Abstract
Bax is cleaved by calpain at aspartate 33 (Asp33) to yield p18 Bax during stress-induced apoptosis. To assess the role of p18 Bax in apoptosis, an ecdysone-inducible expression system was generated. Similar levels of wild-type (WT) and noncleavable Asp33Ala (Asp→Ala) Bax are induced in 293 cells while expression of N-terminal-deleted p18 (Δ1-33) Bax remains low (20% of full-length p21 Bax) due to a reduced half-life (2 hours versus 12 hours for p21 Bax) resulting from increased sensitivity to cathepsin-like proteolytic degradation. Expression of p18 Bax is enhanced to levels comparable to p21 Bax when induction is carried out in the presence of cathepsin inhibitors, Z-Phe-Gly-NHO-Bz or N-Acetyl-Leu-Leu-Met-CHO. Compared with WT Bax, expression of similar levels of p18 Bax and, surprisingly, Asp33Ala Bax more potently induces apoptosis as indicated by increased cytochrome c release, caspase-9/-3 activation, and DNA fragmentation, potentially due to their increased homo-oligomerization in mitochondrial membranes. Studies in A-549, U-937, K-562, and HL-60 cells confirm that inhibition of Bax cleavage results in 25% to 35% reduction of drug-induced apoptosis, while inhibition of p18 Bax degradation enhances apoptosis by 25% to 40%. Results indicate that although cleavage to p18 Bax is not required for Bax to initiate apoptosis, p18 Bax potently accelerates the apoptotic process. (Blood. 2003;102:2605-2614)
Introduction
Apoptosis is mediated through 2 major pathways, the intrinsic pathway and the extrinsic pathway.1 The intrinsic pathway is characterized by mitochondrial dysfunction with release of caspase activators including cytochrome c, followed by activation of caspase-9 and caspase-3 that effect the morphologic signs of apoptosis including membrane blebbing, cell shrinkage, and DNA fragmentation.1,2 The extrinsic apoptotic pathway is initiated at the cell surface through cytokine-induced death receptor-mediated activation of caspase-8 followed by caspase-3 activation.1 It may (type II) or may not (type I) be amplified by additional caspase-9/-3 activation via the intrinsic pathway.3 Interestingly, the link between type I and type II extrinsic apoptosis occurs when caspase-8 induces the proteolytic cleavage of Bid to a truncated Bid (tBid) capable of translocating from cytosol to mitochondrial membranes where tBid stimulates more efficient oligomerization of Bax and activates the intrinsic pathway.4-6 Thus, caspase-mediated proteolytic events not only may destroy structural and functional cellular proteins to produce the morphologic hallmarks of apoptosis but also can act to amplify the apoptotic process.3
Recently it was reported that Bax or Bak is essential for initiating the intrinsic apoptotic pathway.7 The 3-dimensional solution structure of p21 Bax demonstrates a high degree of similarity to that of Bcl-XL8 ; and Bax, Bcl-2, and Bcl-XL have been shown to possess channel-forming activity in artificial membranes.9-12 Evidence indicates that following stress, cytosolic Bax can fully integrate into the outer mitochondrial membrane where it oligomerizes and likely forms death pores that facilitate the release of cytochrome c and other proapoptotic activators from the mitochondrion.13-18 Furthermore, either forced expression of Bax or the addition of purified recombinant Bax to isolated intact mitochondria can trigger the release of cytochrome c, suggesting a direct role for Bax in disrupting mitochondrial integrity.14-17 Interestingly, forced overexpression of Bax in the absence of additional stress treatment does not universally induce apoptosis but may increase sensitivity of the cell to stresses that induce apoptosis, indicating that Bax-mediated killing is subject to regulation. Proteolytic cleavage of Bax was reported to occur in tumor cells treated with various chemotherapeutic agents that activate the intrinsic apoptotic pathway.19-21 Bax was shown to be cleaved at aspartate 33 (Asp33) by calpain to yield a p18 Bax product.22,23 While p18 Bax formation is associated with apoptosis, whether p18 Bax contributes to mitochondrial dysfunction or is merely a proteolytic by-product is not clear.
In addition to caspase-mediated proteolysis that occurs during apoptosis, other cysteine proteases such as calpain and cathepsin may also be activated, but their role in apoptosis is not well defined.24 For example, excessive calpain activation has been reported in Alzheimer disease and muscular dystrophy,25-28 and increased expression and activity of cathepsins have been implicated in malignant progression.29-32 However, the relationship, if any, between Bax proteolysis and calpain/cathepsin action with respect to apoptosis is not clear. Our studies using an inducible Bax expression system indicate that a cathepsin-like cysteine protease is involved in rapidly degrading p18 Bax. However, when p18 Bax is stabilized by addition of a cathepsin inhibitor to cell cultures, p18 Bax is more potent than wild-type (WT) Bax in disrupting mitochondrial membrane integrity and inducing apoptosis. In addition, studies in tumor cell lines confirm that blockade of Bax cleavage significantly retards drug-induced apoptosis, while inhibition of p18 Bax degradation significantly enhances drug-induced apoptosis. Thus, calpain-mediated cleavage of Bax to p18 Bax represents a potential amplification step that can accelerate the apoptotic process following cytotoxic stress. Therefore, either increasing Bax cleavage to p18 Bax or inhibiting p18 Bax degradation by blocking the cathepsin-like protease may represent a novel approach to augment cancer chemotherapy.
Materials and methods
Cell culture
The murine myeloid NFS/N1-H-7 cells were grown as described.33 Human promyelocytic leukemia HL-60, myelogenous leukemia K-562, and histiocytic lymphoma U-937 cells were cultured as described,22 and lung carcinoma A-549 cells were grown in F12K medium (all from American Type Culture Collection [ATCC], Manassas, VA). For inducible Bax expression, human embryonic kidney 293T cells that stably express ecdysone receptor (EcR-293, Invitrogen, Carlsbad, CA) were cultured in 5% CO2 and 95% humidified air at 37°C in Dulbecco modified Eagle medium (DMEM) (Cellgro) containing 10% fetal bovine serum (Gibco, Grand Island, NY), 2 mM glutamine, 1% penicillin-streptomycin (Cellgro, Herndon, VA), and 400 μg/mL phleomycin D1 (Zeocin; Invitrogen).
Drug-induced apoptosis and protease inhibitor studies
A total of 6 × 106 HL-60 cells were cultured in 25-cm2 flasks (Costar, Corning, NY) in 10 mL medium, and camptothecin (Calbiochem, San Diego, CA), a DNA topoisomerase I inhibitor, was added at the indicated concentrations. For protease inhibitor studies, cells were treated with 20 μM Z-Leu-Nle-CHO (calpeptin, calpain inhibitor), or 20 μM N-Acetyl-Leu-Leu-Met-CHO (ALLM; inhibits both calpain and cathepsin), or 20 μM Z-Phe-Gly-NHO-Bz (CI, or cathepsin inhibitor I), or 20 μM proteasome inhibitor lactacystin (all from Calbiochem) for 30 minutes and then challenged with 2.0 μM camptothecin. Control cultures were treated with either the same dose of inhibitor or a corresponding volume of vehicle. Cells were harvested at the indicated times and subjected to protein extraction for immunoblotting with human-specific anti-Bax antibody. DNA fragmentation and cell viability were determined as described in “Cell viability.” HL-60, K-562, U-937, and A-549 cells were treated similarly with 20 μM cisplatin, 50 μM etoposide, or 0.5 μg/mL adriamycin alone or plus the inhibitors.
IL-3 withdrawal from H-7 cells
Bax cDNA constructs
Murine T7-tagged-Bax cDNA was cloned in the pUC19 plasmid. Asp33 and Glu6 of Bax were substituted to create a conservative alteration to alanine (Ala) with a site-directed mutagenesis kit (Clontech, Palo Alto, CA). The p18 Bax (Δ1-33) was created by a deletion mutagenesis using T7-Bax/pUC19 as a template and the mutagenic primer with the sequence 5′-CAAATGGGTCTCGAGATGCGAGCAGGGAGGATG-3′ and selective primer with the sequence 5′-GAGTGCACCATGGGCGGTGTGAAAT-3′. Each single mutant was confirmed by sequencing the cDNA.
Inducible expression of WT and mutant Bax in 293 cells
The ecdysone-inducible expression system (Invitrogen) was utilized. WT, Asp33Ala, p18, and Glu6Ala Bax cDNAs were ligated respectively into the inducible Pind vector and transfected into EcR-293 cells using Lipofectamine (Invitrogen). The transfected EcR-293 cells were selected using both 400 μg/mL phleomycin D1 and 300 μg/mL Gly418 simultaneously. Three separate batch groups of dual-selected 293 cells were generated. For time course studies, 4 × 105 dual-selected cells were cultured in 6-well dishes (Costar) in 2.5 mL dual selection medium for 36 hours to reach about 50% confluency. Expression of Bax was induced by removing media and replacing with fresh medium containing 6 μM inducing agent ponasterone A (PA), an ecdysone analog. For the inhibitor studies, 20 μM calpeptin, or 40 μM ALLM, or 20 μM CI, or 20 μM lactacystin was added at the same time as PA. At the indicated times, cells were collected after trypsinizing for 3 minutes for the purpose of viability assay and Western blotting to detect protein expression as described in “Cell viability.”
Quantifying Bax expression
Following induction, cells were harvested and lysed, and proteins were extracted and quantitated as described.33,36 To determine the expression levels of WT and mutant Bax, equal amounts of protein (100 μg) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by immunoblotting with antibodies including mouse-specific polyclonal anti-Bax serum (nonreactive to human Bax) made against a peptide corresponding to amino acids 28 to 44 of mouse Bax and used at 1:500 dilution (Invitrogen) and mouse anti-T7 tag monoclonal antibody (Novagen, Madison, WI) used at 1:2000 dilution. Densitometry analysis was performed using FluorChem SpotDenso Analysis Tool (Alpha Innotech, San Leandro, CA) to quantify Bax expression.
Cell viability
Inducible Bax expression was performed in the absence or presence of protease inhibitor or 2 μg/mL adriamycin as described in “Inducible expression of WT and mutant Bax in 293 cells.” At the indicated times, cell viability was measured using the trypan blue exclusion method as described.34,37 To determine whether cells underwent apoptosis, DNA was isolated and its oligonucleosomal fragmentation pattern was determined as described.35,38
Bax half-life determination
First, metabolic labeling to measure Bax half-life (t1/2) was carried out when cells had grown to 50% confluent in 60-mm dishes (Falcon, Franklin Lakes, NJ). Cells were washed 3 times with methionine-free DMEM, which was replaced with medium containing 6 μM PA and 0.3 μCi/mL (0.0111 MBq) [35S]methionine, incubated at 37°C for 60 minutes. The medium was then discarded, and fresh medium containing unlabeled methionine plus 6 μM PA was added (time 0). Cells were collected at the indicated times and lysed in 500 μL lysis buffer. Lysates were immunoprecipitated using the mouse-specific anti-Bax antibody and subjected to SDS-PAGE and analyzed by autoradiography. Then, result of the above pulse-chase study was confirmed using the cyclohexamide method.39 A total of 3 × 105 cells were cultured in 6-well dishes in 2.5 mL medium for 36 hours to reach 30% to 40% confluency. Inducible expression was carried out for 20 hours, and then the induction medium was removed and replaced by medium containing 30 μg/mL cyclohexamide to block new protein synthesis (time 0). Cells were harvested at the indicated times and subjected to protein extraction for immunoblotting with the mouse-specific anti-Bax antibody. To determine the effect of ALLM on Bax half-life, induction of Bax and cyclohexamide treatment were performed in the presence of 40 μM ALLM. Densitometry analysis was performed to quantify Bax expression and determine protein half-life.
Caspase-9/-3 cleavage and activation
To assess caspase-3 activity, 1 × 103 cells were cultured in each well of a black 96-well plate (Packard, Meriden, CT) for 36 hours in 100 μL medium to reach about 30% confluency. Inducible Bax expression was performed in the absence or presence of ALLM. Apo-ONE homogeneous caspase-3 activity assay was performed following the manual (Promega, Madison, WI). The assay uses a profluorescent-specific caspase-3 substrate, Z-DEVDR110 (rhodamine 110). To confirm caspase-9/-3 activation, similar experiments were performed with induction medium containing 20 μM caspase-3 inhibitor I (DEVD-CHO) or 20 μM caspase-9 inhibitor II (LEHD-FMK). To determine caspase-9/-3 cleavage, proteins were extracted following Bax induction. Lysates were subjected to SDS-PAGE followed by immunoblotting with antibodies specific to the cleaved caspase-9/-3 (Cell Signaling Technology, Beverly, MA) and full-length procaspase-9/-3 (Santa Cruz Biotechnology, Santa Cruz, CA).
Cytochrome c release
Inducible Bax expression was performed in 100-mm dishes in the absence or presence of ALLM. To assess cytochrome c release, cytosolic and mitochondrial fractions were obtained using a digitonin-based subcellular fractionation technique as described.40-42 Equal amounts of mitochondrial and cytosolic fractions (50 μg) were subjected to SDS-PAGE followed by immunoblotting with an anticytochrome c antibody (BD PharMingen, San Diego, CA).
Alkali extraction of proteins peripherally associated with mitochondrial membranes
Following Bax induction in the presence of 40 μM ALLM, isotonic subcellular fractionation was performed to collect the heavy membrane fraction as described.13,18 The heavy membrane fraction (0.5 mg protein) was divided evenly into 2 fractions. The first fraction was washed once with mitochondrial buffer (MB) and resuspended with 50 μL 2 × SDS-PAGE sample buffer. The second fraction was pelletted and resuspended in freshly prepared 0.1 M Na2CO3, pH 11.5, and incubated on ice for 30 minutes.43 The alkali extracted membrane was then centrifuged at 200 000g for 30 minutes, and the pellet was resuspended with 50 μL 2 × SDS-PAGE sample buffer. Samples were subjected to SDS-PAGE and immunoblotting with anti-Bax antibody. Densitometry analysis was performed to determine the percentage of the alkali-resistant Bax (nonextractable, integral) contained in the mitochondrial membranes with the following calculation: (amount of alkali-resistant Bax contained in the second fraction/amount of Bax in the MB-washed first fraction) × 100.
Cross-linking of Bax in mitochondrial membranes
The heavy membrane fraction (0.5 mg protein) was resuspended in 400 μL MB. Cross-linkers BS3 (Pierce, Rockford, IL) and DSS (Pierce) were added simultaneously to a final concentration of 5 mM.13,18 Incubation was carried out at room temperature for 30 minutes, and then the reaction was quenched by addition of Tris (tris(hydroxymethyl)aminomethane)-HCl, pH 8.0, to a final concentration of 20 mM. The heavy membrane fraction was then lysed, and 40 μg lysate protein was subjected to SDS-PAGE followed by Western blotting with an anti-T7 tag antibody.
Results
Cleavage of Bax to p18 Bax is closely associated with apoptosis induced by drug treatment or growth factor withdrawal
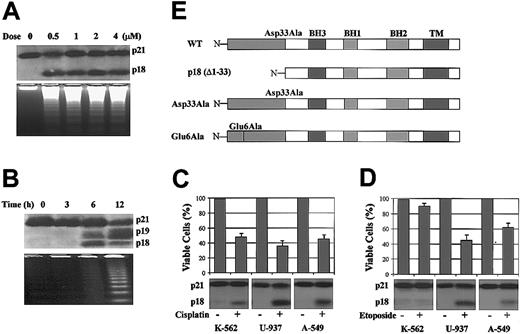
Bax cleavage has been reported to occur in various tumor cell lines undergoing apoptosis following treatment with various chemotherapeutic or biologic agents.19-21 Following treatment with the DNA-damaging agent, camptothecin, HL-60 cells undergo apoptosis in association with Bax cleavage into a p18 product (Figure 1A). Similar results are observed when K-562, U-937, and A-549 cells are treated with cisplatin or etoposide (Figure 1C-D), ruling out a nonspecific or cell type-specific effect for Bax cleavage. Interestingly, Bax is cleaved to p18 Bax in all drug-treated cells undergoing apoptosis except K-562 cells, which are relatively resistant to etoposide (Figure 1D). Collectively, these results suggest a functional role for Bax cleavage in apoptosis. In addition, withdrawal of IL-3 from factor-dependent H-7 cells induces Bax cleavage into both p18 and p19 products (Figure 1B). p18 and p19 Bax are detected using antibodies developed against a peptide corresponding to amino acids 28 to 44 of mouse Bax (Invitrogen; Figure 1B) or residues 43 to 61 of human Bax (BD PharMingen; Figure 1A,C-D). A Bid antibody (BD PharMingen) capable of recognizing both full-length and cleaved human and mouse Bid was used to assess whether Bid was also cleaved under these conditions. Notably, no Bid cleavage was found, although endogenous Bid is highly expressed in the cells (data not shown). Both p18 and p19 Bax products appear by 6 hours following IL-3 withdrawal, a time proceeding cell death as determined by trypan blue staining and DNA fragmentation criteria (Figure 1B). These results suggest that the generation of p18 and p19 Bax may be an early event in the induction of apoptosis and confirm previous reports that Bax is cleaved by calpain at aspartate 33 (Asp33) to yield p18 Bax during apoptosis.22 However, because p19 Bax is observed only in H-7 cells and is not consistently observed following apoptosis-inducing treatments in other cell lines, we have concentrated our studies on the universally observed p18 Bax.
Apoptosis-associated Bax cleavage following drug treatment or IL-3 withdrawal and Bax cDNAs constructs. (A) HL-60 cells were treated with various doses of camptothecin, a DNA topoisomerase I inhibitor, for 20 hours. (B) H-7 cells were deprived of IL-3 for the indicated times. (Upper panels) Proteins were extracted and subjected to SDS-PAGE and Western blotting with anti-Bax antibody. (Lower panels) DNA fragmentations were determined as described in “Materials and methods” to confirm that cells underwent apoptosis. (C) K-562, U-937, and A-549 cells were treated with 20 μM cisplatin for 36 hours. (D) K-562, U-937, and A-549 cells were treated with 50 μM etoposide for 36 hours. Cell viabilities were measured by trypan blue exclusion method. Proteins were extracted and subjected to SDS-PAGE and Western blotting with anti-Bax antibody. Error bars indicate means ± SD (n = 3). (E) WT Bax cDNA was cloned from H-7 cells with a T7 tag at the N-terminal. Asp33Ala, Glu6Ala, and N-terminal-deleted p18 (Δ1-33) Bax cDNA mutants were constructed as described.
Apoptosis-associated Bax cleavage following drug treatment or IL-3 withdrawal and Bax cDNAs constructs. (A) HL-60 cells were treated with various doses of camptothecin, a DNA topoisomerase I inhibitor, for 20 hours. (B) H-7 cells were deprived of IL-3 for the indicated times. (Upper panels) Proteins were extracted and subjected to SDS-PAGE and Western blotting with anti-Bax antibody. (Lower panels) DNA fragmentations were determined as described in “Materials and methods” to confirm that cells underwent apoptosis. (C) K-562, U-937, and A-549 cells were treated with 20 μM cisplatin for 36 hours. (D) K-562, U-937, and A-549 cells were treated with 50 μM etoposide for 36 hours. Cell viabilities were measured by trypan blue exclusion method. Proteins were extracted and subjected to SDS-PAGE and Western blotting with anti-Bax antibody. Error bars indicate means ± SD (n = 3). (E) WT Bax cDNA was cloned from H-7 cells with a T7 tag at the N-terminal. Asp33Ala, Glu6Ala, and N-terminal-deleted p18 (Δ1-33) Bax cDNA mutants were constructed as described.
Ponasterone A (PA)-inducible expression of Bax in 293 cells results in similar levels of WT, Asp33Ala, and Glu6Ala but reduced levels of p18 Bax
WT and mutant Bax cDNAs were constructed and placed under the control of the PA-inducible promoter (Figure 1E). An Asp33Ala (Asp→Ala) Bax mutation was designed to ablate the previously identified calpain cleavage site and prevent cleavage of Bax.22 The Glu6Ala (Glu→Ala) Bax mutation was generated to test whether the Glu6 site is involved in the generation of p19 Bax and served as a control for a single alanine mutation in the N-terminal domain. Inducible expression of Bax protein was quantified by densitometry analysis. Results shown are representative of studies performed 3 times on each of the 3 separate batch groups of WT-, Asp33Ala-, p18-, or Glu6Ala-transfected dual-selected 293 cells. Although there is a low-level “leakiness” of expression of WT, Asp33Ala, and Glu6Ala Bax under noninduced conditions, no such “leakiness” is detected for p18 Bax. Robust expression of Bax is dependent upon the addition of PA. When 6 μM PA is added, expression of WT, Asp33Ala, and Glu6Ala increases approximately 40-fold over the basal level after 24 hours, with the difference between the expression levels of WT, Asp33Ala, and Glu6Ala Bax being less than 10% (Figure 2A). In addition, the expression of endogenous Bax is relatively low in 293 cells and unaffected by PA addition (Figure 3). However, the expression of p18 Bax is only approximately 20% that of WT, Asp33Ala, and Glu6Ala Bax (Figure 2A,C). Similar results were also observed in Cos7 and FDC-P1 cells, ruling out a cell type-specific effect. When transiently expressed in Cos7 cells, p18 Bax expression is about 20% that of WT, Asp33Ala, and Glu6Ala Bax. When stably expressed in FDC-P1 cells, p18 Bax is barely detectable while WT, Asp33Ala, and Glu6Ala Bax are expressed at similar high levels comparable to that of endogenous Bax (data not shown). These results imply that p18 Bax has either increased cytotoxicity or altered protein stability. In addition, the potential cleavage of Bax following induction was assessed. Although the p18 (and p19) cleavage product could be detected in cells expressing WT, Asp33Ala, and Glu6Ala Bax, a significantly reduced amount of p18 Bax is formed from Asp33Ala Bax (Figure 2A,C), indicating that the Asp33Ala mutation successfully inhibits but does not totally block cleavage of Bax to p18 Bax as previously demonstrated in vitro.22 In contrast, the Glu6Ala mutation did not affect the generation of p18 or p19 Bax, indicating that the Glu6 site is not required for Bax cleavage.
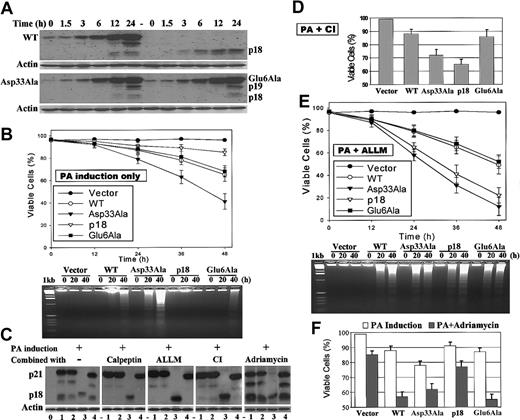
Ponasterone A (PA)-inducible expression of Bax; the effect of cathepsin inhibitors on Bax expression and viability studies. Three separate groups of WT, Asp33Ala, p18, Glu6Ala, or empty vector-transfected 293 cells were used for inducible Bax expression and viability studies as described. (A) Representatives of time course-inducible Bax expressions. Following addition of 6 μM PA, proteins were extracted at the indicated times and subjected to SDS-PAGE and Western blotting with mouse-specific anti-Bax antibody and anti-actin antibody for loading control. (B) Cell viabilities were measured by trypan blue exclusion method following PA-inducible expression of Bax. DNA fragmentations were determined to confirm that cells underwent apoptosis. (C) Twenty-four-hour PA induction of Bax was performed in the presence of 20 μM calpeptin (inhibits calpain) or 40 μM ALLM (inhibits both calpain and cathepsin) or 20 μM cathepsin inhibitor I (CI) or 2 μg/mL adriamycin as described in “Materials and methods.” Lane alignment: 0, vector control; 1, WT; 2, Asp33Ala; 3, p18; 4, Glu6Ala. The representatives of 3 independent experiments were shown. (D) Cell viabilities following 24-hour PA induction of Bax in the presence of CI. (E) Cell viabilities following PA induction of Bax in the presence of ALLM. (F) Cell viabilities following 24-hour combination of PA induction and adriamycin treatment. Error bars in B, D-F indicate means ± SD (n = 3).
Ponasterone A (PA)-inducible expression of Bax; the effect of cathepsin inhibitors on Bax expression and viability studies. Three separate groups of WT, Asp33Ala, p18, Glu6Ala, or empty vector-transfected 293 cells were used for inducible Bax expression and viability studies as described. (A) Representatives of time course-inducible Bax expressions. Following addition of 6 μM PA, proteins were extracted at the indicated times and subjected to SDS-PAGE and Western blotting with mouse-specific anti-Bax antibody and anti-actin antibody for loading control. (B) Cell viabilities were measured by trypan blue exclusion method following PA-inducible expression of Bax. DNA fragmentations were determined to confirm that cells underwent apoptosis. (C) Twenty-four-hour PA induction of Bax was performed in the presence of 20 μM calpeptin (inhibits calpain) or 40 μM ALLM (inhibits both calpain and cathepsin) or 20 μM cathepsin inhibitor I (CI) or 2 μg/mL adriamycin as described in “Materials and methods.” Lane alignment: 0, vector control; 1, WT; 2, Asp33Ala; 3, p18; 4, Glu6Ala. The representatives of 3 independent experiments were shown. (D) Cell viabilities following 24-hour PA induction of Bax in the presence of CI. (E) Cell viabilities following PA induction of Bax in the presence of ALLM. (F) Cell viabilities following 24-hour combination of PA induction and adriamycin treatment. Error bars in B, D-F indicate means ± SD (n = 3).
Comparison of induced Bax and endogenous Bax. (A) Comparison between PA-induced Bax and endogenous Bax in 293 cells. Following induction with 6 μ MPA, proteins were extracted at the indicated times and 100 μg lysate of each time point was subjected to SDS-PAGE and Western blotting with anti-Bax antibody (Cell Signaling Technology; reactive to both the induced mouse Bax and endogenous Bax of human 293 cell); (B) Comparison of endogenous Bax between different cell lines. 100 μg lysate from each cell line was subjected to Western blotting with the anti-Bax antibody described above.
Comparison of induced Bax and endogenous Bax. (A) Comparison between PA-induced Bax and endogenous Bax in 293 cells. Following induction with 6 μ MPA, proteins were extracted at the indicated times and 100 μg lysate of each time point was subjected to SDS-PAGE and Western blotting with anti-Bax antibody (Cell Signaling Technology; reactive to both the induced mouse Bax and endogenous Bax of human 293 cell); (B) Comparison of endogenous Bax between different cell lines. 100 μg lysate from each cell line was subjected to Western blotting with the anti-Bax antibody described above.
Inhibition of a putative cathepsin-like protease increases p18 Bax expression
Expression of p18 Bax can be increased to approximately 80% that of p21 Bax when PA induction is carried out in the presence of N-Acetyl-Leu-Leu-Met-CHO (ALLM; Figure 2C), suggesting that an ALLM-sensitive protease(s) may be involved in p18 Bax degradation. Because ALLM is designed to permeate cells to inhibit both calpain and cathepsin, more selective protease inhibitors were tested in an attempt to distinguish which protease may mediate p18 Bax degradation. The cell-permeable cathepsin inhibitor I (CI), Z-Phe-Gly-NHO-Bz, which does not inhibit calpain,44,45 produces a similar effect to that of ALLM (Figure 2C). However, the cell-permeable calpain inhibitor calpeptin, which does not inhibit cathepsin,25,46 does not affect p18 Bax level (Figure 2C). Moreover, the proteasome inhibitor lactacystin has no effect on p18 Bax expression under these conditions (data not shown). Collectively, these data suggest that an ALLM/CI-sensitive, cathepsin-like cysteine protease may be responsible for p18 Bax degradation and that the proteasome is probably not involved.
Expression of quantitatively similar levels of Asp33Ala or p18 Bax induces higher rates of apoptosis than WT or Glu6Ala Bax
The viabilities of cells expressing WT, Asp33Ala, p18, and Glu6Ala Bax and empty vector-transfected 293 cells were compared following PA induction. Cells expressing WT, Asp33Ala, p18, and Glu6Ala Bax display increased cell death compared with the vector control (Figure 2B). Surprisingly, Asp33Ala Bax is more toxic. That is, nearly 60% of cells expressing Asp33Ala Bax are dead at 48 hours compared with only about 35% for cells expressing either WT or Glu6Ala Bax. Furthermore, in the absence of ALLM or CI, only about 15% of the cells expressing p18 Bax are nonviable, a result consistent with its low level of expression (about 20%) compared with p21 Bax (Figure 2A,C). However, when induction is carried out in the presence of CI for 24 hours, p18 Bax expression increases to the similar level of p21 Bax (Figure 2C), and p18 (or Asp33Ala) Bax-expressing cells undergo significantly higher levels of cell death compared with WT or Glu6Ala Bax (Figure 2D). While the half-life of CI in the culture medium is only about 24 hours,44,45 ALLM is more stable and able to inhibit both calpain and cathepsin. Thus, when ALLM is added during PA induction, p18 Bax expression increases to about 80% that of p21 Bax; furthermore, calpain-mediated cleavage of p21 Bax to p18 Bax is efficiently blocked (Figure 2C). This pharmacologic maneuver allows us to directly compare the potencies of p18 and p21 Bax. Therefore, PA induction was carried out in the presence of ALLM to perform viability and mechanism studies. Under these conditions when similar levels of WT, Asp33Ala, and Glu6Ala Bax are expressed, nearly 90% of the cells expressing Asp33Ala are dead by 48 hours compared with about 50% for cells expressing either WT or Glu6Ala. For cells expressing p18 Bax, 80% are nonviable even though p18 Bax expression is only approximately 80% that of p21 Bax (Figure 2C,E). These data indicate that p18 Bax is a more potent inducer of cell death than WT Bax but can be rapidly degraded by an ALLM/CI-sensitive cathepsin-like protease to nullify its effect. To determine whether cells undergo apoptosis, DNA fragmentation studies were also performed. A typical apoptotic DNA fragmentation ladder is detected in cells expressing WT, Asp33Ala, p18, or Glu6Ala Bax as cell death approaches 20% (Figure 2B,E). No such DNA ladder can be detected for the empty vector-transfected cells treated with either PA alone or PA plus ALLM, indicating that induction of Bax specifically causes apoptosis and that PA or ALLM does not affect the process. Interestingly, ALLM is observed to enhance the proapoptotic function of all Bax forms (Figure 2B,E), probably by increasing their half-life (Figure 4). Notably, p18 and Asp33Ala Bax are equivalent and more potent than WT Bax in inducing apoptosis (Figure 2E). By contrast, no significant difference is observed between the lower levels of apoptosis following WT and Glu6Ala Bax induction.
p18 Bax has a reduced half-life that can be enhanced by ALLM. (A) The half-life of WT and p18 Bax was first determined by [35S]methionine metabolic labeling and pulse-chase as described in “Materials and methods.” (B) The half-life of WT, Asp33Ala, p18, and Glu6Ala Bax was determined again using the cyclohexamide method as described in “Materials and methods.” (C) The effect of ALLM on Bax protein half-life was determined by cyclohexamide method. Half-life was determined by densitometry analysis.
p18 Bax has a reduced half-life that can be enhanced by ALLM. (A) The half-life of WT and p18 Bax was first determined by [35S]methionine metabolic labeling and pulse-chase as described in “Materials and methods.” (B) The half-life of WT, Asp33Ala, p18, and Glu6Ala Bax was determined again using the cyclohexamide method as described in “Materials and methods.” (C) The effect of ALLM on Bax protein half-life was determined by cyclohexamide method. Half-life was determined by densitometry analysis.
Inducible expression of Bax enhances drug-induced apoptosis
We found that 293T cells are somehow resistant to etoposide and cisplatin. When these cells are treated with 2 μg/mL adriamycin for 24 hours, only about 15% of the cells undergo apoptosis. However, adriamycin-induced apoptosis can be significantly enhanced when combined with inducible expression of Bax. While induction of WT (or Glu6Ala) Bax alone for 24 hours causes only about 10% to 15% cell death, when combined with adriamycin treatment about 45% of the cells are dead (Figure 2F). Notably, this apparent synergistic effect is closely correlated with increased formation of p18 product from WT (or Glu6Ala) Bax compared with Asp33Ala Bax (Figure 2C). That is, induction of the cleavage-resistant Asp33Ala Bax leads to a reduced amount of p18 product. In this situation, Asp33Ala Bax has only an additive effect on adriamycin-induced apoptosis, similar to the induction of p18 Bax (Figure 2F). These results suggest that the increased formation of p18 from WT (or Glu6Ala) Bax, presumably caused by additional calpain activation following adriamycin treatment, is the cause of this synergistic increase of apoptosis. Collectively, these data indicate that cleavage of Bax to p18 Bax functions as an amplification step to accelerate the apoptotic process.
p18 Bax has a reduced half-life that can be enhanced by ALLM
The protein half-life (t1/2) of Bax was determined using 2 different methods. A classic pulse-chase study using [35S]methionine to label cellular proteins was performed initially. Results were confirmed using cyclohexamide treatment of cells to block protein synthesis.39 While WT, Asp33Ala, and Glu6Ala Bax have a t1/2 of approximately 12 hours, the t1/2 of p18 Bax is only about 2 hours (Figure 4A-B). When cells were incubated with ALLM, the t1/2 of p18 Bax is increased to about 6 to 8 hours, and that of WT, Asp33Ala, and Glu6Ala Bax is increased to about 24 hours (Figure 4C).
Expression of Asp33Ala or p18 Bax induces increased caspase-9/-3 activation compared with WT or Glu6Ala Bax
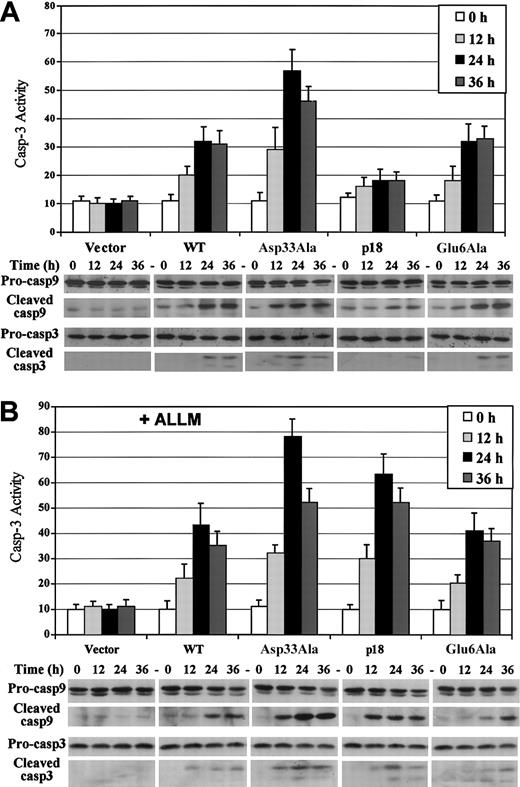
To further assess the rate of apoptosis, caspase-3 activity was measured as described. Following induction of Asp33Ala Bax, caspase-3 activity increases by more than 4-fold over the basal level by 24 hours, while in cells expressing WT or Glu6Ala Bax, caspase-3 activity increases by 2-fold and a 50% increase is observed in cells expressing p18 Bax (Figure 5A). The cleavage of caspase-9/-3 is also an indication of caspase activation.47 Expression of Asp33Ala Bax appears to induce an earlier appearance of cleaved caspase-9/-3 compared with WT or Glu6Ala Bax (Figure 5A). Bax-induced activation of caspase-3 was blocked when caspase-3 inhibitor or caspase-9 inhibitor was added during induction (data not shown), indicating that caspase-9 activation is upstream of caspase-3 activation. However, in cells expressing p18 Bax in the absence of ALLM, caspase cleavage is only minimal and consistent with the low level of apoptosis observed in these cells (Figure 5A). Following induction in the presence of ALLM, the caspase cleavage and activation pattern in p18 Bax-expressing cells quantitatively resembles that for Asp33Ala Bax (Figure 5B). In this case, caspase-3 activity increases by more than 5-fold following p18 Bax expression and nearly 7-fold for Asp33Ala expression, compared with about 3-fold for WT or Glu6Ala Bax. These data confirm that p18 and Asp33Ala Bax are more toxic than WT and Glu6Ala Bax.
Expression of Asp33Ala or p18 Bax induces increased caspase-9/-3 activation compared with WT or Glu6Ala Bax. Caspase-3 activity assay was performed following inducible Bax expression as described in “Materials and methods.” For caspase-9/-3 cleavage studies, proteins were extracted and subjected to SDS-PAGE and Western blotting with cleaved caspase-9/-3-specific antibodies and full-length procaspase-9/-3-specific antibodies, respectively. (A) Time course caspase-3 activity and caspase-9/-3 cleavage following inducible Bax expression. (B) Caspase-3 activity and caspase-9/-3 cleavage following inducible Bax expression in the presence of 40 μM ALLM. Error bars indicate means ± SD (n = 3).
Expression of Asp33Ala or p18 Bax induces increased caspase-9/-3 activation compared with WT or Glu6Ala Bax. Caspase-3 activity assay was performed following inducible Bax expression as described in “Materials and methods.” For caspase-9/-3 cleavage studies, proteins were extracted and subjected to SDS-PAGE and Western blotting with cleaved caspase-9/-3-specific antibodies and full-length procaspase-9/-3-specific antibodies, respectively. (A) Time course caspase-3 activity and caspase-9/-3 cleavage following inducible Bax expression. (B) Caspase-3 activity and caspase-9/-3 cleavage following inducible Bax expression in the presence of 40 μM ALLM. Error bars indicate means ± SD (n = 3).
Expression of Asp33Ala or p18 Bax induces increased cytochrome c release compared with WT or Glu6Ala Bax
For the intrinsic pathway, caspase activation is initiated by cytochrome c release from mitochondria into the cytosol to form the apoptosome.17,48 Studies of cytochrome c release reveal that Asp33Ala Bax expression is associated with an earlier and increased amount of cytochrome c release compared with WT or Glu6Ala Bax (Figure 6A), while in p18 Bax-expressing cells, cytochrome c release is barely detectable in the absence of ALLM. However, as p18 Bax expression increases in the presence of ALLM, a dramatic increase of cytochrome c release is noted that mimics the released level observed in Asp33Ala Bax-expressing cells (Figure 6B). These data strongly support the notion that both p18 and Asp33Ala Bax are more potent in disrupting mitochondrial integrity than WT or Glu6Ala Bax.
Expression of Asp33Ala or p18 Bax induces more cytochrome c release than WT or Glu6Ala Bax. Following inducible expression, cytosolic and mitochondrial fractions were generated using a digitonin-based subcellular fractionation technique. Equal amounts (50 μg) of mitochondrial and cytosolic fractions were subjected to SDS-PAGE and Western blotting with anticytochrome c antibody and, for negative control, antiprohibitin antibody. (A) Time course cytochrome c release following PA-inducible Bax expression. (B) Cytochrome c release at 24 hours following PA-inducible Bax expression in the absence or presence of 40 μM ALLM.
Expression of Asp33Ala or p18 Bax induces more cytochrome c release than WT or Glu6Ala Bax. Following inducible expression, cytosolic and mitochondrial fractions were generated using a digitonin-based subcellular fractionation technique. Equal amounts (50 μg) of mitochondrial and cytosolic fractions were subjected to SDS-PAGE and Western blotting with anticytochrome c antibody and, for negative control, antiprohibitin antibody. (A) Time course cytochrome c release following PA-inducible Bax expression. (B) Cytochrome c release at 24 hours following PA-inducible Bax expression in the absence or presence of 40 μM ALLM.
Mitochondrial targeting and membrane insertion of Bax are not affected by N-terminal cleavage or Asp33Ala mutation
In nonstressed cells, Bax is located mainly in the cytosol where it may be peripherally but not integrally associated with the outer mitochondrial membranes. Following a death stimulus Bax is translocated to mitochondria and becomes integrally associated with the outer mitochondrial membranes.8 This process precedes mitochondrial dysfunction characterized by cytochrome c release.43,49 It has been shown that alkali extraction of mitochondrial membranes can denature the secondary structure of peripherally associated proteins to lead to their separation from the mitochondria.43 This maneuver helps to distinguish whether Bax is peripherally associated with or integrally inserted into mitochondrial membranes. Studies were performed using this technique to determine whether the induced Bax is integrated into mitochondrial membranes (Figure 7). Results reveal that by 12 hours following induction, about 20% to 25% of Bax becomes nonextractable (ie, integral), while more than 95% is nonextractable at 24 hours. This result is not surprising, because Bax overexpression induces cell death in more than 20% of the cells by 24 hours. Compared with WT Bax, however, no enhanced mitochondrial integration affinity was detected for the N-terminal-deleted p18 Bax or Asp33Ala Bax. These data are consistent with a report demonstrating that conformation of the Bax C-terminus determines its mitochondrial targeting and membrane insertion.50
Mitochondrial targeting and membrane insertion of Bax are not affected by N-terminal cleavage or Asp33Ala mutation. Inducible Bax expression was performed in the presence of 40 μM ALLM for the indicated times. The heavy membrane was isolated, and alkali extraction was performed to determine the percentage of the alkali-resistant (integral, nonextractable) Bax contained in the mitochondrial membrane as described in “Materials and methods.” Intermembrane cytochrome c was used as extractable positive control, and inner membrane prohibitin was used as nonextractable negative control.
Mitochondrial targeting and membrane insertion of Bax are not affected by N-terminal cleavage or Asp33Ala mutation. Inducible Bax expression was performed in the presence of 40 μM ALLM for the indicated times. The heavy membrane was isolated, and alkali extraction was performed to determine the percentage of the alkali-resistant (integral, nonextractable) Bax contained in the mitochondrial membrane as described in “Materials and methods.” Intermembrane cytochrome c was used as extractable positive control, and inner membrane prohibitin was used as nonextractable negative control.
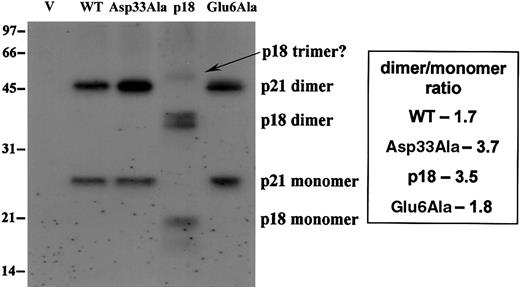
N-terminal cleavage or Asp33Ala mutation appears to enhance homo-oligomerization of Bax in mitochondrial membranes
Alkali extraction indicates that WT, Asp33Ala, p18, and Glu6Ala Bax behave similarly with respect to mitochondrial targeting and membrane insertion. Therefore, cross-linking studies were performed to assess their ability to oligomerize in the mitochondria. Results indicate that a significantly increased amount of Asp33Ala dimers is observed in mitochondria compared with the other Bax forms. Perhaps more importantly, the dimmer-monomer ratio is apparently increased, from about 1.7:1 to 1.8:1 for WT and Glu6Ala Bax to about 3.5:1 to 3.7:1 for p18 and Asp33Ala Bax (Figure 8). We also noted the formation of potential trimers for p18 Bax. These results indicate that both p18 and Asp33Ala Bax may be more efficient at undergoing dimerization or oligomerization in the outer mitochondrial membranes. This could explain their increased proapoptotic potency.
N-terminal cleavage or Asp33Ala mutation appears to enhance homooligomerization of Bax in mitochondrial membranes. Inducible Bax expression was performed in the presence of 40 μM ALLM for 24 hours. The heavy membrane was collected, and cross-linking was performed as described in “Materials and methods.” Then, membranes were lysed and 40μg lysate was analyzed by SDS-PAGE and Western blots with anti-T7 tag antibody.
N-terminal cleavage or Asp33Ala mutation appears to enhance homooligomerization of Bax in mitochondrial membranes. Inducible Bax expression was performed in the presence of 40 μM ALLM for 24 hours. The heavy membrane was collected, and cross-linking was performed as described in “Materials and methods.” Then, membranes were lysed and 40μg lysate was analyzed by SDS-PAGE and Western blots with anti-T7 tag antibody.
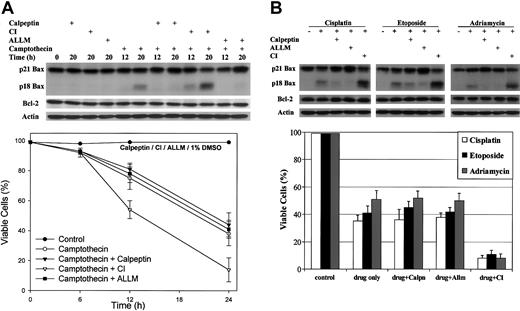
Cathepsin inhibitor I (CI) amplifies drug-induced apoptosis in HL-60 cells
Calpain-mediated cleavage of Bax to p18 Bax was initially observed in HL-60 cells undergoing aopotosis.22
Our results indicate that p18 Bax is more potent than WT Bax to induce apoptosis. However, due to its shorter half-life, the potency of p18 Bax cannot be clearly observed until p18 Bax degradation is inhibited. Because CI can inhibit p18 Bax degradation in 293 cells, we tested the effect of CI, ALLM, and calpeptin on HL-60 cells treated with various cytotoxic drugs. As previously shown, Bax is cleaved to p18 Bax following camptothecin treatment. The presence of CI significantly and specifically increases p18 Bax level but has no effect on Bcl-2 and actin levels (Figure 9A, upper panel). Under these conditions, camptothecin-induced cell death is amplified by more than 25% (Figure 9A, lower panel). Similar results are obtained when HL-60 cells are treated with other antineoplastic agents, including cisplatin, etoposide, or adriamycin. In all cases, CI significantly increases p18 Bax level and amplifies apoptosis by about 25% to 40% (Figure 9B). However, the calpain inhibitor, calpeptin or ALLM, blocks Bax cleavage but has no significant effect on the rate of apoptosis (Figure 9A). The proteasome inhibitor, lactacystin, used as a control, was found to have no significant effect on either p18 Bax level or apoptosis (data not shown), suggesting that the proteasome is not involved in p18 Bax degradation.
Cathepsin inhibitor I (CI) amplifies drug-induced apoptosis in HL-60 cells. (A) HL-60 cells were treated with 2 μM camptothecin only or in the presence of 20 μM calpeptin (calpain inhibitor), or 20 μM cathepsin inhibitor I (CI) or 20 μM ALLM (inhibits calpain and cathepsin) for the indicated times. (B) HL-60 cells were treated with 20 μM cisplatin, or 50 μM etoposide or 0.5 μg/mL adriamycin alone for 20 hours or in the presence of inhibitor. Control cells were left untreated or treated with either the same dose of inhibitor only or a corresponding volume of dimethyl sulfoxide (DMSO). Proteins were extracted and subjected to SDS-PAGE and Western blotting with human-specific anti-Bax antibody, anti-Bcl-2 antibody, and anti-actin antibody for loading control. Cell viabilities were measured by trypan blue exclusion method. Error bars indicate means ± SD (n = 3).
Cathepsin inhibitor I (CI) amplifies drug-induced apoptosis in HL-60 cells. (A) HL-60 cells were treated with 2 μM camptothecin only or in the presence of 20 μM calpeptin (calpain inhibitor), or 20 μM cathepsin inhibitor I (CI) or 20 μM ALLM (inhibits calpain and cathepsin) for the indicated times. (B) HL-60 cells were treated with 20 μM cisplatin, or 50 μM etoposide or 0.5 μg/mL adriamycin alone for 20 hours or in the presence of inhibitor. Control cells were left untreated or treated with either the same dose of inhibitor only or a corresponding volume of dimethyl sulfoxide (DMSO). Proteins were extracted and subjected to SDS-PAGE and Western blotting with human-specific anti-Bax antibody, anti-Bcl-2 antibody, and anti-actin antibody for loading control. Cell viabilities were measured by trypan blue exclusion method. Error bars indicate means ± SD (n = 3).
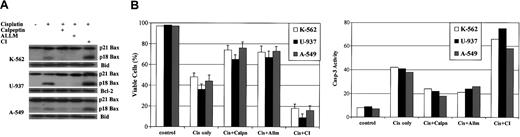
Calpain inhibitors block, while cathepsin inhibitor I (CI) amplifies, drug-induced apoptosis in A-549, K-562, and U-937 cells
When studies are extended to 3 other human tumor cell lines, A-549, K-562, and U-937, results reveal that addition of calpeptin or ALLM also efficiently blocks cleavage of Bax to p18 Bax and significantly inhibits cisplatin-induced apoptosis by 25% to 35% (Figure 10). By contrast, addition of CI significantly increases p18 Bax level and amplifies cisplatin-induced apoptosis by nearly 30% (Figure 10). Similar results are also obtained when etoposide or adriamycin is used (data not shown). Because no significant change has been noted for either Bcl-2 or Bid expression during these studies, the effect on p18 Bax expression appears to be specific. Therefore, these data confirm that p18 Bax expression potently accelerates the apoptotic process following cytotoxic stress.
Calpain inhibitors block while cathepsin inhibitor I (CI) amplifies drug-induced apoptosis in A-549, K-562, and U-937 cells. K-562, U-937, and A-549 cells were treated with 20 μM cisplatin alone or in the presence of 20 μM calpeptin (calpain inhibitor) or 40 μM CI or 20 μM ALLM for 36 hours. Control cells were left untreated or treated with either the same dose of inhibitor only or a corresponding volume of DMSO. (A) Proteins were extracted and subjected to SDS-PAGE and Western blotting with human-specific anti-Bax, anti-Bcl-2, and anti-Bid antibodies. (B) Cell viabilities were measured by the trypan blue exclusion method. Caspase-3 activity was measured as described in “Materials and methods” at 24 hours following the treatments to confirm the viability results. Error bars indicate means ± SD (n = 3).
Calpain inhibitors block while cathepsin inhibitor I (CI) amplifies drug-induced apoptosis in A-549, K-562, and U-937 cells. K-562, U-937, and A-549 cells were treated with 20 μM cisplatin alone or in the presence of 20 μM calpeptin (calpain inhibitor) or 40 μM CI or 20 μM ALLM for 36 hours. Control cells were left untreated or treated with either the same dose of inhibitor only or a corresponding volume of DMSO. (A) Proteins were extracted and subjected to SDS-PAGE and Western blotting with human-specific anti-Bax, anti-Bcl-2, and anti-Bid antibodies. (B) Cell viabilities were measured by the trypan blue exclusion method. Caspase-3 activity was measured as described in “Materials and methods” at 24 hours following the treatments to confirm the viability results. Error bars indicate means ± SD (n = 3).
Discussion
In addition to caspases, other cysteine proteases such as calpain and cathepsin have been implicated in apoptosis.24,51 For example, during drug-induced apoptosis Bax is cleaved by calpain at Asp33 to yield p18 Bax in cells from patients with chronic lymphocytic leukemia.22,23 In an attempt to sort out any physiological function for p18 Bax, our studies employed a conditional expression system to compare a noncleavable Asp33Ala Bax and an N-terminal-deleted p18 (Δ1-33) Bax with WT Bax. Results indicate that p18 Bax and Asp33Ala Bax are equivalent but more potent than WT Bax in disrupting mitochondrial integrity and inducing apoptosis. Because Asp33Ala Bax is not a recognized mutant that occurs in cancers expressing Bax mutations, it may not be physiologically relevant despite its surprisingly enhanced toxicity. However, Asp33Ala Bax may be a helpful tool for dissecting the molecular mechanism of p18 Bax formation and function. Results suggest that Asp33Ala mutation, as well as the N-terminal cleavage from Asp33, may facilitate enhanced oligomerization of Bax in mitochondrial membranes (Figure 8). These results have led us to speculate that the removal of the N-terminal 33 amino acids from Bax may expose the hydrophobic BH3 domain (amino acids 59 to 73), which is required for proapoptotic function and is also reported to be involved in dimer formation.8 Such a conformational change may facilitate Bax oligomerization and potential formation of death pores in the outer mitochondrial membranes that leak cytochrome c into the cytosol to initiate caspase activation (Figure 11). However, additional structural studies will be needed to test this hypothesis. As for the Glu6Ala mutant, it is functionally similar to WT Bax and does not affect the generation of p18 or p19 Bax, indicating that Glu6 is not a cleavage site and the physiological relevance of p19 Bax remains unclear.
Proposed model: calpain-mediated cleavage of Bax to p18 Bax accelerates stress-induced apoptosis, and a cathepsin-like cysteine protease may rapidly degrade p18 Bax. Although full-length p21 Bax can initiate apoptosis through the mitochondrial pathway, p18 Bax is more potent than WT p21 Bax in disrupting mitochondrial integrity and inducing apoptosis. Therefore, calpain-mediated cleavage of Bax to p18 Bax functions as an amplification step that accelerates the apoptotic process following cytotoxic stress. On the other hand, removal of the N-terminal domain of Bax facilitates the rapid degradation of p18 Bax by a cathepsin-like cysteine protease. This specific proteolytic degradation of p18 Bax may be equivalent to a silencing mechanism for this potent form of Bax to delay cell death or promote survival. Thus, in addition to the executionary consequences of caspase activation, noncaspase cysteine proteases such as calpain and the cathepsin-like protease may be involved in regulating apoptosis through sequential proteolytic processing of Bax and p18 Bax.
Proposed model: calpain-mediated cleavage of Bax to p18 Bax accelerates stress-induced apoptosis, and a cathepsin-like cysteine protease may rapidly degrade p18 Bax. Although full-length p21 Bax can initiate apoptosis through the mitochondrial pathway, p18 Bax is more potent than WT p21 Bax in disrupting mitochondrial integrity and inducing apoptosis. Therefore, calpain-mediated cleavage of Bax to p18 Bax functions as an amplification step that accelerates the apoptotic process following cytotoxic stress. On the other hand, removal of the N-terminal domain of Bax facilitates the rapid degradation of p18 Bax by a cathepsin-like cysteine protease. This specific proteolytic degradation of p18 Bax may be equivalent to a silencing mechanism for this potent form of Bax to delay cell death or promote survival. Thus, in addition to the executionary consequences of caspase activation, noncaspase cysteine proteases such as calpain and the cathepsin-like protease may be involved in regulating apoptosis through sequential proteolytic processing of Bax and p18 Bax.
p18 Bax appears to be universally formed in tumor cells undergoing apoptosis induced by various stresses (Figure 1).19-21 Results indicate that although full-length Bax can initiate the mitochondrial apoptotic pathway, p18 Bax is more potent in disrupting mitochondrial integrity. Therefore, we propose that calpain-mediated cleavage of Bax to p18 Bax may represent an amplification step that can accelerate the apoptotic process following cytotoxic stress (Figure 11). In support of this notion, calpain activation has been reported to occur early and is upstream of caspase activation following irradiation, etoposide, or cisplatin treatment.52-54 Under these situations, we have now found that the calpain inhibitors calpeptin and ALLM can block cleavage of Bax to p18 Bax and retard drug-induced apoptosis by about 30% in A-549, K-562, and U-937 cells (Figure 10). This protective effect of calpain inhibitors may appear difficult to reconcile with the findings from the inducible Bax expression in 293 cells. In that case, ALLM apparently increases the half-life of the induced Bax proteins (Figure 4), resulting in increased (about 50% to 100%) accumulation of the induced Bax (Figure 2C), and enhances Bax-induced apoptosis by 15% to 25% (Figure 2B,E). However, these results are not contradictory. Endogenous Bax levels are initially higher in A-549, K-562, and U-937 cells than in 293 cells (Figure 3B), and the relatively higher level of endogenous Bax alone does not trigger apoptosis prior to drug treatment.
Although calpain inhibitors can also result in increased (about 50% to 100%) accumulation of endogenous Bax in these cells (Figures 9 and 10A), it is not sufficient to accelerate cell death. That is, the amount of endogenous Bax increased in these cells is not comparable to that of the induced Bax in 293 cells, in which the primary or even sole cause of death is the forced overexpression of Bax to levels that are more than 5 to 12 times higher than that of endogenous Bax of any of the cell lines tested (Figure 3). Importantly, significant cell death can only be observed in 293 cells after 12 hours (Figure 2B,E), a time when the induced Bax reaches a “threshold” level (about 10-fold that of endogenous Bax).
However, calpain activation in HL-60 cells may occur only subsequent to caspase activation at a late stage of drug-induced apoptosis.37 This could help explain why inhibition of calpain-mediated Bax cleavage fails to affect apoptosis in this particular cell line (Figure 9). Nevertheless, in all 4 tumor cell lines studied, inhibition of p18 Bax degradation by CI significantly enhances apoptosis regardless of the death stimuli applied (Figures 9 and 10). These findings strongly suggest that cleavage of Bax to p18 Bax accelerates stress-induced apoptosis. Interestingly, it has been reported that raloxifene or transforming growth factor-β 1 (TGF-β1) can induce apoptosis through N-terminal cleavage of Bad,55,56 indicating that other proapoptotic regulators may also undergo proteolytic processing. Thus, N-terminal cleavage of some proapoptotic Bcl-2 family members, including Bax, Bad, and Bid,4-6 may represent an activation/amplification signal in the regulation of apoptosis.
Calpain participates in various signaling pathways mediated by Ca2+ and may serve to modulate the activity and function of other proteins by making selective proteolytic cleavages.57 Although a large number of calpain substrates have been demonstrated in vitro,24 the precise physiological functions of calpain have not been clearly identified. Interestingly, calpain activity has been implicated in various aging phenomena, and abnormal activation of calpain occurs in Alzheimer disease.26,27 Therefore, the cleavage of Bax to p18 Bax with potential amplification of the intrinsic apoptosis may be one consequence of calpain activation in some pathologic states.
On the other hand, cleavage of the N-terminal 33 amino acids from Bax sensitizes the p18 Bax product for rapid degradation, which can be inhibited by the cathepsin inhibitors ALLM or CI (Figure 2C). This suggests a role for cathepsin-like protease(s) in p18 Bax degradation. Cathepsins have traditionally been viewed as lysosomal mediators of protein turnover.24 However, recent findings have expanded their role in other fundamental processes including apoptosis.51,58 For example, increased expression and activity of cathepsins have been observed in malignant progression of various cancers.29,30 Therefore, the selective degradation of p18 Bax by such a protease may be equivalent to a “silencing” mechanism for this potent form of Bax (Figure 11). Such a regulatory mechanism could serve to delay the cell death process in the event that calpain activation occurs inappropriately. Thus, we speculate that some cathepsin-like proteases may be involved in regulating the rate of apoptosis rather than simply mediating protein turnover. For example, during tumor invasion the rapid degradation of p18 Bax by the cathepsin-like protease might promote tumor cell survival during hypoxia stress, while other cathepsins may work to remodel extracellular matrix.29,30 These cooperative actions among such proteases may be essential for malignant progression. If there is a role for a cathepsin or a cathepsin-like protease in the metabolism of Bax and the regulation of apoptosis, therapeutic targeting with the intention to inhibit this protease and enhance p18 Bax expression may be a clinically useful strategy to augment cancer chemotherapy. This view is supported by the results indicating that CI can significantly amplify drug-induced apoptosis by inhibiting p18 Bax degradation (Figures 9 and 10). Although we cannot rule out the possibility that other proteases sensitive to ALLM and CI may be involved in p18 Bax degradation, these results suggest that a cathepsin-like protease is one candidate.
Taken together, our data suggest that calpain and a cathepsin-like protease may serve to regulate apoptosis through sequential proteolytic processing of Bax and p18 Bax. Thus, in addition to the executionary consequences of caspase activation, the apoptotic process may be regulated by other cysteine proteases such as calpain and cathepsin-like protease(s) whose aberrant activation and function may have pathologic consequences in tumorigenesis and neurodegenerative diseases.
Prepublished online as Blood First Edition Paper, June 19, 2003; DOI 10.1182/blood-2003-01-0211.
Supported by National Institutes of Health grant CA44649-11.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Mary Schleissing for her assistance in the caspase-3 activity assay and Dr Richard Bennett for his advice and critical reading of the manuscript.


![Figure 4. p18 Bax has a reduced half-life that can be enhanced by ALLM. (A) The half-life of WT and p18 Bax was first determined by [35S]methionine metabolic labeling and pulse-chase as described in “Materials and methods.” (B) The half-life of WT, Asp33Ala, p18, and Glu6Ala Bax was determined again using the cyclohexamide method as described in “Materials and methods.” (C) The effect of ALLM on Bax protein half-life was determined by cyclohexamide method. Half-life was determined by densitometry analysis.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/7/10.1182_blood-2003-01-0211/6/m_h81935030004.jpeg?Expires=1767769997&Signature=O6qqKHNfRVOp8sVr6sp-jp7y492S9PQb3CLRhnf8Osd9MFMdYl10oiBlRj7g5GBYQtO84jDYotbhs0zWFBrh-IIBIr0ZzAdyp4Na2E9m4L3CX8JYRZ1hMOIUF9Tw9QdvX9geEqlqw5KaGcfjLj1oHNZ4O9p6IOojthWLwaV6qSFohuc11NaXP4eIStBWXivN9FZvprx5kXXQwbdAhn-eydmuptn4wg8JLtSVRI3SVM3MyP7Yh8-Ez87W55LbK5h2gWRvSTnj3wAh36FlpknCdezJG8hkQM-sxWzCD8-Z0SBPeHD8yLy9ezdte~LAoi2QTc6gOt3OaJBwngyg~pb3MA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal