Transplant type, % | |||||
| Myeloablative | 74.6 | 76.3 | 67.9 | 73.8 | 45.4 |
| Nonmyeloablative | 25.4 | 23.7 | 32.1 | 26.2 | 54.6 |
| HLA matching, % | |||||
| Matched-related | 49.6 | 53.6 | 34.0 | 40.4 | 9.1 |
| Matched-unrelated | 35.6 | 31.3 | 52.8‡ | 47.6 | 72.7 |
| Mismatched-related | 4.6 | 5.7 | 0 | — | — |
| Mismatched-unrelated | 10.2 | 9.4 | 13.2 | 11.9 | 18.2 |
| Stem cell source, % | |||||
| Bone marrow | 62.1 | 62.1 | 62.3 | 66.7 | 45.4 |
| Peripheral blood | 37.9 | 37.9 | 37.7 | 33.3 | 54.6 |
| Conditioning regimen, % | |||||
| Myeloablative | |||||
| Cyclophosphamide and TBI | 70.8 | 72.5 | 64.1 | 69.0 | 45.4 |
| Other | 3.8 | 3.8 | 3.8 | 4.8 | — |
| Nonmyeloablative | |||||
| Busulfan and fludarabine | 25.4 | 23.7 | 32.1 | 26.2 | 54.6 |
| Risk group, % | |||||
| Low | 30.3 | 30.8 | 28.3 | 31.0 | 18.2 |
| High | 69.7 | 69.2 | 71.7 | 69.0 | 81.8 |
| CMV serology of donor/recipient, % | |||||
| D-/R- | 46.3 | 46.3 | 46.2 | 43.9 | 54.5 |
| D-/R+ | 15.7 | 16.7 | 11.5 | 9.8 | 18.2 |
| D+/R- | 20.8 | 21.2 | 19.2 | 19.5 | 18.2 |
| D+/R+ | 17.2 | 15.8 | 23.1 | 26.8 | 9.1 |
| GVHD prophylaxis, % | |||||
| Methotrexate | 53.8 | 54.0 | 52.8 | 54.8 | 45.4 |
| Cyclosporine | 45.8 | 45.0 | 49.1 | 47.6 | 54.6 |
| Tacrolimus | 40.5 | 39.3 | 45.3 | 45.2 | 45.4 |
| Sirolimus | 16.3 | 15.6 | 18.9 | 19.1 | 18.2 |
| MMF | 4.9 | 4.7 | 5.7 | 2.4 | 18.2 |
| T-cell manipulation | 18.6 | 20.4 | 11.3 | 14.3 | 0 |
| Corticosteroids | 37.9 | 35.1 | 49.1 | 42.9 | 72.7 |
| GVHD treatment, %* | |||||
| Corticosteroids | — | — | 100 | 100 | 100 |
| Cyclosporine | — | — | 54.7 | 52.5 | 63.6 |
| Tacrolimus | — | — | 64.2 | 61.9 | 72.7 |
| Sirolimus | — | — | 20.8 | 14.3 | 45.4 |
| MMF | — | — | 52.8 | 47.6 | 72.7 |
| Daclizumab | — | — | 54.7 | 47.6 | 81.8 |
| Organ-specific GVHD grade III or IV, %* | |||||
| Gastrointestinal | — | — | 35.9 | 21.4 | 90.9§ |
| Cutaneous | — | — | 43.4 | 42.9 | 45.4 |
| Hepatic | — | — | 39.6 | 42.9 | 27.3 |
| Median cumulative prednisone-equivalents, mg/kg (IQR)* | — | — | 112.9 (71.0-146.4) | 111.2 (57.2-150.9) | 132.5 (98.0-146.1) |
| Empiric antifungal use, %*† | |||||
| Amphotericin B | — | — | 69.8 | 69.1 | 72.7 |
| Fluconazole | — | — | 60.4 | 54.8 | 81.8 |
| Total patient-days | 83 244 | 69 992 | 13 252 | 11 448 | 1804 |
| Median follow-up, d (IQR) | 252 (121-493) | 269 (137-507) | 196 (103-363)‡ | 219 (87-401) | 143 (121-198) |
| Median survival, d (95% CI) | 397 (276-871 +) | 503 (366-871 +) | 196 (143-336)‡ | 224 (144-871 +) | 143 (121-198)§ |
Variable . | Cohort . | No and nonsevere GVHD . | Severe GVHD . | Non-infliximab recipients . | Infliximab recipients . |
|---|---|---|---|---|---|
| No. of patients | 264 | 211 | 53 | 42 | 11 |
| Median age, y (range) | 44 (18-66) | 44 (18-66) | 46 (20-62) | 46 (23-60) | 47 (20-62) |
| Transplant type, % | |||||
| Myeloablative | 74.6 | 76.3 | 67.9 | 73.8 | 45.4 |
| Nonmyeloablative | 25.4 | 23.7 | 32.1 | 26.2 | 54.6 |
| HLA matching, % | |||||
| Matched-related | 49.6 | 53.6 | 34.0 | 40.4 | 9.1 |
| Matched-unrelated | 35.6 | 31.3 | 52.8‡ | 47.6 | 72.7 |
| Mismatched-related | 4.6 | 5.7 | 0 | — | — |
| Mismatched-unrelated | 10.2 | 9.4 | 13.2 | 11.9 | 18.2 |
| Stem cell source, % | |||||
| Bone marrow | 62.1 | 62.1 | 62.3 | 66.7 | 45.4 |
| Peripheral blood | 37.9 | 37.9 | 37.7 | 33.3 | 54.6 |
| Conditioning regimen, % | |||||
| Myeloablative | |||||
| Cyclophosphamide and TBI | 70.8 | 72.5 | 64.1 | 69.0 | 45.4 |
| Other | 3.8 | 3.8 | 3.8 | 4.8 | — |
| Nonmyeloablative | |||||
| Busulfan and fludarabine | 25.4 | 23.7 | 32.1 | 26.2 | 54.6 |
| Risk group, % | |||||
| Low | 30.3 | 30.8 | 28.3 | 31.0 | 18.2 |
| High | 69.7 | 69.2 | 71.7 | 69.0 | 81.8 |
| CMV serology of donor/recipient, % | |||||
| D-/R- | 46.3 | 46.3 | 46.2 | 43.9 | 54.5 |
| D-/R+ | 15.7 | 16.7 | 11.5 | 9.8 | 18.2 |
| D+/R- | 20.8 | 21.2 | 19.2 | 19.5 | 18.2 |
| D+/R+ | 17.2 | 15.8 | 23.1 | 26.8 | 9.1 |
| GVHD prophylaxis, % | |||||
| Methotrexate | 53.8 | 54.0 | 52.8 | 54.8 | 45.4 |
| Cyclosporine | 45.8 | 45.0 | 49.1 | 47.6 | 54.6 |
| Tacrolimus | 40.5 | 39.3 | 45.3 | 45.2 | 45.4 |
| Sirolimus | 16.3 | 15.6 | 18.9 | 19.1 | 18.2 |
| MMF | 4.9 | 4.7 | 5.7 | 2.4 | 18.2 |
| T-cell manipulation | 18.6 | 20.4 | 11.3 | 14.3 | 0 |
| Corticosteroids | 37.9 | 35.1 | 49.1 | 42.9 | 72.7 |
| GVHD treatment, %* | |||||
| Corticosteroids | — | — | 100 | 100 | 100 |
| Cyclosporine | — | — | 54.7 | 52.5 | 63.6 |
| Tacrolimus | — | — | 64.2 | 61.9 | 72.7 |
| Sirolimus | — | — | 20.8 | 14.3 | 45.4 |
| MMF | — | — | 52.8 | 47.6 | 72.7 |
| Daclizumab | — | — | 54.7 | 47.6 | 81.8 |
| Organ-specific GVHD grade III or IV, %* | |||||
| Gastrointestinal | — | — | 35.9 | 21.4 | 90.9§ |
| Cutaneous | — | — | 43.4 | 42.9 | 45.4 |
| Hepatic | — | — | 39.6 | 42.9 | 27.3 |
| Median cumulative prednisone-equivalents, mg/kg (IQR)* | — | — | 112.9 (71.0-146.4) | 111.2 (57.2-150.9) | 132.5 (98.0-146.1) |
| Empiric antifungal use, %*† | |||||
| Amphotericin B | — | — | 69.8 | 69.1 | 72.7 |
| Fluconazole | — | — | 60.4 | 54.8 | 81.8 |
| Total patient-days | 83 244 | 69 992 | 13 252 | 11 448 | 1804 |
| Median follow-up, d (IQR) | 252 (121-493) | 269 (137-507) | 196 (103-363)‡ | 219 (87-401) | 143 (121-198) |
| Median survival, d (95% CI) | 397 (276-871 +) | 503 (366-871 +) | 196 (143-336)‡ | 224 (144-871 +) | 143 (121-198)§ |
Abbreviations and some variables are explained in Table 1. — indicates calculation not applicable.
Only treatment for patients with severe GVHD is shown. Percentages do not add up to 100% because patients received multiple simultaneous or sequential interventions; some patients were switched from one calcineurin to another due to drug adverse effects.
Prescription of antifungals at any point after transplantation or onset of GVHD before a diagnosis of probable or proven IFI was documented as empiric use.
Two-sided P < .05 when compared with patients in the cohort who had no or nonsevere GVHD.
Two-sided P < .05 when compared with patients with severe GVHD who did not receive infliximab.
Patients diagnosed with severe GVHD received multiple medications that always included corticosteroids at an initial dose of at least 2 mg/kg/d, tapered to response, and the addition or increase in dose of a calcineurin inhibitor or sirolimus. A frequently used third medication in patients with severe GVHD was daclizumab,18 a humanized monoclonal anti–interleukin-2 receptor α-chain antibody, which was given at a dose of 1 mg/kg every third day for at least 3 doses to 29 patients (54.7%). Many of these patients received other treatments: 43.4% (23 patients) received MMF and 20.8% (11 patients) received infliximab.
Infliximab was administered a median of 33 days after the initial diagnosis of acute GVHD (range, 3-154 days; IQR, 10-53 days). Patients received a median of 2 doses of 10 mg/kg on a weekly basis (range, 1-9 doses). When compared with patients who did not receive infliximab, recipients were more likely to have severe gastrointestinal GVHD (organ grade 4 in 9 of 11 infliximab recipients versus 5 of 42 in nonrecipients; P = .0002). They had a higher proportion of nonmyeloablative HSC transplants and unrelated donors, but these differences were not significant. There was a nonsignificant trend for these patients to have received MMF and corticosteroids as GVHD prophylaxis.
IFIs in the cohort
Eighteen proven or probable IFIs13 not due to Candida species were diagnosed in the cohort during the observation period; the incidence proportion for the cohort was 6.8% after a median follow-up time of 252 days. Invasive aspergillosis was diagnosed in 16 patients. Ten cases were biopsy or autopsy proven and 6 were probable based on compatible thoracic computed tomography and positive cultures from bronchoalveolar lavage or sputum. The diagnosis was made before death in 15 of these 16 cases. Disseminated zygomycosis was diagnosed in 2 patients; both cases were diagnosed at autopsy after a rapidly fatal illness. One of these patients received deferoxamine therapy for transfusion-related hemosiderosis. The median time to a diagnosis of IFI in the cohort was 135 days after HSCT (IQR, 81-211 days). Three patients were diagnosed before day 30 after HSCT; 2 patients were diagnosed between day 31 and 100. The remaining patients were diagnosed after day 100. Of note, 4 patients (1.52%) developed candidemia during follow-up, but were not included in this analysis. No protocol-driven antifungal prophylaxis was used.
IFI rates in the cohort
The incidence proportions, incidence rates (IRs), and crude incidence rate ratios (IRRs) of IFIs stratified according to baseline characteristics of the cohort are presented in Table 3. The cohort's IFI IR was 0.22 cases/1000 patient-days after transplantation. When stratified by GVHD severity, the IR for patients who developed severe GVHD was 0.78 cases/1000 patient-days compared with 0.11 cases/1000 patient-days among patients who had no or grades I to II GVHD (IRR, 6.85; 95% CI, 2.70-17.4). Baseline characteristics associated with a significant increase in the IR of IFI in the cohort on univariate analyses were receiving an HSC transplant from an unrelated donor (IRR, 6.65; 95% CI, 1.92-23.0) and undergoing nonmyeloablative HSCT (IRR, 4.49; 95% CI, 1.77-11.4). Use of MMF and corticosteroids as GVHD prophylaxis was associated with an increased crude IRR, although these characteristics were collinear with reception of a nonmyeloablative HSC transplant. Likewise, methotrexate was associated with a lower risk of IFI (IRR, 0.34; 95% CI, 0.12-0.95), but this was collinear with reception of a myeloablative HSC transplant.
IFI risk in the HSCT cohort according to selected baseline characteristics
Characteristic . | No. . | IFI (%)* . | Days after HSCT . | IR (95% CI)† . | IRR (95% CI)‡ . |
|---|---|---|---|---|---|
| Cohort | 264 | 18 (6.8) | 82 629 | 0.22 (0.13-0.34) | — |
| Sex | |||||
| Female | 107 | 7 (6.5) | 34 346 | 0.20 (0.11-0.41) | — |
| Male | 157 | 11 (7.0) | 48 283 | 0.23 (0.08-0.42) | 1.12 (0.43-2.88) |
| Age, y | |||||
| 18-44 | 135 | 6 (4.4) | 46 861 | 0.13 (0.05-0.28) | — |
| 45-66 | 129 | 12 (9.3) | 35 768 | 0.34 (0.17-0.59) | 2.62 (0.98-6.98) |
| Transplant type | |||||
| Myeloablative | 197 | 8 (4.1) | 64 642 | 0.12 (0.05-0.24) | — |
| Nonmyeloablative | 67 | 10 (14.9) | 17 987 | 0.56 (0.27-1.02) | 4.49 (1.77-11.4) |
| Relationship to donor | |||||
| Related | 143 | 3 (2.1) | 47 156 | 0.06 (0.01-0.19) | — |
| Unrelated | 121 | 15 (12.4) | 35 473 | 0.42 (0.24-0.70) | 6.65 (1.92-23.0) |
| Stem cell source | |||||
| Bone marrow | 164 | 9 (5.5) | 57 717 | 0.16 (0.07-0.30) | — |
| Peripheral blood | 100 | 9 (9) | 24 912 | 0.36 (0.17-0.69) | 2.32 (0.92-5.84) |
| Risk group | |||||
| Low risk | 80 | 3 (3.8) | 30 824 | 0.10 (0.02-0.28) | — |
| High risk | 184 | 15 (8.2) | 51 805 | 0.29 (0.16-0.48) | 2.98 (0.86-10.3) |
| CMV serology of donor/recipient | |||||
| Any seropositive | 137 | 10 (7.3) | 41 868 | 0.24 (0.12-0.44) | 1.30 (0.50-3.42) |
| D-/R- | 118 | 7 (5.9) | 38 105 | 0.18 (0.07-0.38) | — |
| GVHD prophylaxis | |||||
| Methotrexate | 142 | 5 (3.5) | 43 886 | 0.11 (0.04-0.27) | 0.34 (0.12-0.95) |
| No methotrexate | 122 | 13 (10.7) | 38 743 | 0.34 (0.18-0.57) | — |
| Cyclosporine | 121 | 13 (10.7) | 41 729 | 0.31 (0.17-0.53) | 2.55 (0.91-7.15) |
| No cyclosporine | 143 | 5 (3.5) | 40 900 | 0.12 (0.04-0.29) | — |
| Tacrolimus | 107 | 2 (1.9) | 27 257 | 0.07 (0.01-0.27) | 0.25 (0.06-1.10) |
| No tacrolimus | 157 | 16 (10.2) | 55 372 | 0.29 (0.17-0.47) | — |
| Sirolimus | 43 | 1 (2.3) | 11 492 | 0.09 (0.00-0.49) | 0.36 (0.05-2.74) |
| No sirolimus | 221 | 17 (7.7) | 71 137 | 0.24 (0.14-0.38) | — |
| MMF | 13 | 3 (23.1) | 2 719 | 1.10 (0.23-3.22) | 5.88 (1.70-20.3) |
| No MMF | 251 | 15 (6.0) | 79 910 | 0.19 (0.11-0.31) | — |
| T-cell manipulation | 49 | 3 (6.1) | 16 245 | 0.18 (0.04-0.54) | 0.82 (0.24-2.82) |
| No T-cell manipulation | 215 | 15 (7.0) | 66 384 | 0.23 (0.13-0.37) | — |
| Corticosteroids | 100 | 11 (11) | 29 578 | 0.37 (0.19-0.67) | 2.82 (1.09-7.27) |
| No corticosteroids | 164 | 7 (4.3) | 53 051 | 0.13 (0.05-0.27) | — |
| GVHD severity | |||||
| No and nonsevere GVHD | 211 | 8 (3.8) | 69 881 | 0.11 (0.05-0.23) | 6.85 (2.70-17.4) |
| Severe GVHD | 53 | 10 (18.9) | 12 748 | 0.78 (0.38-1.44) | — |
Characteristic . | No. . | IFI (%)* . | Days after HSCT . | IR (95% CI)† . | IRR (95% CI)‡ . |
|---|---|---|---|---|---|
| Cohort | 264 | 18 (6.8) | 82 629 | 0.22 (0.13-0.34) | — |
| Sex | |||||
| Female | 107 | 7 (6.5) | 34 346 | 0.20 (0.11-0.41) | — |
| Male | 157 | 11 (7.0) | 48 283 | 0.23 (0.08-0.42) | 1.12 (0.43-2.88) |
| Age, y | |||||
| 18-44 | 135 | 6 (4.4) | 46 861 | 0.13 (0.05-0.28) | — |
| 45-66 | 129 | 12 (9.3) | 35 768 | 0.34 (0.17-0.59) | 2.62 (0.98-6.98) |
| Transplant type | |||||
| Myeloablative | 197 | 8 (4.1) | 64 642 | 0.12 (0.05-0.24) | — |
| Nonmyeloablative | 67 | 10 (14.9) | 17 987 | 0.56 (0.27-1.02) | 4.49 (1.77-11.4) |
| Relationship to donor | |||||
| Related | 143 | 3 (2.1) | 47 156 | 0.06 (0.01-0.19) | — |
| Unrelated | 121 | 15 (12.4) | 35 473 | 0.42 (0.24-0.70) | 6.65 (1.92-23.0) |
| Stem cell source | |||||
| Bone marrow | 164 | 9 (5.5) | 57 717 | 0.16 (0.07-0.30) | — |
| Peripheral blood | 100 | 9 (9) | 24 912 | 0.36 (0.17-0.69) | 2.32 (0.92-5.84) |
| Risk group | |||||
| Low risk | 80 | 3 (3.8) | 30 824 | 0.10 (0.02-0.28) | — |
| High risk | 184 | 15 (8.2) | 51 805 | 0.29 (0.16-0.48) | 2.98 (0.86-10.3) |
| CMV serology of donor/recipient | |||||
| Any seropositive | 137 | 10 (7.3) | 41 868 | 0.24 (0.12-0.44) | 1.30 (0.50-3.42) |
| D-/R- | 118 | 7 (5.9) | 38 105 | 0.18 (0.07-0.38) | — |
| GVHD prophylaxis | |||||
| Methotrexate | 142 | 5 (3.5) | 43 886 | 0.11 (0.04-0.27) | 0.34 (0.12-0.95) |
| No methotrexate | 122 | 13 (10.7) | 38 743 | 0.34 (0.18-0.57) | — |
| Cyclosporine | 121 | 13 (10.7) | 41 729 | 0.31 (0.17-0.53) | 2.55 (0.91-7.15) |
| No cyclosporine | 143 | 5 (3.5) | 40 900 | 0.12 (0.04-0.29) | — |
| Tacrolimus | 107 | 2 (1.9) | 27 257 | 0.07 (0.01-0.27) | 0.25 (0.06-1.10) |
| No tacrolimus | 157 | 16 (10.2) | 55 372 | 0.29 (0.17-0.47) | — |
| Sirolimus | 43 | 1 (2.3) | 11 492 | 0.09 (0.00-0.49) | 0.36 (0.05-2.74) |
| No sirolimus | 221 | 17 (7.7) | 71 137 | 0.24 (0.14-0.38) | — |
| MMF | 13 | 3 (23.1) | 2 719 | 1.10 (0.23-3.22) | 5.88 (1.70-20.3) |
| No MMF | 251 | 15 (6.0) | 79 910 | 0.19 (0.11-0.31) | — |
| T-cell manipulation | 49 | 3 (6.1) | 16 245 | 0.18 (0.04-0.54) | 0.82 (0.24-2.82) |
| No T-cell manipulation | 215 | 15 (7.0) | 66 384 | 0.23 (0.13-0.37) | — |
| Corticosteroids | 100 | 11 (11) | 29 578 | 0.37 (0.19-0.67) | 2.82 (1.09-7.27) |
| No corticosteroids | 164 | 7 (4.3) | 53 051 | 0.13 (0.05-0.27) | — |
| GVHD severity | |||||
| No and nonsevere GVHD | 211 | 8 (3.8) | 69 881 | 0.11 (0.05-0.23) | 6.85 (2.70-17.4) |
| Severe GVHD | 53 | 10 (18.9) | 12 748 | 0.78 (0.38-1.44) | — |
— indicates reciprocal rate not shown for clarity.
Percent indicates incidence proportion, independent of person-time at risk contributed.
Cases/1000 patient-days of observation from HSCT date (day 0). Confidence intervals were calculated using the Haenszel method.
IRR indicates crude incidence rate ratio, when compared to complementary characteristic in the preceding row. Confidence intervals were calculated using the Byar method.
IFI rates among patients with severe GVHD
Given that only patients who developed severe GVHD would be considered for infliximab and other immunomodulator use, we calculated the IR, crude IRR, as well as the unadjusted and adjusted hazard ratios for IFI from the onset of GVHD in this subcohort to address the possible IFI risk associated with infliximab use and to identify other possible IFI risk factors. Table 4 presents these data stratified by baseline characteristics, treatments received to control GVHD, empiric antifungal used, and organ-specific maximum GVHD grade.
Univariate and multivariate analysis of IFI risk in patients with severe GVHD
Characteristic . | No. . | IFI (%) . | Days after GVHD* . | IR†(95% CI) . | IRR (95% CI) . | Unadjusted HR‡ . | P . | Adjusted HR§ . | P . |
|---|---|---|---|---|---|---|---|---|---|
| Severe GVHD | 53 | 10 (18.9) | 10 115 | 0.99 (0.48-1.82) | — | — | — | — | — |
| Sex | |||||||||
| Female | 21 | 4 (19.1) | 5255 | 0.76 (0.21-1.95) | — | — | — | — | — |
| Male | 32 | 6 (18.8) | 4900 | 1.22 (0.45-2.67) | 1.61 (0.45-5.70) | 1.29 | .69 | — | — |
| Age, y | |||||||||
| 18-44 | 23 | 3 (13.0) | 4240 | 0.71 (0.15-2.07) | — | — | — | — | — |
| 45-66 | 30 | 7 (23.3) | 5915 | 1.18 (0.48-2.44) | 1.67 (0.43-6.47) | 1.58 | .51 | — | — |
| Transplant type | |||||||||
| Myeloablative | 36 | 2 (5.6) | 7227 | 0.28 (0.03-0.99) | — | — | — | — | — |
| Nonmyeloablative | 17 | 8 (47.1) | 2928 | 2.73 (1.18-5.38) | 9.87 (2.10-46.5) | 8.54 | .007 | 8.05 (1.56-41.4) | .013 |
| Relationship to donor | |||||||||
| Related | 18 | 2 (11.1) | 4394 | 0.46 (0.06-1.64) | — | — | — | — | — |
| Unrelated | 35 | 8 (22.9) | 5761 | 1.39 (0.60-2.74) | 3.05 (0.65-14.4) | 2.87 | .18 | — | — |
| Stem cell source | |||||||||
| Bone marrow | 33 | 3 (9.1) | 6647 | 0.45 (0.09-1.32) | — | — | — | — | — |
| Peripheral blood | 20 | 7 (35) | 3508 | 2.00 (0.80-4.11) | 4.42 (1.14-17.1) | 3.52 | .07 | — | — |
| Risk group | |||||||||
| Low risk | 15 | 1 (6.7) | 3540 | 0.28 (0.01-1.57) | — | — | — | — | — |
| High risk | 38 | 9 (23.7) | 6615 | 1.36 (0.62-2.59) | 4.82 (0.61-38.0) | 3.93 | .20 | — | — |
| CMV serology of donor/recipient | |||||||||
| Any seropositive | 28 | 5 (17.9) | 6526 | 0.77 (0.25-1.79) | 0.63 (0.17-2.36) | 0.77 | .7 | — | — |
| D-/R- | 24 | 4 (16.7) | 3313 | 1.21 (0.33-3.09) | — | — | — | — | — |
| GVHD treatments | |||||||||
| Cumulative prednisone-equivalents | |||||||||
| 1-100 mg/kg | 21 | 1 (4.8) | 2359 | 0.42 (0.01-2.36) | — | — | — | — | — |
| 101-200 mg/kg | 26 | 7 (26.9) | 5350 | 1.31 (0.53-2.70) | — | — | — | — | — |
| 201-300 mg/kg | 4 | 2 (50) | 1088 | 1.84 (0.22-6.64) | — | — | — | — | — |
| 301-400 mg/kg | 2 | 0 (0) | 1358 | — | — | — | — | — | — |
| Cyclosporine | 29 | 8 (27.6) | 6939 | 1.15 (0.50-2.27) | 3.35 (0.71-15.8) | 3.56 | 0.11 | — | — |
| No cyclosporine | 24 | 2 (8.3) | 5809 | 0.34 (0.04-1.24) | — | — | — | — | — |
| Tacrolimus | 34 | 3 (8.8) | 7030 | 0.43 (0.09-1.25) | 0.19 (0.05-0.74) | 0.21 | 0.02 | — | — |
| No tacrolimus | 19 | 7 (36.8) | 3125 | 2.24 (0.90-4.61) | — | — | — | — | — |
| Sirolimus | 11 | 2 (18.2) | 1493 | 1.34 (0.16-4.84) | 1.45 (0.31-6.83) | 1.25 | 0.78 | — | — |
| No sirolimus | 42 | 8 (19.1) | 8662 | 0.92 (0.40-1.82) | — | — | — | — | — |
| MMF | 28 | 8 (28.6) | 5056 | 1.58 (0.68-3.12) | 4.03 (0.86-19.0) | 3.36 | 0.13 | — | — |
| No MMF | 25 | 2 (8) | 5099 | 0.39 (0.05-1.42) | — | — | — | — | — |
| Daclizumab | 29 | 4 (13.8) | 4720 | 0.85 (0.23-2.17) | 0.77 (0.22-2.72) | 0.74 | 0.64 | — | — |
| No daclizumab | 24 | 6 (25) | 5435 | 1.10 (0.41-2.41) | — | — | — | — | — |
| Infliximab | 11 | 5 (45.5) | 737 | 6.78 (2.20-15.8) | 12.8 (3.70-44.1) | 30.4 | 0.002 | 13.6 (2.29-80.2) | .004 |
| No infliximab | 42 | 5 (11.9) | 9418 | 0.53 (0.17-1.24) | — | — | — | — | — |
| Empiric amphotericin B | 37 | 7 (18.9) | 5309 | 1.32 (0.53-2.72) | 2.13 (0.55-8.24) | 1.93 | 0.34 | — | — |
| No empiric amphotericin B | 16 | 3 (18.8) | 4846 | 0.62 (0.13-1.81) | — | — | — | — | — |
| Empiric fluconazole | 32 | 8 (25) | 5938 | 1.35 (0.58-2.65) | 2.84 (0.60-13.4) | 2.16 | 0.34 | — | — |
| No empiric fluconazole | 21 | 2 (9.5) | 4217 | 0.47 (0.06-1.71) | — | — | — | — | — |
| Organ-specific GVHD | |||||||||
| Gastrointestinal 3 or 4 | 19 | 6 (31.6) | 1950 | 3.08 (1.13-6.71) | 6.31 (1.78-22.4) | 4.56 | 0.02 | 3.46 (0.67-17.9) | .14 |
| No gastrointestinal 3 or 4 | 34 | 4 (11.8) | 8205 | 0.49 (0.13-1.25) | — | — | — | — | — |
| Hepatic 3 or 4 | 21 | 2 (9.5) | 3233 | 0.62 (0.08-2.23) | 0.54 (0.11-2.52) | 0.50 | 0.38 | — | — |
| No hepatic 3 or 4 | 32 | 8 (25) | 6922 | 1.16 (0.50-2.27) | — | — | — | — | — |
| Cutaneous 3 or 4 | 23 | 5 (21.7) | 4141 | 1.21 (0.39-2.81) | 1.45 (0.42-5.02) | 1.47 | 0.55 | — | — |
| No cutaneous 3 or 4 | 30 | 5 (16.7) | 6014 | 0.83 (0.27-1.94) | — | — | — | — | — |
Characteristic . | No. . | IFI (%) . | Days after GVHD* . | IR†(95% CI) . | IRR (95% CI) . | Unadjusted HR‡ . | P . | Adjusted HR§ . | P . |
|---|---|---|---|---|---|---|---|---|---|
| Severe GVHD | 53 | 10 (18.9) | 10 115 | 0.99 (0.48-1.82) | — | — | — | — | — |
| Sex | |||||||||
| Female | 21 | 4 (19.1) | 5255 | 0.76 (0.21-1.95) | — | — | — | — | — |
| Male | 32 | 6 (18.8) | 4900 | 1.22 (0.45-2.67) | 1.61 (0.45-5.70) | 1.29 | .69 | — | — |
| Age, y | |||||||||
| 18-44 | 23 | 3 (13.0) | 4240 | 0.71 (0.15-2.07) | — | — | — | — | — |
| 45-66 | 30 | 7 (23.3) | 5915 | 1.18 (0.48-2.44) | 1.67 (0.43-6.47) | 1.58 | .51 | — | — |
| Transplant type | |||||||||
| Myeloablative | 36 | 2 (5.6) | 7227 | 0.28 (0.03-0.99) | — | — | — | — | — |
| Nonmyeloablative | 17 | 8 (47.1) | 2928 | 2.73 (1.18-5.38) | 9.87 (2.10-46.5) | 8.54 | .007 | 8.05 (1.56-41.4) | .013 |
| Relationship to donor | |||||||||
| Related | 18 | 2 (11.1) | 4394 | 0.46 (0.06-1.64) | — | — | — | — | — |
| Unrelated | 35 | 8 (22.9) | 5761 | 1.39 (0.60-2.74) | 3.05 (0.65-14.4) | 2.87 | .18 | — | — |
| Stem cell source | |||||||||
| Bone marrow | 33 | 3 (9.1) | 6647 | 0.45 (0.09-1.32) | — | — | — | — | — |
| Peripheral blood | 20 | 7 (35) | 3508 | 2.00 (0.80-4.11) | 4.42 (1.14-17.1) | 3.52 | .07 | — | — |
| Risk group | |||||||||
| Low risk | 15 | 1 (6.7) | 3540 | 0.28 (0.01-1.57) | — | — | — | — | — |
| High risk | 38 | 9 (23.7) | 6615 | 1.36 (0.62-2.59) | 4.82 (0.61-38.0) | 3.93 | .20 | — | — |
| CMV serology of donor/recipient | |||||||||
| Any seropositive | 28 | 5 (17.9) | 6526 | 0.77 (0.25-1.79) | 0.63 (0.17-2.36) | 0.77 | .7 | — | — |
| D-/R- | 24 | 4 (16.7) | 3313 | 1.21 (0.33-3.09) | — | — | — | — | — |
| GVHD treatments | |||||||||
| Cumulative prednisone-equivalents | |||||||||
| 1-100 mg/kg | 21 | 1 (4.8) | 2359 | 0.42 (0.01-2.36) | — | — | — | — | — |
| 101-200 mg/kg | 26 | 7 (26.9) | 5350 | 1.31 (0.53-2.70) | — | — | — | — | — |
| 201-300 mg/kg | 4 | 2 (50) | 1088 | 1.84 (0.22-6.64) | — | — | — | — | — |
| 301-400 mg/kg | 2 | 0 (0) | 1358 | — | — | — | — | — | — |
| Cyclosporine | 29 | 8 (27.6) | 6939 | 1.15 (0.50-2.27) | 3.35 (0.71-15.8) | 3.56 | 0.11 | — | — |
| No cyclosporine | 24 | 2 (8.3) | 5809 | 0.34 (0.04-1.24) | — | — | — | — | — |
| Tacrolimus | 34 | 3 (8.8) | 7030 | 0.43 (0.09-1.25) | 0.19 (0.05-0.74) | 0.21 | 0.02 | — | — |
| No tacrolimus | 19 | 7 (36.8) | 3125 | 2.24 (0.90-4.61) | — | — | — | — | — |
| Sirolimus | 11 | 2 (18.2) | 1493 | 1.34 (0.16-4.84) | 1.45 (0.31-6.83) | 1.25 | 0.78 | — | — |
| No sirolimus | 42 | 8 (19.1) | 8662 | 0.92 (0.40-1.82) | — | — | — | — | — |
| MMF | 28 | 8 (28.6) | 5056 | 1.58 (0.68-3.12) | 4.03 (0.86-19.0) | 3.36 | 0.13 | — | — |
| No MMF | 25 | 2 (8) | 5099 | 0.39 (0.05-1.42) | — | — | — | — | — |
| Daclizumab | 29 | 4 (13.8) | 4720 | 0.85 (0.23-2.17) | 0.77 (0.22-2.72) | 0.74 | 0.64 | — | — |
| No daclizumab | 24 | 6 (25) | 5435 | 1.10 (0.41-2.41) | — | — | — | — | — |
| Infliximab | 11 | 5 (45.5) | 737 | 6.78 (2.20-15.8) | 12.8 (3.70-44.1) | 30.4 | 0.002 | 13.6 (2.29-80.2) | .004 |
| No infliximab | 42 | 5 (11.9) | 9418 | 0.53 (0.17-1.24) | — | — | — | — | — |
| Empiric amphotericin B | 37 | 7 (18.9) | 5309 | 1.32 (0.53-2.72) | 2.13 (0.55-8.24) | 1.93 | 0.34 | — | — |
| No empiric amphotericin B | 16 | 3 (18.8) | 4846 | 0.62 (0.13-1.81) | — | — | — | — | — |
| Empiric fluconazole | 32 | 8 (25) | 5938 | 1.35 (0.58-2.65) | 2.84 (0.60-13.4) | 2.16 | 0.34 | — | — |
| No empiric fluconazole | 21 | 2 (9.5) | 4217 | 0.47 (0.06-1.71) | — | — | — | — | — |
| Organ-specific GVHD | |||||||||
| Gastrointestinal 3 or 4 | 19 | 6 (31.6) | 1950 | 3.08 (1.13-6.71) | 6.31 (1.78-22.4) | 4.56 | 0.02 | 3.46 (0.67-17.9) | .14 |
| No gastrointestinal 3 or 4 | 34 | 4 (11.8) | 8205 | 0.49 (0.13-1.25) | — | — | — | — | — |
| Hepatic 3 or 4 | 21 | 2 (9.5) | 3233 | 0.62 (0.08-2.23) | 0.54 (0.11-2.52) | 0.50 | 0.38 | — | — |
| No hepatic 3 or 4 | 32 | 8 (25) | 6922 | 1.16 (0.50-2.27) | — | — | — | — | — |
| Cutaneous 3 or 4 | 23 | 5 (21.7) | 4141 | 1.21 (0.39-2.81) | 1.45 (0.42-5.02) | 1.47 | 0.55 | — | — |
| No cutaneous 3 or 4 | 30 | 5 (16.7) | 6014 | 0.83 (0.27-1.94) | — | — | — | — | — |
Days of follow-up after onset of GVHD, censored for IFI, death, or end of follow-up period.
Cases/1000 GVHD patient-days of observation from onset of GVHD. Confidence intervals were calculated using the Haenszel method.
Unadjusted hazard ratio (HR) obtained from univariate time-dependent Cox model.
Adjusted HR from multivariate time-dependent Cox model, with 95% CI shown in parentheses. Values shown are from covariates retained in final model. See text for details.
The overall IFI IR among patients with severe GVHD was 0.99 cases/1000 GVHD patient-days (95% CI, 0.48-1.82). Among baseline characteristics, nonmyeloablative HSCT was associated with a significantly increased IFI IR of 2.73 cases/1000 GVHD patient-days, whereas myeloablative HSC transplant recipients who developed severe GVHD had an IFI IR of 0.28 cases/1000 GVHD patient-days (IRR, 9.87; 95% CI, 2.10-46.5). Characteristics of nonmyeloablative HSCT protocols, such as conditioning regimen, receiving peripheral blood stem cells, and cyclosporine use for GVHD prophylaxis, were also associated with a higher risk of IFI. Underlying hematologic diagnosis and CMV serostatus had no impact on the risk of IFI.
Of 10 patients with severe GVHD who developed an IFI, 5 had received infliximab. When patients with severe GVHD were stratified by infliximab use, patients who received infliximab had an IFI IR of 6.78 cases/1000 GVHD patient-days compared with an IR of 0.53 cases/1000 GVHD patient-days in those with severe GVHD who did not receive infliximab (IRR, 12.8; 95% CI, 3.70-44.1).
Only one infliximab recipient developed CMV DNAemia and was treated pre-emptively. Use of methotrexate and tacrolimus for GVHD prophylaxis was associated with a lower risk of IFI, although these were collinear with myeloablative transplantation. Use of MMF for GVHD prophylaxis was associated with an increased risk of IFI in the cohort, but it was not significantly associated with IFI in patients who received MMF to treat severe GVHD (data not shown).
The median cumulative dose of prednisone-equivalents for all patients with severe GVHD was 112.9 mg/kg (IQR, 71.0-146.4). Cumulative doses were similar in those who received infliximab and those who did not receive infliximab (132.5 versus 111.2 mg/kg; P = .53).
The median cumulative dose of prednisone-equivalents among patients with severe GVHD who developed an IFI was higher than that of patients who did not develop an IFI (140.7 versus 102.8 mg/kg; P = .047). The median cumulative dose of prednisone among infliximab recipients who developed an IFI was similar to the dose in nonrecipients (135.4 versus 180.8 mg/kg; P = .84). When IFI IR was calculated by 100 mg/kg prednisone-equivalent increments, there was a nonsignificant increased trend for increased IFI risk with higher accumulated doses of corticosteroids. Daclizumab use was not associated with an increased IFI IR.
Empiric antifungal use was common in patients who developed severe GVHD; amphotericin B formulations were given to 69.8% at some point after transplantation, whereas 60.4% received fluconazole. Fluconazole use, but not amphotericin B, was higher in patients who eventually received infliximab, but this was not significant (81.8% versus 54.8%; P = .17). IFI IR was not signifi-cantly different among patients who received empiric antifungal therapy.
Patients with gastrointestinal organ-specific GVHD grade 3 or 4 had an IFI IR of 3.08 cases/1000 GVHD patient-days, whereas other patients with severe GVHD but without grade 3 or 4 gastrointestinal involvement had an IFI IR of 0.49 cases/1000 GVHD patient-days (IRR, 6.31; 95% CI, 1.78-22.4).
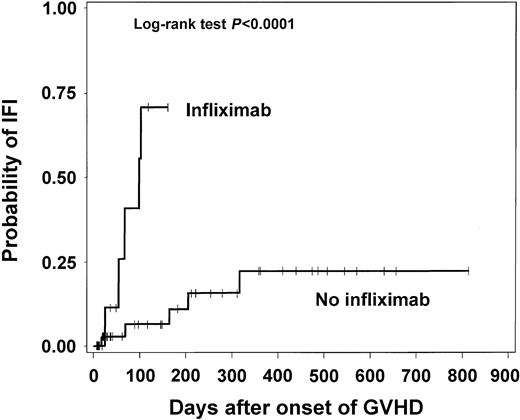
The time to IFI from the onset of GVHD among patients with severe GVHD stratified by infliximab use is presented in Figure 1. There was a significantly higher probability of IFI in the infliximab recipients (P < .0001). The results were similar when patients were stratified according to GVHD organ-specific severity.
Time to IFI from onset of GVHD among HSC transplant recipients who developed severe GVHD.
Time to IFI from onset of GVHD among HSC transplant recipients who developed severe GVHD.
Multivariate analysis of risk of IFI in patients with severe GVHD
A time-dependent Cox regression analysis model for developing IFI in patients with severe GVHD was developed. Univariate hazard ratios (HRs) were calculated for all possible IFI risk factors as shown in Table 4. Only characteristics with an unadjusted HR P values of less than .20 were considered in the multivariate model.
Variables that were collinear with a nonmyeloablative HSCT described were not included separately, and given that 10 IFIs were being analyzed, 2 covariates with the highest HR and P values of less than .05 were retained in the final model.19 Given that infliximab was given preferentially to patients with severe gastrointestinal GVHD and that gastrointestinal organ-specific grade 3 or 4 was found to be significantly associated with IFI on univariate Cox, this covariate was kept in the final model to minimize confounding by indication, even though it became nonsignificant in the presence of other covariates modeled. The adjusted HR of infliximab use, as a time-dependent covariate, was 13.6 (P = .004; 95% CI, 2.29-80.2); the adjusted HR of nonmyeloablative HSCT was 8.05 (P = .013; 95% CI, 1.56-41.4). The adjusted HR of severe gastrointestinal organ-specific grade 3 or 4 GVHD was 3.46 in the presence of infliximab use and transplant type as covariates (P = .14). The time to IFI hazard function plots of infliximab exposure showed increasing hazard over time.
To address the robustness of these findings and address possible surveillance bias,20 the data were also censored on March 1, 2002, when the possible association of infliximab with IFI was first proposed at our institution. The findings and estimates of risk were similar (data not shown).
Survival
The median survival of the whole cohort at the end of follow-up was 397 days (95% CI, 276-871+). When stratified according to GVHD severity, the median survival of patients with severe GVHD was significantly lower than that of patients with no or nonsevere GVHD (196 versus 503 days after HSCT; P = .004; Table 2). Among patients with severe GVHD, the median survival of infliximab recipients (143 days; 95% CI, 121-198) was signifi-cantly lower than that of nonrecipients (224 days; 95% CI, 144-842+; P = .028).
Discussion
As the benefits of TNF-α blockade with infliximab have become more clear, such as in the therapy for Crohn disease21 and rheumatoid arthritis,22 there is also increasing clinical experience that suggests that this pathway is critical in the defense against certain pathogens.
TNF-α is the principal mediator of acute inflammation after microbial challenge, secreted mainly by activated mononuclear phagocytes and, among other effects, stimulates the recruitment of neutrophils and monocytes and activates these cells to eradicate microbes.23 Nagai and colleagues showed that interferon-γ and TNF-α infusions were protective in mice with invasive aspergillosis.24 Th-1 responses, of which TNF-α is a major cytokine, enhance phagocyte effector cell function and seem to be important in successful alveolar clearance of conidia.25
Infliximab is a humanized monoclonal anti–TNF-α antibody; it binds to high-affinity soluble and transmembrane forms of TNF-α and inhibits the binding of TNF-α with its receptors. In experimental models, administration of anti–TNF-α antibody resulted in delayed and less intense pyogenic abscess formation,26 and increased susceptibility to Histoplasma capsulatum.27
Moreover, patients who received infliximab for treatment of Crohn disease or rheumatoid arthritis had an unusually high proportion of extrapulmonary and disseminated tuberculosis28 and histoplasmosis,29 possibly due to defective granuloma formation and absence of TNF-α–mediated macrophage apoptosis.28
Anti–TNF-α therapy with murine monoclonal antibodies was used to treat steroid-resistant GVHD and it appeared to be particularly useful if there was intestinal involvement.30 However, there was no improvement in survival due either to GVHD recurrence or complications, perhaps related to the dose of antibody used and its short half-life.
Couriel and colleagues reported that a weekly dose of 10 mg/kg infliximab in patients with steroid-refractory GVHD resulted in an 80% response rate in 37 patients with gastrointestinal involvement.10 Death occurred in 60% of the patients with acute GVHD and it was attributed to GVHD in the majority. Infectious complications were not mentioned in their report. Based on these data, other centers have reported similar GVHD response rates in small series of patients, but with IFI incidence proportions as high as 50%.31-34
In the 2-year observational cohort presented here, the incidence proportion of non-Candida IFI was 6.8% of patients who received an HSC transplant. When stratified by acute GVHD severity, the incidence proportion was 18.9% in those with severe GVHD versus 3.8% in those who had no or nonsevere GVHD (risk ratio, 4.98). However, a more accurate measurement of risk that accounts for different exposures and follow-up is the IR. In the present cohort, the IFI IR was 0.78 cases/1000 patient-days of follow-up in those who developed severe GVHD, and 0.11 cases/1000 patient-days in those who had no or grades I to II GVHD (IRR, 6.85). When GVHD time at risk was considered, patients who received infliximab to control severe GVHD had an IFI IR of 6.78 cases/1000 GVHD patient-days, which was significantly higher than that of other patients with severe GVHD (0.53 cases/1000 GVHD patient-days; IRR, 12.8).
The time to IFI analysis demonstrated that the cumulative probability of IFI was significantly higher and the median time to IFI was shorter (67 versus 164 days) in infliximab recipients when compared with other patients with severe GVHD (Figure 1). The IFI HR remained significantly elevated at 13.6 when infliximab was considered as a time-dependent variable in the Cox model for patients with severe GVHD in which nonmyeloablative HSCT and gastrointestinal predominant GVHD were included as covariates. This is the first report to establish such an association in this population as well as a quantified estimate of risk of IFI due to infliximab.
Corticosteroid treatment is a risk factor for IFI in HSCT and in other populations.4,35 Given that all patients with severe GVHD receive corticosteroids as initial standard treatment, cumulative corticosteroid administration adjusted to body weight was used to estimate its risk for IFI.36 Patients with severe GVHD who developed an IFI had received higher cumulative doses of prednisone-equivalents (median, 140.7 versus 102.8 mg/kg; P = .047), but when IFI cases were stratified by infliximab use, cumulative prednisone-equivalent doses were not significantly lower in infliximab recipients (median, 135.4 versus 180.8 mg/kg in nonrecipients; P = .84). Although subject to overfitting19 and some degree of collinearity with time to IFI, the estimated HR of corticosteroid exposure was 1.83 per 100 mg/kg prednisone-equivalents in the Cox model for patients with severe GVHD (P = .31).
Patients with severe GVHD are treated with multiple medications to control rejection besides corticosteroids, including calcineurin inhibitors, sirolimus, MMF, and daclizumab.2,37 Many of them were used simultaneously or sequentially. Given the collinearity of several of these treatments with the allocated protocols and the relatively few events in the cohort, we were unable to model the specific contribution of each of these medications to IFI risk beyond the univariate analysis presented.18 Although Willenbacher and colleagues suggested an increased risk of infection in patients who received daclizumab for the treatment of GVHD,38 these infections seemed to be largely driven by CMV disease and its complications; they reported only one case of invasive aspergillosis among 16 patients treated.38 We did not observe an increased IFI risk among patients who received daclizumab for treatment of acute GVHD.
Several points are worth mentioning regarding the use of infliximab in this population. Infliximab administration at 10 mg/kg on a weekly basis is higher than the dosing scheme administered to patients with other conditions (5-10 mg/kg at 2- to 8-week intervals).21,22 Preliminary experience with adalimumab, another anti–TNF-α antibody similar to infliximab, has suggested that susceptibility to infection is dose dependent.28 Dose-ranging trials to address the optimal therapeutic window of infliximab in patients with GVHD are needed.
Another strategy that should be formally explored and that has been adopted at our institution is the possibility of pre-emptive systemic antifungal use in patients who require infliximab to control refractory GVHD. A systemic antifungal with activity against Aspergillus species and other molds that is tailored to organ involvement, toxicity, and drug interactions could be given during this period of highest risk. Although small, an encouraging initial experience involving such approach was recently presented.39 This should be coupled to more strict environmental protective measures after patients leave the hospital to avoid community exposures.40 Alternatively, in the future, these patients could be monitored systematically with molecular fungal markers,13 and targeted pre-emptive therapy initiated accordingly.
In addition to the risk of infliximab, the independent risk of IFI with nonmyeloablative HSCT deserves mention. As shown in Table 1, patients who underwent nonmyeloablative transplantation were older, received more matched-unrelated grafts, and had a larger proportion of diagnoses considered high risk. The incidence proportion (14.9% versus 4.1%) and IR (0.56 versus 0.12 cases/1000 patient-days; IRR, 4.49; 95% CI, 1.77-11.4) for IFI in nonmyeloablative HSCT were elevated compared with myeloablative HSCT. This excess risk was observed in those nonmyeloablative HSC transplant recipients who also developed severe acute GVHD (1.94 versus 0.14 cases/1000 patient-days; IRR, 13.5; 95% CI, 2.86-63.5). In the multivariate analysis, nonmyeloablative HSCT remained a significant risk factor in patients with severe GVHD (HR, 8.05).
The high proportion of IFI after nonmyeloablative transplantation has been observed previously. Walsh and colleagues recently presented their experience of IFI in 48 patients who underwent nonmyeloablative HSCT41 ; 15.8% developed a non-Candida IFI. A logistic regression model identified high-grade GVHD, cumulative steroid doses of more than 10 mg/kg, and non-CMV viral respiratory infections as risk factors within the population.41 Similarly, Hagen and colleagues reported an incidence of proven or probable IFI of 23.8% among 31 consecutive nonmyeloablative HSC transplant recipients at their institution over a 2.5-year period.42 They identified severe GVHD, high-dose corticosteroid use, recurrent neutropenia, and relapsed hematologic disease at the time of transplantation as risk factors in an univariate analysis.42 Junghanss and colleagues reported a matched control study on the infections of 56 consecutive nonmyeloablative HSC transplant recipients matched by CMV serostatus, donor characteristics, and stem-cell source to contemporary myeloablative HSC transplant recipients cared for at their institution.43 They observed an incidence proportion of 15% of invasive aspergillosis in the nonmyeloablative cases and an incidence proportion of 9% in the myeloablative controls; these proportions were similar (P = .30). Their multivariate analysis identified CMV disease, high-risk underlying hematologic malignancy, and age older than 40 years as significant risk factors.43
Residual confounding by indication20 cannot be completely excluded given the novelty of both nonmyeloablative HSCT and infliximab use in the present observational cohort, even after adjusting the HR estimates to gastrointestinal organ-specific GVHD, but the magnitude of the IFI cumulative probability, IRR, and HR estimates suggest a true association.
In conclusion, infliximab is associated with a high risk of IFI when used in patients with severe GVHD. In addition, nonmyeloablative HSCT is an emergent risk factor for IFI, especially in patients who develop severe GVHD. The determinants of this excess risk should be explored further and alternative conditioning regimens and modes of GVHD prophylaxis and treatment should be considered. As targeted immunomodulatory therapies become available, such as monoclonal antibodies, the opportunity and obligation to define the unintended immune consequences is on us. These observations will lead to an improved understanding of human immunology and allow optimization of patient care.
Prepublished online as Blood First Edition Paper, July 10, 2003; DOI 10.1182/blood-2003-01-0267.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are thankful to Deborah Yokoe, Anushua Sinha, and the infection control department at BWH/DFCI for their invaluable assistance and comments; to Bijal Parikh and Qiheng Yang for their work on data extraction and management; and to John Page for his assistance and review of the statistical analysis.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal