Abstract
Women are conferred with greater immunologic and survival benefits compared to men. Female sex steroids contribute to this sexual dimorphism. Furthermore, during human pregnancy when female sex hormones are elevated, neutrophil apoptosis is delayed. This study examines the specific effects of estradiol and progesterone on neutrophil apoptosis and function in healthy adult men and women. We also examined the contribution of these hormones to the persistence and resolution of an inflammatory response. Spontaneous apoptosis was significantly decreased in women compared with men. Physiologic doses of estradiol and progesterone caused a further delay in spontaneous apoptosis in both men and women but did not diminish Fas antibody-induced apoptosis. The delay in apoptosis was mediated at the level of the mitochondria with decreased release of cytochrome c, which may alter caspase cleavage and activity. There were no associated alterations in neutrophil CD11b, but production of reactive oxygen intermediates (ROIs) in women was increased. Thus, female sex hormones mediate delayed neutrophil apoptosis in both sexes and enhance female intracellular production of ROIs. Modulating hormonal responses may be an effective therapeutic tool in combating inflammatory diseases. (Blood. 2003;102:2653-2659)
Introduction
From conception to senescence, women have a significant survival advantage.1 Men are more susceptible to sepsis and subsequent morbidity and mortality than women of reproductive age.2-4 The incidence of sepsis in postmenopausal women increases to levels almost equal to those seen in age-matched men.5,6 The exact mechanism mediating this sexual dimorphism is unclear. However, female sex hormones have been implicated because they modulate the immune system under normal and stress conditions.7 Following trauma or hemorrhage, female mice maintain their immune function, whereas male mice have significantly depressed responses.8 Estradiol has been shown to be protective in organ ischemia-reperfusion injury and shock by preventing androgen-induced immunosuppression in male animals.9-12 Thus, female sex hormones may be a useful adjunct in preventing trauma-induced immunosuppression and the associated increased susceptibility to sepsis.13 Estrogen treatment, testosterone depletion, and testosterone receptor antagonists improve outcome in male animals following trauma and sepsis.14 The exact role of changes in the estradiol-testosterone ratio in immune function requires further clarification. Estrogens are also cited as having a protective role in neurodegenerative and cardiac diseases through a variety of mechanisms including blockade of oxidation, antagonism of nitric oxide synthase activity,15 and interference with the apoptotic process in a variety of cell systems.
Altered neutrophil apoptosis has been implicated in the pathogenesis of several inflammatory conditions.16,17 Excessively delayed neutrophil apoptosis is associated with the systemic inflammatory response syndrome (SIRS). This syndrome is also characterized by activated neutrophils with increased production of reactive oxygen intermediates (ROIs) and associated tissue damage, which may result in multiple organ failure.2,18
Previous studies have confirmed that neutrophils from women at term pregnancy have a significant delay in apoptosis,19 which may contribute to their physiologic neutrophilia.20 Progesterone and estradiol levels rise throughout pregnancy and may peak at a 100-fold higher than normal nonpregnant values.21 In view of these findings, the aggravated morbidity experienced by the postmenopausal woman, and the widespread use of natural or synthetic ligands of steroid receptors in fertility control and cancer endocrine therapy, we further investigated the impact of female hormones on neutrophil survival and function. We hypothesized that female sex steroids may mediate the female immunologic advantage by altering neutrophil survival and function. The objectives of this study were to investigate the effects of estradiol and progesterone on normal male and female neutrophil apoptosis and function and the mechanisms involved in these processes.
Materials and methods
Reagents and antibodies
Dulbecco modified Eagle medium (DMEM), penicillin, streptomycin solution, l-glutamine, and fetal calf serum (FCS) were from Gibco Life Technologies (Paisley, United Kingdom); dextran T-500 and Ficoll from Pharmacia (Buckinghamshire, United Kingdom); E-Lyse from Cardinal Associates (Santa Fe, NM); pro and active caspase 9 antibody from Becton Dickinson (Cambridge, United Kingdom); pro and active caspase 3 antibody from Santa Cruz Biotechnology (Santa Cruz, CA); and dihydrorhodamine 123 (DHR) from Molecular Probes (Eugene, OR). CD11b (LeuTM-15) phycoerythrin (PE)-labeled monoclonal antibody (mAb) was from Becton Dickinson (San Jose, CA); monoclonal anti-h-FAS (CD95) fluorescein from R&D Systems (Minneapolis, MN); monoclonal agonistic Fas (CD95) antibody (CH-11) from Immunotech (Marseille, France); caspase 3 substrate Ac-DEVD-AMC and caspase 9 substrate Ac-LEHD-AMC from Biomol Laboratories (Plymouth Meeting, PA); rabbit antimanganese superoxide dismutase (MnSOD) polyclonal antibody from Stress-gen (Victoria, BC, Canada); ICI 182 780 (Faslodex) from Tocris (Bristol, United Kingdom); charcoal dextran-treated fetal bovine serum (Perbio-sciences, Cheshire, United Kingdom); water-soluble estradiol, progesterone, RU486, and all remaining chemicals unless otherwise stated were from Sigma-Aldrich (Dorset, United Kingdom).
Neutrophil isolation
Neutrophils were isolated from healthy male (n = 26) and female (n = 26) volunteers with an age range of 26 to 33 years; fully informed consent was given by all volunteers. Institutional ethical committee approval from the Mater Misericordiae Hospital was received for the study period. The technique used was dextran (3%) sedimentation and centrifugation through a discontinuous Ficoll gradient; red blood cells were lysed using E-Lyse.20 The neutrophil pellet was resuspended in DMEM culture medium supplemented with 10% FCS, 1% glutamine, 1% penicillin/streptomycin solution, and amphotericin B at a concentration of 1 × 106 cells/mL. Cells were incubated in polypropylene tubes (Falcon/Becton Dickinson, Cambridge, United Kingdom) to prevent adherence. Neutrophil purity as assessed by size and granularity on flow cytometry was consistently more than 95%.
Quantification of apoptosis
Spontaneous apoptosis of neutrophils was quantified by flow cytometry as the percent of cells with hypodiploid DNA.20,21 Cells (1 × 106/mL) were centrifuged at 130g for 5 minutes and then gently resuspended in 400 μL hypotonic fluorochrome solution (200 mL phosphate-buffered saline [PBS], 10 mg propidium iodide, 3.4 mM sodium citrate, 1 mM Tris [tris(hydroxymethyl)aminomethane], 0.1 mM EDTA [ethylenediaminetetraacetic acid], 0.1% Triton X-100). They were placed on ice for 10 minutes before they were analyzed using the Coulter Epics XL-MCL cytofluorometer (Miami, FL). A minimum of 5000 events were collected and analyzed. Apoptotic nuclei were distinguished from normal nuclei by their hypodiploid DNA and debris was excluded from analysis by raising the forward threshold. All measurements were performed under the same instrument settings.
Quantification of cell surface antigen expression
The expression of CD11b and Fas antigens on the surface of neutrophils was measured by flow cytometry; 500 μL cells (1 × 106/mL) were treated with 10 μL PE-CD11b or control antibody and incubated at 4°C for 20 minutes. The cells were washed 3 times with 400 μL cold PBS at 130g for 10 minutes and finally resuspended in 400 μL Isoton II solution and stored on ice before they were analyzed by flow cytometry. Similarly, 500 μL neutrophils (1 × 106cells/mL) were stained with 5 μL Fas antibody (50 μg/mL) and centrifuged at 150g for 5 minutes. The supernatant was aspirated, the pellet resuspended in 400 μL PBS, and the mean channel fluorescence assessed by flow cytometry.
Western blot analysis
Total protein was isolated from 9 × 106 human neutrophils using NP-40 isolation solution (0.5% NP-40, Tris 10 mM, pH 8.0, 60 mM KCL, 1 mM EDTA, pH 8.0, 1 mM DTT [dithiothreitol], 10 mM phenylmethylsulfonyl fluoride [PMSF], 1 μM leupeptin, 1 μM aprotinin, and 2 μM pepstatin).Total protein was measured by the Bradford assay protein detection kit (Bio-Rad, Hercules, CA) and loaded at 50 μg/well. Samples were then run on 12% sodium dodecyl sulfate (SDS)-polyacrylamide gradient gel (140 V for 60 minutes), and electrophoretically transferred to Immobilon P (Millipore, Bedford, MA; 100 V, 80 minutes). The remaining gel was stained after transfer with Coomassie blue solution to confirm equal loading. Blots were incubated with rabbit antihuman caspase 3 antibody (1:1000) or rabbit antihuman caspase 9 antibody (1:1000) in 1% bovine serum albumin (BSA), triethanolamine-buffered saline (TBS), and 0.1% Tween 20 for 1 hour at room temperature. The blots were then incubated with horseradish peroxidase-conjugated antirabbit IgG at 1:5000 dilution for 1 hour.
Isolated neutrophils were prepared for cytochrome c evaluation by initially pelleting the cells followed by resuspension in 100 μL CLAMI buffer (250 mM sucrose, 70 mM KCL in PBS) containing 200 μg/mL digitonin. Following a 5-minute incubation on ice, the cells were pelleted at 1000g for 5 minutes at 4°C. The supernatant or cytosolic fraction was stored at -70°C until 30 μL was run on Western blot. The pellet containing the mitochondrial fraction was resuspended in 100 μL universal immunoprecipitation buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 2 mM EDTA, 2 mM ethylene glycol tetraacetic acid [EGTA], 0.2% Triton X-100, 0.3% NP-40, 1 mM PMSF, leupeptin, aprotinin) for 10 minutes on ice. The solution was then pelleted at 10 000g for 10 minutes at 4°C and the supernatant or mitochondrial fraction stored at -70°C until 30 μL was run on a Western blot. The anti-cytochrome c antibody (1:1000) was a generous gift from Dr Seamus Martin (Trinity College, Dublin, Ireland). The secondary antibody used was horseradish peroxidase-conjugated antirabbit IgG at 1:5000 dilution for 1 hour.
MnSOD is used as a specific marker of the mitochondrial fraction of the cell lysate22 and confirmed the purity of the cytosolic and mitochondrial fractions. Blots were incubated with the antibody at 1:1000 and the secondary antibody used was horseradish peroxidase-conjugated antirabbit IgG at 1:5000 dilution for 1 hour.
All blots were developed using the enhanced chemiluminescence system. The density of each band was measured using the UN-SCAN-ITgel Version 5.1 program (Silk Scientific, Orem, UT).
Respiratory burst activity
Generation of ROIs was evaluated by flow cytometry using the technique of Smith and Wiedemann23 Neutrophils (500 μL [1 × 106/mL]) were incubated with DHR 123 (100 μM) at 37°C for 10 minutes. Cells were stimulated with 1 μL (16 μM) phorbol 12-myrisate 13-acetate (PMA) for 20 minutes at 37°C. The reaction was then halted by placing samples on ice. Neutrophil fluorescence intensity was assessed by flow cytometry and expressed as Ln mean channel fluorescence (LnMCF). DHR 123 has been shown to detect mainly intracellular H2O2 and OH radical production.23
Caspase extraction and activity assay
Cell lysates were prepared from 10 × 106 cells using caspase isolation buffer (25 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.8, 5 mM MgCl2, 1 mM EDTA, 10 mM leupeptin, 5 mM pepstatin, 100 mM PMSF, and 10 mM DTT) and caspase incubation buffer (100 mM HEPES, pH 7.5, 10% sucrose, 0.1% CHAPS [3[(3-cholamidopropyl)dimethylammonio]-1-propane-sulfonic acid], 10 mM Leupeptin, 5 mM pepstatin, 100 mM PMSF, and 10 mM DTT). Aliquots of the lysates (40 μL) were diluted in caspase incubation buffer (40 μL) and 20 mM (5 μL) Ac-LEHD-AMC (caspase 9) or 20 mM Ac-DEVD-AMC (caspase 3) and incubated for 1 hour at 3°C in a 96-well Costar plate (Corning, NY). The release of AMC fluorescent tag was measured using a cytofluorometer II (Perkin Elmer Biosciences, Birchwood Science, Park North, Warrington, United Kingdom) at 380 nm excitation and 460 nm emission. The difference between specific caspase activity at 0 and 1 hour was calculated and expressed as caspase activity per microgram protein.24
Statistics
Statistical analysis was carried out using ANOVA one-way analysis of variance with Student-Newman correction and Student t test using Minitab statistical software 13.32 (http://www.minitab.com). Significance was assumed for P values less than .05. Results are expressed as mean ± SD unless stated otherwise.
Results
Sex differences in neutrophil apoptosis and the role of estradiol and progesterone
Human neutrophils die spontaneously by the process of apoptosis both in vivo and in vitro. This study demonstrated a significant delay in the spontaneous rates of apoptosis after 24 hours of in vitro incubation in healthy women of reproductive age compared with age-matched men (Figure 1). The addition of physiologic levels of estradiol (E2) and progesterone (P) further delayed female neutrophil apoptosis and reduced the rates of apoptosis in male neutrophils in a dose-dependent manner. There was no significant additive effect when these steroid hormones were added in combination over the same physiologic range (Figure 2).
Sex differences in spontaneous neutrophil apoptosis. Neutrophils (1 × 106 cells/mL) were isolated from healthy male (n = 26) and female (n = 26) volunteers and incubated in vitro for 24 hours. Percentage neutrophil apoptosis was evaluated by propidium iodide DNA staining and flow cytometry. *P < .05 versus men. Error bars indicate mean ± SD.
Sex differences in spontaneous neutrophil apoptosis. Neutrophils (1 × 106 cells/mL) were isolated from healthy male (n = 26) and female (n = 26) volunteers and incubated in vitro for 24 hours. Percentage neutrophil apoptosis was evaluated by propidium iodide DNA staining and flow cytometry. *P < .05 versus men. Error bars indicate mean ± SD.
Dose-dependent effects of estradiol, progesterone, or both in combination on spontaneous neutrophil apoptosis. Neutrophils (1 × 106 cells/mL) isolated from male (▪; n = 10) and female (♦; n = 10) controls were incubated in vitro with increasing physiologic doses (10-12 to 10-7 g/mL) of estradiol (A), progesterone (B), or both in combination (C). Propidium iodide DNA-binding staining using flow cytometry assessed neutrophil apoptosis at 24 hours. *P < .05 versus control (Con). Error bars indicate mean ± SD.
Dose-dependent effects of estradiol, progesterone, or both in combination on spontaneous neutrophil apoptosis. Neutrophils (1 × 106 cells/mL) isolated from male (▪; n = 10) and female (♦; n = 10) controls were incubated in vitro with increasing physiologic doses (10-12 to 10-7 g/mL) of estradiol (A), progesterone (B), or both in combination (C). Propidium iodide DNA-binding staining using flow cytometry assessed neutrophil apoptosis at 24 hours. *P < .05 versus control (Con). Error bars indicate mean ± SD.
Testosterone is converted to dihydrotestosterone (DHT) in the cell by 5α-reductase. DHT is the active metabolite mediating androgenic effects in the cell. Testosterone can also be converted to estradiol by aromatase. Testosterone-induced responses would therefore not give a true indication of androgenic effects. We studied DHT (10-9 M), which had no significant effect on male (control = 24.2% ± 0.5%; DHT = 23.0% ± 2.5%; n = 4) and female (control = 16.0% ± 4.1%; DHT = 12.2% ± 3.0%; n = 4) neutrophil apoptosis at physiologic doses. In addition, coincubation of DHT with estradiol or progesterone did not alter the female sex steroid hormone-mediated delay in neutrophil apoptosis (results not shown).
The effects of estradiol and progesterone on neutrophil function
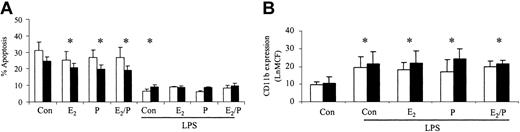
The ability of these hormones to stimulate increases in neutrophil function was also assessed. There was no significant difference in control CD11b expression or ROI activity between male and female neutrophils. CD11b expression was not significantly altered in response to estradiol and progesterone in male or female volunteers (Figure 3A). There was no significant alteration in baseline ROIs in men and women but PMA-induced respiratory burst was increased in women in response to estradiol and progesterone (Figure 3B-C). Female neutrophils had an increased baseline ROI production on coincubation with estradiol and progesterone in combination and therefore the Δ difference between baseline and PMA-stimulated was reduced. (Figure 3B). The ability of these hormones to prime for an additive response to lipopolysaccharide (LPS) was also investigated. Estradiol and progesterone did not increase the ability of LPS to delay neutrophil apoptosis or to increase CD11b expression (Figure 4). CD33 and CD34 were also evaluated as markers of altered neutrophil maturity and were unchanged by these female sex hormones (data not shown). Therefore, physiologic levels of estradiol and progesterone alone or in combination do not appear to alter neutrophil CD11b expression despite causing an increase in female ROI production and delaying apoptosis in both sexes.
Effects of estradiol and progesterone on markers of inflammatory cell activation. Normal neutrophils (1 × 106 cells/mL) were incubated for 6 hours with estradiol, progesterone, or both in combination (10-8 g/mL). Cells were then assessed for CD11b (A) using a PE-labeled mAb and the mean channel fluorescence analyzed using flow cytometry. □ indicates male (n = 8); and ▪ indicates female (n = 8). (B) ROI activity was assessed in neutrophils preincubated with sex steroids for 6 hours and then stimulated with DHR (baseline [BL]) alone or in addition to PMA (respiratory burst [RB]). Results were expressed as the Ln mean channel fluorescence ± SEM. (C) Results were also expressed as the Δ difference of the Ln mean channel fluorescence between DHR-treated cells with and without PMA stimulation. *P < .05 versus control (male, n = 8; female, n = 8). Error bars indicate mean ± SD.
Effects of estradiol and progesterone on markers of inflammatory cell activation. Normal neutrophils (1 × 106 cells/mL) were incubated for 6 hours with estradiol, progesterone, or both in combination (10-8 g/mL). Cells were then assessed for CD11b (A) using a PE-labeled mAb and the mean channel fluorescence analyzed using flow cytometry. □ indicates male (n = 8); and ▪ indicates female (n = 8). (B) ROI activity was assessed in neutrophils preincubated with sex steroids for 6 hours and then stimulated with DHR (baseline [BL]) alone or in addition to PMA (respiratory burst [RB]). Results were expressed as the Ln mean channel fluorescence ± SEM. (C) Results were also expressed as the Δ difference of the Ln mean channel fluorescence between DHR-treated cells with and without PMA stimulation. *P < .05 versus control (male, n = 8; female, n = 8). Error bars indicate mean ± SD.
Effects of estradiol and progesterone on the priming effect. Normal neutrophils (1 × 106 cells/mL) were incubated for 1 hour either with or without estradiol, progesterone, or both in combination (10-8 g/mL), before the addition of LPS (1 μg/mL). Cells were then assessed for percentage apoptosis by propidium iodide DNA staining after 24 hours (A) or CD11b expression using a PE-labeled mAb after 6 hours and expressed as Ln mean channel fluorescence (B). □ indicates male (n = 6) and ▪ indicates female controls (n = 6). *P < .05 versus control. Error bars indicate mean ± SD.
Effects of estradiol and progesterone on the priming effect. Normal neutrophils (1 × 106 cells/mL) were incubated for 1 hour either with or without estradiol, progesterone, or both in combination (10-8 g/mL), before the addition of LPS (1 μg/mL). Cells were then assessed for percentage apoptosis by propidium iodide DNA staining after 24 hours (A) or CD11b expression using a PE-labeled mAb after 6 hours and expressed as Ln mean channel fluorescence (B). □ indicates male (n = 6) and ▪ indicates female controls (n = 6). *P < .05 versus control. Error bars indicate mean ± SD.
Female sex steroid hormone receptor antagonists reverse the delay in apoptosis
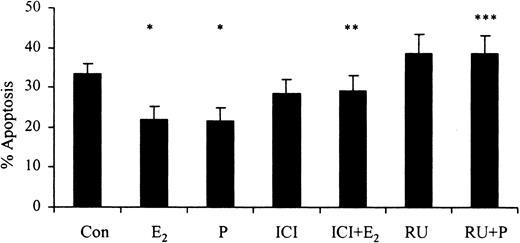
The progesterone receptor antagonist RU486 (10-6 M) reversed progesterone-induced (10-7g/mL) delays in apoptosis. Similarly, the selective estrogen receptor antagonist ICI 182 780 (10-8 M) reversed the estradiol-mediated (10-7 g/mL) delay in apoptosis after 24 hours (Figure 5). This demonstrates that the delay in apoptosis associated with female sex steroids is mediated through their respective steroid hormone receptors.
The effects of inhibitors of female sex hormones on neutrophil apoptosis. Neutrophils (1 × 106 cells/mL) were incubated with estradiol, progesterone, or both (10-7 g/mL) for 24 hours. RU486 (RU; 10-6 M) was added at time 0 hours and percentage apoptosis was assessed by flow cytometry at 24 hours (n = 11). ICI 182 780 (ICI; 10-8 M) was added at time 0 hours and percentage apoptosis was assessed by flow cytometry at 24 hours following propidium iodide staining (n = 8). All experiments used both male and female subjects. *P < .05 versus control; **P < .05 versus estradiol; ***P < 0.05 versus progesterone (mean ± SEM).
The effects of inhibitors of female sex hormones on neutrophil apoptosis. Neutrophils (1 × 106 cells/mL) were incubated with estradiol, progesterone, or both (10-7 g/mL) for 24 hours. RU486 (RU; 10-6 M) was added at time 0 hours and percentage apoptosis was assessed by flow cytometry at 24 hours (n = 11). ICI 182 780 (ICI; 10-8 M) was added at time 0 hours and percentage apoptosis was assessed by flow cytometry at 24 hours following propidium iodide staining (n = 8). All experiments used both male and female subjects. *P < .05 versus control; **P < .05 versus estradiol; ***P < 0.05 versus progesterone (mean ± SEM).
Mechanism of female sex hormone-mediated delay in neutrophil apoptosis
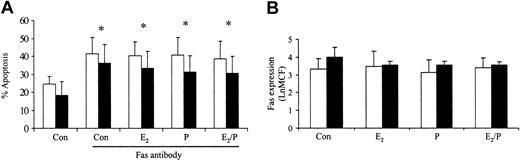
Previous studies in our laboratory have demonstrated that delays in neutrophil apoptosis are associated with resistance to Fas antibody-induced apoptosis and inhibition of caspase activity.20 To determine how estradiol and progesterone delay apoptosis, we investigated their effects on Fas antibody-induced apoptosis. Preincubation for 1 hour with estradiol or progesterone had no effect on Fas antibody-induced apoptosis (Figure 6A). This response was not mediated by Fas expression on the surface of the neutrophil, because this was unchanged by these steroid hormones (Figure 6B). Etoposide-induced apoptosis was also not reversed by estradiol and progesterone (data not shown).
Effects of estradiol and progesterone on Fas-induced apoptosis and Fas receptor expression. Neutrophils were incubated with estradiol, progesterone, or both (10-8 g/mL) for 6 hours. (A) Fas antibody was added and the percentage apoptosis assessed at 24 hours by propidium iodide staining and flow cytometry. □ indicates male (n = 10) and ▪ indicates female (n = 10). (B) Neutrophils were incubated with Fas antibody and mean channel fluorescence (LnMCF) was subsequently assessed by flow cytometry. □ indicates male (n = 5) and ▪ indicates female (n = 5). Error bars indicate mean ± SD.
Effects of estradiol and progesterone on Fas-induced apoptosis and Fas receptor expression. Neutrophils were incubated with estradiol, progesterone, or both (10-8 g/mL) for 6 hours. (A) Fas antibody was added and the percentage apoptosis assessed at 24 hours by propidium iodide staining and flow cytometry. □ indicates male (n = 10) and ▪ indicates female (n = 10). (B) Neutrophils were incubated with Fas antibody and mean channel fluorescence (LnMCF) was subsequently assessed by flow cytometry. □ indicates male (n = 5) and ▪ indicates female (n = 5). Error bars indicate mean ± SD.
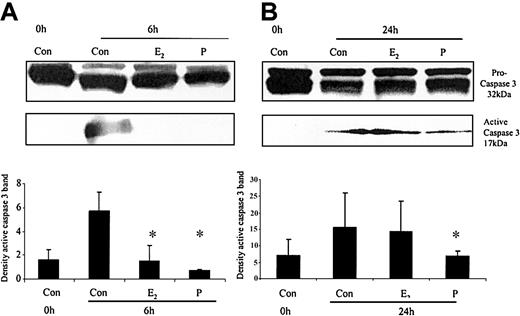
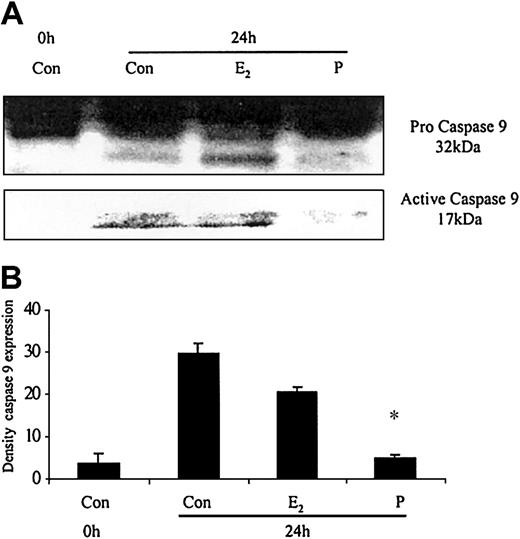
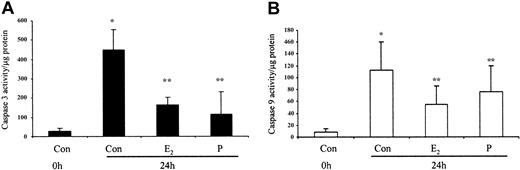
The ability of these hormones to block spontaneous caspase activity was then assessed. The expression of the (active) proteolytic cleavage product of caspase 3 protein was increased following 6 and 24 hours of neutrophil incubation in vitro. Neutrophils incubated with progesterone or estradiol demonstrated a significant decrease in active caspase 3 protein expression at 6 hours that was confirmed by densitometry (Figure 7A). Progesterone but not estradiol treatment did result in a decrease in active caspase 3 protein expression at 24 hours (Figure 7B). Neutrophils incubated with progesterone demonstrated a decrease in active caspase 9 (Figure 8). There was a smaller decrease in active caspase 9 following incubation with estradiol at 24 hours (Figures 7A and 8). To further validate altered active caspase protein expression we assessed caspase activity using fluorescent substrates. Both caspase 3 and 9 activities were reduced at 24 hours by estradiol and progesterone (Figure 9).
The effects of estradiol and progesterone on spontaneous caspase 3 activation at 6 and 24 hours assessed by Western blot and densitometry. Total cellular protein was extracted at 6 and 24 hours from control neutrophils and cells incubated with estradiol or progesterone (10-8 g/mL). (A) Pro and active caspase 3 were assessed by Western blot analysis. These blots are representative of one of at least 3 separate experiments. (B) Densitometry was performed and the density of each band (percent of total pixels) expressed as the mean ± SD of all blots. *P < .05 versus control at 6 hours.
The effects of estradiol and progesterone on spontaneous caspase 3 activation at 6 and 24 hours assessed by Western blot and densitometry. Total cellular protein was extracted at 6 and 24 hours from control neutrophils and cells incubated with estradiol or progesterone (10-8 g/mL). (A) Pro and active caspase 3 were assessed by Western blot analysis. These blots are representative of one of at least 3 separate experiments. (B) Densitometry was performed and the density of each band (percent of total pixels) expressed as the mean ± SD of all blots. *P < .05 versus control at 6 hours.
The effects of estradiol and progesterone on spontaneous caspase 9 activation at 24 hours assessed by Western blot and densitometry. Total cellular protein was extracted at 24 hours from control neutrophils and cells incubated with estradiol or progesterone (10-8 g/mL). (A) Pro and active caspase 9 were assessed by Western blot analysis. These blots are representative of 1 of at least 3 separate experiments. (B) Densitometry was performed and the density of each band (percent of total pixels) expressed as the mean ± SD of all blots. *P < .05 versus control at 6 hours.
The effects of estradiol and progesterone on spontaneous caspase 9 activation at 24 hours assessed by Western blot and densitometry. Total cellular protein was extracted at 24 hours from control neutrophils and cells incubated with estradiol or progesterone (10-8 g/mL). (A) Pro and active caspase 9 were assessed by Western blot analysis. These blots are representative of 1 of at least 3 separate experiments. (B) Densitometry was performed and the density of each band (percent of total pixels) expressed as the mean ± SD of all blots. *P < .05 versus control at 6 hours.
Spontaneous caspase 3 and 9 activation by estradiol and progesterone at 24 hours using fluorescent caspase substrates. Cell lysates were prepared from 10 × 106 cells using caspase isolation and incubation buffers. Aliquots of the lysates were diluted in caspase incubation buffer and Ac-DEVD-AMC (caspase 3; panel A) or Ac-LEHD-AMC (caspase 9; panel B). The release of AMC fluorescent tag was measured using a cytofluorometer at 0 and 1 hour and specific activity was measured as the difference between 0 and 1 hour results and expressed as caspase activity per microgram protein. Each graph represents at least 5 separate experiments performed in duplicate (mean ± SEM). *P < .05 versus control at 0 hours; **P < .05 versus control at 24 hours. (A) Men, n = 2; women, n = 3. (B) Men, n = 3; women, n = 3.
Spontaneous caspase 3 and 9 activation by estradiol and progesterone at 24 hours using fluorescent caspase substrates. Cell lysates were prepared from 10 × 106 cells using caspase isolation and incubation buffers. Aliquots of the lysates were diluted in caspase incubation buffer and Ac-DEVD-AMC (caspase 3; panel A) or Ac-LEHD-AMC (caspase 9; panel B). The release of AMC fluorescent tag was measured using a cytofluorometer at 0 and 1 hour and specific activity was measured as the difference between 0 and 1 hour results and expressed as caspase activity per microgram protein. Each graph represents at least 5 separate experiments performed in duplicate (mean ± SEM). *P < .05 versus control at 0 hours; **P < .05 versus control at 24 hours. (A) Men, n = 2; women, n = 3. (B) Men, n = 3; women, n = 3.
Cytochrome c release and female sex steroid hormones
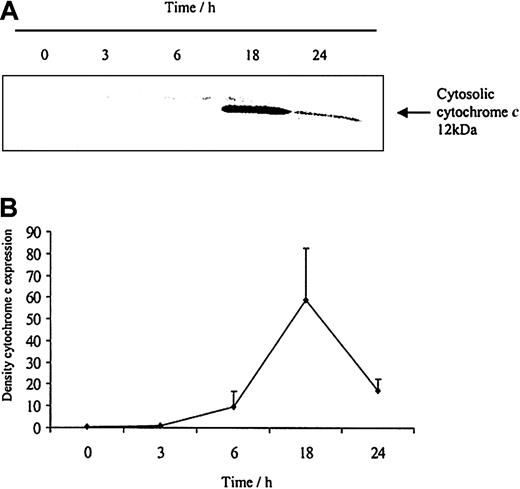
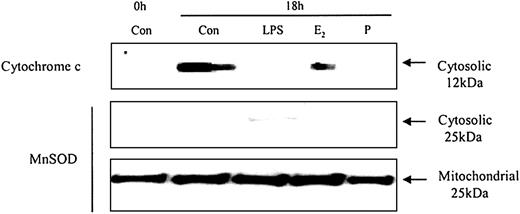
To understand how these caspases are activated we examined the effects of neutrophil aging on spontaneous release of cytochrome c from the mitochondria. Cytochrome c is an activator of the caspase cascade and triggers neutrophil apoptosis.25 This is confirmed in Figure 10, which demonstrates that progressive cytosolic cytochrome c release in aging neutrophils corresponds to increasing neutrophil apoptosis. LPS (1 μg/mL) inhibited the release of cytochrome c and previous studies have also confirmed LPS-induced inhibition of apoptosis, as well as caspase 3 and 9 activity.20 In addition, estradiol and progesterone diminished mitochondrial cytochrome c release into the cytosol (Figure 11).
The release of cytochromecfrom aging neutrophils on Western blot and densitometry. Cytoplasmic fractions were extracted from human neutrophils after 0, 3, 6, 18, and 24 hours of aging in vitro. (A) Cytosolic cytochrome c release was assessed using Western blot analysis. The blot represents one of 3 separate experiments. (B) Densitometry of the 3 blots was performed and the density of each band (percent of total pixels) expressed as mean ± SEM.
The release of cytochromecfrom aging neutrophils on Western blot and densitometry. Cytoplasmic fractions were extracted from human neutrophils after 0, 3, 6, 18, and 24 hours of aging in vitro. (A) Cytosolic cytochrome c release was assessed using Western blot analysis. The blot represents one of 3 separate experiments. (B) Densitometry of the 3 blots was performed and the density of each band (percent of total pixels) expressed as mean ± SEM.
The effects of estradiol and progesterone on spontaneous cytochrome c release: Western blot and the expression of MnSOD in cytosolic and mitochondrial fractions. Cytoplasmic fractions were also extracted at 18 hours from control neutrophils and cells incubated with LPS, estradiol, progesterone, or both (10-8 g/mL). Cytosolic cytochrome c release was assessed using Western blot analysis. Cytoplasmic and mitochondrial fractions were also stained with MnSOD polyclonal antibody. Each blot represents 1 of 3 separate independent experiments.
The effects of estradiol and progesterone on spontaneous cytochrome c release: Western blot and the expression of MnSOD in cytosolic and mitochondrial fractions. Cytoplasmic fractions were also extracted at 18 hours from control neutrophils and cells incubated with LPS, estradiol, progesterone, or both (10-8 g/mL). Cytosolic cytochrome c release was assessed using Western blot analysis. Cytoplasmic and mitochondrial fractions were also stained with MnSOD polyclonal antibody. Each blot represents 1 of 3 separate independent experiments.
Discussion
In this study, we demonstrated that neutrophil survival was significantly increased in women. Estradiol and progesterone delayed neutrophil apoptosis, although functional activity remained intact. This represents a possible mechanism for the elevated systemic neutrophil count seen in women compared with men.26 The link between the timing of maximum female neutrophil counts during normal menstruation and elevated estradiol and progesterone levels has been established.27,28 Similarly, neutrophils isolated from pregnant women show a more exaggerated delay in neutrophil apoptosis.29 This is reflected in the marked neutrophilia associated with term pregnancy30 and coincides with significantly elevated levels of female steroid hormones.19
Estrogen has been shown to have pro- and anti-inflammatory properties depending on the cell type involved. Both cell proliferation and cell death have been described in the setting of breast cancer in response to sex steroids.31 Female sex hormones have been shown to delay apoptosis in the peripheral blood mononuclear cells of women with normal menstrual cycles,32 B cells,33 and endothelial cells.34 Tumor necrosis factor (TNF)-induced apoptosis in monoblastoid cells is also delayed by these hormones.35 Estrogen also has proinflammatory effects on the uterus and promotes neutrophil influx. In addition, progesterone can antagonize proinflammatory estrogenic activities via the progesterone receptor.36
The specific receptor antagonists, ICI 182 780 and RU486, reversed the delays in apoptosis following coincubation with exogenous estradiol and progesterone, respectively. Recently, both estradiol receptor (ER) α and β have been described on the surface of neutrophils from men and women. However, although both receptor subtypes are up-regulated by 17β-estradiol (10-8 M) in premenopausal women, only ERα expression was enhanced in men.37,38 These studies with the respective specific receptor antagonists, ICI 182 780 and RU486, demonstrate that the delay in apoptosis is a receptor-mediated effect. Estradiol- and progesterone-mediated interactions may therefore be involved in the transcriptional or translational regulation of antiapoptotic genes.
To further understand the role of male sex steroids and sex in neutrophil apoptosis we incubated neutrophils with DHT. Testosterone is converted to DHT in the cell by 5α-reductase and by aromatase to estradiol. These enzymes have been shown to have a sex-dependent distribution in immune cells. T cells from women have displayed aromatase activity and male-derived T cells have increased 5α-reductase activity.39 This, in turn, may mediate the cellular immunosuppression seen in men following trauma-hemorrhage. Neither aromatase nor 5α-reductase activities have been described in the mature neutrophil, although HL60 cells and progenitor hematopoietic CD34 cells have aromatase activity.40 Because testosterone can be converted to estradiol by aromatase and would therefore not give a true indication of androgenic effects, we studied DHT rather than testosterone. DHT had no significant effect on male and female neutrophil apoptosis in physiologic doses.
PMA-induced ROI release was enhanced by estradiol and progesterone in women. The combination of these hormones elevated the baseline ROI production. Both increased41,42 and decreased ROI43-46 production have been reported in response to female sex hormones. Increased ROI production was found when an intracellular dye was used, whereas decreased ROIs were found in studies measuring extracellular ROI release. Our study used the intracellular dye DHR 123. This is consistent with the published data that describe an enhanced intracellular ROI production in neutrophils from pregnant women, when estradiol and progesterone are at maximum physiologic levels.47 Differences between the PMA-induced ROI production with these sex hormones in men and women may be due to a sex-specific alteration in the neutrophil phenotype. ER subtypes are differentially expressed on male and female neutrophils exposed to estradiol.38 Sex differences have also been described in the expression of protein kinase C, which is a primary target for PMA. This may explain the differences between the PMA-induced responses.48 Neutrophil CD11b expression in men and women was unaffected by physiologic doses of female sex hormones. These hormones had no priming effect on CD11b expression or delays in apoptosis following activation with LPS.
Despite the effects of these hormones on spontaneous apoptosis they had no effect on Fas antibody-induced apoptosis. This is in contrast to LPS and granulocyte-macrophage colony-stimulating factor (GM-CSF), which delay apoptosis and prevent the induction of Fas antibody-induced apoptosis.20 The delay in neutrophil apoptosis with estradiol and progesterone is relatively subtle and not as exaggerated as that seen with LPS and GM-CSF. An inhibitory effect may be seen at a lower dose of Fas antibody. This may partially explain the failure of these hormones at physiologic doses to reverse Fas-induced apoptosis or to completely inhibit caspase activity.
The mechanisms by which these hormones delay spontaneous neutrophil apoptosis have yet to be fully elucidated. The current study demonstrated that after 6 hours of estradiol and progesterone treatment there was an inhibition of the proteolytic activation of caspase 3, an important activator of the apoptotic cascade. At 24 hours the decreased activity of caspase 3 was not associated with a significant decrease in the corresponding active protein expression. This may indicate the ability of these hormones to delay but not completely inhibit apoptosis. Estradiol has previously been shown to induce an undefined inhibitor of active caspases through a receptor-mediated nongenomic pathway in neurons.49 Estrogen withdrawal stimulates the processing of caspase 3 to its active form in chick oviduct, culminating in the induction of apoptosis.50 Decreased apoptosis in MCF-7 breast cancer cell line51 and in B cells33 is believed to be due to increased Bcl-2 expression. The fact that the active protein is expressed but the activity is inhibited and apoptosis delayed would indicate that these hormones inhibit the functional activity of the caspase. However, because similar effects are seen with caspase 9 activity, we investigated cytochrome c release and the mitochondria as a site for apoptotic inhibition.
Cytochrome c is an important activator of the caspase cascade in the neutrophil20 and is released from the mitochondria to initiate spontaneous apoptosis.25 Caspase activation in the neutrophil requires 100-fold less cytochrome c release than other cells such as Jurkats.25 We believe that even though we did not detect cytochrome c at the earlier time points, undetectable quantities are released from the mitochondria enabling activation of the caspases. Both estradiol and progesterone had an inhibitory effect on cytochrome c release indicating the ability of these hormones to regulate apoptosis at the level of the mitochondria and thus preventing caspase activation and apoptosis. Therefore, the apparent time delay in the up-regulation of cytochrome c at 18 hours compared with the caspase 3 activity at 6 hours may be due to late detection by the relatively insensitive method of Western blotting.
These studies demonstrate a novel and interesting paradox whereby estradiol increases neutrophil survival without an increase in neutrophil activation. This would be immunologically advantageous by enhancing the number of circulating neutrophils available to combat infection. The neutrophil phenotype in this case is also different from the extreme immune activation seen in SIRS.18 Female sex hormones have been shown to induce a significant female survival advantage in a number of disease states including neurodegeneration, cardiac disease, and sepsis.5,9,10 These beneficial effects may be partially mediated by the effects of these hormones on the immune system.
The mechanism and use of these hormones as an adjunctive treatment in sepsis or shock warrant further investigation. This study also highlights the importance of equal male-to-female ratios in control samples used in future neutrophil research. The effect of therapeutic sex steroid hormone agonists and antagonists on immune function is often overlooked and we suggest that further study is required to assess the impact of immune modulation on disease progression. In conclusion, estradiol and progesterone increase neutrophil survival while preserving normal function. We postulate that by altering the phenotype of the neutrophil these hormones prepare the female host for potential inflammatory insults.
Prepublished online as Blood First Edition Paper, June 5, 2003; DOI 10.1182/blood-2003-02-0649.
Supported by grants from the Health Research Board of Ireland (Clinical Research Fellowship, E.J.M.), the Mater College (Dublin, Ireland), and the Children's Research Fund, Our Lady's Hospital for Sick Children.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We wish to thank all the laboratory and hospital staff who donated blood samples to this project. We also acknowledge the generous gift of anti-cytochrome c antibody from Prof Seamus Martin, Molecular Cell Biology Laboratory, Department of Genetics, Smurfit Institute, Trinity College, Dublin. We are grateful to Dr Colm Morrissey, Mater Misericordiae Hospital, Conway Institute for his critical reading of this paper.



![Figure 3. Effects of estradiol and progesterone on markers of inflammatory cell activation. Normal neutrophils (1 × 106 cells/mL) were incubated for 6 hours with estradiol, progesterone, or both in combination (10-8 g/mL). Cells were then assessed for CD11b (A) using a PE-labeled mAb and the mean channel fluorescence analyzed using flow cytometry. □ indicates male (n = 8); and ▪ indicates female (n = 8). (B) ROI activity was assessed in neutrophils preincubated with sex steroids for 6 hours and then stimulated with DHR (baseline [BL]) alone or in addition to PMA (respiratory burst [RB]). Results were expressed as the Ln mean channel fluorescence ± SEM. (C) Results were also expressed as the Δ difference of the Ln mean channel fluorescence between DHR-treated cells with and without PMA stimulation. *P < .05 versus control (male, n = 8; female, n = 8). Error bars indicate mean ± SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/7/10.1182_blood-2003-02-0649/6/m_h81934998003.jpeg?Expires=1769094447&Signature=czxA6dApM~0ilWyScCLhd7-VMXwYyJPLO~fB0~z-DT7msfVVpe9~UJUjmNXM6QMa9CcrYK6cmu9k8UAD-Xb4dzU3OEgZDP8OQuqXJCYCt6XnZZXHpdRCamqKWV0KEnnsKKFAbBLrBoWDCql~1i8lnEcL7iyZYd5kjvxu08y1im00MCr2dqI6Gk0eh5oksoClxALuEJ7QvTxn4fsLUUkEaX-JSSYMKAbaRNlMhG8ygVEOAvQa-YutC0w5YF61UrEYBzKn4AgMTnvrXIM4DVXuQfSl1ppud5-nsYGUVXw3NeplrWJS1Qth8GRSsEdDUPLyASXxOjL9eLhxTl85RQ~d-g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal