Abstract
Protease-activated receptors (PARs) are stimulated by proteolytic cleavage of their extracellular domain, unmasking a new N-terminus acting as tethered ligand. Whereas the role of PARs in platelets is well known, their presence and function in human monocytes and other antigen-presenting cells has not been characterized. Here it is demonstrated that human peripheral monocytes and monocyte-derived macrophages and dendritic cells differentially express PARs. Human monocytes express mainly PAR1 and less PAR3. Differentiation of monocytes into macrophages by either macrophage colony-stimulating factor (M-CSF) or granulocyte-macrophage colony-stimulating factor (GM-CSF) elicits enhanced expression of PAR1, PAR2, and PAR3. In contrast, dendritic cells differentiated from monocytes by GM-CSF and interleukin-4 (IL-4) strongly down-regulated PAR1, PAR2, and PAR3, both at the mRNA and the protein level. Down-regulation of the PAR expression was apparently due to IL-4, because treatment of macrophages with IL-4 caused down-regulation of PAR1, PAR2, and PAR3. PAR4 mRNA expression remained undetectable in any of the cell types investigated. Stimulation of PAR1, PAR2, and PAR3 with thrombin, trypsin, or established receptor-activating peptides (PAR-APs) triggered cytosolic Ca2+ responses, indicating functionally active PARs. Further, stimulation of monocytes or macrophages with thrombin or PAR1-AP, but not with PAR2-or PAR4-AP, triggers expression of monocyte chemoattractant protein-1 (MCP-1) both at the mRNA and the protein level. These data demonstrate that differentiation of human monocytes is associated with differential expression of functionally active PARs that mediate distinct regulatory functions in inflammation and atherogenesis. (Blood. 2003;102:2645-2652)
Introduction
Apart from its pivotal role in hemostasis, thrombin is involved in signaling pathways that modulate inflammatory reactions. Tissue factor expression during inflammation promotes the generation of thrombin that stimulates a variety of blood and vascular cells including platelets, monocytes, and endothelial and smooth muscle cells.1-3
The cellular activity of thrombin and some other proteases is mediated mainly through the family of protease-activated receptors (PARs) belonging to the G protein-coupled receptors. Proteolytic cleavage of the N-terminal exodomain of the receptor unmasks a new N-terminus, the so-called tethered ligand that appears to initiate signaling by interaction with the second extracellular loop of the receptor. Synthetic receptor-activating peptides (APs) mimicking the newly formed N-terminal sequence of the receptor activate PARs independently of protease activity and receptor cleavage.1-3 So far, the PAR family comprises 4 members, PAR1, PAR3, and PAR4 activated by thrombin, and PAR2 activated by factor Xa, trypsin, and mast cell tryptase, but not by thrombin; PAR4 may be additionally activated by trypsin.3 Apart from direct signaling, cofactor-assisted signaling of PARs has been observed, for example, in murine platelets, where PAR3 has been shown to serve as coreceptor of PAR4.4,5
PARs may regulate and modulate monocyte functions. Thus, thrombin stimulates release of cytokines such as interleukin-1β (IL-1β) and IL-6 in monocytes,6 and thrombin as well as PAR1-AP elicit IL-8 release in U937 cells,7 where thrombin also induces actin polymerization and Ca2+ mobilization.8 Similarly, PAR1-AP was found to trigger Ca2+ mobilization in human monocytes.9 Thrombin also elicits monocyte chemotaxis, which might, however, be mediated through a different receptor, because it occurs independently of the proteolytic activity of thrombin.10 Although PAR1 expression has been demonstrated in the monocytic cell line U937,6 it has not yet been confirmed in human monocytes.
Serine proteases act at the interface between coagulation and inflammation. Interestingly, PAR1-AP selectively increases vascular permeability leading to edema formation. Moreover, the thrombin inhibitor hirulog was able to inhibit the carrageenan-induced paw edema.11 The local expression of PARs may be regulated by the inflammatory process. Indeed, PAR1 is abundant in the synovial lining of patients with rheumatoid and osteoarthritis.12 PARs have also been implicated in the development of the atherosclerotic plaque. In human arteries PAR1 is highly expressed in regions rich in macrophages and smooth muscle cells such as atherosclerotic lesions.13 In addition, the amount of thrombin present in human neointima is apparently sufficient to induce proliferation of smooth muscle cells.14 Further evidence for the functional relevance of these findings is provided by studies showing that adenoviral expression of the thrombin inhibitor hirudin reduces neointima formation after arterial injury.15 Finally, balloon angioplasty triggers strong expression of PAR1 in cells from the area of restenosis.12 These data imply an important role of PAR1 in inflammation, atherogenesis, and restenosis and suggest PARs as a therapeutic target.
Upon migration from the bloodstream, human peripheral monocytes mature into macrophages that perform an array of crucial functions ranging from phagocytosis of infectious agents to T-cell activation by antigen presentation.16 When exposed to appropriate signals, monocytes can differentiate into dendritic cells. After encountering infectious agents that may also play a role in atherogenesis,17,18 dendritic cells are major antigen-presenting cells.16
Considering the significance of monocytes and monocyte-derived antigen-presenting cells for pathological processes, such as chronic inflammation and atherosclerosis, we have investigated the expression and functional activity of PARs in monocytes, macrophages, and dendritic cells. We demonstrate that human peripheral monocytes, macrophages, and dendritic cells differentially express functionally active PAR1, PAR2, and PAR3, but not PAR4. Activation of these receptors is linked to distinct cellular functions as exemplified by the induction of the potent C-C chemokine MCP-1 in monocytes and macrophages by PAR1-AP, but not by any of the other PAR-APs tested. We further demonstrate a negative regulation of PAR expression by the immunoregulatory cytokine IL-4.
Materials and methods
Materials
Anti-PAR1 mouse monoclonal antibodies (mAbs) SPAN12, WEDE15, and anti-CD41 were purchased from Immunotech (Marseille, France). Polyclonal anti-PAR3 and anti-PAR4 antibodies as well as SAM11 mAbs against PAR2 were from Santa Cruz Biotechnology (Santa Cruz, CA), and CD14 as well as CD1a mAbs were from Pharmingen (Heidelberg, Germany). CD68 mAb was from Dako (Glostrup, Denmark) and CD83 mAb from Serotec (Oxford, United Kingdom). Phycoerythrin (PE)-conjugated donkey anti-mouse and anti-rabbit IgG F(ab)2 were purchased from Dianova (Hamburg, Germany). Recombinant human tumor necrosis factor (TNF)-α, macrophage colony-stimulating factor (M-CSF), and interleukin-4 (IL-4) were from R&D Systems (Minneapolis, MN). Histopaque 1077, probenecid, endotoxin-free bovine serum albumin (BSA), N-formyl-Met-Leu-Phe (FMLP), thrombin (specific activity 1690 U/mg), and control mouse IgG were from Sigma (St Louis, MO). Tissue culture media were from Invitrogen (Carlsbad, CA). Recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF) was from Novartis (Basel, Switzerland). Trypsin (specific activity, 2.5 U/mg) was from Calbiochem (San Diego, CA), and recombinant hirudin from Aventis (Frankfurt, Germany). PAR1-AP (TFLLRNPNDK) and PAR-specific primers were from Interactiva (Ulm, Germany), PAR2-AP (SLIGKVamide) and PAR4-AP (GYPGQV) were from Bachem (Heidelberg, Germany). Fura-2 acetoxymethyl ester was from Molecular Probes (Eugene, OR). Other chemicals were of analytical grade.
Cell isolation and cell differentiation
Human peripheral monocytes were isolated by Percoll gradient centrifugation as previously described.19,20 To assure immediate inactivation of possible traces of thrombin during blood collection, 0.75 μM recombinant hirudin was added. For cytosolic Ca2+ measurements and flow cytometric analysis, preparations with 94% or more CD14+ cells were used; contaminating cells were lymphocytes. Flow cytometry of CD14+ cells stained with anti-CD41 antibodies did not reveal any platelet contamination. For RNA isolation, Percoll-isolated monocytes were additionally purified by anti-CD14 MACS magnetic micro beads (Miltenyi Biotec, Bergisch Gladbach, Germany) yielding more than 98% CD14+ cells.
Monocytes used for differentiation were isolated from buffy coats that were diluted with washing buffer (phosphate-buffered saline [PBS] containing 2 mM EDTA [ethylenediaminetetraacetic acid] and 0.5% endotoxin-free BSA, pH 7.4), layered over 15 mL Histopaque 1077, and centrifuged at 750g for 20 minutes at 20°C. The mononuclear cell layer was removed and washed 3 times (150g for 10 minutes at 20°C). Washed mononuclear cell pellets were resuspended in RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum (FCS), plated in 10-cm tissue-culture dishes, and permitted to adhere for 45 minutes at 37°C. Nonadherent cells were removed by washing the plates vigorously 3 times with PBS. Macrophages were differentiated from adherent monocytes in the presence of M-CSF (15 ng/mL) or, in some experiments, GM-CSF (1000 U/mL).21 Differentiation of monocytes with GM-CSF (1000 U/mL) and IL-4 (25 ng/mL) gave rise to dendritic cells.21 Media were changed every 48 hours. Macrophages were used after 7 days of culture, whereas dendritic cells were used after 6 days. Differentiation of monocytes into macrophages and dendritic cells was confirmed by flow cytometric analysis of CD14, CD68, and CD1a.22 Platelets were isolated as previously described.23
Semiquantitative reverse transcription-polymerase chain reaction analysis
For reverse transcription-polymerase chain reaction (RT-PCR) mRNA was isolated with oligo-dT25 magnetic beads (Dynal, Oslo, Norway) according to the manufacturer's instructions. One microgram of mRNA, oligo(dT), random hexamers, and the reverse transcriptase Super Script II (Invitrogen) were used for the RT reactions. PAR1-specific primers were 5′-TACGCCTCTATCTTGCTCATGAC-3′ (sense) and 5′-TTTGTGGGTCCGAAGCAAAT-3′ (antisense), yielding a PCR product with an expected size of 453 base pair (bp). Primers for PAR2, PAR3, and PAR4 were according to Kahn et al.24 PAR1 was amplified with 28 cycles (94°C for 30 seconds, 55°C for 30 seconds, 72°C for 60 seconds), and 34 cycles were used to amplify PAR2 and PAR3. For PAR4, 35 cycles (94°C for 30 seconds, 55°C for 30 seconds, 72°C for 30 seconds) were used. RT-PCR analysis of CD41 was performed as described.25 RT-PCR of MCP-1 was performed according to Seino et al.26 Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) used for normalization was amplified with 28 cycles (94°C for 30 seconds, 55°C for 30 seconds, and 72°C for 60 seconds).
To exclude any genomic DNA contamination, all RNA samples were pretreated with DNase I (Invitrogen) for 30 minutes at 25°C, followed by 5 minutes at 75°C to stop the reaction. The identity of the PCR products was confirmed by sequencing (Abi Prism 310, Applied Biosystems, Foster City, CA).
Analysis of protein expression
For flow cytometry, cells were incubated with 1 μg/mL mAbs for 1 hour at 4°C. After washing, the cells were incubated with PE-conjugated anti-mouse IgG F(ab)2 (1:100) for 1 hour at 4°C in the dark. After a final washing step, cells were analyzed using a FACScan (BD Biosciences, San Jose, CA). Polyclonal PAR3 antibodies were raised in rabbits against a synthetic peptide (RGAPPNSFEEFPFamide) corresponding to the N-terminal sequence of PAR3.27 Rabbit antibodies were visualized by donkey anti-rabbit PE-conjugated F(ab)2.
For Western blot analysis 2 × 106 monocytes/assay were lysed and resolved by polyacrylamide gel electrophoresis (PAGE) as previously described.28 Blots were immunostained with SPAN12 and SAM11 for PAR1 and PAR2, respectively. For PAR3 staining the affinity-purified polyclonal antibodies were used. Antibody complexes were detected using the SuperSignal West Pico enhanced chemiluminescence detection kit (Pierce, Rockford, IL) with subsequent exposure to Hyperfilm ECL (Amersham Biosciences, Freiburg, Germany); the antibodies yielded single bands.
Measurement of cytosolic calcium levels
Cells were washed and resuspended in HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid)-buffered salt solution (in mM: 25 HEPES, 3.5 KCl, 125 NaCl, 1.2 MgCl2, 5 NaHCO3, 5 glucose, 5 Na-pyruvat, pH 7.4) containing 0.1% bovine serum albumin. Monocytes and dendritic cells were incubated with 2 μM Fura-2 acetoxymethyl ester for 30 minutes at 37°C. Macrophages were loaded for 60 minutes at 37°C in HEPES-buffered salt solution additionally conditioned with 2.5 mM probenecid.29 The calcium indicator Fura-2 was retained in these cells only in the presence of probenecid, suggesting that the Fura-2 efflux was mediated by an organic anion transporter. Cells were washed 3 times and finally resuspended in the buffer solution supplemented with 1.2 mM CaCl2. One million cells were transferred to a microcuvette and allowed to equilibrate at 37°C for 3 minutes prior to fluorescence measurement in a PTI Fluorescence Detection System (Photon Technology International, Lawrenceville, NJ). Cells were challenged with thrombin, trypsin, or corresponding PAR-APs. FMLP 10 nM was used as a positive control. Fura-2 fluorescence was measured at 2 wavelengths, with excitation at 340 and 380 nm and emission at 510 nm. The ratio of the fluorescence at the 2 wavelengths is proportional to the calcium concentration in nM:
where Rmax and Rmin are the fluorescence ratio values under saturating and Ca2+-free conditions, respectively, and Sf2/Sb2 is the ratio of fluorescence values for Ca2+-bound/Ca2+-free indicator measured at the wavelength 380 nm used to monitor the Ca2+-free indicator. The Kd of Fura-2 for Ca2+ is 224 nM.30
Expression of MCP-1
Monocytes or macrophages incubated in RPMI 1640 supplemented with 10% FCS were stimulated with thrombin or various PAR-APs for 8 and 24 hours for mRNA and protein expression, respectively. Antibody blockade of thrombin cleavage with WEDE15 was performed as described.31 Because M-CSF itself triggers MCP-1 expression, the macrophages were allowed to rest in the absence of M-CSF for 24 hours before PAR stimulation. MCP-1 was assayed in cell-free supernatants using enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN).
Statistics
Data shown represent mean ± SEM where applicable. Statistical significance was calculated with the Newman-Keuls test. Differences were considered significant for P < .05.
Results
Human peripheral monocytes express PAR1 and PAR3
Expression of PARs was analyzed in freshly isolated human peripheral monocytes. RT-PCR was performed with mRNA isolated from monocytes that were additionally purified with anti-CD14 magnetic bead separation. The purity of these monocyte preparations was always more than 98% CD14+ cells. Platelet contamination was excluded by analysis of CD41 that was undetectable both by RT-PCR and flow cytometry (data not shown).
Primers specific for PAR1, PAR2, and PAR3 yielded bands of the expected respective size (Figure 1A); the identity of the amplification products was confirmed by automated sequencing (data not shown). Whereas only a faint band of PAR2 amplification product was observed (Table 1), PAR4 could not be detected at all despite the fact that the respective primers amplified PAR4 in purified platelets (Figure 2). Analysis of monocyte RNA without reverse transcriptase did not lead to amplification of any bands, indicating that the PCR products obtained were not due to genomic DNA contamination (data not shown).
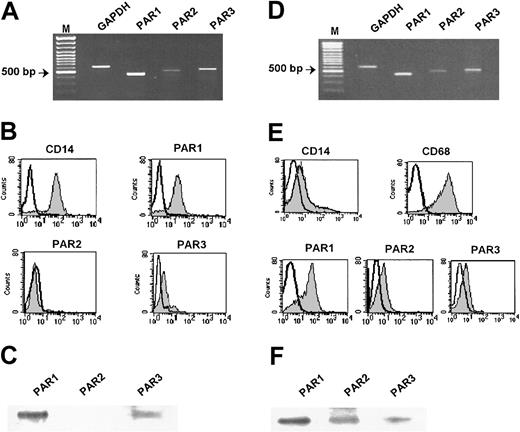
Expression of protease-activated receptors in human peripheral monocytes and in monocyte-derived human macrophages. (A) Monocytes. RT-PCR analysis of PAR1, PAR2, and PAR3 mRNA expression in CD14+ monocytes. GAPDH was used for normalization. (B) Monocytes. Flow cytometric analysis of PAR1, PAR2, and PAR3 expression using specific mAb for PAR1 (SPAN12), PAR2 (SAM11), and polyclonal antibody for PAR3 (shaded peaks). Nonshaded peaks represent cells stained with isotype-matched control antibodies. (C) Monocyte lysates were prepared and subjected to Western blot analysis with the appropriate antibodies. (D) Macrophages. RT-PCR analysis of PAR1, PAR2, and PAR3 mRNA expression in macrophages differentiated from monocytes for 7 days in the presence of 15 ng/mL M-CSF. (E) Macrophages. Flow cytometric analysis of PAR1, PAR2, and PAR3 in human macrophages was performed as described in Figure 1B. CD14 and CD68 antigens were used as differentiation markers (shaded peaks). (F) Macrophage lysates were prepared and subjected to Western blot analysis with the appropriate antibodies. M indicates marker. Results of 1 of 6 independent experiments are shown.
Expression of protease-activated receptors in human peripheral monocytes and in monocyte-derived human macrophages. (A) Monocytes. RT-PCR analysis of PAR1, PAR2, and PAR3 mRNA expression in CD14+ monocytes. GAPDH was used for normalization. (B) Monocytes. Flow cytometric analysis of PAR1, PAR2, and PAR3 expression using specific mAb for PAR1 (SPAN12), PAR2 (SAM11), and polyclonal antibody for PAR3 (shaded peaks). Nonshaded peaks represent cells stained with isotype-matched control antibodies. (C) Monocyte lysates were prepared and subjected to Western blot analysis with the appropriate antibodies. (D) Macrophages. RT-PCR analysis of PAR1, PAR2, and PAR3 mRNA expression in macrophages differentiated from monocytes for 7 days in the presence of 15 ng/mL M-CSF. (E) Macrophages. Flow cytometric analysis of PAR1, PAR2, and PAR3 in human macrophages was performed as described in Figure 1B. CD14 and CD68 antigens were used as differentiation markers (shaded peaks). (F) Macrophage lysates were prepared and subjected to Western blot analysis with the appropriate antibodies. M indicates marker. Results of 1 of 6 independent experiments are shown.
Expression of PAR mRNA in human monocytes, macrophages, and dendritic cells
. | PAR-1 . | PAR-2 . | PAR-3 . |
|---|---|---|---|
| Monocytes | 88.60 ± 19.70 | 5.80 ± 3.70 | 26.40 ± 11.40 |
| Macrophages | 100 ± 6.35 | 17.10 ± 5.60 | 56.10 ± 18.40 |
| Dendritic cells | 17.60 ± 2.20 | 2.50 ± 0.75 | 8.30 ± 2.10 |
. | PAR-1 . | PAR-2 . | PAR-3 . |
|---|---|---|---|
| Monocytes | 88.60 ± 19.70 | 5.80 ± 3.70 | 26.40 ± 11.40 |
| Macrophages | 100 ± 6.35 | 17.10 ± 5.60 | 56.10 ± 18.40 |
| Dendritic cells | 17.60 ± 2.20 | 2.50 ± 0.75 | 8.30 ± 2.10 |
PAR mRNA was analyzed by semiquantitative RT-PCR and normalized to GAPDH. Results are expressed in arbitrary units and are mean ± SEM of 6 independent experiments.
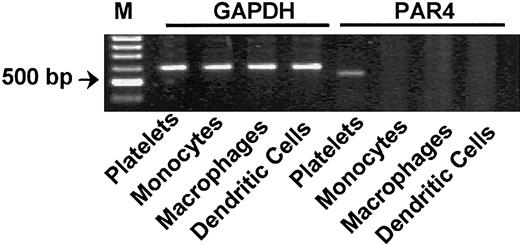
Human peripheral monocytes, macrophages, and dendritic cells do not express protease-activated receptor 4 mRNA. RT-PCR analysis did not detect PAR4 mRNA expression on either CD14+ monocytes or macrophages or dendritic cells. Human platelets were used as a positive control. GAPDH was used for normalization. M indicates marker. Results of 1 of 3 independent experiments are shown.
Human peripheral monocytes, macrophages, and dendritic cells do not express protease-activated receptor 4 mRNA. RT-PCR analysis did not detect PAR4 mRNA expression on either CD14+ monocytes or macrophages or dendritic cells. Human platelets were used as a positive control. GAPDH was used for normalization. M indicates marker. Results of 1 of 3 independent experiments are shown.
PAR expression was further analyzed by flow cytometry using PAR1, PAR2, and PAR3-specific antibodies. Monocytes bound the anti-PAR1 mAb SPAN12, but basically no anti-PAR2 mAb SAM1132 (Figure 1B). Similar results were obtained with WEDE15 and S14.8.2, mAbs targeting different epitopes of PAR1 and PAR2, respectively (data not shown). In addition, monocytes bound anti-PAR3 antibodies; their binding was blocked in the presence of the peptide (100 μM) used for immunization (data not shown). Similar results were obtained with commercial PAR3 antibodies (data not shown). The observed PAR expression profile was further confirmed by Western blot analysis of monocyte lysates demonstrating expression of mainly PAR1 and significantly less PAR3, whereas PAR2 remained undetectable (Figure 1C). These results provide the first demonstration of PAR expression in human peripheral monocytes.
Monocyte-derived macrophages express PAR1, PAR2, and PAR3
Depending on the cytokine environment, blood monocytes differentiate into macrophages or dendritic cells, as defined by changes in cell morphology, surface antigen expression, and cell function. We further investigated the effects of differentiation on PAR expression in macrophages. Figure 1D shows that macrophages differentiated by 15 ng/mL M-CSF express PAR1, PAR2, and PAR3 mRNA (Table 1). As in monocytes, PAR4 mRNA remained undetectable by RT-PCR in these cells (Figure 2).
Flow cytometric analysis of CD14 and CD68 expression confirmed the macrophage phenotype of these cells (Figure 1E). Macrophages express primarily PAR1 and less PAR2 and PAR3 (Figure 1E). Macrophages differentiated with 1000 U/mL GM-CSF showed a similar pattern of PAR expression (data not shown). Western blot analysis of PARs in macrophage lysates showing single bands of the expected size confirmed the flow cytometric data (Figure 1F).
Down-regulation of PAR1, PAR2, and PAR3 in dendritic cells
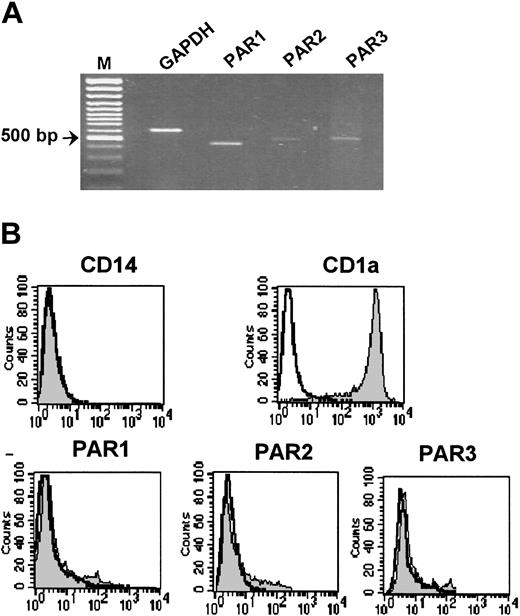
PAR expression was further analyzed in dendritic cells that had been differentiated from monocytes by a combination of 1000 U/mL GM-CSF and 25 ng/mL IL-4. Differentiation of monocytes into dendritic cells was confirmed by up-regulation of CD1a expression and down-regulation of CD14 expression (Figure 3B). PAR expression at the transcriptional level was examined by semiquantitative RT-PCR (Figure 3A). Small amounts of PAR1, PAR3, and traces of PAR2 mRNA were detectable in dendritic cells, yet they were profoundly reduced compared with monocytes and especially to macrophages (Table 1). As in monocytes and macrophages, PAR4 mRNA expression could not be detected in dendritic cells (Figure 3).
Expression of protease-activated receptors in human dendritic cells. (A) RT-PCR analysis shows PAR1, PAR2, and PAR3 mRNA expression in dendritic cells differentiated from monocytes for 6 days in the presence of 1000 U/mL GM-CSF and 25 ng/mL IL-4. GAPDH was used for normalization. (B) Flow cytometric analysis of PAR1, PAR2, and PAR3 in human dendritic cells was performed as described in Figure 1. CD14 and CD1a antigens were used as differentiation markers. Nonshaded peaks represent cells stained with isotype-matched control antibodies. M indicates marker. Results of 1 of 3 independent dendritic cell preparations are shown.
Expression of protease-activated receptors in human dendritic cells. (A) RT-PCR analysis shows PAR1, PAR2, and PAR3 mRNA expression in dendritic cells differentiated from monocytes for 6 days in the presence of 1000 U/mL GM-CSF and 25 ng/mL IL-4. GAPDH was used for normalization. (B) Flow cytometric analysis of PAR1, PAR2, and PAR3 in human dendritic cells was performed as described in Figure 1. CD14 and CD1a antigens were used as differentiation markers. Nonshaded peaks represent cells stained with isotype-matched control antibodies. M indicates marker. Results of 1 of 3 independent dendritic cell preparations are shown.
In spite of expression of small amounts of mRNA, protein expression of PAR1, PAR2, and PAR3 could not be detected on the surface of dendritic cells by flow cytometric analysis (Figure 3B), suggesting both transcriptional and translational regulation.
These data indicate that PAR1, PAR2, and PAR3 are differentially expressed on human monocytes, macrophages, and dendritic cells. The only difference between macrophages differentiated with GM-CSF and dendritic cells was the presence of IL-4. Therefore, the data suggested that the differential PAR expression on macrophages and dendritic cells might be IL-4 dependent.
IL-4 down-regulates PAR1, PAR2, and PAR3 expression in macrophages
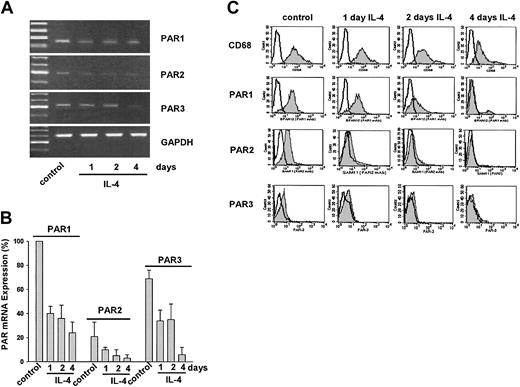
Indeed, treatment of macrophages for an additional 4 days with 25 ng/mL IL-4 induced a time-dependent down-regulation of PAR1, PAR2, and PAR3 mRNA. All PARs exhibited the lowest level of mRNA expression after 4 days of IL-4 treatment (Figure 4A-B). In agreement with the mRNA expression data, flow cytometric analysis demonstrated that IL-4 time dependently down-regulates expression of PAR1, PAR2, and PAR3 in macrophages (Figure 4C). Down-regulation of PAR mRNA became detectable already after 1 day of IL-4 exposure. After 4 days, PAR1, PAR2, and PAR3 protein expression was completely down-regulated, although there was still some mRNA mainly of PAR1. The decrease in PAR protein expression was accompanied by a similar though incomplete down-regulation of CD68 expression (Figure 4C). In parallel, the dendritic cell markers CD40 and HLA-DR were up-regulated (data not shown). However, IL-4 did not up-regulate CD1a expression in macrophages (data not shown), indicating that in response to IL-4, the cells acquired a follicular dendritic cell phenotype.
IL-4 down-regulates protease-activated receptor mRNA expression in monocyte-derived human macrophages. (A) RT-PCR analysis shows PAR mRNA expression in macrophages treated with 25 ng/mL IL-4 for 1, 2, or 4 days. (B) Semiquantitative analysis of the mRNA expression. PAR mRNA was normalized to GAPDH mRNA. PAR1 expression was set to 100% in each experiment. Results are mean ± SEM of 3 independent experiments. (C) Flow cytometric analysis of PARs on macrophages treated with 25 ng/mL of IL-4 for 1, 2, or 4 days. CD68 was used as macrophage-specific marker. Nonshaded peaks represent cells stained with isotype-matched control antibodies. Results of 1 of 3 independent experiments are shown.
IL-4 down-regulates protease-activated receptor mRNA expression in monocyte-derived human macrophages. (A) RT-PCR analysis shows PAR mRNA expression in macrophages treated with 25 ng/mL IL-4 for 1, 2, or 4 days. (B) Semiquantitative analysis of the mRNA expression. PAR mRNA was normalized to GAPDH mRNA. PAR1 expression was set to 100% in each experiment. Results are mean ± SEM of 3 independent experiments. (C) Flow cytometric analysis of PARs on macrophages treated with 25 ng/mL of IL-4 for 1, 2, or 4 days. CD68 was used as macrophage-specific marker. Nonshaded peaks represent cells stained with isotype-matched control antibodies. Results of 1 of 3 independent experiments are shown.
Calcium signaling of PARs in monocytes and macrophages
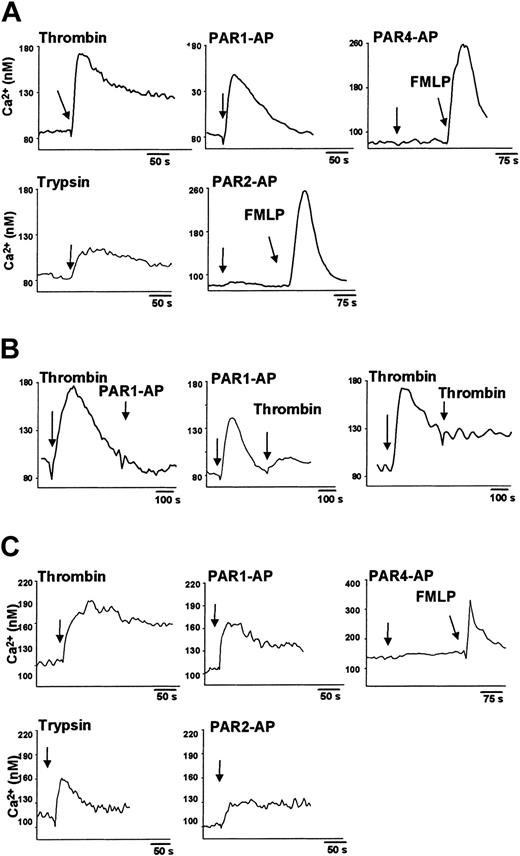
To examine whether the PARs expressed are functionally active, we analyzed cytosolic Ca2+ levels using Fura-2. Monocytes or macrophages were stimulated with thrombin, trypsin, and, by mimicking the tethered ligands, with PAR-specific APs. At the end of each experiment, 10 nM FMLP was applied as positive control.
In freshly isolated human monocytes 10 U/mL thrombin as agonist for PAR1 and PAR3, elicited a cytosolic Ca2+ increase of approximately 85 nM (Figure 5A). Smaller increases in cytosolic Ca2+ also were detected in the presence of 1 U/mL thrombin (data not shown). PAR1-AP (300 μM) triggered a cytosolic Ca2+ rise that was only slightly lower than that induced by 10 U/mL thrombin. As expected on the basis of the gene expression data, monocytes failed to respond to PAR4-AP (Figure 5A). Additional concentrations of PAR4-AP up to 600 μM yielded the same negative results (data not shown). Exposure of monocytes to 100 nM trypsin that activates PAR2, but also PAR1,32,33 induced a small Ca2+ response. By contrast, 300 μM PAR2-AP did not trigger any cytosolic Ca2+ elevation in monocytes, which responded, however, to the positive control 10 nM FMLP (Figure 5A). Pretreatment of monocytes with 10 U/mL thrombin totally abolished the response to 300 μM PAR1-AP (Figure 5B). Conversely, preincubation of monocytes with PAR1-AP 300 μM decreased, but did not abolish, the cytosolic Ca2+ response to 10 U/mL thrombin. As anticipated, thrombin stimulation caused desensitization to a second thrombin exposure (Figure 5B).
Protease-activated receptor ligands increase cytosolic Ca2+ levels in human peripheral monocytes and monocyte-derived macrophages. (A) Monocytes loaded with 2 μM Fura-2 acetoxymethyl ester for 30 minutes were stimulated (arrows) with 10 U/mL thrombin, 100 nM trypsin, 10 nM FMLP, or with 300 μM of each PAR-AP. (B) In desensitization experiments, monocytes were sequentially stimulated either with thrombin (10 U/mL) and PAR1-AP (300 μM) or thrombin (10 U/mL). (C) Macrophages loaded with 2 μM Fura-2 acetoxymethyl ester for 60 minutes were stimulated with thrombin, trypsin, PAR-AP, or FMLP, as indicated. Results of 1 of 3 independent experiments are shown.
Protease-activated receptor ligands increase cytosolic Ca2+ levels in human peripheral monocytes and monocyte-derived macrophages. (A) Monocytes loaded with 2 μM Fura-2 acetoxymethyl ester for 30 minutes were stimulated (arrows) with 10 U/mL thrombin, 100 nM trypsin, 10 nM FMLP, or with 300 μM of each PAR-AP. (B) In desensitization experiments, monocytes were sequentially stimulated either with thrombin (10 U/mL) and PAR1-AP (300 μM) or thrombin (10 U/mL). (C) Macrophages loaded with 2 μM Fura-2 acetoxymethyl ester for 60 minutes were stimulated with thrombin, trypsin, PAR-AP, or FMLP, as indicated. Results of 1 of 3 independent experiments are shown.
Challenge of macrophages with 10 U/mL thrombin also elicited a cytosolic Ca2+ response. Similarly, stimulation with 300 μM PAR1-AP resulted in an increase of cytosolic Ca2+ levels. In agreement with the expression data and in line with the monocyte results, 300 μM PAR4-AP did not induce any increase in cytosolic Ca2+ (Figure 5C). In contrast to monocytes, 300 μM PAR2-AP triggered a cytosolic Ca2+ increase in macrophages, as did 100 nM trypsin (Figure 5C).
Consistent with the lack of PAR protein expression, dendritic cells did not react to 10 U/mL thrombin or to 100 nM trypsin. They also did not respond to 300 μM of PAR1-AP, PAR2-AP, or PAR4-AP. Yet these cells responded very well to 10 nM FMLP (data not shown).
Activation of PAR1 stimulates induction of MCP-1
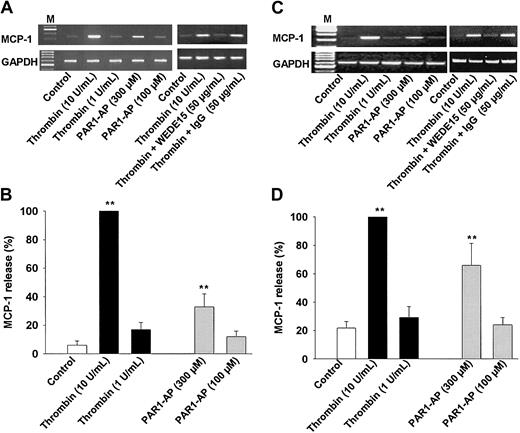
Thrombin is known to stimulate the p38 mitogen-activated protein kinase (MAPK) signaling pathway, which we and others have previously shown to be essential for MCP-1 induction.28,34,35 In order to strengthen the functional significance of PAR activation, we have investigated whether any of the PAR-AP might elicit MCP-1 induction in monocytes. Indeed, both thrombin and PAR1-AP induced a concentration-dependent expression of MCP-1 mRNA, with thrombin being more potent than PAR1-AP (Figure 6A). The activation of MCP-1 mRNA expression by thrombin was nearly abolished by the PAR1-blocking antibody WEDE1531 (Figure 6A), indicating signaling through PAR1. None of the other APs for PAR2 or PAR4 induced any significant induction of MCP-1 expression (data not shown). These results correlated with the release of MCP-1 by monocytes stimulated either with thrombin or PAR1-AP for 24 hours (Figure 6B). Again, the other APs did not trigger any significant release of MCP-1 (data not shown).
PAR1 activation stimulates expression of MCP-1 in human monocytes and monocyte-derived human macrophages. (A) Monocytes. MCP-1 mRNA expression. Cells incubated in the presence or absence of thrombin, PAR1-AP, WEDE15, or control IgG for 8 hours; mRNA was extracted and subjected to RT-PCR. GAPDH transcript levels were used for normalization. Results of 1 of 3 experiments are shown. (B) Monocytes. MCP-1 release. Cells were incubated in the presence or absence of the stimuli for 24 hours before MCP-1 release was analyzed by ELISA; results are the mean ± SEM of 6 independent experiments; 100% = 1.18 ± 0.34 ng/106 cells. (C) Macrophages. MCP-1 mRNA expression. Experimental conditions were the same as for monocytes. (D) Macrophages. MCP-1 release. Cells were treated as described for monocytes. Results are the mean ± SEM of 4 independent experiments. 100% = 5.2 ± 1.2 ng/106 cells. **P < .01 versus control (B, D).
PAR1 activation stimulates expression of MCP-1 in human monocytes and monocyte-derived human macrophages. (A) Monocytes. MCP-1 mRNA expression. Cells incubated in the presence or absence of thrombin, PAR1-AP, WEDE15, or control IgG for 8 hours; mRNA was extracted and subjected to RT-PCR. GAPDH transcript levels were used for normalization. Results of 1 of 3 experiments are shown. (B) Monocytes. MCP-1 release. Cells were incubated in the presence or absence of the stimuli for 24 hours before MCP-1 release was analyzed by ELISA; results are the mean ± SEM of 6 independent experiments; 100% = 1.18 ± 0.34 ng/106 cells. (C) Macrophages. MCP-1 mRNA expression. Experimental conditions were the same as for monocytes. (D) Macrophages. MCP-1 release. Cells were treated as described for monocytes. Results are the mean ± SEM of 4 independent experiments. 100% = 5.2 ± 1.2 ng/106 cells. **P < .01 versus control (B, D).
Analogous experiments were performed with macrophages that basically yielded identical results except that macrophages generally released much higher amounts of MCP-1, even under basal conditions already. Both thrombin and PAR1-AP induced concentration-dependent expression of MCP-1 mRNA that in the case of thrombin stimulation was again significantly reduced by the PAR1-neutralizing mAb WEDE15 (Figure 6C). Exposure of macrophages for 24 hours to thrombin or PAR1-AP was followed by release of MCP-1 into the media (Figure 6D). Also in these cells, the AP for PAR2 or PAR4 did not induce MCP-1 expression (data not shown), demonstrating that there is no redundancy among PAR activation and that MCP-1 induction is PAR1 specific.
Discussion
Considerable evidence implies an important role for thrombin in inflammation,12,36-39 yet there are no data on the expression and function of PARs in mononuclear phagocytes crucial for chronic inflammatory reactions.40 To investigate PAR expression in monocytes, we have used highly purified monocyte preparations, where platelet contamination as a putative source of PARs24 could be excluded. Further, we studied the PAR expression as well as its function in antigen-presenting cells such as macrophages and dendritic cells.
Consistent with the mRNA data, analysis of the cell surface expression of PARs revealed that PAR1 is the most abundant member of the PAR family both in monocytes and macrophages. As analyzed by flow cytometry, the expression of PAR3 on monocytes and macrophages is very low compared to that of PAR1. Macrophages were the only cells expressing detectable PAR2 on their membrane; the small amounts of PAR2 mRNA in monocytes could possibly be induced by the isolation procedure. In dendritic cells PAR mRNA was profoundly down-regulated, and these cells did not express any detectable PAR on their cell membrane, suggesting differentiation-associated down-regulation both at the transcriptional and translational level. Consistent with the lack of PAR expression, dendritic cells did not respond to thrombin or any of the PAR-APs. This finding suggests that cellular functions of dendritic cells might not be regulated through PAR activation, for example, by thrombin. In line with this, we were unable to find any evidence in the literature for thrombin-mediated regulation of dendritic cell function, although immunohistochemical studies identified some coagulation factors including prothrombin in close vicinity to follicular dendritic cells.41
The regulation of the PAR expression is not well understood. Activated PAR1 is internalized by phosphorylation-dependent mechanisms and subsequently degraded in lysosomes.3 In endothelial cells PAR1 expression is up-regulated at the transcriptional level by thrombin via the Gi-linked Ras/MAPK cascade.42 Yet, in this cell type PAR1 expression is unaffected by proinflammatory mediators, such as lipopolysaccharide, TNF-α, or IL-1α.43 Hence, it was suggested that regulation of PAR1 expression is nonadaptive. On the other hand, activation of endothelial cells with proinflammatory mediators resulted in up-regulation of PAR2 mRNA.43 Currently, there exist no reports on any negative regulation of PARs and, therefore, our observation, that IL-4 down-regulates PAR expression is of particular importance.
IL-4 produced by Th2 lymphocytes is known to exert anti-inflammatory effects on monocytes or macrophages. IL-4 acts through activation of the Jak/STAT signaling pathway and inhibits release of proinflammatory cytokines by monocytes.44-47 Our data demonstrate that IL-4 also rapidly down-regulates the expression of mRNA for PAR1, PAR2, and PAR3 accompanied by a decrease in the cell surface expression of these receptors. The latter may somewhat precede that of mRNA, suggesting that regulation at the level of translation also may be involved. The negative regulation of PARs by IL-4 might point to opportunities for therapeutic modulation of PAR expression.
Although activation of PARs triggers a rapid increase in cytosolic Ca2+ concentration in platelets, smooth muscle cells, and transfected cell lines,2 the data concerning thrombin-induced signaling in human monocytes remain controversial. It has been reported that thrombin does not trigger any cytosolic Ca2+ increase in these cells,48 but also that monocytes, similar to platelets, respond to thrombin or PAR1-AP with an intracellular Ca2+ increase.9 This discrepancy could be due to cleavage of thrombin receptors by trace amounts of thrombin generated during blood collection when citrate was used as anticoagulant. In our hands it was important to employ the anticoagulant EDTA and the thrombin antagonist hirudin.49 The use of EDTA may, however, be a double-edged sword as it is the best anticoagulant to prevent platelet activation, yet at the expense that it also dampens monocyte activation.50 This could easily explain why in comparison to other cell systems rather high amounts of thrombin and PAR-AP were required for the monocyte stimulation. Further, incubation of the cells in serum-containing media could also reduce, due to their antithrombin III contents, at least part of the thrombin-mediated activation.51
As expected, the stimulation of monocytes with thrombin desensitized the cells to subsequent thrombin or PAR1-AP activation. Vice versa, pretreatment of monocytes with PAR1-AP at 300 μM decreased the thrombin-mediated signaling. The thrombin-induced cytosolic Ca2+ increase was at least in its initial phase due mainly to activation of PAR1, because a similar Ca2+ increase was observed after treatment with PAR1-AP. However, in the latter case, the Ca2+ signal was somewhat smaller, very rapid, and decayed within 100 seconds. This suggests that the later sustained phase of the thrombin-mediated signal could proceed through another receptor, possibly PAR3. However, experiments with murine PAR3 provided evidence that this receptor does not signal directly, but in cooperation with PAR4, leading to the proposal that PAR3 might serve as coreceptor for PAR4.52 Consistent with this proposal, PAR3 overexpressed in COS 7 cells is activated in terms of cytosolic Ca2+ increase, when the cells were stimulated with thrombin but not in response to an AP mimicking the newly formed N-terminus.27 It is intriguing that in human monocytes and macrophages we could not detect PAR4 either by RT-PCR or by flow cytometry or by stimulation with PAR4-AP53 precluding any PAR4 costimulatory activity; this finding is not consistent with the paradigm of a coreceptor function of PAR3 in these cells. Similarly, transcription of mRNA for PAR3 in the absence of that for PAR4 was found in human neutrophils and eosinophils.54 The putative functional relevance of PAR3 in monocytes and macrophages is currently subject to intense investigation.
Consistent with the lack of PAR2 membrane receptors, PAR2-AP did not trigger cytosolic Ca2+ increase in monocytes. The rise in Ca2+ level observed in monocytes treated with trypsin (100 nM) is probably due to activation of PAR1.32,33,55 By contrast, macrophages express PAR1, PAR2, and PAR3. Although PAR2 is only moderately expressed by macrophages, PAR2-AP elicits a small cytosolic Ca2+ increase in these cells suggesting that at variance with monocytes, PAR2 might modulate macrophage functions.
Calcium signaling has been implicated in the regulation of a wide variety of cellular responses including adhesion, cell motility, gene expression, and proliferation.56 The amplitude and duration of the Ca2+ signals can vary significantly, which ensures the selective transmission of the signal to the Ca2+-regulated proteins.56 Thus, in T lymphocytes NF-κB and Jun N-terminal kinase are specifically stimulated by transient Ca2+ signals with high amplitude, while nuclear factor of activated T cells (NFAT) is activated by long-lasting Ca2+ signals with low amplitude.57 Cytosolic Ca2+ oscillations are apparently important for the efficiency and specificity of gene expression; they reduce the effective Ca2+ threshold for activating transcription factors, thereby increasing signal detection at low levels of stimulation.58 Ca2+ signaling in monocytes treated with PAR agonists might suffice to stimulate the IL-8 promoter, which has been shown to be activated by increased cytosolic Ca2+.58 Consistent with this hypothesis, it has recently been reported that thrombin and PAR1-AP induce IL-8 production in human monocytic U937 cells.7
The chemokine MCP-1 is expressed by a variety of cells, including LPS-stimulated monocytes and thrombin-activated vascular smooth muscle and endothelial cells.34,35,59-61 Therefore, we investigated whether PAR stimulation might induce monocyte MCP-1 expression. Indeed, we could show for the first time that stimulation of PAR1 either with thrombin or the PAR1-AP triggers expression of MCP-1 in human monocytes. The thrombin-mediated stimulation was apparently due to PAR1 activation, as it could be strongly inhibited by the PAR1-blocking mAb WEDE15.31 By contrast, none of the other PAR-APs had any significant effect, indicating that specific PAR subtypes are linked to distinct cellular responses. Analogous results though at a somewhat higher level were obtained with macrophages, further supporting the notion that MCP-1 expression is dependent on PAR1 activation. This mechanism is pathophysiologically relevant, as it has only recently been shown that thrombin triggers MCP-1 and IL-6 expression in a murine model of peritoneal inflammation.62 MCP-1 acts as a potent chemoattractant for monocytes, activated T cells, and NK cells63,64 and is believed to be an important signaling molecule in the development of atherosclerosis.59 As shown by the PAR1-mediated MCP-1 expression in human monocytes and macrophages, activation of PARs on monocytes and macrophages is likely to contribute to the development of inflammation and atherosclerosis.
Taken together, our data demonstrate differential expression of functionally active protease-activated receptors in human monocytes and monocyte-derived antigen-presenting cells. As exemplified by the PAR1-mediated expression of the chemokine MCP-1 in human monocytes and macrophages, different PARs do not seem to convey redundant signaling, rather they trigger distinct cellular reactions. Moreover, this is the first report on a negative regulation of PAR expression in human cells. Our findings provide novel insights into the link between thrombin and inflammation.
Prepublished online as Blood First Edition Paper, June 12, 2003; DOI 10.1182/blood-2002-08-2497.
Supported by the Deutsche Forschungsgemeinschaft.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The monoclonal antibodies S14.8.2 and SAM11 ascites fluid were generous gifts from Dr V. Ramakrishnan, COR Therapeutics, South San Francisco, CA, and Dr L. F. Brass, University of Philadelphia, PA. The technical assistance of J. Marinacci and A. Schüle is gratefully acknowledged.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal