Abstract
While most anaplastic lymphoma kinase (ALK)-positive non-Hodgkin lymphomas (NHLs) are of T-cell lineage, a small number of B-lineage tumors with plasmablastic morphology and expression of the full-length ALK protein have been described in the literature. All of these reported tumors lacked the NPM-ALK fusion transcript. There is controversy regarding the existence of ALK fusion-positive B-cell NHL, with many investigators contending that ALK fusions are expressed uniquely in T- or null-cell lymphomas. Here we describe 2 well-characterized cases of ALK-positive B-cell lymphoma expressing the NPM-ALK fusion. Both tumors occurred in pediatric patients and showed poor response to chemotherapy. Each had plasmablastic morphology, showed immunoglobulin A restriction, and was ALK positive and CD30- by immunohistochemistry. One tumor showed the t(2;5)(p23;q35) chromosomal translocation by conventional cytogenetics. Both were positive for NPM-ALK by reverse transcriptase-polymerase chain reaction. Thus, ALK-positive plasmablastic B-cell lymphomas are more heterogeneous at the molecular level than previously recognized. (Blood. 2003;102:2642-2644)
Introduction
ALK-positive anaplastic large cell lymphoma (ALCL) is classified by the World Health Organization (WHO) as a subtype of T-lineage non-Hodgkin lymphoma characterized by expression of the anaplastic lymphoma kinase (ALK) and of CD30.1 In approximately 70% of these tumors,2 ALK overexpression is the result of the t(2;5)(p23;q35) chromosomal translocation that leads to fusion of the nucleophosmin, NPM, gene at 5q35 to the ALK gene at 2p23.3 In 1997, Delsol et al described an unusual subtype of large B-cell lymphoma with plasmablastic morphology and expression of full-length ALK. Clinically, these tumors occurred most often in older adult patients and had a poor response to chemotherapy.4 By immunohistochemistry (IHC), the tumor cells were CD20-, immunoglobulin A (IgA) restricted and lacked CD30 expression. A significant subset of those tumors also expressed weak CD4 and CD57. All of these tumors were negative for the NPM-ALK transcript. The ALK expression, as detected by IHC, had a membrane and speckled cytoplasmic distribution, different from the homogeneous cytoplasmic and/or nuclear pattern characteristic for the classic T/null-lineage ALCL containing NPM-ALK.5 To date, a total of 10 cases of ALK-positive B-cell lymphoma of this type has been described in the literature.4,6 The WHO classification has placed these tumors under the large B-cell lymphoma category, where they are designated as “diffuse large B-cell lymphomas with expression of full-length ALK.”1
We describe the clinicopathologic features of 2 cases of large B-cell lymphoma with plasmablastic morphology and expression of the NPM-ALK transcript. One of these cases was included in a series of patients previously reported by our institution.7
Study design
Patients
The patients reported here were identified through a search of the St Jude Department of Pathology files for NPM-ALK-positive tumors that were CD30- and/or of B-cell lineage. Ethical approval of the institutional review board of St Jude Children's Research Hospital was obtained prior to the medical records review for these 2 patients.
Histologic examination. Examination was performed on formalin- and B5-fixed, paraffin-embedded tissue sections that were stained with hematoxylin and eosin (H&E).
Immunohistochemical staining. Staining was performed on formalin-fixed paraffin-embedded tissue sections following heat-induced epitope retrieval using the avidin-biotin peroxidase technique and the Ventana Nexes IHC Staining System (Ventana, Tucson, AZ) or DAKO Autostainer (DAKO, Glostrup, Denmark). Antibody specificities, along with their sources were as follows: ALK-1, CD3, CD4, CD8, CD30, CD79a, epithelial membrane antigen (EMA), Epstein-Barr virus latent membrane protein-1 (EBV-LMP1), immunoglobulin (alpha, mu, and gamma heavy chains); (DAKO), CD20, CD45, CD45RO/ubiquitin carboxy-terminal hydrolase L1 (UCHL-1), CD56 (Ventana), and CD57 (Novocastra, Newcastle upon Tyne, United Kingdom).
In situ hybridization. In situ hybridization studies for EBV (EBV-encoded RNA [EBER]) and immunoglobulin kappa and lambda light chains were performed on formalin-fixed paraffin-embedded tissue sections following pretreatment with proteinase K, using specific fluorescein-conjugated RNA probes, the DAKO PNA ISH detection kit (all from DAKO), and the Nexes Discovery staining system (Ventana).
Reverse transcriptase-polymerase chain reaction (RT-PCR) for ALK and NPM-ALK
Total RNA extracted from frozen (patient 1) or paraffin-embedded (patient 2) tissue was reverse-transcribed using random primer (Promega, Madison, WI). The cDNA quality was checked by amplifying a 321-bp portion of the NPM gene using the primers NPM/F (5′-TCGATGGACATGGACATGAGC-3′) and NPM/R (5′-ATGCACTGGCCCTGAACCACAC-3′).
Detection of NPM-ALK mRNA. RT-PCR was performed in a standard reaction using the primers 5′ NPM/F and 3′ ALK-3 (5′-CGAGGTGCGGAGCTTGCTCAGC-3′).
Detection of ALK mRNA. Transcripts encoding the cytoplasmic portion of ALK (3′ ALK) were detected using primers based on the ALK cDNA sequence, ALK-4201/F: 5′-GCTTTGCTGGCAAGACCTCCTCC-3′ (positions 4201-4123 of the full-length normal ALK cDNA; clone RMS17-2), and ALK-4342/R:5′-GGCTTGGGTCGTTGGGCATTC-3′ (positions 4342-4321). Transcripts encoding the extracellular portion of ALK (5′ALK) were detected using 2 primers, ALK-EC1:5′-CCATCTCCTTCTCCTGATTATTTT-3′ (positions 1611-1634), and ALK-EC2: 5′-CACTGCAGACAAGCTGGGGTT-3′ (positions 2162-2142).
Results and discussion
Patient 1
A 16-year-old black male presented with a 5-cm mass of the scalp and parietal bone, fatigue, and a 5-pound weight loss. Additional evaluation showed cervical, axillary, and inguinal lymphadenopathy, and multiple lytic skeletal lesions. A biopsy of the scalp mass showed large B-cell lymphoma. The patient was treated according to LMB89 chemotherapy protocol10 with poor initial response. He developed recurrent disease 4 months after completion of chemotherapy. Weekly vinblastine, intrathecal chemotherapy, and palliative radiation therapy led to only a partial response. The patient died of disease 2 years from initial diagnosis.
Patient 2
A 10-year-old white male presented with a laryngeal supraglottic mass and cervical and submandibular lymphadenopathy. Additional evaluation showed no other sites of disease. The laryngeal mass was resected and showed large B-cell lymphoma. The patient was enrolled in the POG8719 protocol,11 with persistent disease (in the larynx and cervical lymph nodes) at the end of induction and consolidation therapy. He then received radiation therapy (total 39 Gy) to the sites of persistent disease and 3 courses of DAHP (dexamethasone, cytarabine, cisplatinum), with complete response. He is currently in complete continuous remission, 13 years from initial diagnosis.
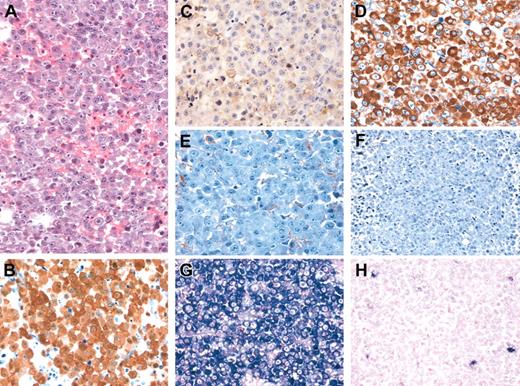
The tumors from both patients showed similar histologic features, closely resembling the morphology and immunophenotype described by Delsol et al4 and Reichard et al.6 Both tumors had a diffuse growth pattern largely replacing the underlying tissue in the scalp lesion and the laryngeal tumor, and partially effacing the architecture of involved lymph node, with an interfollicular distribution (in a lymph node obtained from patient 2 following chemotherapy). Tumor cells were predominantly plasmablastic in appearance (Figure 1A). Occasional large, bizarre, multinucleated cells and variable numbers of mature plasma cells were present throughout the tumors.
Morphologic and immunophenotypic features of ALK-positive large B-cell lymphoma. Characteristic plasmablastic and immunoblastic morphology (H&E staining) (A). On immunohistochemical staining (B-F), the tumor strongly expresses ALK, with a diffuse cytoplasmic and nuclear pattern (B), CD79, with a weak and focal membrane pattern (C), and cytoplasmic immunoglobulin (Ig) alpha heavy chains (D) and lacks expression of Ig gamma (E) and Ig mu (F) heavy chains. (Immunoperoxidase staining with hematoxylin counter-stain, panels B-F.) By in situ hybridization for immunoglobulin lambda (G) and kappa (H) light chain RNA the tumor shows lambda light chain restriction. Original magnification, × 400.
Morphologic and immunophenotypic features of ALK-positive large B-cell lymphoma. Characteristic plasmablastic and immunoblastic morphology (H&E staining) (A). On immunohistochemical staining (B-F), the tumor strongly expresses ALK, with a diffuse cytoplasmic and nuclear pattern (B), CD79, with a weak and focal membrane pattern (C), and cytoplasmic immunoglobulin (Ig) alpha heavy chains (D) and lacks expression of Ig gamma (E) and Ig mu (F) heavy chains. (Immunoperoxidase staining with hematoxylin counter-stain, panels B-F.) By in situ hybridization for immunoglobulin lambda (G) and kappa (H) light chain RNA the tumor shows lambda light chain restriction. Original magnification, × 400.
Immunophenotypically, these tumors expressed the leukocyte common antigen and were positive for ALK, CD79a (weak, focal), IgA, and EMA. The ALK staining was homogeneous, nuclear, and cytoplasmic in patient 2 (Figure 1) and only cytoplasmic in patient 1 (data not shown). By in situ hybridization, there was immunoglobulin light chain restriction (Ig kappa in patient 1 and Ig lambda in patient 2). The tumors were negative for CD30, CD3, CD8, CD20, CD56, CD57, IgM, IgD, and EBV (EBER and LMP-1). On staining for CD4, the tumor from patient 2 was positive, while the one from patient 1 was negative.
Cytogenetic analysis was available only in patient 2 (performed from lymph node biopsy material obtained following chemotherapy), with the following karyotype: 45, XY, der(1;11)(q10;q10), t(2;5)(p23;q35)[3]/46, XY [10].
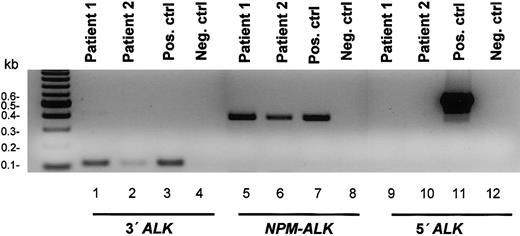
In both tumors, RT-PCR demonstrated the presence of the NPM-ALK and the 3′ALK transcripts (the latter encoding for the intracellular portion of ALK and present in the NPM-ALK fusion transcript) and was negative for the 5′ALK mRNA (encoding for the extracellular portion of the ALK protein) (Figure 2).
RT-PCR analysis demonstrating the presence of theNPM-ALKfusion transcript and the absence of mRNA for the extracellular portion of ALK in 2 cases of ALK-positive B-cell lymphoma. RT-PCR was performed on total RNA extracted from the patient tissue samples, a known positive control, and a known negative control. The presence of amplifiable RNA was confirmed by RT-PCR amplification of a 321-bp portion of the ubiquitously expressed NPM gene. The analysis demonstrates the presence of 429-bp NPM-ALK and 121-bp 3′ALK (encoding for the intracellular portion of ALK, also present in the NPM-ALK) transcripts and is negative for the 552-bp 5′ALK transcript (encoding for the extracellular portion of ALK).
RT-PCR analysis demonstrating the presence of theNPM-ALKfusion transcript and the absence of mRNA for the extracellular portion of ALK in 2 cases of ALK-positive B-cell lymphoma. RT-PCR was performed on total RNA extracted from the patient tissue samples, a known positive control, and a known negative control. The presence of amplifiable RNA was confirmed by RT-PCR amplification of a 321-bp portion of the ubiquitously expressed NPM gene. The analysis demonstrates the presence of 429-bp NPM-ALK and 121-bp 3′ALK (encoding for the intracellular portion of ALK, also present in the NPM-ALK) transcripts and is negative for the 552-bp 5′ALK transcript (encoding for the extracellular portion of ALK).
To our knowledge the cases described here represent the first report of NPM-ALK-positive plasmablastic large B-cell lymphoma. A single case of ALK-positive, CD30-, IgA kappa-restricted B-cell lymphoma that was reported to be t(2;5) positive but NPM-ALK negative by RT-PCR was mentioned in a larger series of cases by Gascoyne et al.12 It is possible that the NPM-ALK was not detected in that case due to breakpoint heterogeneity that prevented amplification with the set of primers used in that study.
The 2 cases of NPM-ALK-positive plasmablastic lymphoma described here are morphologically, immunophenotypically, and clinically very similar to the cases previously described by Delsol et al.4 However, the present cases differ markedly in their ALK staining pattern from the latter tumors, showing diffuse staining versus a membrane and speckled pattern. The existence of NPM-ALK-positive B-cell tumors is not surprising in light of the observation that enforced expression of NPM-ALK in mice has been reported to induce T-lineage lymphoblastic lymphomas or B-lineage plasmacytic/plasmablastic tumors with high frequency.13-15
The cases presented here, very similar to the previously described ALK-positive plasmablastic lymphomas (currently classified as diffuse large B-cell lymphomas with expression of full-length ALK), suggest that this family of tumors is molecularly more heterogeneous than previously thought and should be more appropriately designated as “diffuse large B-cell lymphoma expressing ALK.”
Prepublished online as Blood First Edition Paper, June 19, 2003; DOI 10.1182/blood-2003-04-1095.
Supported in part by the National Institutes of Health (grants CA21765 and CA69129) (S.W.M.) and by the American Lebanese Syrian Associated Charities (ALSAC) of St Jude Children's Research Hospital.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal