Abstract
An unbalanced translocation der(1;7)(q10; p10) is a nonrandom chromosomal aberration commonly observed in myelodysplastic syndrome and acute myeloid leukemia. We molecularly analyzed the breakpoints of der(1;7)(q10;p10) by quantitative fluorescent in situ hybridization (FISH) analyses using centromeric satellite DNAs mapped to chromosomes 1 and 7 as probes. We found that the signal intensities of 2 centromere alphoid probes, D1Z7 on chromosome 1 and D7Z1 on chromosome 7, were almost invariably reduced on the derivative chromosome compared with those on their normal counterparts. These results suggest that this translocation results from the recombination between the 2 alphoids, which was further confirmed by fiber FISH experiments. Because the relative reduction in the intensities of D1Z7 and D7Z1 signals on the derivative chromosomes was highly variable among patients, it was estimated that the breakpoints in these patients were randomly distributed over several megabase pairs within each alphoid cluster except for its extreme end to the short arm. Our results provide a novel insight into the structural basis for generation of this translocation as well as its leukemogenic roles. (Blood. 2003;102:2597-2604)
Introduction
The unbalanced whole-arm translocation of chromosome 1 (chr1) and chromosome 7 (chr7), der(1;7)(q10;p10), is a nonrandom chromosomal abnormality first described by Geraedts et al.1 It is commonly found in myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) and less frequently in myeloproliferative disorders, involving all subgroups of MDS and AML. Since more than half of the patients had a history of previous antitumor chemotherapy (especially containing alkylating agents) and/or radiotherapy or occupational exposure to toxic agents, it has been causally related to secondary or therapy-related MDS or AML.2-9 In MDS, as many as 6% of the patients are reported to have this karyotypic abnormality. They typically present trilineage morphologic myelodysplasia in the bone marrow and pancytopenia in the peripheral blood and have a high rate of progression to AML with generally poor prognosis.10,11
This unbalanced translocation, typically described as 46, XY (or XX), +1, der(1;7)(q10;p10), has 2 prominent cytogenetic characteristics: (1) it retains only 1 of 2 possible derivative chromosomes, containing 1q and 7p; and (2) there are 2 copies of apparently normal chr1 and only 1 copy of normal chr7, resulting in trisomy of 1q and monosomy of 7q (Figure 1). In the early fluorescent in situ hybridization (FISH) studies, several authors pointed out the dicentric nature of this translocation, in a sense that the signals of the 2 alphoids from chr1 and chr7 are colocalized on the derivative chromosome. Alitalo et al12 reported that FISH signals of D1Z5 and D7Z2 coexisted on the der(1;7)(q10;p10), and others13,14 also described that D1Z5 and D7Z1 cohybridized to the derivative chromosome in this translocation. However, no further molecular analysis, especially concerning exact locations of the breakpoints and their distribution, has been conducted to date.
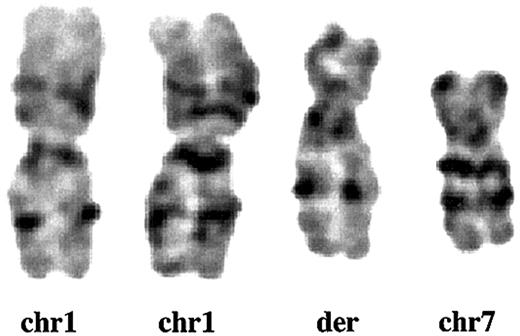
Partial karyotype of der (1;7)(q10;p10). GTG-banding analysis from Pt 1 shows that the whole long arm of chromosome 1 (chr1) and the whole short arm of chromosome 7 (chr7) fuse to constitute der(1;7)(q10;p10) (der).
Partial karyotype of der (1;7)(q10;p10). GTG-banding analysis from Pt 1 shows that the whole long arm of chromosome 1 (chr1) and the whole short arm of chromosome 7 (chr7) fuse to constitute der(1;7)(q10;p10) (der).
In this paper, in order to disclose the genomic mechanism generating der(1;7)(q10;p10), we focused on the centromere alphoid sequences on chr1 and chr7 and dissected the structural alterations associated with this translocation by the 2-color FISH method with simultaneous measurements of FISH signals.
Patients, materials, and methods
Clinical samples
A total of 27 bone marrow samples were collected from patients carrying der(1;7)(q10;p10) and subjected to FISH analysis. The patients' profiles are summarized in Table 1. All patients gave their informed consent to the sample collection and to the biologic analyses included in the present study according to the Declaration of Helsinki. Giemsa trypsin G-banding (GTG-banding) analysis was performed according to the standard protocol.15
Cytogenetic profile of patients
Patient . | Age, y/sex . | Diagnosis . | Karyotype . |
|---|---|---|---|
| 1 | 45/M | MDS (RA) | 46,XY, + 1,der(1;7) (q10;p10), add(10)(q?)[20] |
| 2 | 74/M | MDS (RA) | 46,XY, + 1,der(1;7) (q10;p10)[18] |
| 3 | 67/M | MDS (RA) | 46,XY, + 1,der(1;7) (q10;p10)[9]/46, XY[6] |
| 4 | 74/M | MDS (RA) | 46,XY, + 1,der(1;7) (q10;p10), del(20)(q11)[12]/45, idem,−20[4] |
| 5 | 66/M | MDS, MF | 46,XY, + 1,der(1;7) (q10;p10)[5]/47, idem, + 8[13] |
| 6* | 58/M | MDS (RA) | 46,XY, + 1,der(1;7) (q10;p10)[3]/46, XY[18] |
| 7* | 53/F | MDS | 46,XX, + 1,der(1;7) (q10;p10)[12]/46,X,−X, + 1,der(1;7)(q10;p10), + der(1;7)(q10;p10), −2, +5, +6, −8, −12, +14, −16, −17, +18, +21[1]/46, XX[7] |
| 8* | 70/M | MDS (RA) | 46,XY, + 1,der(1;7) (q10;p10)[18]/46, XY[2] |
| 9* | 67/M | MDS (RA) | 46,XY, + 1,der(1;7) (q10;p10)[1]/47, idem, + 82/20/46, idem, del(20)(q11)[2]/47, idem, + 8, del(20)(q11)[15] |
| 10 | 51/M | MDS (RAEB) | 46,XY, + 1,der(1;7) (q10;p10)[19]/45, X,−Y, idem[1] |
| 11 | 53/M | MDS | 46,XY, + 1,der(1;7) (q10;p10)[20] |
| 12 | 68/M | MDS | 46,XY, + 1,der(1;7) (q10;p10)[10]/46, XY[10] |
| 13 | 49/M | MDS (RA) | 46,XY, + 1,der(1;7) (q10;p10)[12]/46, XY[8] |
| 14 | 49/M | AML (M0) | 46,XY, + 1,der(1;7) (q10;p10)[20] |
| 15* | 68/M | AML (M2) | 46,XY, + 1,der(1;7) (q10;p10)[5]/47, idem, + 8[16] |
| 16 | 88/M | MDS (RA) | 47,XY, + 1,der(1;7) (q10;p10), del(20)(q?), del(20)[9]/46, XY[10] |
| 17 | 56/M | MDS (RA) | 46,XY, + 1,der(1;7) (q10;p10)[18]/46, XY[4] |
| 18* | 47/M | MF | 46,XY, + 1,der(1;7) (q10;p10)[16]/46, XY[2] |
| 19 | 58/M | MDS (RA) | 46,XY, + 1,der(1;7) (q10;p10)[3]/47, idem, + 8[16]/46, XY[2] |
| 20* | 74/M | MDS (RA) | 46,XY, + 1,der(1;7) (q10;p10)[13]/46, XY[1] |
| 21* | 68/M | MDS (RAEB-t) | 46,XY, + 1,der(1;7) (q10;p10)[14]/46, XY[6] |
| 22 | 54/M | MDS (RA) | 46,XY, + 1,der(1;7) (q10;p10)[2]/46, XY[18] |
| 23 | 72/M | MF | 46,XY, + 1,der(1;7) (q10;p10)[1]/46, XY,del(13)(q10q21)[4]/46, XY[14] |
| 24* | 57/M | MM | 46,XY, + 1,der(1;7) (q10;p10)[5]/46, XY[16] |
| 25* | 70/M | MDS (RA) | 46,XY, + 1,der(1;7) (q10;p10)[9] |
| 26 | 72/M | AML (M0) | 46,XY, + 1,der(1;7) (q10;p10)[17]/45, idem, −14[1]/45, idem, −21[1]/46, XY[1] |
| 27 | 58/M | MDS/AML | 47,XY, + 1,der(1;7) (q10;p10), + 8[4]/47, idem, del (20)(q11)[1]/46, XY[1] |
Patient . | Age, y/sex . | Diagnosis . | Karyotype . |
|---|---|---|---|
| 1 | 45/M | MDS (RA) | 46,XY, + 1,der(1;7) (q10;p10), add(10)(q?)[20] |
| 2 | 74/M | MDS (RA) | 46,XY, + 1,der(1;7) (q10;p10)[18] |
| 3 | 67/M | MDS (RA) | 46,XY, + 1,der(1;7) (q10;p10)[9]/46, XY[6] |
| 4 | 74/M | MDS (RA) | 46,XY, + 1,der(1;7) (q10;p10), del(20)(q11)[12]/45, idem,−20[4] |
| 5 | 66/M | MDS, MF | 46,XY, + 1,der(1;7) (q10;p10)[5]/47, idem, + 8[13] |
| 6* | 58/M | MDS (RA) | 46,XY, + 1,der(1;7) (q10;p10)[3]/46, XY[18] |
| 7* | 53/F | MDS | 46,XX, + 1,der(1;7) (q10;p10)[12]/46,X,−X, + 1,der(1;7)(q10;p10), + der(1;7)(q10;p10), −2, +5, +6, −8, −12, +14, −16, −17, +18, +21[1]/46, XX[7] |
| 8* | 70/M | MDS (RA) | 46,XY, + 1,der(1;7) (q10;p10)[18]/46, XY[2] |
| 9* | 67/M | MDS (RA) | 46,XY, + 1,der(1;7) (q10;p10)[1]/47, idem, + 82/20/46, idem, del(20)(q11)[2]/47, idem, + 8, del(20)(q11)[15] |
| 10 | 51/M | MDS (RAEB) | 46,XY, + 1,der(1;7) (q10;p10)[19]/45, X,−Y, idem[1] |
| 11 | 53/M | MDS | 46,XY, + 1,der(1;7) (q10;p10)[20] |
| 12 | 68/M | MDS | 46,XY, + 1,der(1;7) (q10;p10)[10]/46, XY[10] |
| 13 | 49/M | MDS (RA) | 46,XY, + 1,der(1;7) (q10;p10)[12]/46, XY[8] |
| 14 | 49/M | AML (M0) | 46,XY, + 1,der(1;7) (q10;p10)[20] |
| 15* | 68/M | AML (M2) | 46,XY, + 1,der(1;7) (q10;p10)[5]/47, idem, + 8[16] |
| 16 | 88/M | MDS (RA) | 47,XY, + 1,der(1;7) (q10;p10), del(20)(q?), del(20)[9]/46, XY[10] |
| 17 | 56/M | MDS (RA) | 46,XY, + 1,der(1;7) (q10;p10)[18]/46, XY[4] |
| 18* | 47/M | MF | 46,XY, + 1,der(1;7) (q10;p10)[16]/46, XY[2] |
| 19 | 58/M | MDS (RA) | 46,XY, + 1,der(1;7) (q10;p10)[3]/47, idem, + 8[16]/46, XY[2] |
| 20* | 74/M | MDS (RA) | 46,XY, + 1,der(1;7) (q10;p10)[13]/46, XY[1] |
| 21* | 68/M | MDS (RAEB-t) | 46,XY, + 1,der(1;7) (q10;p10)[14]/46, XY[6] |
| 22 | 54/M | MDS (RA) | 46,XY, + 1,der(1;7) (q10;p10)[2]/46, XY[18] |
| 23 | 72/M | MF | 46,XY, + 1,der(1;7) (q10;p10)[1]/46, XY,del(13)(q10q21)[4]/46, XY[14] |
| 24* | 57/M | MM | 46,XY, + 1,der(1;7) (q10;p10)[5]/46, XY[16] |
| 25* | 70/M | MDS (RA) | 46,XY, + 1,der(1;7) (q10;p10)[9] |
| 26 | 72/M | AML (M0) | 46,XY, + 1,der(1;7) (q10;p10)[17]/45, idem, −14[1]/45, idem, −21[1]/46, XY[1] |
| 27 | 58/M | MDS/AML | 47,XY, + 1,der(1;7) (q10;p10), + 8[4]/47, idem, del (20)(q11)[1]/46, XY[1] |
RA indicates refractory anemia; MF, myelofibrosis; RAEB, refractory anemia with excess blasts; M0, minimally differentiated acute myeloblastic leukemia; M2, acute myeloblastic leukemia with maturation; RAEB-t, refractory anemia with excess blasts in transformation; and MM, multiple myeloma.
Therapy-related cases.
DNA probes
The screening of bacterial artificial chromosome (BAC) or P1-based artificial chromosome (PAC) clones was previously described.16 DNA from BACs or PACs was extracted using the Large-Construct Kit (QIAGEN, Tokyo, Japan). A D1Z7 clone, pE25.a, was purchased from American Type Culture Collection (ATCC; Manassas, VA). A clone of D7Z1, called D7Z16mer, was obtained from polymerase chain reaction (PCR) amplification with primers spanning the dimer and the tetramer of the published D7Z1 sequences (National Center for Biotechnology Information [NCBI] accession: M16087, M16101). The sequences of the primers were 5′-TCCACTTGCAAATTCCACAA-3′ and 5′-TGGATATATGGACCGCATTG-3′. The probes of satellite III (SatIII) and D7Z2 were also generated by PCR. Primers for SatIII (GenBank database X60726) were 5′-TCCATTCCAGTCCATTCGAT-3′ and 5′-AATCATCATCCAACGGAAGC-3′ and those for D7Z2 (NCBI accession: G31642) were 5′-CTGGAGGCGGATATTAGGGT-3′ and 5′-CTGGGAATACTTCTGTCTAT-3′. All the plasmid DNAs were extracted using QIAGEN Plasmid Maxi Kit (QIAGEN). Three directly labeled probes, CEP 1/5 (Spectrum Orange), CEP 1 (Spectrum Orange), and CEP 7 (Spectrum Green), which detect the sequences of D1Z7, D1Z5, and D7Z1, respectively, were purchased from VYSIS (Downers Grove, IL).
FISH analysis
FISH experiments on metaphase chromosomes and interphase nuclei were performed as described17 with some modifications in posthybridization washes, which consisted of 2 × standard saline citrate (SSC)/50% formamide at 37°C for 15 minutes followed by 2 × SSC and 1 × SSC at room temperature for 15 minutes each. Biotin- and digoxigenin-labeled probes were detected with avidin-fluorescein isothiocyanate (FITC; Roche, Mann-heim, Germany) and anti-digoxigenin-rhodamine Fab fragments (Roche), respectively. For fiber FISH experiments, the released chromatin fibers were prepared as described18 and hybridized with 2 directly labeled probes, CEP 1/5 and CEP 7, following the procedures recommended by the manufacturer. Samples were examined with a Nikon E800 epi-fluorescence microscope (Tokyo, Japan) at × 1000 magnification, and the FISH images were captured to 8-bit-depth image files using a Kodak KAF1400 thermoelectronically cooled charge-coupled device (CCD) camera (Roper Scientific, Tucson, AZ) and the SimpleVision software (Digital Scientific, Cambridge, United Kingdom) through a triple-band-pass filter, which allowed rhodamine, FITC, and DAPI (4,6 diamidino-2-phenylindole) signals to be captured without any image displacement. For each experiment, more than 30 metaphase images taken under exact focus conditions were stored. The whole intensity of each signal was automatically computed as the sum of pixel intensities under the indicated area by using the IP lab/PathVysion Extras software (Scanalytics, Fairfax, VA), and finally the average was taken from the 30 measurements together with calculation of the standard deviation. The allelic origin of the 7p and 1q components of the der(1;7)(q10;p10) was determined by comparing the signal intensity of polymorphic satellite elements on chr1 (SatIII) and on chr7 (D7Z2), which were safely apart from the breakpoints and their sequences were expected to be completely reserved on the der(1;7)(q10; p10) in tumor cells.
Southern blot analysis
Neo1 and An4, Neo7 and NT18, Neo2, Neo16, and Neo19, were monochromosomal human-mouse hybrid cell lines containing human chromosome 1, 7, 2, 16, and 19, respectively, and were purchased from the JCRB Cell Bank (Japanese Collection of Research Bioresources, Osaka, Japan). Genomic DNAs were extracted from the cell lines19 and subjected to Southern blot analysis essentially as described.20 High-stringency hybridization was done at 53°C for 16 to 18 hours in hybridization solution containing 50% formamide, 5 × SSC, and 1 × Denhaldt solution. The final wash was performed at 65°C in 0.1 × SSC/0.1% sodium dodecyl sulfate (SDS) for 20 minutes.
Results
Breakpoint mapping of der(1;7)(q10;p10) by FISH
Initially, in order to map the breakpoint of der(1;7)(q10;p10), we repeatedly performed FISH experiments using a series of probes already mapped to, or newly isolated from, the pericentric regions of chr1 and chr7. Finally, the breakpoints were mapped between D1Z1 and D1S3445 on chr1 and between D7Z2 and sWSS295 on chr7, when no more BAC probes could be obtained to extend the contigs because of a heavy load of repetitive sequences around these regions (data not shown). It was noted, however, that the FISH signals of D1Z7 (probe pE25.a) on the derivative chromosome always looked weaker compared with those on normal chr1s in most cases, raising a possibility that the breakpoints on chr1 might be within the D1Z7 alphoid region. On the other hand, several BAC clones mapped to the pericentromeric region of chr7 seemed to produce a reduced FISH signal on the derivative chromosome when compared with the signal on the normal chr7 in tumor cells. Subsequent database analyses and Southern blot experiments revealed that these BAC clones characteristically had varying contents of D7Z1 alphoid sequences (data not shown), suggesting that the breakpoint on chr7 might be associated with the D7Z1 alphoid cluster.
Identification of the allelic chr1 and chr7 counterparts of the derivative chromosome
Both D1Z7 and D7Z1 belong to the centromere alpha satellite DNAs (or alphoid DNA), which are characterized by a large block of tandem repeats (a subset) spanning from 250 kilobase (kb) to 5000 kb, depending on chromosomes.19,21-24 Each alphoid subset is composed of highly ordered repeats of multimers, which consist of homologous 171-base pair (bp) monomer units. According to their sequence homology and array structure, alphoid subsets can be divided into 5 different suprachromosomal families.21 Because alphoid DNAs typically show extensive polymorphism regarding the number of their tandem repeats,25-27 we had to determine the exact allelic origin of the derivative chromosome before properly evaluating the reduction of the D1Z7 and D7Z1 signals on this chromosome, as the intensity of these FISH signals directly depends on their cluster length.
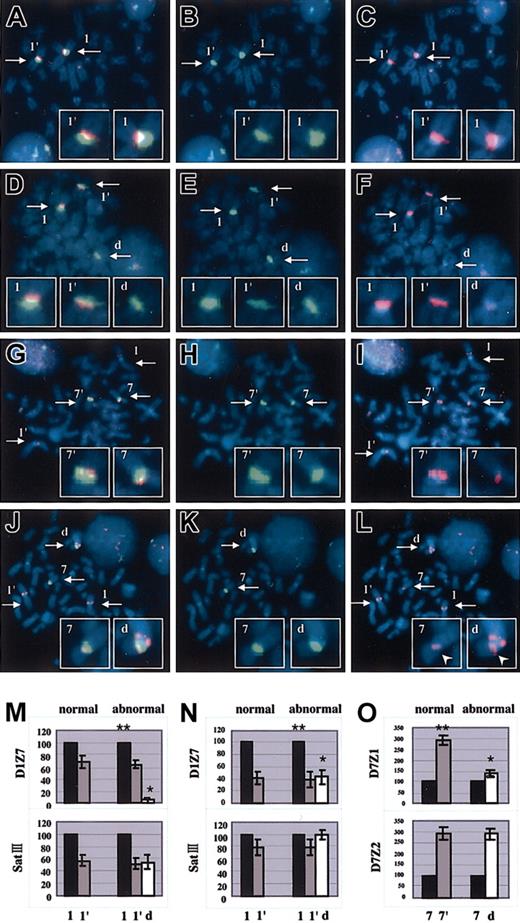
To this end, we used the other 2 repetitive sequences as the allelic references, SatIII on 1q11 to q1221,28,29 and D7Z2 on the short-arm side of D7Z1 on chr7,30,31 which also exhibit a prominent allelic polymorphism. When FISH was performed using D1Z7 and SatIII probes, as shown in Figure 2A-F and 2M, the origins of the 2 homologous chr1s could be distinguished by the relative intensity of their SatIII signals, which were uniform among all the metaphases from normal as well as abnormal (tumor) cells (Table 2), indicating that the 2 apparently normal chr1s in tumor cells have different allelic origins and did not result from duplication of either of the 2. Of note is that the SatIII signal on the derivative chromosome always had the same intensity as either of the 2 SatIII signals on the normal chr1s. Similarly, using D7Z2 probes, relative intensities of the 2 D7Z2 signals in abnormal and, if existed, normal metaphases were also uniform (Figure 2G-L,O; Table 3), showing that the short-arm portion of the derivative chromosome and the remaining normal chr7 had different allelic origins. Given these measurements, we could determine the origins of chr1 and chr7 portions of the derivative chromosome using SatIII and D7Z2 as allelic markers, respectively, and thus quantitatively evaluate the alteration of the signal intensities on the derivative chromosome on an allelic basis.
Representative results of 2-color FISH experiments and comparison of signal intensities in centromere alphoids. Two-color FISH was performed with SatIII (green) and D1Z7 (red) probes for normal (A-C) and abnormal (D-F) metaphases in Pt 6 and with D7Z2 (red), 97G24 (red), and D7Z1 (green) probes for normal (G-I) and abnormal (J-L) metaphases in Pt 16. 97G24 is a PAC probe located on 1q13 that helps detect trisomy of 1q. Images for FITC (B,E,H,K), Rhodamin (C,F,I,L), and both (A,D,G,J) were separately captured through a single triple-band-pass filter with an appropriate first-pass filter using a synchronized wheel filter device. 1 and 1′, 7 and 7′, and d indicate different alleles of chr1, chr7, and derivative chromosome, respectively. Captured images were subjected to measurement of signal intensities. Original magnification, × 1000. Intensity of the D1Z7 signal on derivative chromosome (*) is grossly reduced from its original intensity (**), as determined using SatIII intensity as a reference in Pt 6 (M) and Pt 8 (N). Likewise, D7Z1 signal on derivative chromosome (*) is also shown to decrease when compared with its original signal (**), as determined using D7Z2 intensity in Pt 16 (O). Arrows in panels A-L indicate the centromeres of chromosome 1, chromosome 7, or the derivative chromosome. Arrowheads in panel L point to the D7Z2 signals in order to distinguish these signals from the red signals of 97G24. Error bars represent the SD from 30 measurements in 1 patient.
Representative results of 2-color FISH experiments and comparison of signal intensities in centromere alphoids. Two-color FISH was performed with SatIII (green) and D1Z7 (red) probes for normal (A-C) and abnormal (D-F) metaphases in Pt 6 and with D7Z2 (red), 97G24 (red), and D7Z1 (green) probes for normal (G-I) and abnormal (J-L) metaphases in Pt 16. 97G24 is a PAC probe located on 1q13 that helps detect trisomy of 1q. Images for FITC (B,E,H,K), Rhodamin (C,F,I,L), and both (A,D,G,J) were separately captured through a single triple-band-pass filter with an appropriate first-pass filter using a synchronized wheel filter device. 1 and 1′, 7 and 7′, and d indicate different alleles of chr1, chr7, and derivative chromosome, respectively. Captured images were subjected to measurement of signal intensities. Original magnification, × 1000. Intensity of the D1Z7 signal on derivative chromosome (*) is grossly reduced from its original intensity (**), as determined using SatIII intensity as a reference in Pt 6 (M) and Pt 8 (N). Likewise, D7Z1 signal on derivative chromosome (*) is also shown to decrease when compared with its original signal (**), as determined using D7Z2 intensity in Pt 16 (O). Arrows in panels A-L indicate the centromeres of chromosome 1, chromosome 7, or the derivative chromosome. Arrowheads in panel L point to the D7Z2 signals in order to distinguish these signals from the red signals of 97G24. Error bars represent the SD from 30 measurements in 1 patient.
Relative intensities of D1Z7 and Satlll signals on the derivative chromosome and its allelic equivalent
. | D1Z7 . | . | . | Satlll . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | Normal cells . | Tumor cells . | . | Normal cells . | Tumor cells . | . | ||||
| Patient . | chr1* . | chr1* . | der . | chr1* . | chr1* . | der . | ||||
| 1 | ND | 124.9 (18.5) | 3.8 (2.6) | ND | 166.6 (64.2) | 168.2 (59.5) | ||||
| 2 | ND | 80.8 (9.5) | 17.5 (5.5) | ND | 50.2 (17.4) | 48.9 (11.5) | ||||
| 3 | 190.0 (27.3) | 188.3 (24.5) | 37.9 (10.7) | 57.9 (8.8) | 57.2 (10.8) | 56.0 (10.3) | ||||
| 4 | ND | 127.7 (17.6) | 15.9 (7.9) | ND | 41.3 (8.7) | 41.8 (10.2) | ||||
| 5 | ND | 128.0 (19.1) | 7.0 (4.7) | ND | 68.7 (13.3) | 68.3 (12.6) | ||||
| 6 | 68.5 (9.2) | 64.3 (7.8) | 5.7 (3.3) | 56.3 (9.0) | 51.5 (8.4) | 53.7 (11.4) | ||||
| 7 | 163.4 (24.5) | 158.2 (39.0) | 28.8 (14.7) | 46.7 (5.3) | 45.9 (9.2) | 46.1 (7.6) | ||||
| 8 | 225.5 (46.9) | 233.8 (57.2) | 104.9 (37.0) | 129.9 (23.4) | 131.9 (25.4) | 130.1 (22.0) | ||||
| 9 | ND | 75.6 (8.3) | 43.5 (7.0) | ND | 65.0 (4.8) | 69.0 (6.3) | ||||
| 10 | ND | 191.6 (41.2) | 31.3 (12.9) | ND | 48.1 (6.6) | 48.9 (7.7) | ||||
| 11 | ND | 64.3 (9.6) | 37.4 (8.9) | ND | 266.5 (59) | 265.6 (52.2) | ||||
| 12 | 124.7 (14.8) | 122.2 (14.5) | 15.6 (4.2) | 52.3 (6.5) | 49.5 (6.5) | 49.0 (8.7) | ||||
| 13 | 152.6 (15.8) | 149.2 (12.4) | 51.7 (9.3) | 171.5 (24.4) | 174.1 (30.6) | 176.1 (30.5) | ||||
| 14 | ND | 140.2 (13.1) | 50.7 (7.7) | ND | 21.2 (4.6) | 19.3 (7.1) | ||||
| 15 | ND | 149.5 (13.3) | 56.6 (9.7) | ND | 269.3 (43.3) | 270.1 (38.0) | ||||
| 16 | 78.2 (6.1) | 78.1 (8.4) | 33.3 (8.3) | 167.5 (11.6) | 169.3 (18.5) | 170.4 (18.1) | ||||
| 17 | 130.8 (9.5) | 129.2 (9.8) | 8.4 (2.7) | 75.2 (6.5) | 72.1 (7.1) | 72.1 (6.8) | ||||
| 18 | ND | 70.7 (7.1) | 10.2 (7.0) | ND | 63.2 (8.2) | 63.2 (8.0) | ||||
| 19 | ND | 149.7 (14.9) | 24.8 (6.6) | ND | 60.9 (10.3) | 62.1 (9.8) | ||||
| 20 | 64.7 (9.1) | 65.3 (6.5) | 33.4 (3.7) | 45.3 (7.0) | 43.4 (6.5) | 43.1 (8.4) | ||||
| 21 | 153.6 (8.7) | 158.5 (18.1) | 69.1 (10.0) | 51.7 (4.2) | 50.8 (10.0) | 50.8 (9.3) | ||||
| 22 | 149.4 (10.8) | 143.4 (19.0) | 31.3 (12.6) | 20.8 (2.8) | 21.7 (3.6) | 20.6 (4.7) | ||||
| 23 | 185.0 (10.7) | 179.0 (17.5) | 45.3 (13.9) | 138.9 (6.5) | 137.1 (13.1) | 136.7 (17.1) | ||||
| 24 | 137.4 (6.1) | 142.8 (13.0) | 58.5 (14.2) | 34.9 (2.8) | 35.2 (4.4) | 35.8 (6.3) | ||||
| 25 | ND | 160.5 (16.0) | 51.6 (7.9) | ND | 52.9 (7.5) | 52.6 (6.6) | ||||
| 26 | ND | 192.9 (8.7) | 22.7 (6.0) | ND | 22.0 (1.8) | 22.7 (2.7) | ||||
| 27 | 52.9 (4.9) | 53.8 (5.5) | 0 | 45.7 (3.1) | 45.1 (6.1) | 47.4 (6.2) | ||||
. | D1Z7 . | . | . | Satlll . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | Normal cells . | Tumor cells . | . | Normal cells . | Tumor cells . | . | ||||
| Patient . | chr1* . | chr1* . | der . | chr1* . | chr1* . | der . | ||||
| 1 | ND | 124.9 (18.5) | 3.8 (2.6) | ND | 166.6 (64.2) | 168.2 (59.5) | ||||
| 2 | ND | 80.8 (9.5) | 17.5 (5.5) | ND | 50.2 (17.4) | 48.9 (11.5) | ||||
| 3 | 190.0 (27.3) | 188.3 (24.5) | 37.9 (10.7) | 57.9 (8.8) | 57.2 (10.8) | 56.0 (10.3) | ||||
| 4 | ND | 127.7 (17.6) | 15.9 (7.9) | ND | 41.3 (8.7) | 41.8 (10.2) | ||||
| 5 | ND | 128.0 (19.1) | 7.0 (4.7) | ND | 68.7 (13.3) | 68.3 (12.6) | ||||
| 6 | 68.5 (9.2) | 64.3 (7.8) | 5.7 (3.3) | 56.3 (9.0) | 51.5 (8.4) | 53.7 (11.4) | ||||
| 7 | 163.4 (24.5) | 158.2 (39.0) | 28.8 (14.7) | 46.7 (5.3) | 45.9 (9.2) | 46.1 (7.6) | ||||
| 8 | 225.5 (46.9) | 233.8 (57.2) | 104.9 (37.0) | 129.9 (23.4) | 131.9 (25.4) | 130.1 (22.0) | ||||
| 9 | ND | 75.6 (8.3) | 43.5 (7.0) | ND | 65.0 (4.8) | 69.0 (6.3) | ||||
| 10 | ND | 191.6 (41.2) | 31.3 (12.9) | ND | 48.1 (6.6) | 48.9 (7.7) | ||||
| 11 | ND | 64.3 (9.6) | 37.4 (8.9) | ND | 266.5 (59) | 265.6 (52.2) | ||||
| 12 | 124.7 (14.8) | 122.2 (14.5) | 15.6 (4.2) | 52.3 (6.5) | 49.5 (6.5) | 49.0 (8.7) | ||||
| 13 | 152.6 (15.8) | 149.2 (12.4) | 51.7 (9.3) | 171.5 (24.4) | 174.1 (30.6) | 176.1 (30.5) | ||||
| 14 | ND | 140.2 (13.1) | 50.7 (7.7) | ND | 21.2 (4.6) | 19.3 (7.1) | ||||
| 15 | ND | 149.5 (13.3) | 56.6 (9.7) | ND | 269.3 (43.3) | 270.1 (38.0) | ||||
| 16 | 78.2 (6.1) | 78.1 (8.4) | 33.3 (8.3) | 167.5 (11.6) | 169.3 (18.5) | 170.4 (18.1) | ||||
| 17 | 130.8 (9.5) | 129.2 (9.8) | 8.4 (2.7) | 75.2 (6.5) | 72.1 (7.1) | 72.1 (6.8) | ||||
| 18 | ND | 70.7 (7.1) | 10.2 (7.0) | ND | 63.2 (8.2) | 63.2 (8.0) | ||||
| 19 | ND | 149.7 (14.9) | 24.8 (6.6) | ND | 60.9 (10.3) | 62.1 (9.8) | ||||
| 20 | 64.7 (9.1) | 65.3 (6.5) | 33.4 (3.7) | 45.3 (7.0) | 43.4 (6.5) | 43.1 (8.4) | ||||
| 21 | 153.6 (8.7) | 158.5 (18.1) | 69.1 (10.0) | 51.7 (4.2) | 50.8 (10.0) | 50.8 (9.3) | ||||
| 22 | 149.4 (10.8) | 143.4 (19.0) | 31.3 (12.6) | 20.8 (2.8) | 21.7 (3.6) | 20.6 (4.7) | ||||
| 23 | 185.0 (10.7) | 179.0 (17.5) | 45.3 (13.9) | 138.9 (6.5) | 137.1 (13.1) | 136.7 (17.1) | ||||
| 24 | 137.4 (6.1) | 142.8 (13.0) | 58.5 (14.2) | 34.9 (2.8) | 35.2 (4.4) | 35.8 (6.3) | ||||
| 25 | ND | 160.5 (16.0) | 51.6 (7.9) | ND | 52.9 (7.5) | 52.6 (6.6) | ||||
| 26 | ND | 192.9 (8.7) | 22.7 (6.0) | ND | 22.0 (1.8) | 22.7 (2.7) | ||||
| 27 | 52.9 (4.9) | 53.8 (5.5) | 0 | 45.7 (3.1) | 45.1 (6.1) | 47.4 (6.2) | ||||
The allelic equivalent of the derivative chromosome for chr1 was determined by comparing the relative intensities of Satlll signals within normal and abnormal metaphases. Then the intensities of D1Z7 and Satlll on the derivative chromosome and its equivalent normal chr1 were expressed as relative values to those on their homologous chr1, where the latter were set to 100. The mean values calculated from 30 measurements for normal and abnormal metaphases are presented, and the standard deviations are given in parentheses.
der indicates the derivative chromosome; and chr1*, the normal chr1 from which the derivative chromosome was generated. ND indicates that there is either no detectable normal metaphase in the samples or that the normal metaphases are too little to get a reliable statistical estimation.
Relative intensities of D7Z1 and D7Z2 signals on the derivative chromosome and its allelic equivalent
. | D7Z1 . | . | D7Z2 . | . | ||
|---|---|---|---|---|---|---|
| Patient . | norm . | der . | norm . | der . | ||
| 3 | 130.2 (11.3) | 72.2 (9.1) | 283.0 (40.4) | 277.5 (38.5) | ||
| 6 | 303.7 (9.2) | 182.4 (19.3) | 69.7 (8.3) | 72.1 (8.2) | ||
| 7 | 133.0 (14.7) | 46.4 (8.4) | 49.5 (6.9) | 51.8 (7.5) | ||
| 8 | 115.0 (11.1) | 88.6 (5.9) | 48.0 (15.1) | 48.6 (9.3) | ||
| 12 | 80.2 (9.0) | 55.4 (8.7) | 364.5 (74.8) | 364.2 (70.4) | ||
| 13 | 222.6 (16.6) | 168.8 (16.5) | 245.1 (23.0) | 254.3 (29.2) | ||
| 16 | 293.3 (25.1) | 144.6 (8.3) | 293.0 (30.3) | 290.3 (25.0) | ||
| 17 | 231.4 (22.0) | 210.2 (8.6) | 233.0 (19.4) | 246.1 (16.0) | ||
| 20 | 220.7 (15.3) | 133.3 (4.0) | 64.4 (4.0) | 62.8 (2.0) | ||
| 21 | 225.5 (18.9) | 103.1 (12.3) | 177.0 (8.7) | 173.1 (14.8) | ||
| 22 | 76.3 (4.0) | 61.8 (1.6) | 181.9 (16.9) | 182.3 (9.7) | ||
| 23 | 246.8 (22.1) | 118.7 (6.7) | 55.2 (4.3) | 54.8 (5.5) | ||
| 24 | 144.2 (5.1) | 84.1 (4.0) | 74.3 (3.8) | 75.5 (2.0) | ||
| 27 | 50.1 (4.6) | 49.3 (4.7) | 172.3 (19.8) | 179.6 (27.8) | ||
. | D7Z1 . | . | D7Z2 . | . | ||
|---|---|---|---|---|---|---|
| Patient . | norm . | der . | norm . | der . | ||
| 3 | 130.2 (11.3) | 72.2 (9.1) | 283.0 (40.4) | 277.5 (38.5) | ||
| 6 | 303.7 (9.2) | 182.4 (19.3) | 69.7 (8.3) | 72.1 (8.2) | ||
| 7 | 133.0 (14.7) | 46.4 (8.4) | 49.5 (6.9) | 51.8 (7.5) | ||
| 8 | 115.0 (11.1) | 88.6 (5.9) | 48.0 (15.1) | 48.6 (9.3) | ||
| 12 | 80.2 (9.0) | 55.4 (8.7) | 364.5 (74.8) | 364.2 (70.4) | ||
| 13 | 222.6 (16.6) | 168.8 (16.5) | 245.1 (23.0) | 254.3 (29.2) | ||
| 16 | 293.3 (25.1) | 144.6 (8.3) | 293.0 (30.3) | 290.3 (25.0) | ||
| 17 | 231.4 (22.0) | 210.2 (8.6) | 233.0 (19.4) | 246.1 (16.0) | ||
| 20 | 220.7 (15.3) | 133.3 (4.0) | 64.4 (4.0) | 62.8 (2.0) | ||
| 21 | 225.5 (18.9) | 103.1 (12.3) | 177.0 (8.7) | 173.1 (14.8) | ||
| 22 | 76.3 (4.0) | 61.8 (1.6) | 181.9 (16.9) | 182.3 (9.7) | ||
| 23 | 246.8 (22.1) | 118.7 (6.7) | 55.2 (4.3) | 54.8 (5.5) | ||
| 24 | 144.2 (5.1) | 84.1 (4.0) | 74.3 (3.8) | 75.5 (2.0) | ||
| 27 | 50.1 (4.6) | 49.3 (4.7) | 172.3 (19.8) | 179.6 (27.8) | ||
The allelic equivalent of the derivative chromosome for chr7 was determined by comparing the relative intensities of D7Z2 signals within normal and abnormal metaphases. Then the intensities of D7Z1 and D7Z2 on the derivative chromosome and its equivalent normal chr7 were expressed as relative values to those on their homologous chr7, where the latter were set to 100. The mean values calculated from 30 measurements for normal and abnormal metaphases are presented, and the standard deviations are given in parentheses. We were able to obtain the information regarding the signal reduction of D7Z1 from only 14 of the 27 patients because in the remaining cases, there were either no normal metaphases in their samples or the numbers of normal metaphase were too small to make a reliable statistical assessment.
der indicates the derivative chromosome; and norm, the normal chr7 from which the derivative chromosome was generated.
Reduction of the D1Z7 and D7Z1 signal intensities on the derivative chromosome
Comparison of the D1Z7 signals based on the SatIII marker showed that the signal intensity of D1Z7 on the derivative chromosome was clearly reduced from that of the normal counterpart to the varying extent in all but one (patient [Pt] 27) of the 27 samples examined (the ratios of D1Z7 and SatIII signals are summarized in Table 2). Even when the D1Z7 signal on one of the normal chr1s seemed weaker than that on the derivative chromosome, the signal reduction in the latter could also be detected when compared properly with its authentic normal counterpart (Figure 2N). In the same manner the reduction of the D7Z1 signal on the derivative chromosome was also demonstrated, except for Pt 27, using D7Z2 as an allelic reference, though the comparison was more complicated and, in principle, only possible for those samples that contain normal metaphases (Table 3). These observations strongly suggest that the breakpoints of der(1;7)(q10:p10) are located within the D1Z7 alphoid cluster on chr1 and D7Z1 on chr7.
FISH analysis of D1Z5 alphoid clusters
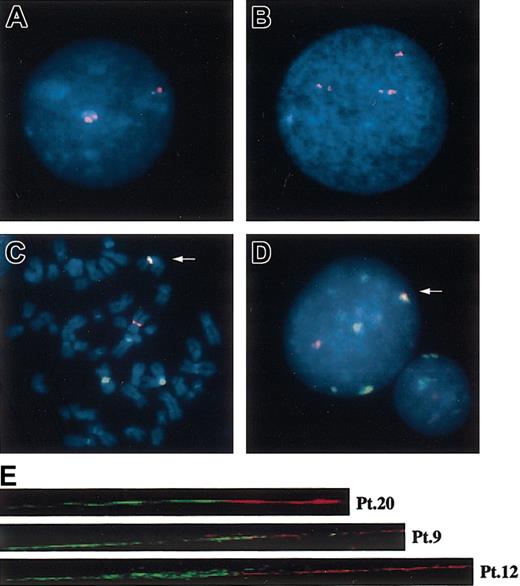
D1Z5 is another kind of alphoid on the centromere of chr1 and is clustered on both sides of the D1Z7 alphoid.17,32 Results from interphase FISH and fiber FISH experiments suggest that there seems to be no intervening sequences between these alphoids.17 When the interphase nuclei from normal samples were hybridized with a D1Z5 probe (CEP1), the 2 separate clusters of D1Z5 could be clearly recognized as a pair of doublet signals with different signal intensities (Figure 3A). In contrast, in all other tumor samples except for Pt 27, in addition to the 2 pairs of the doublet signals corresponding to the 2 normal chr1s, there was another signal having the intensity comparable to that of the larger signal in one of the 2 doublets (Figure 3B). This indicated that the smaller D1Z5 cluster was lost on the derivative chromosome and that the breakpoint of der(1;7)(q10.p10) exists between the 2 D1Z5 clusters, most likely within the D1Z7 cluster.
FISH analysis with the patients using various centromeric alphoid probes. Interphase FISH with a D1Z5 probe, CEP1, in normal (A) and abnormal (B) cells. Two-color FISH with pE25.a (green) and D7Z16mer (red) with tumor cells, showing almost completely overlapped signals (arrows) on metaphase (C) and interphase (D) nuclei. Original magnification, × 1000. (E) Fiber FISH analysis of der(1;7)(q10;p10) using a D1Z7 probe, CEP1/5 (red), and a D7Z1 probe, CEP7 (green), visualizing direct connection of both alphoids on the same DNA fiber.
FISH analysis with the patients using various centromeric alphoid probes. Interphase FISH with a D1Z5 probe, CEP1, in normal (A) and abnormal (B) cells. Two-color FISH with pE25.a (green) and D7Z16mer (red) with tumor cells, showing almost completely overlapped signals (arrows) on metaphase (C) and interphase (D) nuclei. Original magnification, × 1000. (E) Fiber FISH analysis of der(1;7)(q10;p10) using a D1Z7 probe, CEP1/5 (red), and a D7Z1 probe, CEP7 (green), visualizing direct connection of both alphoids on the same DNA fiber.
Breakpoint in Pt 27
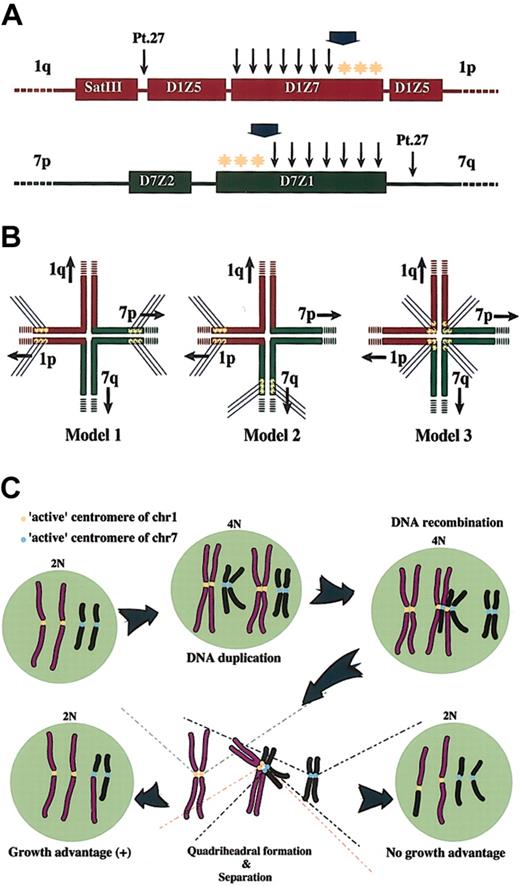
Pt 27 was considered an exceptional case because no visible D1Z7 or D1Z5 signal was detected on the derivative chromosome, whereas the D7Z1 and SatIII signals seemed completely preserved (Tables 2-3; data not shown). This suggested that, in this particular case, the breakpoint should be mapped between the larger cluster of D1Z5 and SatIII on chr1 and between D7Z1 and the 7q tail on chr7 (Figure 4A).
Breakpoint mapping in der(1;7)(q10;p10) and proposed mechanism generating this translocation. (A) Breakpoints are widely distributed within D1Z7 and D7Z1. The ends to the short arm within both alphoids (*) are free from recombinations. A broad arrow indicates the hypothetical critical point on each alphoid. A recombination that occurs beyond this point might compromise segregation of recombined chromosomes and eventually result in their loss. Thin arrows represent the locations of the breakpoints; yellow stars indicate the hypothetic active centromere. (B) Three possible models for the quadriheadral formation with relation to relative location of active centromeres to the breakpoint. Active centromeres may be either contralaterally (Model 1), ipsilaterally (Model 2), or centrally (Model 3) positioned. For the sake of stable maintenance of sister chromatids, the contralateral model might be favored, and only this seems to be compatible with the real distribution of the breakpoints and with allelic distribution in this unbalanced translocation. (C) Proposed mechanism of generation of 46, XY (or XX), +1, der(1;7)(q10;p10). Active centromere sequences on chr1 and chr7 are indicated as yellow and blue circles, respectively. For simplicity, some features of chromosomes are not always as they really are. For example, sister chromatids are depicted separately from before recombination, which should be tightly paired and stuck to each other through cohesion molecules.
Breakpoint mapping in der(1;7)(q10;p10) and proposed mechanism generating this translocation. (A) Breakpoints are widely distributed within D1Z7 and D7Z1. The ends to the short arm within both alphoids (*) are free from recombinations. A broad arrow indicates the hypothetical critical point on each alphoid. A recombination that occurs beyond this point might compromise segregation of recombined chromosomes and eventually result in their loss. Thin arrows represent the locations of the breakpoints; yellow stars indicate the hypothetic active centromere. (B) Three possible models for the quadriheadral formation with relation to relative location of active centromeres to the breakpoint. Active centromeres may be either contralaterally (Model 1), ipsilaterally (Model 2), or centrally (Model 3) positioned. For the sake of stable maintenance of sister chromatids, the contralateral model might be favored, and only this seems to be compatible with the real distribution of the breakpoints and with allelic distribution in this unbalanced translocation. (C) Proposed mechanism of generation of 46, XY (or XX), +1, der(1;7)(q10;p10). Active centromere sequences on chr1 and chr7 are indicated as yellow and blue circles, respectively. For simplicity, some features of chromosomes are not always as they really are. For example, sister chromatids are depicted separately from before recombination, which should be tightly paired and stuck to each other through cohesion molecules.
Interphase and fiber FISH analysis
As expected from these results, pE25.a (D1Z7) and D7Z16mer (D7Z1) signals were observed largely overlapped on metaphase chromosomes as well as in interphase nuclei of der(1;7)(q10;p10) samples in dual-color FISH experiments (Figure 3C-D). To improve the FISH resolution and to further confirm the direct recombination between D1Z7 and D7Z1 in der(1;7)(q10;p10), we performed fiber FISH analysis. As shown in Figure 3E, the D1Z7 (the red beads) and the D7Z1 (the green beads) signals were found directly connected on the same DNA fiber in 10 tumor samples examined (Pt 2-Pt 9, Pt 12, and Pt 20).
Distribution of the breakpoints within the alphoid regions
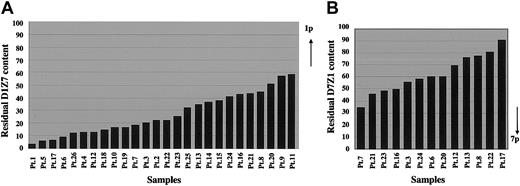
To investigate the characteristics of this translocation more in detail, we focused on the residual proportions of D1Z7 and D7Z1 signals on the derivative chromosome, which could provide a gross estimation for the relative locations of the breakpoints within each cluster. As summarized in Figure 5, the relative reduction in D1Z7 and D7Z1 sequences on the derivative chromosome was different from patient to patient. Considering each alphoid cluster is estimated to be several megabase pairs in length, our results suggest that the breakpoints on chr1 and chr7 are not clustered but widely distributed within the D1Z7 and D7Z1 sequences among these patients.
Relative reduction of D1Z7 and D7Z1 contents on the derivative chromosome in different samples. The remaining proportions of D1Z7 (A) and D7Z1 (B) alphoids are depicted based on the measurements of FISH signals. They show great variations from patient to patient, indicating the wide distribution of the breakpoints within each alphoid in der(1;7)(q10;p10). Note that the extreme ends to the short arm within both alphoids are retained, and therefore are devoid of breakpoints. As indicated by the arrows, the 1p portion of D1Z7 and the 7q portion of D7Z1 are lost on the derivative chromosome.
Relative reduction of D1Z7 and D7Z1 contents on the derivative chromosome in different samples. The remaining proportions of D1Z7 (A) and D7Z1 (B) alphoids are depicted based on the measurements of FISH signals. They show great variations from patient to patient, indicating the wide distribution of the breakpoints within each alphoid in der(1;7)(q10;p10). Note that the extreme ends to the short arm within both alphoids are retained, and therefore are devoid of breakpoints. As indicated by the arrows, the 1p portion of D1Z7 and the 7q portion of D7Z1 are lost on the derivative chromosome.
Similarity of the 2 involved alphoid subsets
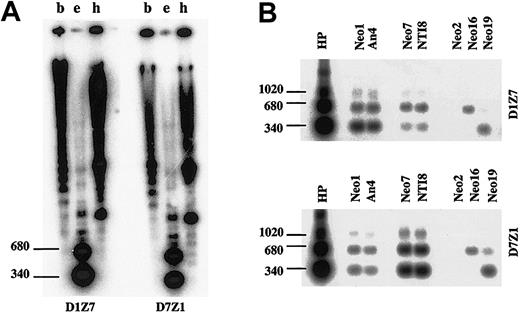
When hybridized to the human genomic DNA at high stringency, D1Z7 (pE25.a) and D7Z1 (D7Z16mer) produced almost an identical hybridization pattern (Figure 6A). To separately evaluate the specific hybridization of each probe to different homologous alphoids on different human chromosomes, the DNAs from a series of monochromosomal human-mouse hybrid cells were examined by Southern blot analysis using both probes. Under the same stringent condition, D1Z7 probe strongly hybridized to chr1 but also, to a lesser extent, to chr7, creating the similar hybridization pattern with 3 major bands of 340-bp dimer, 680-bp tetramer, and 1020-bp hexamer (Figure 6B). D7Z1 also created the similar hybridization pattern with stronger hybridization to chr7 than to chr1. While chr16 and chr19 contain alphoids of the same suprachromosomal family 1 as D1Z7 and D7Z1,20 the hybridization patterns in chr16 and chr19 were substantially different. No hybridization was detected in chr2, which has no suprachromosomal family 1 alphoid. These results indicated that D1Z7 and D7Z1 were more similar than other alphoids of suprachromosomal family 1, not only in their sequence contents but also in their higher-order array structures.
Similarity of the 2 involved alphoid subsets. Southern blot analysis of D1Z7 (pE25.a) and D7Z1 (D7Z16mer) alphoids in total human genome (A) digested with BamHI (b), EcoRI (e), and HindIII (h), as well as in DNAs from human monochromosomal mouse hybrid cells containing chromosome 1 (Neo1 and An4), 7 (Neo7 and NTI8), 2 (Neo2), 16 (Neo16), or 19 (Neo19) digested with EcoRI (B). Locations of dimmer (340 bp), tetramer (680 bp), and hexamer (1020 bp) are indicated to the left. D1Z7 and D7Z1 created a similar hybridization pattern with some cross hybridization with each other.
Similarity of the 2 involved alphoid subsets. Southern blot analysis of D1Z7 (pE25.a) and D7Z1 (D7Z16mer) alphoids in total human genome (A) digested with BamHI (b), EcoRI (e), and HindIII (h), as well as in DNAs from human monochromosomal mouse hybrid cells containing chromosome 1 (Neo1 and An4), 7 (Neo7 and NTI8), 2 (Neo2), 16 (Neo16), or 19 (Neo19) digested with EcoRI (B). Locations of dimmer (340 bp), tetramer (680 bp), and hexamer (1020 bp) are indicated to the left. D1Z7 and D7Z1 created a similar hybridization pattern with some cross hybridization with each other.
Discussion
On exploring the centromeric fusion in this unbalanced translocation, the conventional methods for identification of chromosomal breakpoints, such as Southern blot analysis, were not available due to the highly repetitive and polymorphic features of the pericentric sequences. Nevertheless, it was these features that allowed us the other approach to molecularly delineate the structure of der(1;7)(q10; p10); variations of the large cluster lengths of the involved alphoid sequences can be easily detected and compared on an allelic basis by measuring the intensity of polymorphic satellite signals. The reproducibility of our signal measurements was satisfactory enough to get a reliable estimation on the alterations of the signal intensity. Although the relationship between the intensity of FISH signals and the alphoid length might not be linear, it was still expected to be monotonic. Given this monotonic relationship, we could grossly estimate the relative reduction in the length of both involved alphoid clusters. According to this estimation, the proportion of shortening of each alphoid sequence is highly variable, indicating that the breakpoints of der(1;7)(q10;p10) translocation were widely distributed within D1Z7 on chr1 and D7Z1 on chr7, although they seemed to spare the extreme end to the short arm within both alphoids (Figure 5). Therefore, it seems unlikely that there exists a unique gene target at or near those breakpoints, and the leukemogenic potential of this translocation may well be ascribed to altered gene dosages resulting from trisomy 1q and/or monosomy 7q, well-known chromosomal abnormalities found in MDS/AML (7q-) as well as many solid cancers (+1q), even though the critical gene targets for these deletion and duplication remain to be unveiled.
FISH analysis in this study also provided additional information as to the structure of the alphoids on chr1, their order being 1p-D1Z5 (smaller cluster)-D1Z7-D1Z5 (larger cluster)-1q. Of interest is that this translocation generates a derivative chromosome that contains 4 alphoid subsets at its centromeric region, 2 from chr1 and the other 2 from chr7 (mapped as 7p-D7Z2-partial D7Z1-partial D1Z7-the larger cluster of D1Z5-1q). This is the first report in the literature that 4 alphoid subsets coexist on a single centromere. Further investigations will be required to understand how this multi-alphoid centromere can function and be maintained in eukaryotic cells.
With regard to the exact sequences that participate in this centromeric fusion, our fiber FISH results strongly indicated that D1Z7 and D7Z1 were directly involved in the DNA recombination. This is also supported by the fact that the centromere alphoid clusters are highly ordered tandem arrays without interruptions by other elements19,32,33 except at their marginal regions of the cluster.34,35 In addition, both alphoids have extremely high structural homology, which is estimated to be about 90% at a unit component level. Both are composed of dimer and tetramer repeat units, each of which is defined by EcoRI sites. This extreme similarity in their higher-order array structures was also confirmed by our Southern blot experiment (Figure 6). These particular similarities in the unit component as well as higher-order structures seem to make both alphoids especially prone to be recombined to each other.
Although the exact mechanism through which this alphoid recombination takes place is still unclear, we may postulate that it is mediated by an error that occurred during DNA repair processes because, clinically, der(1;7)(q10;p10) has been closely associated with secondary MDS/AML that arises after heavy doses of chemotherapy, especially of alkylating agents, the well-known antitumor drugs that cause DNA double-strand breaks (DSBs).36,37 As the excessive accumulation of DSBs imposed on either D1Z7 or D7Z1, cells would recruit the repair processes, which are thought to erroneously mediate recombination between these highly homologous alphoid sequences. Given that the initiating DSBs occur randomly, it might be expected that the chromosome having a larger centromere alphoid content would be involved more frequently. In fact, in our case series, the der(1;7) tended to be originated from the homologous chromosome with a larger alphoid content. Participation of the chromosome allele with a larger amount of D1Z7 and D7Z1 was observed in 19 of 26 and 11 of 13 samples, respectively.
From a cytogenetic point of view, der(1;7)(q10;p10) has a consistent feature in that it contains 2 apparently normal chr1s and only one copy of normal chr7, and our analysis has clearly demonstrated that the 2 apparently normal chr1s as well as the remaining chr7 and the 7p of the derivative chromosome in the tumor cells always have different allelic origins. To explain this prominent cytogenetic feature we could assume that the recombination occurs during or after DNA synthesis (Figure 4). In this model, a double-stranded DNA breakage taking place on one of the just-duplicated daughter chromatids of chr1 or chr7 evokes a recombination repair process, during which an error occurs to misconnect the D1Z7 and D7Z1 sequences and leads to the quadriradial formation involving the 2 pairs of daughter chromatids (Figure 4B-C). At this stage, there may be several possibilities as to the way of kinetocore formation and separation of duplicated chromatids, as shown in Figure 4B. This process (mitosis) is thought to involve a number of well-coordinated interactions of DNA and proteins, and, for the time being, we could not know its exact mechanism. However, the cytogenetic profile of the der(1; 7)(q10;p10) seems to be most simply explained by Model 1 shown in Figure 4B, in which “active” or “critical” centers of kinetocore formation on the replicated chr1 and chr7 are both polarized to the short arm of each chromosome. According to this model, the quadriradial daughter chromatids, also previously proposed by Morrison-DeLap et al,5 will be separated following the usual mechanism of mitosis. Of the 2 possible ways of chromatid distributions only one will result in the unbalanced translocation with uneven distribution of chromosomal materials, which might confer a growth advantage to the cells that inherit der(1;7)(q10; p10) (Figure 4C). Although in the budding yeast, only a 125-bp sequence ensures the complete centromere functions including kinetocore attachment, spindle formation, and successful chromatid separation, little has been known about the essential part of mammalian centromeres. In order to explain the unique karyotypic feature of der(1;7)(q10;p10), we hypothesize existence of the sequences related to this active centromere function and kinetocore formation to the centromere region polarized to the short arm of each chromosome. There is no definite evidence for this hypothesis but there are some rationales: first of all, this gives us the simplest explanation. Once the quadriradial structure is formed, the cytogenetic configuration of der(1;7)(q10;p10) under interest will be directly generated without involvement of any other abnormalities in mitotic machinery. Second, our model also holds true for Pt 27, an exceptional case in terms of the location of the breakpoints, because the quadriradial chromatids (Figure 4A-B, Model 1) in Pt 27 are expected to retain the whole of the D7Z1 and D7Z2 clusters on its 7p arm and the whole of the D1Z7 and D1Z5 clusters on its 1p arm. In this case we could more safely conclude that the active centromeres are localized to the 7p and 1p arms. Finally, as shown in Figure 5, the breakpoints strangely spare the short-arm ends of both D1Z7 and D7Z1, where we presumed the hypothetical active centromere elements are localized.
In conclusion, we disclosed the molecular characteristics of der(1;7)(q10:p10), one of the most common forms of chromosomal abnormalities found in secondary MDS/AML. It directly involves 2 centromere alphoids of its participating chromosomes, and the underlying mechanism that gives rise to this translocation seems to be closely related to the structural similarities of both alphoids and their association with centromere functions. It requires further investigation of human centromere structure and its functions to fully understand the entire pathogenesis, which may serve as prevention of this translocation with poor prognosis.
Prepublished online as Blood First Edition Paper, June 19, 2003; DOI 10.1182/blood-2003-01-0031.
Supported in part by Grant-in-Aid for Scientific Research (KAKENHI 13307029).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Dr Huntington F. Willard for providing us the chromosome 7 alpha satellite probes that were used to confirm the results in our paper.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal